Abstract
This microreview provides a compilation of synthetic approaches and total syntheses of pinnatoxin A in a survey of the literature up to early 2010. Pinnatoxin A is the first discovered and representative member of a fascinating group of potent marine toxins that share a spiroimine subunit as a unifying structural element.
Keywords: Natural products, Total synthesis, Marine toxins, Spiroimine natural products, Pinnatoxin, Spiro compounds
1. Introduction
Pinnatoxin A is an early member of the growing family of nonproteinaceous macrocyclic marine toxins, currently consisting of pinnatoxins (Figure 1), pteriatoxins (Figure 2), spirolides and gymnodimines (Figure 3), and spiro-prorocentrimine.[1] Numerous shellfish poisoning instances have been attributed to these bioactive alkaloids, which have established themselves as appealing synthetic targets. The producing organism responsible for the biogenesis of pinnatoxins is presently unknown, and continuing efforts are being devoted towards the synthesis of these compounds to provide viable access to the materials for biological testing.[1,7]
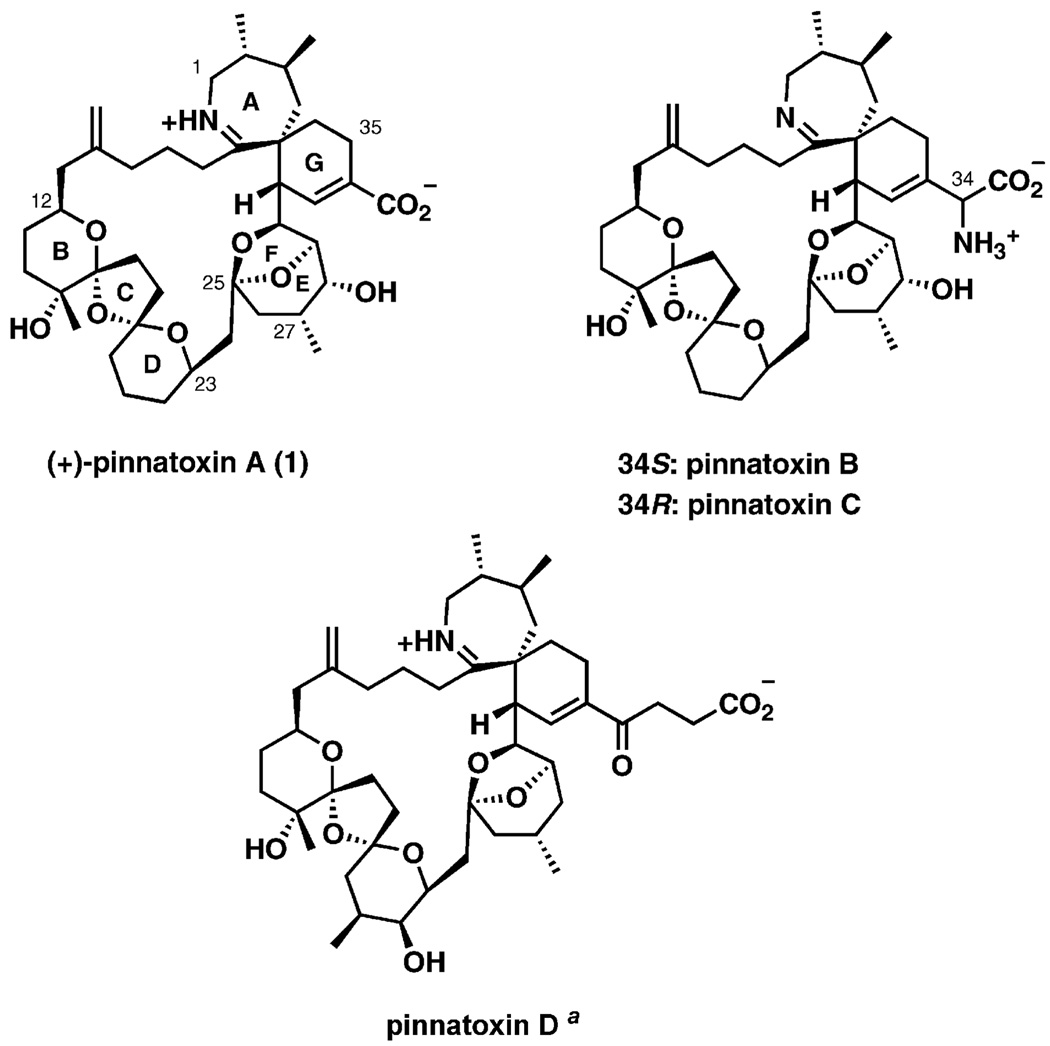
Figure 1.
Spiroimine marine toxins: pinnatoxins A–D. [a] The structure of pinnatoxin D given in the literature is inconsistent. Shown above is a structure based on the original proposal presented by Uemura[2] and confirmed by follow-up work from Kitching.[3]
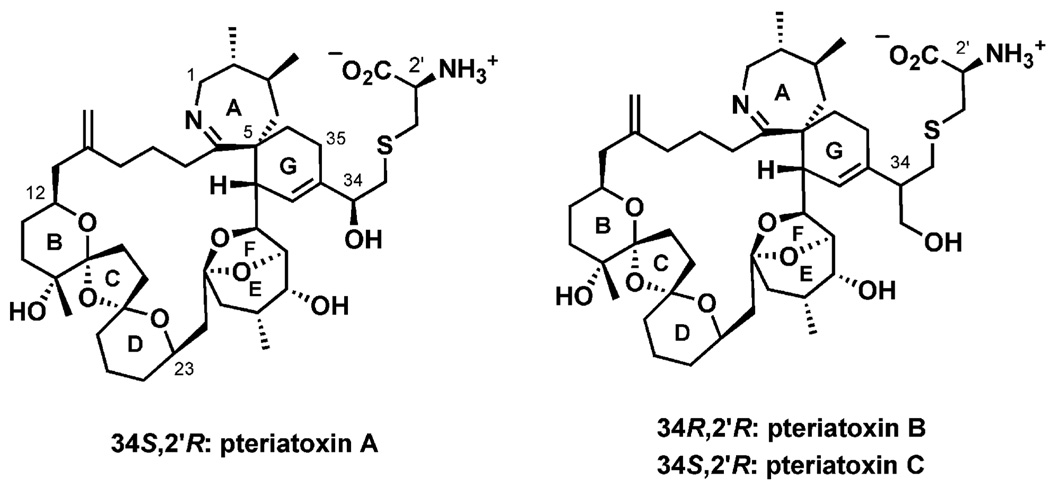
Figure 2.
Spiroimine marine toxins: pteriatoxins A–C.
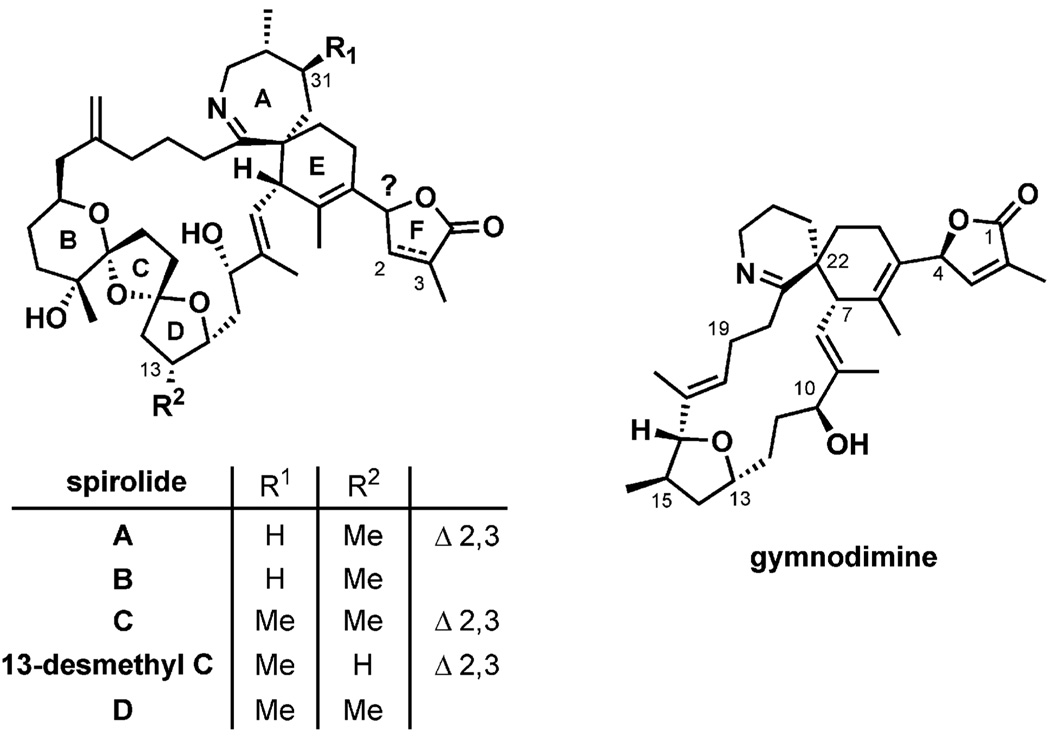
Figure 3.
Spiroimine marine toxins: spirolides A–D and gymnodimine.
Among anisomyarian clams, the bivalve family Pinnidea has received increased attention over the last two decades, being the culprit responsible for an outbreak of shellfish poisoning in China and Japan.[4] Subsequent investigation of the frequently consumed adductor muscle of the shellfish led to the isolation from Pinna attenuata of a bioactive extract described as pinnatoxin A, which is reported to be a Ca2+ channel activator.[4]
Pinna has a worldwide distribution, inhabiting shallow and warm tropical seas, although certain species are restricted to the Indo-Pacific region.[5] One of these species is Pinna muricata, viscera of which (45 kg) were collected in Okinawa, Japan, eventually leading to the isolation of pinnatoxins A (3.5 mg), B and C (1.2 mg as a mixture), and D (2.0 mg), along with the structural characterization of pinnatoxin A by detailed NMR and positive ion ESI MS/MS analysis.[6] The biological activities of the spiroimines, including pinnatoxins, have been briefly documented:[7] for pinnatoxins, in vitro toxicity to isolated cells was found to be low, whereas acute intraperitoneal toxicity in mice was found to be notable for a 1:1 mixture (as isolated) of pinnatoxins B and C (LD99 = 22 µgkg−1).[8] Similarly, natural (+)-pinnatoxin A is a rapidly acting toxin characterized by the rapid onset of symptoms closely followed by death (LD99 = 180 µgkg−1),[9] whereas (−)-pinnatoxin A was inactive in mouse assays in doses of up to 100 µg[28] (5000 µgkg−1 bodyweight).[7] Pinnatoxin D showed the weakest acute toxicity (LD99 = 400 µgkg−1), but the strongest cytotoxicity against the murine leukemia cell line P388 (IC50 = 2.5 µgmL−1).[1b]
Harmful algal blooms (HABs) may give rise to catastrophic fish deaths due to the toxic metabolites produced from phytoplankton;[10] of particular note are dinoflagellates of the genus Alexandrium, which have caused several cases of paralytic shellfish poisoning in humans.[11] Filter-feeding bivalves accumulate lethal doses of algal toxins after feeding on the toxic phytoplankton, and it is probable that pinnatoxin A found in Pinna muricata has its origin in a similar mechanism, although evidence for this is circumstantial.[12] It has been shown that the dinoflagellate Alexandrium ostenfeldii, collected from field samples of aquaculture sites in south-eastern Nova Scotia, Canada, is the producing organism for spirolides.[13] Spirolides, containing the macrocycle-nested spiroimine moiety, are close analogues of the pinnatoxins, and after studies of the hydrolytically labile spirolides A and B it has been suggested that the spiroimine subunit is the pharmacophore of the toxins.[14,15]
Continuing efforts focusing on the identification of toxins responsible for diarrhetic, paralytic, and neurotoxic shellfish poisoning (DSP, PSP, and NSP, respectively) have led to the characterization of other spiroimine marine toxins. In 1994, after unprecedented NSP coinciding with HABs of the dinoflagellate Gymnodinium cf. mikimotoi, gymnodimine was isolated from oysters (Tiostrea chilensis, 3 kg) collected at Foveaux Strait, South Island, New Zealand. Gymnodimine was also isolated from cultured G. cf. mikimotoi (60 L), thereby assigning its origin as a metabolite from the dinoflagellate that is likely to be in the oysters’ food chain.[16] Again, gymnodimine exhibited high toxicity by intraperitoneal injection in mice (LD50 = 96 µgkg−1).[17] More gymodimine analogues have been found subsequently, albeit in much smaller quantities.[18] In 2001, another spiroimine, spiro-prorocentrimine (LD99 = 2500 µgkg−1),[19] was isolated from a benthic Prorocentrum species from Taiwan. Spiro-prorocentrimine is closely related to the other nitrogenous polyether macrocycles prorocentrolide[20] and prorocentrolide B.[21] Although these polar, lipid-soluble metabolites have not been linked to human poisoning, benthic marine dinoflagellates in the genus Prorocentrum sp. algae are well known for the biogenesis of toxins such as okadaic acid and derivatives leading to cases of DSP.[22] In the same year, the extremely potent pteriatoxins, with macrocyclic cores identical to those of the pinnatoxins, were isolated from 75% aqueous ethanol extracts of viscera (82 kg) of another oyster: the Okinawan bivalve Pteria penguin.[23] Three isomers – pteriatoxin A (LD99 = 100 µgkg−1) and pteriatoxins B and C as a 1:1 mixture (LD99 = 8 µgkg−1) – were identified and their stereochemistry was designated after their synthesis.[24]
Pinnatoxins possess a common 27-membered carbocyclic backbone composed of a unique 6,7-azaspiro-linked imine fragment (AG ring), a bridged 5,6-bicycloketal (EF ring), and a 6,5,6-dispiroketal (BCD ring),[25] as well as varying functional group substitution at C21, C22, C28, and C33. Along with its isolation and structural characterization, Uemura proposed a biosynthetic pathway to pinnatoxin A through a Diels–Alder cycloaddition to install the G ring together with formation of the macrocycle. The polycyclic ether could thus arise from a linear polyketide. Feeding studies on Alexandrium ostenfeldii demonstrated that most of the carbons in the macrocycle of 13-desmethyl spirolide C are polyketide-derived and that a glycine unit is incorporated intact in the imine.[26]
This microreview documents the synthetic studies and total syntheses of pinnatoxin A, covering the literature up to early 2010. The account of the strategies employed to construct the characteristic spiroimine macrocycles begins with the first total synthesis of (−)-pinnatoxin A, the unnatural enantiomer, accomplished by Kishi’s group and continues in chronological order, describing contributions from the Murai, Inoue–Hirama, Zakarian, and Nakamura–Hashimoto groups.
2. Early Studies of Pinnatoxins
2.1 Kishi’s Synthesis of (−)-Pinnatoxin A (1998)
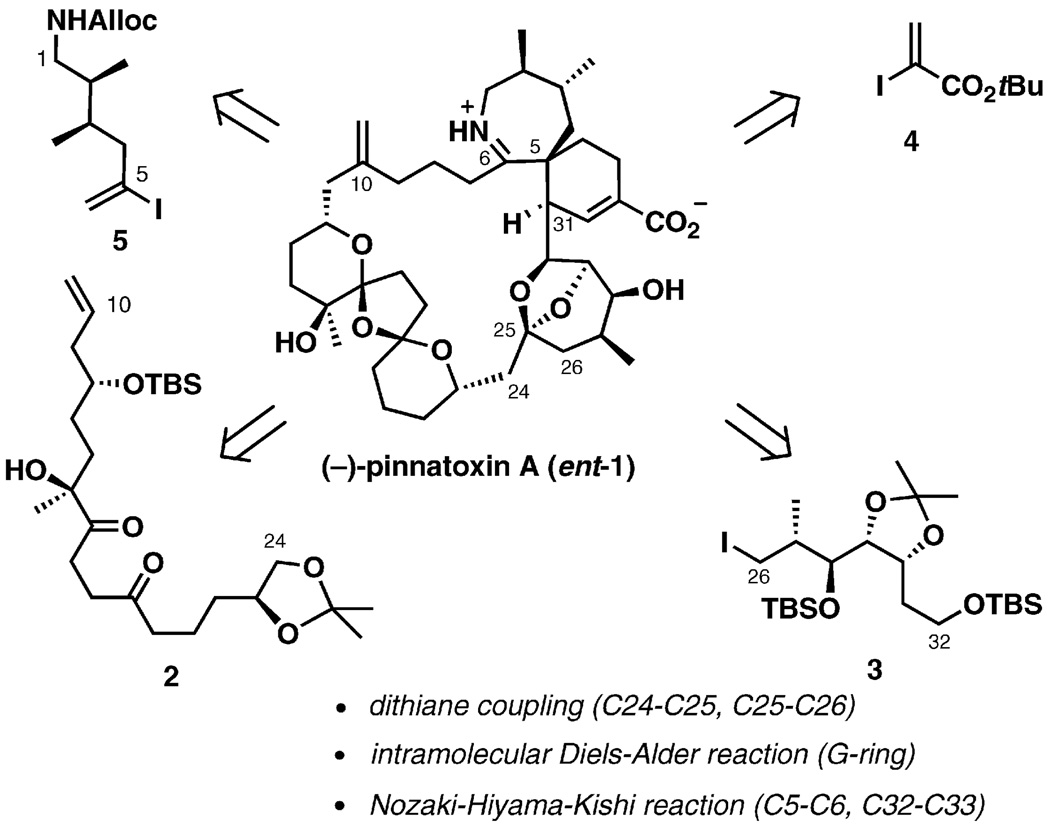
The biosynthetic pathway to pinnatoxin A proposed by Uemura served as the basis for the first total synthesis of the unnatural enantiomer of the toxin (ent-1), which established the absolute stereochemistry of 1.[28] The toxicity of the unnatural enantiomer was shown to be low to none. An extension of this synthesis has recently provided a complete stereochemical assignment for pinnatoxins B and C[27] and for the related pteriatoxins A–C.[24] The total synthesis of the unnatural enantiomer of pinnatoxin A by Kishi and coworkers set a precedent both for the power and for the limitations of the Diels–Alder approach to the cyclohexene ring of the macrocycle. Inspired by Uemura’s proposal, Kishi’s group envisioned a synthesis plan in which the AG-spiroimine ring system would be assembled through an intramolecular Diels–Alder reaction followed by imine formation (Scheme 1). It was intended that a dithiane-based coupling would form the C24–C25 and C25–C26 bonds and that the Nozaki–Hiyama–Kishi reaction would be employed twice to incorporate the fragments 4 and 5.
Scheme 1.
Synthesis plan for (−)-pinnatoxin A.
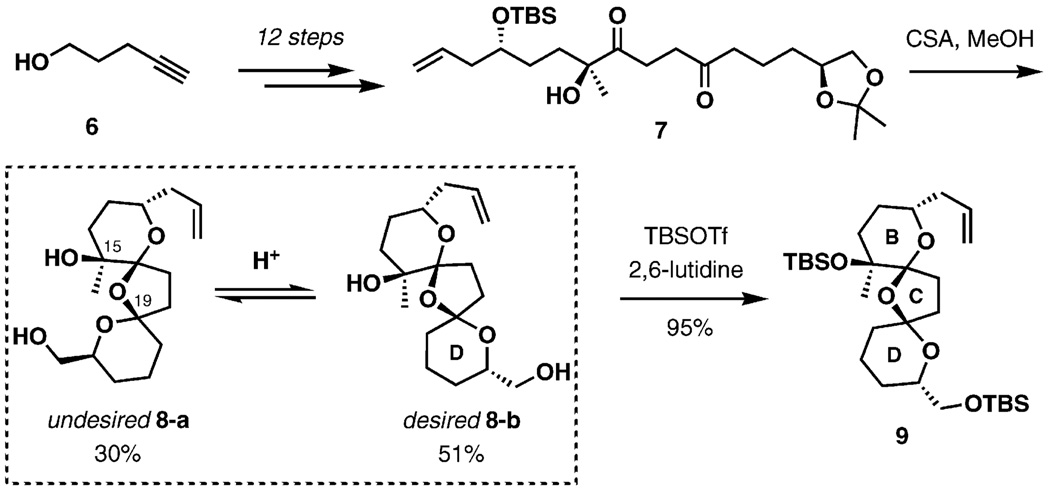
To begin the synthesis, the diketone 7 was efficiently prepared from pent-4-yn-1-ol in 12 steps (Scheme 2). Removal of the tert-butyldimethylsilyl (TBS) and acetonide groups in 7 with the aid of camphorsulfonic acid (CSA) yielded the desired tricyclic spiroacetal 8-b and its C19-epimer 8-a in 51% and 30% yields, respectively. As suggested previously by Murai and co-workers,[32] the formation of the C19-epimer was primarily due to hydrogen bonding between the tertiary hydroxy group and the D ring oxygen. Murai determined that acid-catalyzed spiroketalization of the elaborated C10–C24 fragment favored the undesired diastereomer at C19. Molecular mechanics calculations based on the AMBER force field indicate that the unnatural diastereomer, with the free hydroxy group positioned at C15, is 3.2 kcalmol−1 more stable than the natural isomer. This energy difference is minimized to 1.8 kcalmol−1 if the C15 hydroxy group is protected as its TBS ether. It has been demonstrated that the 8-a/8-b ratio is controlled by acid, solvent, and addition of metal ion effects. With this in mind, the epimers were consequently subjected to acidic equilibration conditions. Silylation of the tertiary hydroxy in the mixture of 8-a and 8-b with tert-butyldimethylsilyl triflate (TBSOTf) afforded the configurationally more stable 9 in 95% yield.
Scheme 2.
Synthesis of the BCD-spiroketal.
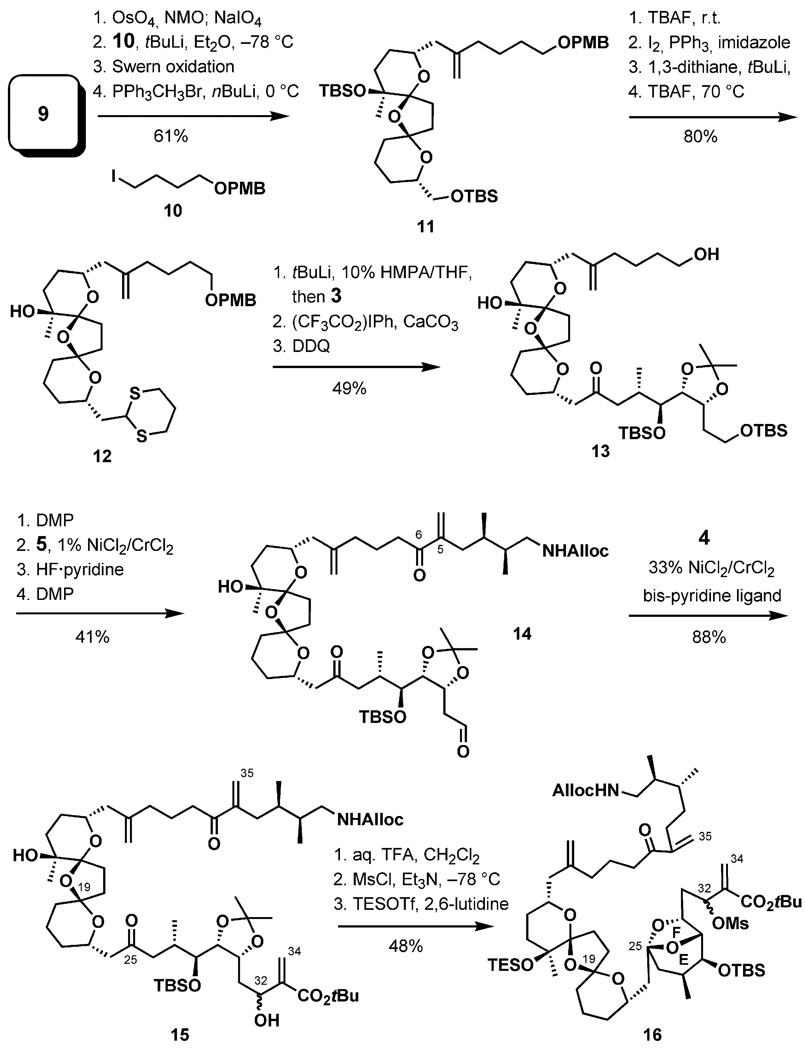
Conversion of the double bond in 9 to form the aldehyde, followed by four-carbon chain elongation and a Wittig olefination, delivered 11. The dithiane was installed in four steps to give 12 in 80% overall yield (Scheme 3). Fragment coupling was accomplished by alkylation of the lithiated dithiane 12 with the iodide 3, followed by two oxidative deprotection steps to deliver 13 in 49% yield over the three steps. After oxidation of the primary hydroxy group in 13 to the aldehyde, the termini of the bisketal fragment were modified by two successive Nozaki–Hiyama–Kishi reactions, first involving iodide 5 to forge the C5–C6 bond, and then iodide 3, to form the C32–C33 bond. Hydrolysis of the acetonide led to the formation of the EF-bridged ketal (16) and a C19-epimer. Fortunately, this position could be re-epimerized to the original configuration upon silylation with triethylsilyl trifluoromethanesulfonate (TESOTf).
Scheme 3.
Synthesis of the substrate for the intramolecular Diels–Alder reaction.
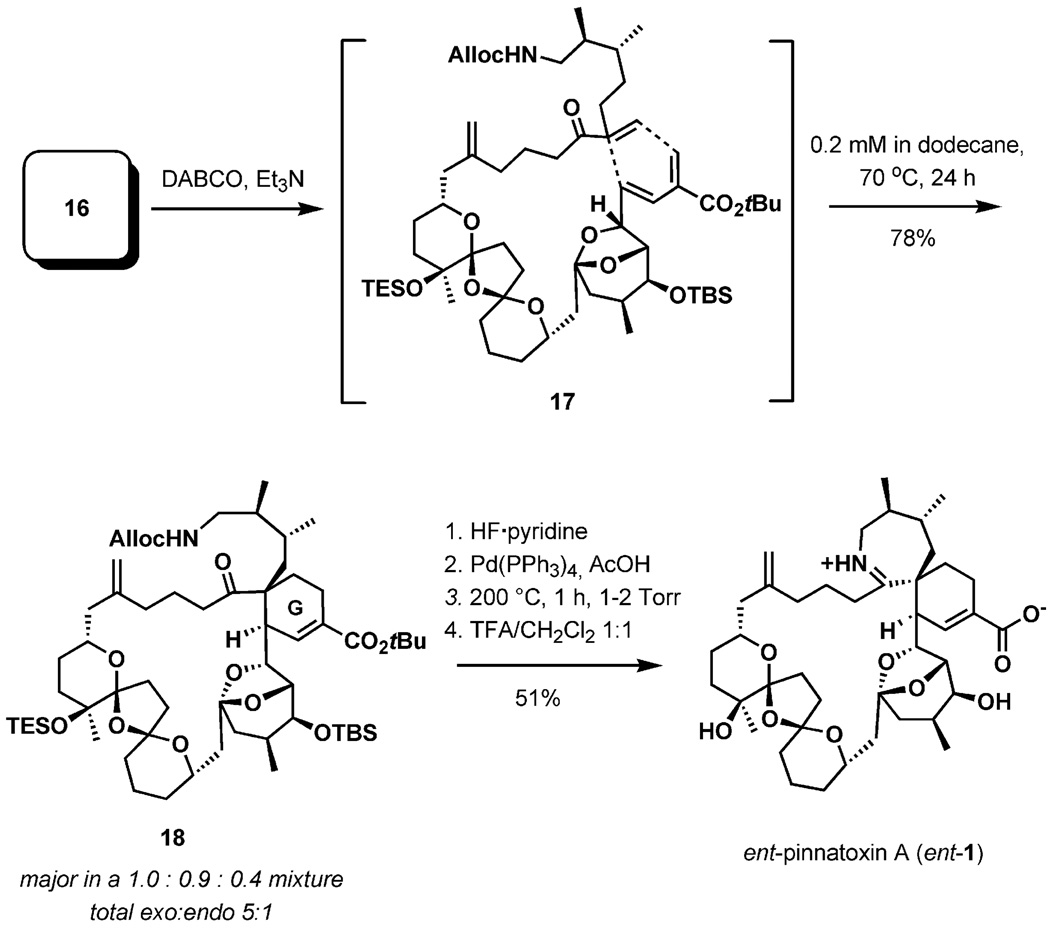
SN2′ displacement of the C32 allylic mesylate and removal of the C31 proton afforded the key diene for the intramolecular Diels–Alder reaction (Scheme 4). The results of optimization experiments concluded that the exo/endo ratio could be enhanced by adjustment of the solvent and temperature. The regioselective reaction thus proceeded at a temperature of 70 °C in dodecane (0.2 mm) to afford a 1.0:0.9:0.4 ratio of Diels–Alder adducts with an overall 5:1 exo/endo ratio.
Scheme 4.
Endgame.
Perhaps the most intriguing discovery of this total synthesis is that the final imine ring closure of the amino ketone required a strong thermal activation (200 °C, 1 h, 70% yield, Scheme 4). This observation, in conjunction with the hydrolytic stability of pinnatoxin A, implies that there is a large energy barrier for the interconversion of the cyclic imine to the amino ketone. In the final step, acidic treatment of the tert-butyl ester formed synthetic (−)-pinnatoxin A in 70% yield, establishing the absolute stereochemistry of natural (+)-pinnatoxin A (1).
2.2 Synthetic Studies on Pinnatoxins by Murai’s Group (1997–2002)
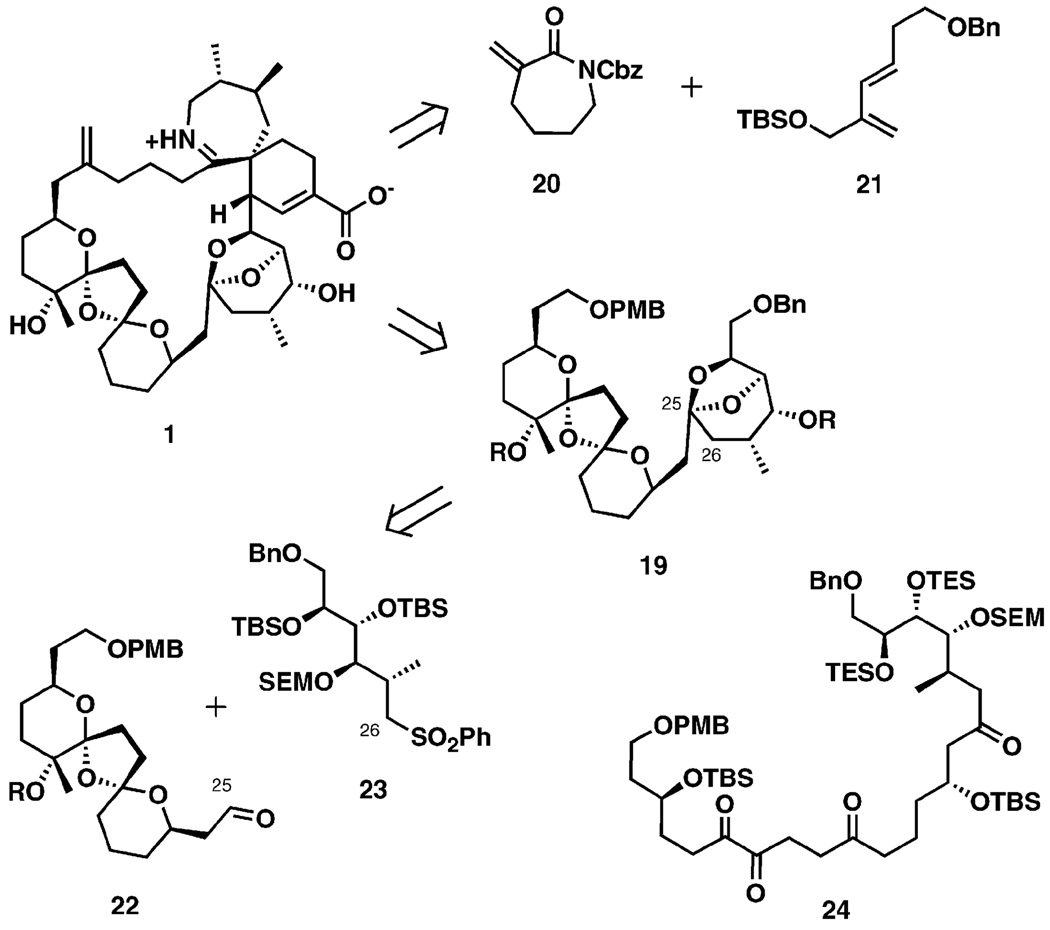
Murai and co-workers developed a convergent approach to the BCDEF ring polyether fragment (Scheme 5). The C25–C26 bond was to be constructed through a Julia coupling reaction joining fragments 22 and 23, with most of the chiral centers installed through Sharpless asymmetric epoxidation (AE).[29a] In an optimized approach, a one-step formation of a pentacyclic system related to 19 from the triketone 24 was later developed.[29b] Subsequently, enantioselective Diels–Alder cyclization chemistry between the α-methylene caprolactam 20 and the functionalized diene 21 was investigated as an approach to the spiroimine ring system.[29d]
Scheme 5.
Target fragments of pinnatoxins.
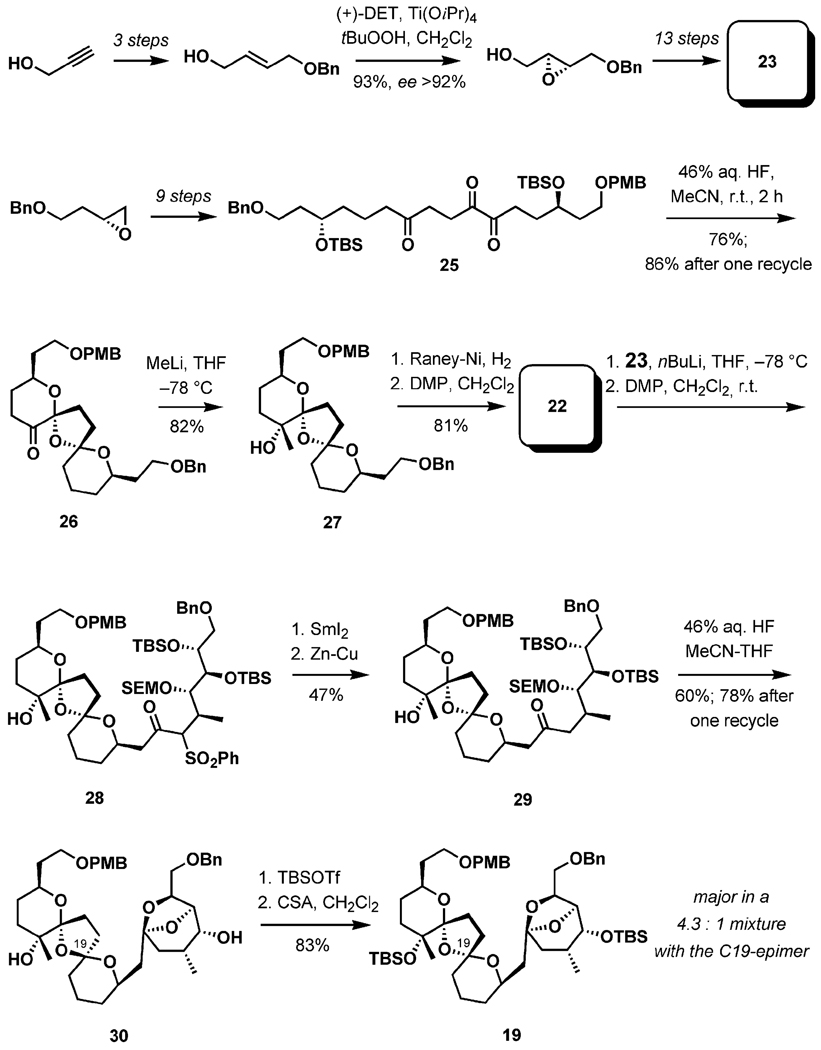
In initial studies, the sulfone 23 could be accessed in a straightforward 17-step sequence from propargyl alcohol (Scheme 6).[29b] Two consecutive Sharpless AE reactions, followed by regioselective reductive opening of the epoxides, efficiently installed the hydroxylated stereogenic centers. The bis-ketal 22 was prepared by conversion of (R)-2-[2-(benzyloxy)ethyl]oxirane into the triketone 25 by a nine-step process. Removal of silyl ethers with 46% aqueous HF in acetonitrile resulted in a stereoselective ketalization, giving 26 as a major product in 76% yield along with a number of isomeric and partially cyclized byproducts that could be recycled. Addition of methyllithium was also selective, and the aldehyde 22 was accessed in two additional steps. Julia coupling followed by a two-step reductive desulfonation gave 29. Upon ketalization to the EF ketal, complete C19 epimerization was observed during desilylation with HF in MeCN/THF; however, the stereochemistry could be corrected under acidic conditions after the installation of TBS ethers at the tertiary and secondary hydroxy groups, completing the synthesis of the BCDEF-pentacyclic triketal 19.
Scheme 6.
Spiroketalization.
Because both ketal fragments (BCD- and EF-) could be procured under similar conditions, a more convenient method for the formation of two intramolecular acetals was elucidated in 2001.[29c] The BCD-tricyclic spiroketal and the EF-bicyclic ketal were constructed simultaneously from the tetraketo precursor. As shown in Scheme 7, a Julia coupling between the sulfone 31 and the aldehyde 32, followed by desulfonation and oxidation, generated the ketone 33, which was in turn coupled with the sulfone 23 in a similar fashion. A perruthenate-mediated oxidation of the alkyne yielded the tetraketo precursor 24. Upon exposure to HF·pyridine in MeCN, the desired pentacyclic system was produced as a single isomer in 71% yield (83% after recycling of partially cyclized byproducts). With THF as a solvent, however, a 1:2:1 (31-a/31-b/31-c) mixture of the isomers was produced. The authors interpreted the substantial difference in reactivity by invoking kinetic control in THF, whereas thermodynamic control was observed with the reaction in acetonitrile.[30]
Scheme 7.
Synthesis of the BCDEF ring.
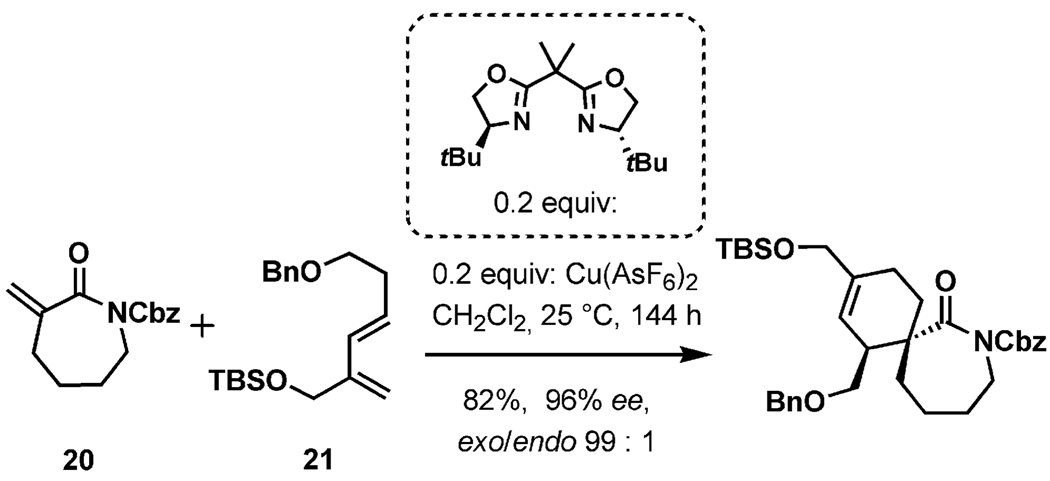
In 2002, Murai described an approach to the simplified spiroimine ring system of pinnatoxins.[29d] As illustrated in Scheme 8, the fragment could be generated by an asymmetric exo-selective Diels–Alder reaction between the α-methylene caprolactam 20 and the diene 21 in the presence of a chiral complex of CuII as a Lewis acid. Under optimized conditions, the spirocyclic adduct was generated in 82% yield with very high enantioselectively and exo-selectivity, which also makes it applicable to the syntheses of other marine spiroimine toxins.
Scheme 8.
Diastereoselective formation of the spiroimine ring through a copper-bisoxazoline-catalyzed Diels–Alder reaction.
3. Recent Total Syntheses of Pinnatoxin A
3.1 Formal Synthesis of Pinnatoxin A by the Inoue–Hirama Group (2004)
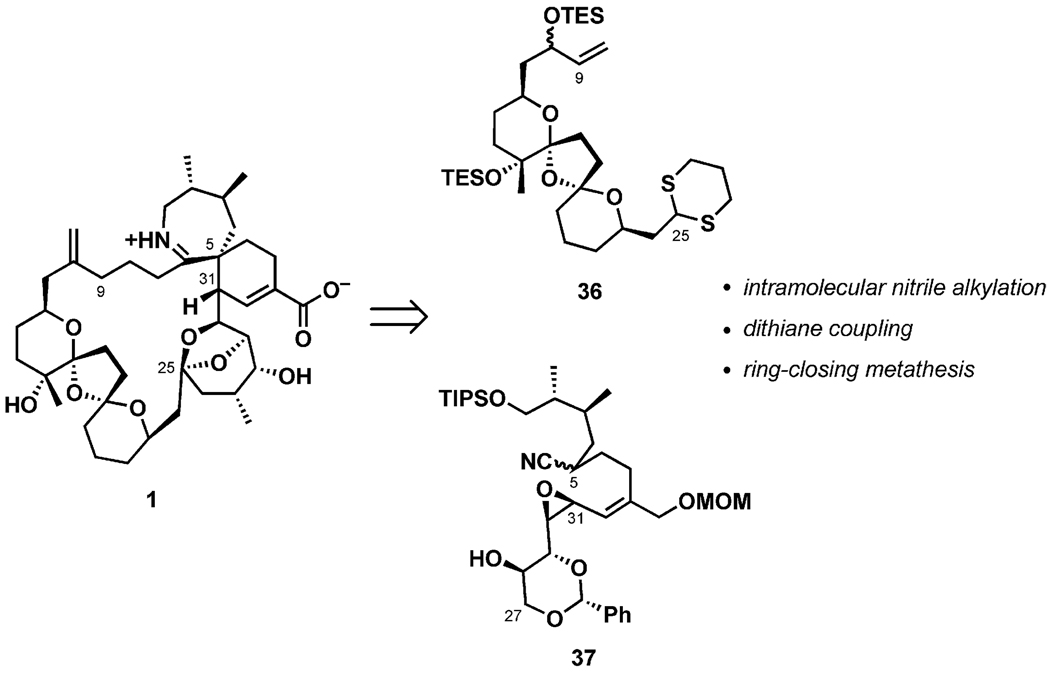
In 2004, the Inoue–Hirama group reported a formal total synthesis of (+)-pinnatoxin A (1) based on a convergent route.[31] As shown in Scheme 9, it was envisioned that two complex fragments (36 and 37) would be joined together through a dithiane alkylation reaction at C25. Intramolecular cyclization of an epoxy nitrile in 37 would set the stereochemistry of the C5 quaternary center. Finally, the 27-membered carbocycle would be formed by a ring-closing olefin metathesis reaction.
Scheme 9.
Synthetic strategy for (+)-pinnatoxin A (1).
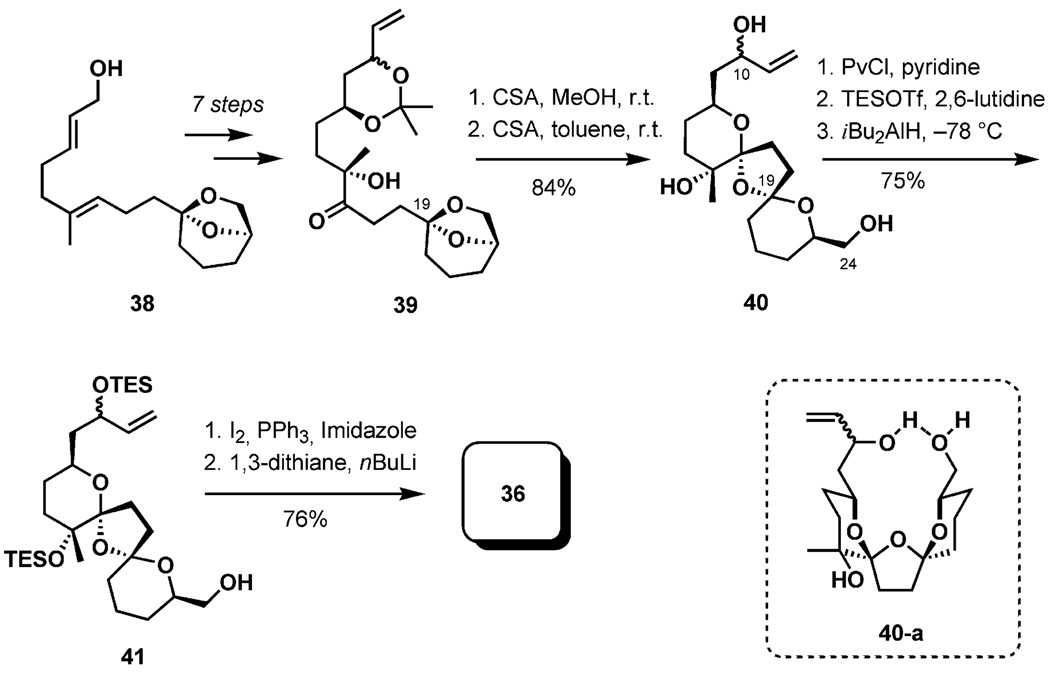
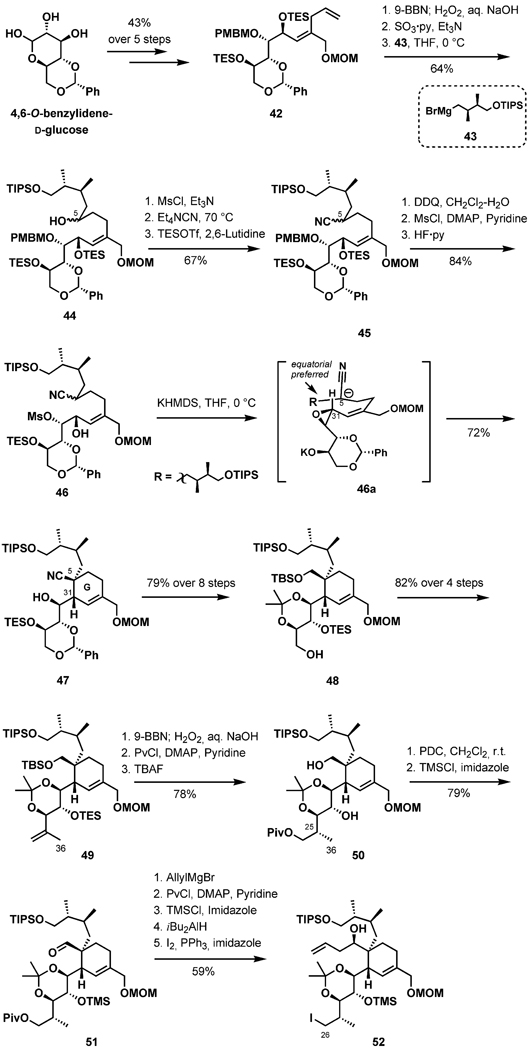
Construction of the BCD-bis-spiroketal 36 in an enantioselective fashion began with a Sharpless AE reaction of the allylic alcohol 38 in the presence of (+)-diethyl tartrate and proceeding through a Parikh–Doering oxidation to yield the ketone 39 in 41% overall yield after seven standard transformations (Scheme 10). The acetonide in the bis-spiroketalization precursor 39 was initially removed with CSA in methanol at room temperature. Subsequently, substitution of the solvent with the less polar toluene preferentially afforded the desired bis-ketal diastereomer (out of four possible stereoisomers) in 84% yield. Intramolecular hydrogen bonding between the terminal hydroxy groups at C10 and C24 was suggested as a rationale for the observed stereoselectivity in the ketalization (40-a).[32] Protection of the OH-10 and OH-15 groups with TESOTf, conversion of the OH-15 into the iodide, and installation of the dithiane ring furnished the BCD-fragment 36 in a direct five-step sequence (stereochemistry confirmed by NOE).
Scheme 10.
Preparation of the BCD ring.
The approach to fragment 52 commenced with 4,6-O-benzylidene-d-glucose,[33] which was advanced to the MOM ether 42 in five routine steps (Scheme 11). The alcohol 44 was accessed after chemoselective hydroboration/oxidation of the terminal olefin in 42, followed by Grignard addition of 43. Subsequent mesylation, substitution, and a series of protecting group manipulations afforded the epoxy-nitrile 46.
Scheme 11.
Synthesis of fragment 52.
The G ring was assembled stereoselectively upon exposure to an excess of KN(SiMe3)2, to afford 47 exclusively in 72% yield. This notable transformation proceeds through the transition state 46a, in which the large branched carbon chain at C5 adopts an equatorial orientation, furnishing the C5 quaternary and C31 tertiary centers selectively with the desired configuration.[34] The diol 47 was elaborated to the alcohol 48 in preparation for the stereoselective installation of the C36 methyl group (Scheme 11). After Parikh–Doering oxidation of the alcohol, methyl Grignard addition, and further oxidation, the resulting ketone was treated with Tebbe reagent to generate the exo-olefin 49 in 82% overall yield. Regio- and stereoselective hydroboration of the diene, followed by pivaloyl ester formation and regioselective desilylation, smoothly afforded 50 with the correct stereochemistry at C25. Selective oxidation of the diol at the primary OH group with PDC and subsequent installation of the TMS ether yielded 51. After addition of allylmagnesium bromide to the C6 aldehyde and protecting group transformations, the free alcohol at C26 was converted into the iodide in anticipation of the coupling of fragments 36 and 52.
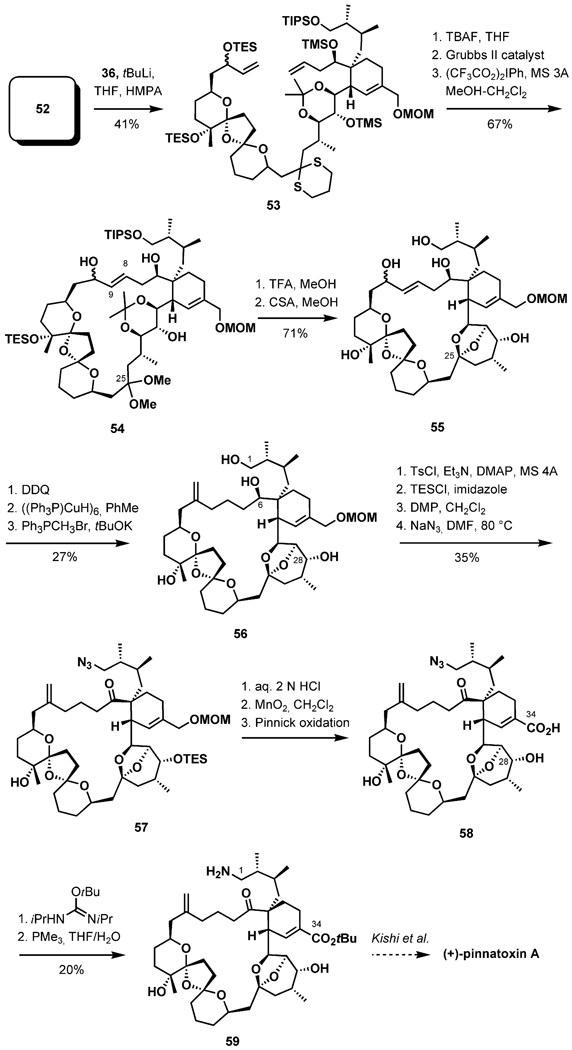
The unification of the two complex fragments was initiated by lithiation of the dithiane 36, followed by the addition of a pre-cooled solution of the iodide 52, delivering the coupled product in 41% isolated yield along with recovered 36 (44%, Scheme 12). It was experimentally determined that the silyl protecting groups in 53 were detrimental for the ring-closing metathesis reaction, presumable due to steric hindrance. After selective cleavage of the TES and two TMS groups with TBAF, the RCM reaction was achieved in the presence of the second-generation Grubbs catalyst, favoring formation of the E olefin. The dithiane was then converted into the dimethyl acetal with use of the Stork reagent. En route to the EF ring system, the macrocycle 54 was subjected to acidic conditions, accomplishing protecting group cleavage and EF-ketalization while leaving the trioxadispiroacetal intact. Regioselective oxidation of the allylic alcohol with DDQ generated the α,β-unsaturated ketone, which underwent a 1,4 reduction of the C8=C9 bond with the Stryker reagent. A Wittig reaction installed the exo-methylene group at C10 to provide 56. Protecting group transformations and replacement of the primary hydroxy group gave rise to the azide 57. Hydrolytic removal of the methoxymethyl ether and subsequent oxidation to the carboxylic acid at C34 provided 58. Attempts to utilize the Staudinger reduction/aza-Wittig cyclization to convert the azide intermediate directly into 1 were unsuccessful, but the azide was alternatively reduced to an amino group in two steps to provide Kishi’s intermediate, thus completing the formal synthesis of (+)-pinnatoxin A.
Scheme 12.
Endgame.
3.2 Total Synthesis of Pinnatoxin A by the Nakamura–Hashimoto Group (2008)
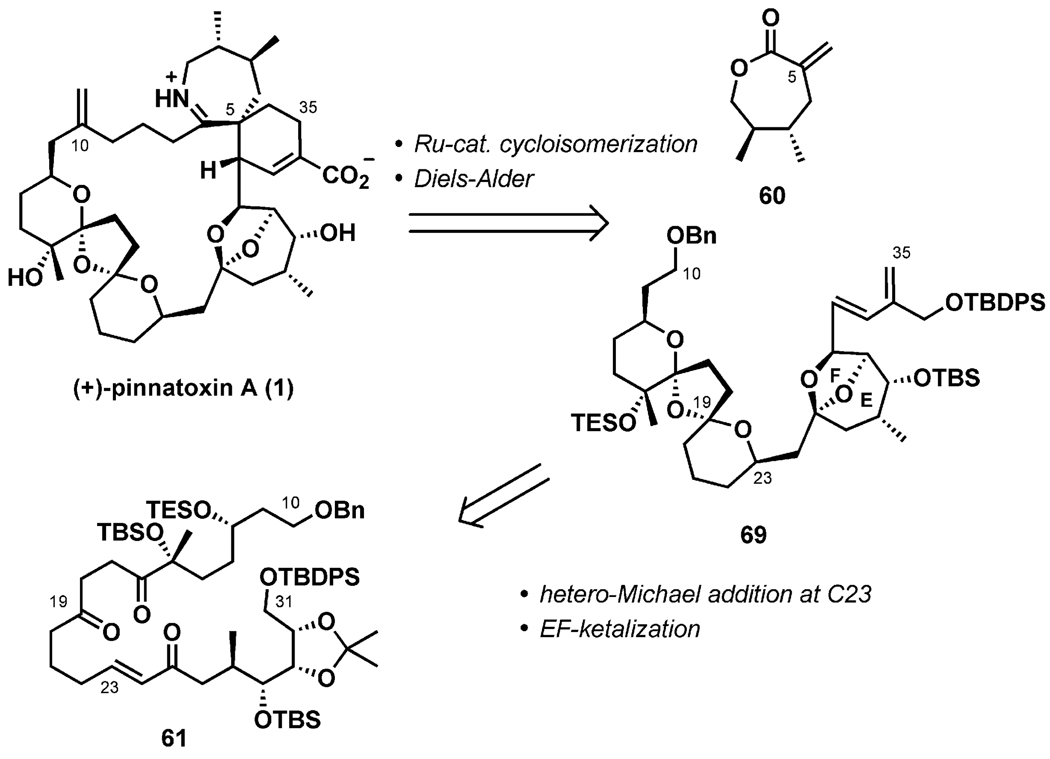
The Nakamura–Hashimoto group reported the synthesis of (+)-pinnatoxin A (1) in 2008[35] as the culmination of dedicated studies.[36,37] The synthesis strategy, outlined in Scheme 13, exploits the Diels–Alder reaction, mirroring that of Uemura’s biosynthetic proposal. Late stages of the synthesis were to include an exo-selective Diels–Alder reaction utilizing the α-methylene lactone 60 as a dienophile for the construction of the G ring, as well as a Ru-catalyzed cycloisomerization to construct the 27-membered carbocyclic ring, culminating with the formation of the seven-membered cyclic imine by self-catalyzed dehydration of an advanced ketoamino acid.
Scheme 13.
Synthesis plan.
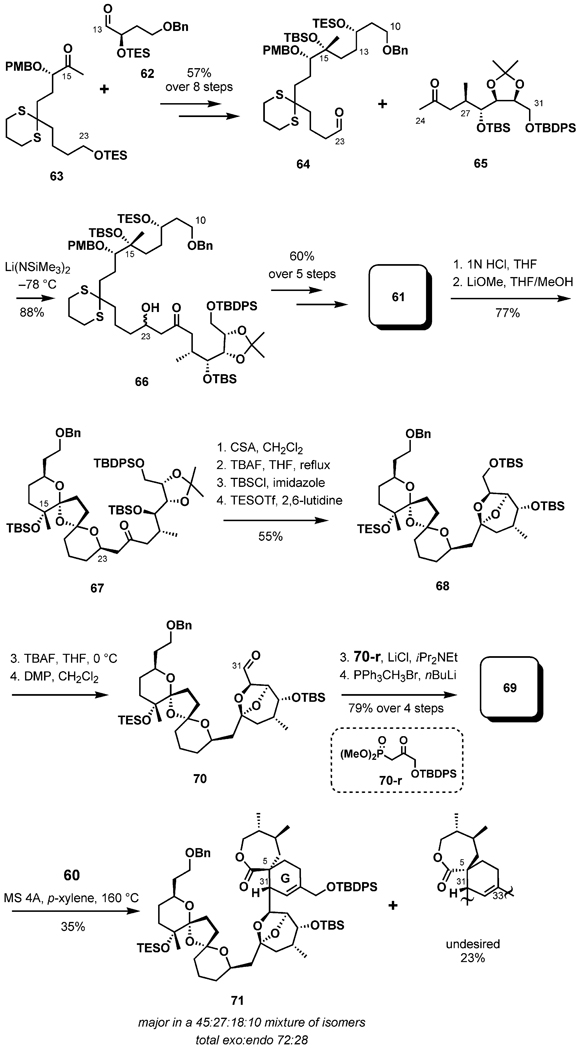
Nakamura and Hashimoto envisaged an approach to the 6,5,6-dispiroketal (BCD ring) system involving a stereoselective tandem double hemiketal formation/intramolecular hetero-Michael addition process. This strategy should have the advantage of generating a chiral center from an enone in the conjugate addition step under thermodynamic control. After the desired dispiroketalization, the synthesis of the entire C10–C31 pentacyclic ketal fragment (BCDEF ring) should also be achievable.
The aldehyde 62 and the methyl ketone 63 were each prepared by a series of conventional transformations and coupled in an aldol condensation with the aid of LiHMDS and ZnCl2 in THF (Scheme 14).[36] After an additional seven steps, the C10–C23 aldehyde fragment 64 was ultimately accessed. The construction of the C23–C24 linkage was based on a fragment assembly by another aldol reaction. The lithium enolate derived from the ketone 65 [prepared in six steps from a known starting material involving a conjugate anti-addition of MeCu(CN)Li in the presence of BF3·OEt2 to establish the C27 stereogenic center] reacted with the aldehyde to give a mixture of diastereomeric aldol adducts 66 in 88% yield. Five standard steps subsequently yielded the target triketone 61 in 60% yield. With the key substrate to hand, dispiroketalization through a tandem double hemiketal formation/hetero-Michael addition process was attempted by an established protocol. Treatment of the triketone with aqueous HCl (1 n) selectively liberated the C12 hydroxy group, yielding an equilibrium mixture of hydroxy triketones and hemiketals. Upon treatment with lithium methoxide in THF/MeOH (10:1) at room temperature, the desired dispiroketal was formed in 77% yield, accompanied by a 14% yield of other diastereomers. Removal of the acetonide group in 67 with CSA in dichloromethane and simultaneous ketalization afforded the C10–C31 fragment 68, containing the BCDEF pentacyclic ring system, as a single isomer in 55% yield after silylation steps.
Scheme 14.
Synthesis of the Diels–Alder adduct.
Protecting group exchange was necessary because the C15-TBS ether could not be cleaved during the later stages of the synthesis.[35] Selective removal of the C31-TBS ether with TBAF at 0 °C, followed by immediate oxidation with Dess–Martin periodinane buffered with pyridine, yielded the desired aldehyde 70. Homologation by a Horner–Wadsworth–Emmons reaction with β-ketophosphonate 70-r under Masamune conditions yielded the diene 69 in 72% yield over four steps to set the stage for the critical Diels–Alder reaction. Regioselective cycloaddition with the α-methylene lactone 60 in p-xylene at 160 °C in a sealed tube yielded a separable mixture of stereoisomers in a 45:27:18:10 ratio.[35] The selectivity observed was exo (72:28), however, with poor diastereofacial selection (63:37), again underscoring the stereochemical challenges associated with the Diels–Alder approach. The desired adduct 71 was isolated in 35% yield.
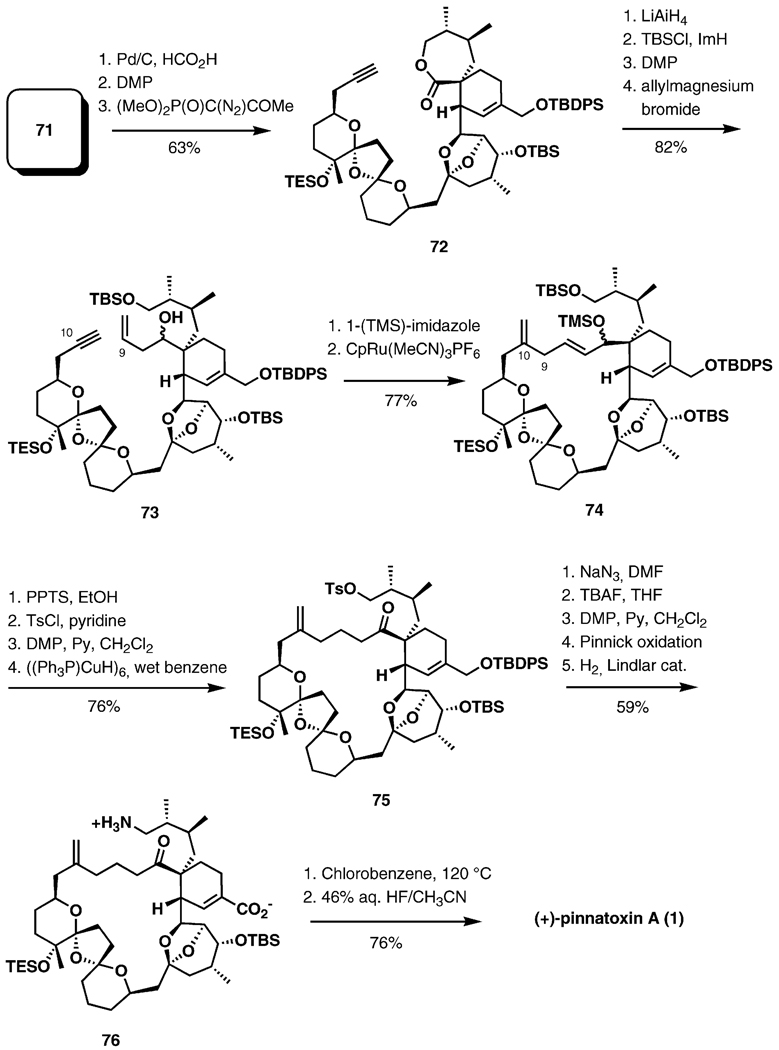
Selective removal of the benzyl group at C10, followed by Dess–Martin oxidation and treatment with the Ohira–Bestmann reagent in the presence of K2CO3 in MeOH, afforded the alkyne 72 in 63% over three steps (Scheme 15). The lactone moiety was reduced with LiAlH4 to the diol, which was first monosilylated at the C1 hydroxy group and then oxidized with the Dess–Martin reagent, and the resulting aldehyde was treated with allylmagnesium bromide to afford 73. The cycloisomerization precursor was furnished after silylation of the secondary neopentylic hydroxy group with 1-(trimethylsilyl)imidazole. The cyclization product 74 was obtained upon exposure of 73 to [CpRu-(MeCN)3]PF6 (10 mol-%, acetone, 50 °C), in 79% yield, with complete regioselectivity, and with no dimerization. The ketone 75 was furnished after the selective removal of silyl protecting groups at C1 and C6 with pyridinium p-toluenesulfonate in EtOH, tosylation of the primary alcohol, oxidation of the allylic alcohol at C6, and a final conjugate reduction with the Stryker reagent in wet benzene, in 76% yield over four steps. Displacement of the tosylate with azide, followed by selective removal of the C34-TBDPS ether with TBAF, two consecutive oxidations (Dess–Martin, Pinnick), and hydrogenation with the Lindlar catalyst, efficiently provided the ketoamino acid 76. One of the most important original contributions of this synthesis is the development of a direct cyclization of the ketamino acid 76 to the cyclic imine under thermolysis conditions (chlorobenzene, 120 °C, 18 h, 74% yield, 84% yield based on recovered starting material). The silyl groups were removed with HF in aqueous MeCN to complete the synthesis of pinnatoxin A.
Scheme 15.
Endgame.
3.3 Total Synthesis of Pinnatoxin A by Zakarian’s Group (2008)
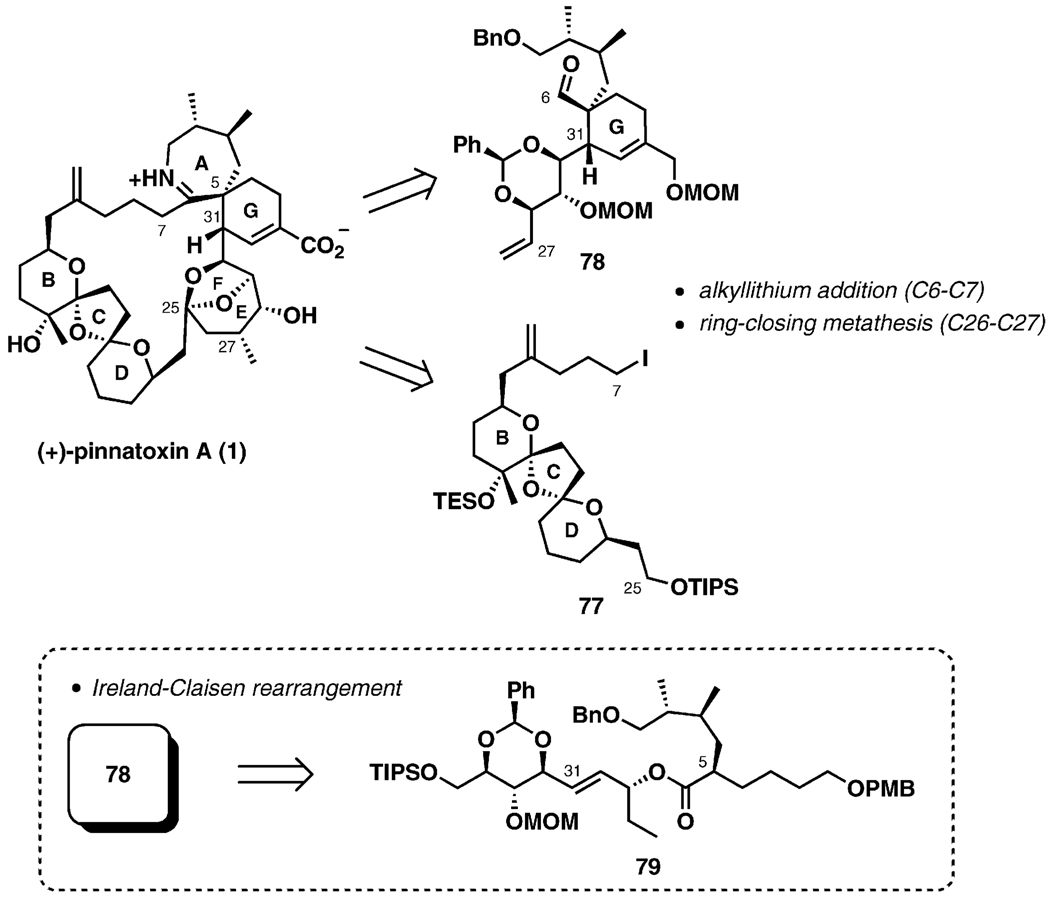
Zakarian’s group described a series of synthetic studies culminating in the total synthesis of the natural enantiomer of pinnatoxin A in 2008.[38] The synthesis plan is based on a convergent strategy calling for the preparation of the two large fragments 77 and 78 (Scheme 16). It was anticipated that the joining of the fragments by alkyllithium addition to the C6 aldehyde of 78 would be followed by 27-membered macrocycle formation upon ring-closing metathesis with subsequent elaboration of the EF ketal. The acyclic Ireland–Claisen rearrangement of the complex ester 79 is a dominant feature of this synthesis plan, illustrating a departure from the Diels–Alder approach with the goal of improving stereocontrol for the introduction of the adjacent quaternary and tertiary stereogenic centers at C5 and C31.
Scheme 16.
General synthesis plan.
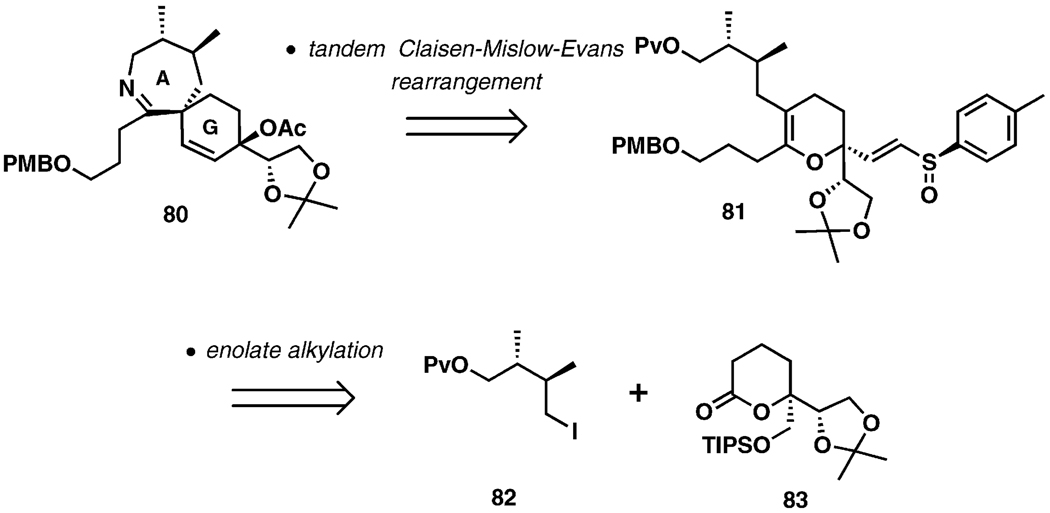
Scheme 16 represents an early approach to the synthesis of the spirocyclic imine fragment characteristic of pinnatoxins and pteriatoxins. This route employed a cascade sigma-tropic process that utilizes the Claisen and Mislow–Evans rearrangements to construct the quaternary chiral center at the core of the AG-spiroimine.[39,40] It was envisioned that a tandem sigmatropic rearrangement of the vinylic sulfoxide 80 (Scheme 17), derived from 82 and 83, would deliver the desired fragment.
Scheme 17.
Initial approach to the AG-spiroamine ring.
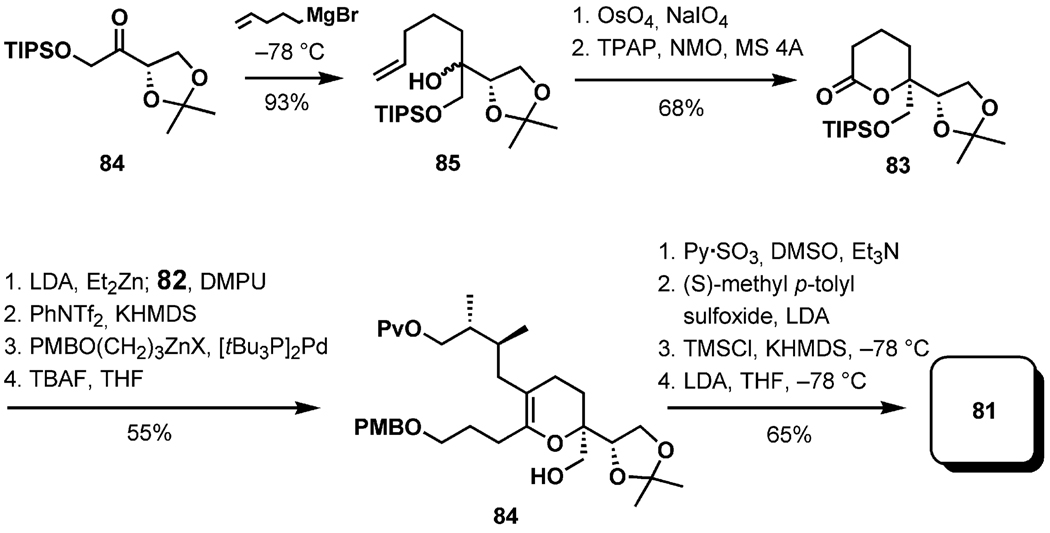
The synthesis of 81 began with the addition of pent-4-enylmagnesium bromide to the ketone 84, readily available from ascorbic acid in four steps (Scheme 18). The lactone 83 was obtained by cleavage of the double bond and oxidation of the lactol. Alkylation of the zincate enolate generated from 83 with the iodide 82, prepared from (2R,3R)-dimethylsuccinic acid,[45] afforded the expected product as a 5:1 mixture of diastereomers. Formation of the triflate followed by Negishi coupling yielded the dihydropyran 84, which was advanced to the sulfoxide 81 by standard transformations.
Scheme 18.
Synthesis of the vinyl sulfoxide 81.
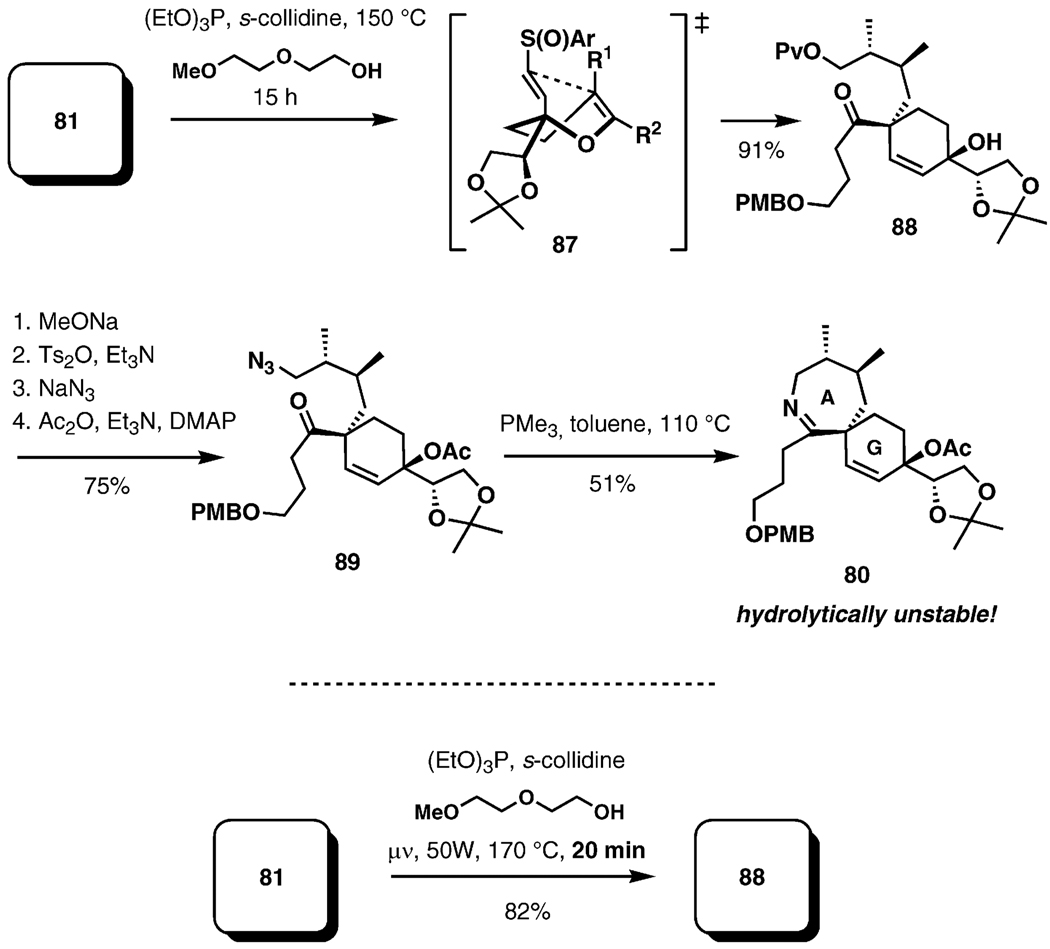
As illustrated in Scheme 19, upon heating of the starting material in the presence of triethyl phosphite and s-collidine as a buffer in a high-boiling alcohol at 150 °C, the rearrangement results in smooth formation of the quaternary C5 chiral center and a stereoselective installation of a tertiary allylic alcohol, presumably proceeding through transition state 87. Both diastereomers at the sulfoxide stereogenic center proved to be suitable substrates for the tandem sigmatropic rearrangement. The incorporation of imine ring closure into the reaction cascade through an aza-Wittig reaction was not productive. However, the imine 80 could be obtained in a separate event after a series of transformations culminating in a Staudinger reduction of the azide and an aza-Wittig cyclization (PMe3, PhMe, 110 °C).
Scheme 19.
Cascade Claisen–Mislow–Evans rearrangement.
This key reaction cascade can also be performed in a microwave-assisted version, with significantly reduced reaction times.[40] The formation of the spiroimine 80 presented an opportunity to explore the stability of the spiroimine moiety outside the macrocyclic pinnatoxin framework, in which it displays uncharacteristic stability towards hydrolysis. It was found that 80 is readily hydrolyzed upon exposure to moisture,[40] suggesting that the entire macrocyclic structure of pinnatoxin A is necessary to ensure stability of the spiroimine.
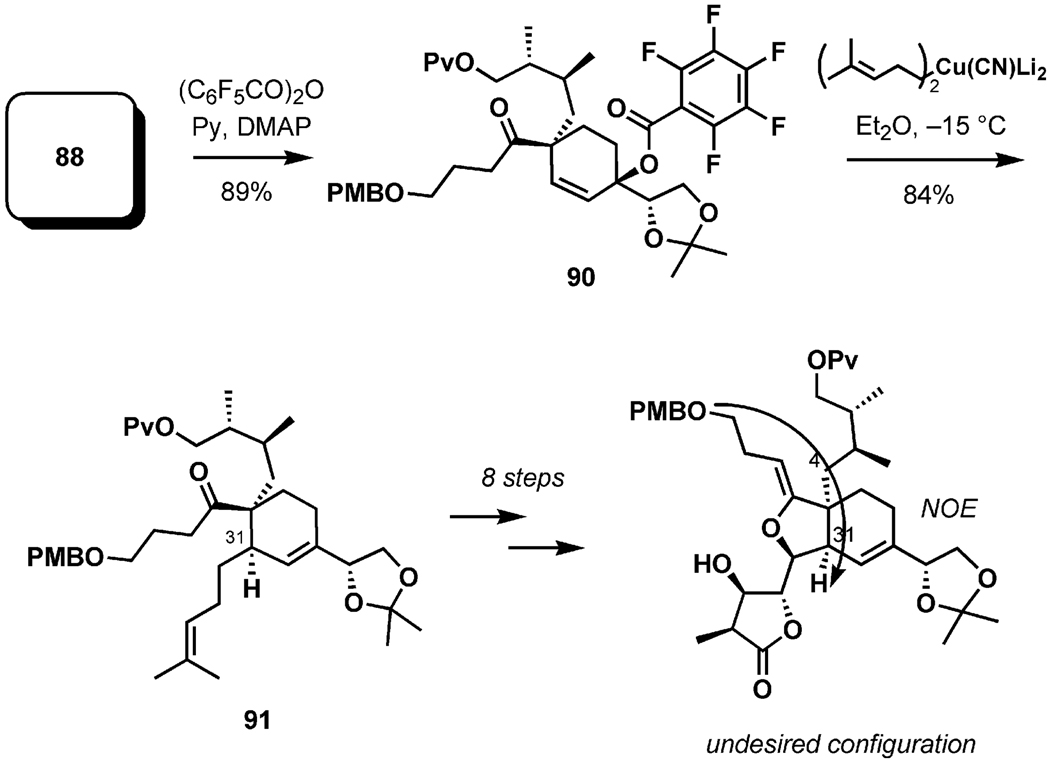
Although this tandem sigmatropic reaction sequence was found to be potentially suitable for the synthesis of (+)-pinnatoxin A (1), it was limited in scope, preventing its application in syntheses of the spirolides or of gymnodimine.[46] Another serious obstacle was encountered during attempts to introduce the requisite C31 side chain. After significant experimentation, it was found that cuprate substitution held promise. When the pentafluorobenzoate 90 (Scheme 20), generated from 88 in high yield, was treated with dilithium bis(4-methylbut-3-enyl)cyanocuprate, a single regio- and stereoisomeric product (91) was isolated in good yield. It was initially assumed, on the basis of multiple precedent and preliminary NOE data, that 91 had the correct configuration at C31. However, after an extensive eight-step elaboration to a polycyclic advanced intermediate, it was conclusively established by extensive NOE correlations that the C31 stereochemistry introduced during the cuprate substitution was incorrect.[41]
Scheme 20.
Lithium cuprate addition.
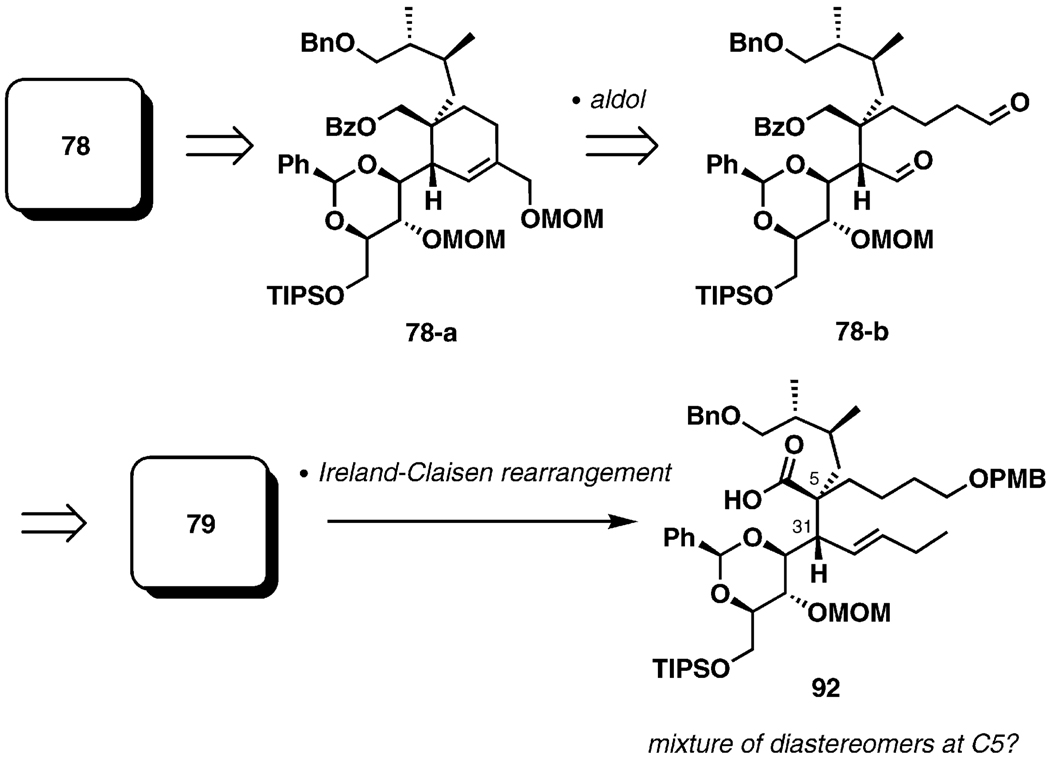
Scheme 21 outlines the second-generation strategy for spiroimine assembly. As in the original approach, introduction of the C5 and C31 stereogenic centers early in the synthesis was anticipated, in order to alleviate some imminent challenges associated with the formation of these chiral centers. In this case, however, the Ireland–Claisen rearrangement was selected as a central transformation for the new strategy. In order to accommodate the key sigmatropic process, the cyclohexene ring (ring G) had to be deconstructed into an acyclic fragment. In a forward sense, an aldol cyclocondensation was deemed an optimal reaction to build the six-membered ring. Subsequent analysis was based on straightforward transformations to reach the Ireland–Claisen chemistry via 78-b (see Schemes 16 and 21).
Scheme 21.
New strategy for spiroamine formation.
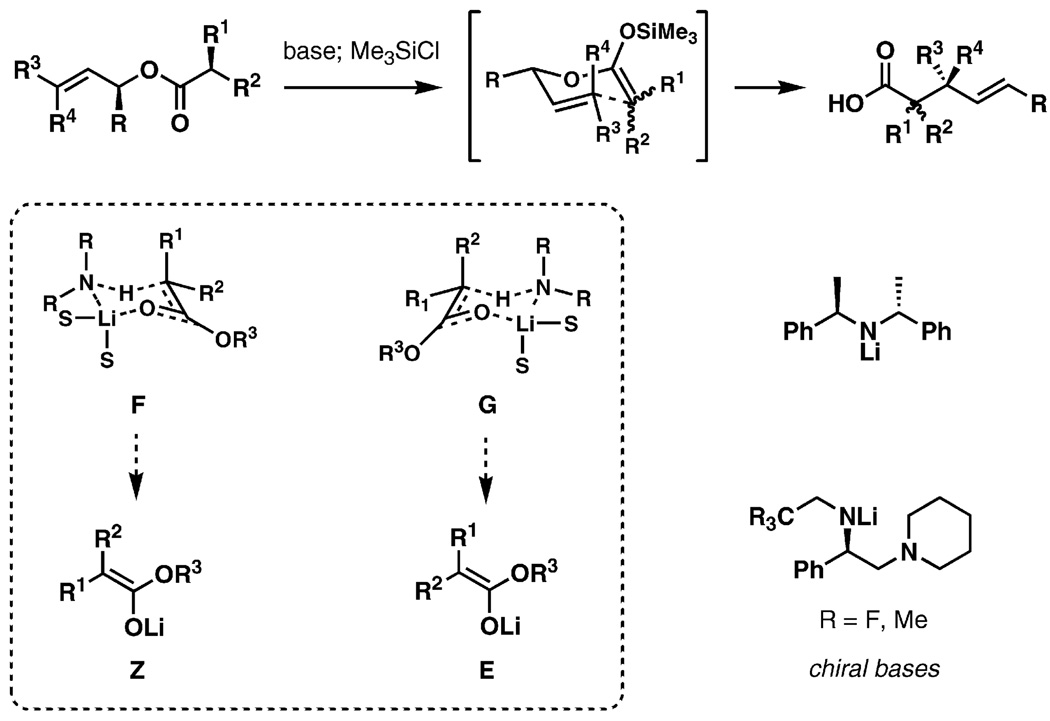
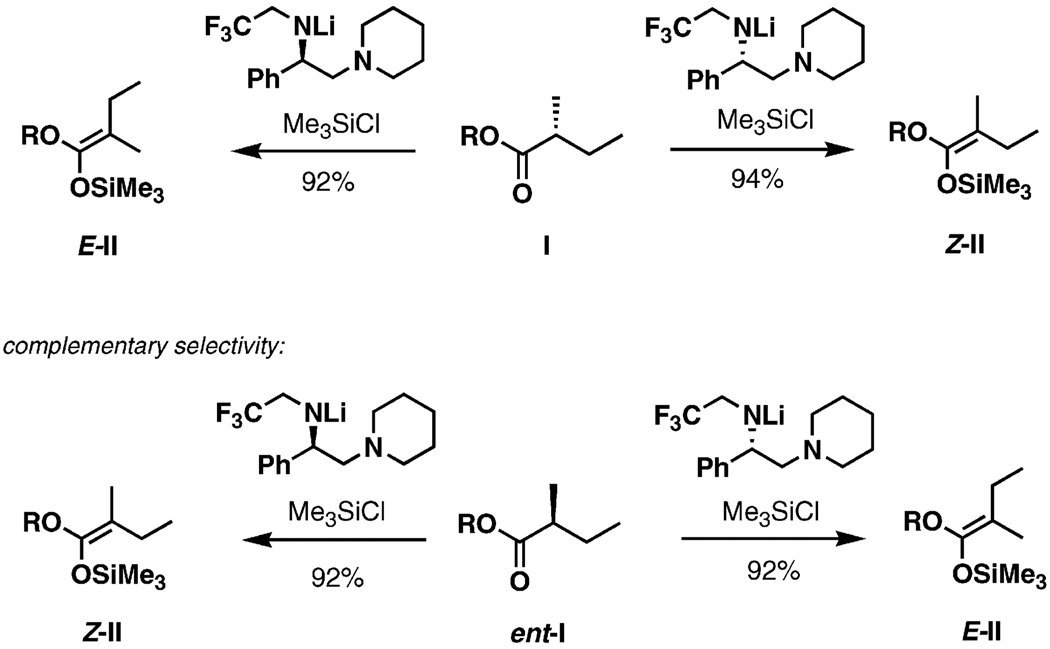
An apparent deficiency of the new plan is the key step itself: from prior knowledge one would expect a mixture of diastereomers at C5 from the Ireland–Claisen rearrangement of the ester 79. This shortcoming created an opportunity to explore and to develop methodology for diastereoselective Ireland–Claisen rearrangements of complex α-branched acyclic esters (Figure 4).[42] It is well-established that diastereocontrol in the Ireland–Claisen rearrangement is predictably correlated to the geometric selectivity of enolate formation, so the enoliation step became the focus of the method development. Ireland and co-workers proposed a simple model for enolization of carbonyl compounds with lithium amides based on chair-like transition structures (F and G, Figure 4).[43] If this model is applied to stereodefined esters, it can be hypothesized that the two diastereomeric transition structures would lead to different geometric isomers upon proton transfer (F→Z, G→E). Traditional achiral bases would be unlikely to exert a preference for the formation of the six-membered chair-like structures, but the process might be effectively controllable by application of chiral bases. The ready availability of a selection of chiral amines gives this approach further appeal.
Figure 4.
Transition structures of the Ireland–Claisen rearrangement.
In practice, it has been demonstrated that chiral bases as shown in Figure 4 enable highly stereoselective generation of α,α-disubstituted enolates. The examples in Scheme 22 serve to illustrate the stereochemical course of the enolization process, providing a empirical model with which to predict the geometries of enolates based on chirality matching between the substrate and the chiral base. Application of the enolization technique in the Ireland–Claisen rearrangement has been described.[42]
Scheme 22.
Chirality of the base dictates stereoselectivity.
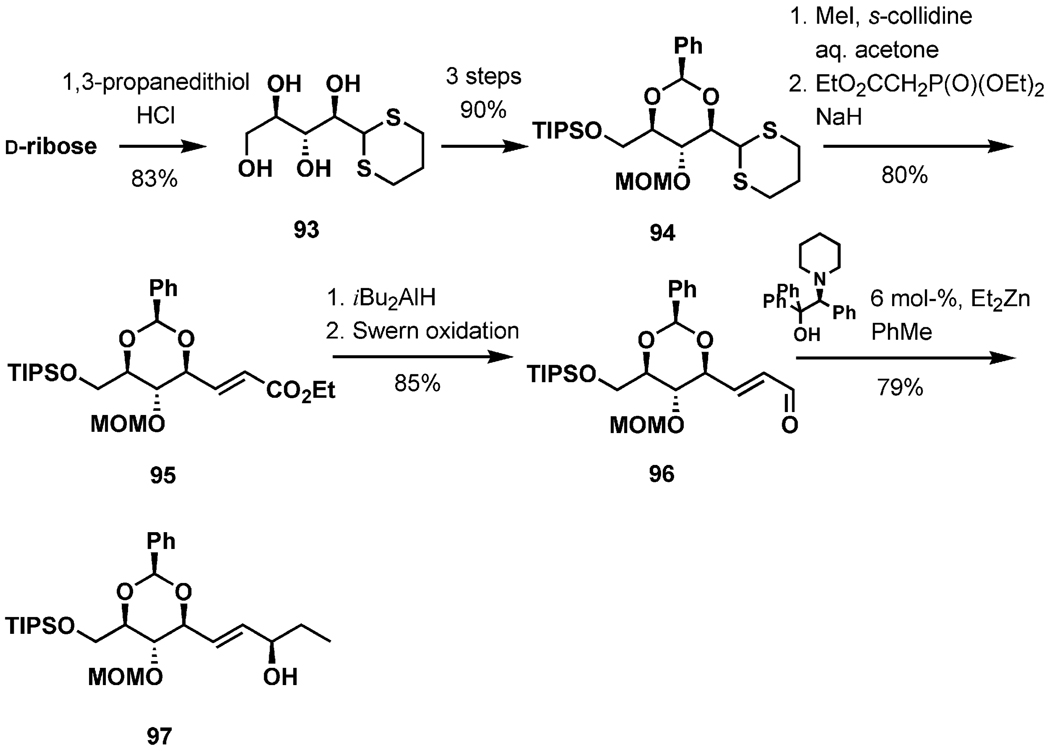
The new enolization method having been developed, its application in the intricate venue of the total synthesis of pinnatoxin A was pursued. The dithioacetal 92 (Scheme 23), derived from d-ribose in one step, was modified by protecting group chemistry to unmask the aldehyde group in preparation for the Horner–Emmons olefination. Reduction of the ester to the allylic alcohol and subsequent Swern oxidation furnished the α,β-unsaturated aldehyde 96, which was converted into the target allylic alcohol 97 by enantioselective addition of diethylzinc catalyzed by (S)-1,1,2-triphenyl-2-(piperidin-1-yl)ethanol.
Scheme 23.
Preparation of the allylic alcohol 97.
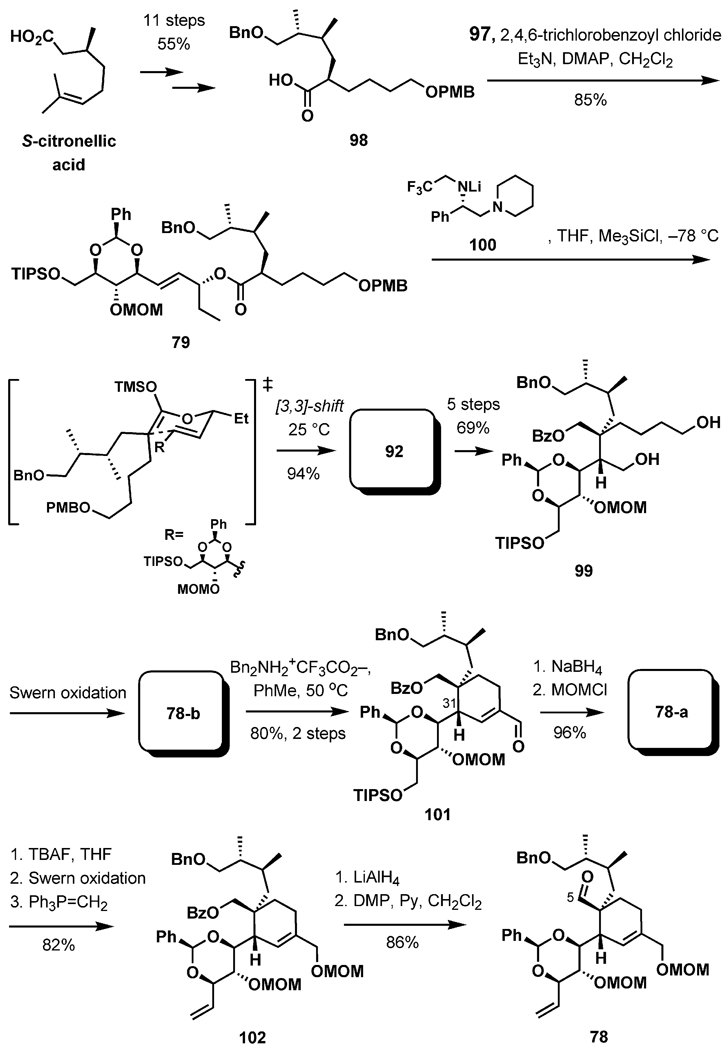
Esterification of the alcohol 97 with the carboxylic acid 98 [11 steps from (S)-citronellic acid] under the Yamaguchi conditions provided the ester 79 in 85% yield (Scheme 24). In the key event, enolization of 79 in the presence of the chiral Koga-type base 100 proved to be highly stereoselective, forming the Z enolate, which underwent a smooth [3,3]-sigmatropic rearrangement to the acid 92 at room temperature after trapping as a silyl ketene acetal. The chiral base could be recovered in almost quantitative yield by extraction with acid.
Scheme 24.
Application of the [3,3] sigmatropic shift.
The product of the Ireland–Claisen rearrangement, containing the requisite C5 and C31 stereogenic centers formed in a single step, was advanced to the diol 99 in five steps (69% yield). Double oxidation of the two primary hydroxy groups under Swern reaction conditions provided the dialdehyde 78-b, which underwent a completely regioselective aldol cyclocondensation to compound 101 upon treatment with dibenzylammonium trifluoroacetate at slightly elevated temperatures. Above 50 °C, epimerization at C31 became notable, but no epimerization was observed at lower temperatures. Reduction of the aldehyde and protection as a methoxymethyl ether provided 78-a, which was advanced by deprotection (TIPS), oxidation, olefination to 102, and finally by reductive debenzoylation and oxidation to the C5 aldehyde 78.
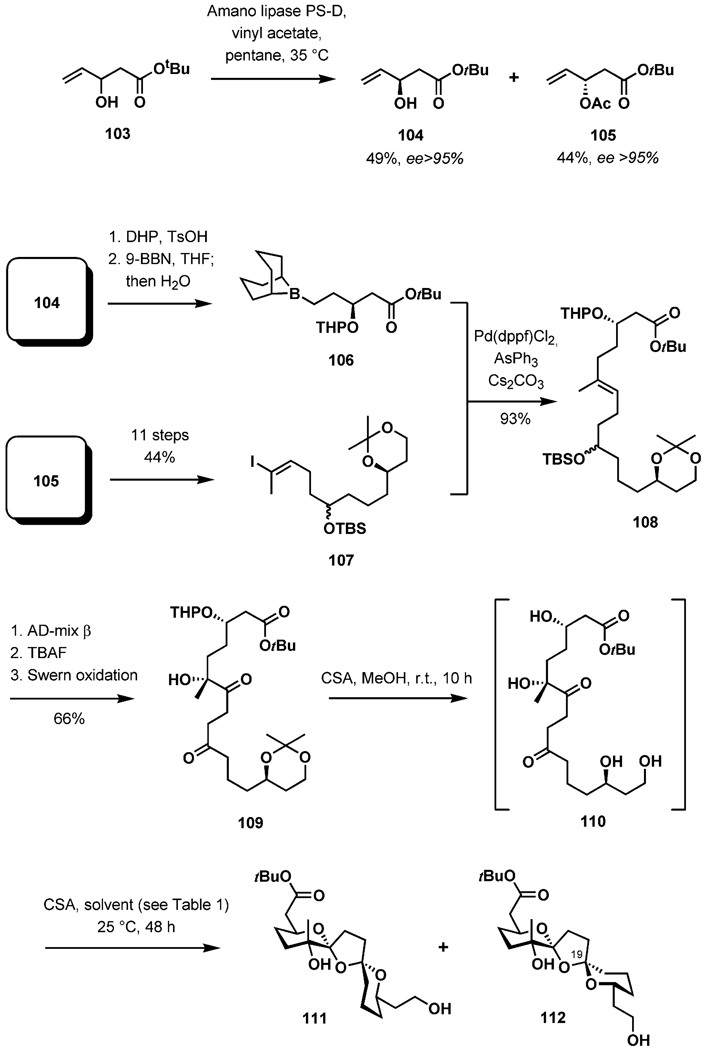
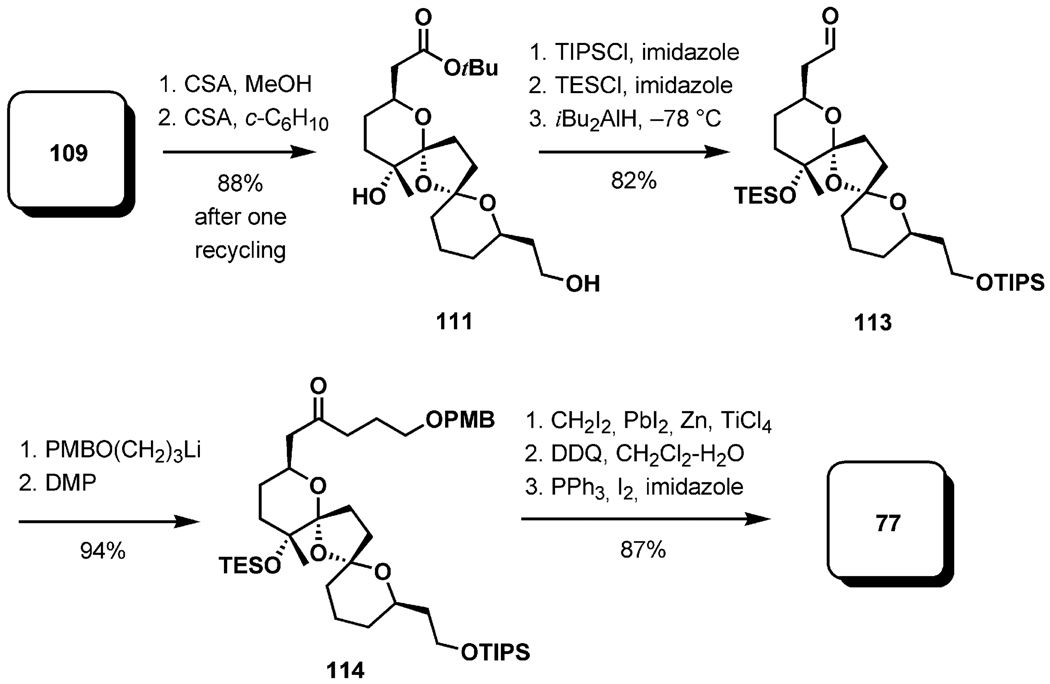
The central transformation in the synthesis of the BCD-dispiroketal was the thermodynamically controlled ketalization of the diketo diol precursor 110 (Scheme 25).[44] The influence of solvent polarity on the selectivity of the process in favor of the thermodynamically preferred product became the focus of the study. Solvent effects on the extents, and occasionally even the positions, of anomeric equilibria in simple systems have been known for a long time.[47] It has been demonstrated that the anomeric effect is reinforced in solvents of low polarity. To explore this phenomenon in the context of the BCD-spiroketal synthesis, a racemic mixture of the allylic alcohols 103 was resolved by enzymatic acetylation with immobilized Amano lipase PS-D. The resulting products could easily be separated by chromatography. The alcohol 104 was advanced to the borane 106 (used in situ), whereas the acetate 105 was converted into the iodoalkene 107 (11 steps, 44% yield). These intermediates were reunited in a palladium-catalyzed cross-coupling, delivering 108 in high yield. A three-step sequence involving dihydroxylation, silyl ether removal, and oxidation furnished the diketone 109; methanolysis of this under acidic conditions set the stage for the study of dispiroketalization. In line with expectations, increasingly higher yields of the fully stabilized diastereomer 111 were isolated with decreasing solvent polarity. The reactions were allowed to continue for 2 d to ensure that the thermodynamic equilibrium has been reached. Under optimal conditions, a 10:1 ratio of 111 and 112 was achieved in cyclohexane (Table 1).
Scheme 25.
Preparation of the BCD-spiroketal precursor.
Table 1.
Solvent effects on spiroketalization.
| Entry | Solvent | 111 (% yield) | 112 (% yield) |
|---|---|---|---|
| 1 | methanol | 49 | 22 |
| 2 | dichloromethane | 62 | 23 |
| 3 | toluene | 63 | 13 |
| 4 | cyclohexane | 78 | 8 |
The synthesis of the BCD-dispiroketal fragment was completed in eight additional steps as shown in Scheme 26. The ester 111 was produced in 88% yield after a single recycling step. Protection of the free hydroxy groups as silyl ethers and reduction of the ester with iBu2AlH gave the aldehyde 113 in 82% yield. Chain-extension, oxidation, olefination, oxidative debenzylation, and iododehydroxylation completed the synthesis of the BCD-bisketal fragment 77.
Scheme 26.
Completion of fragment 77.
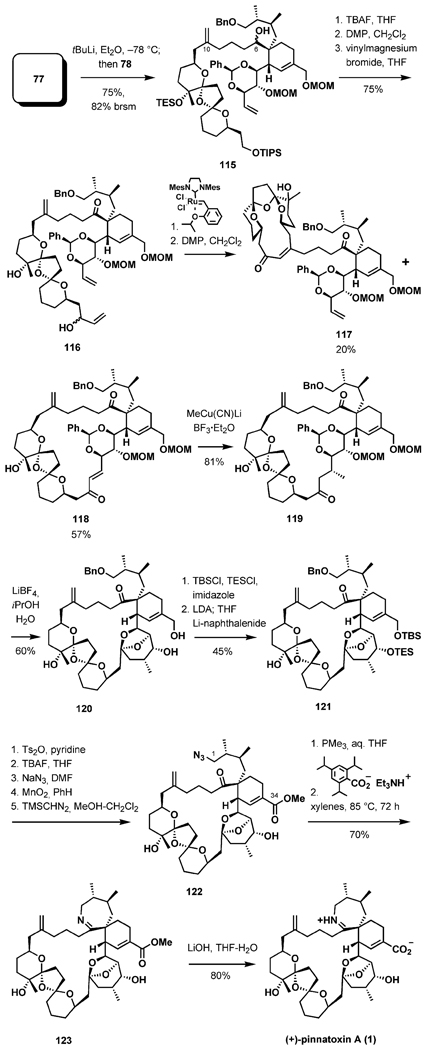
In one of the major events in the synthesis, the two advanced building blocks were combined by direct addition of a complex lithium reagent generated from 77 to the aldehyde 78 (Scheme 27). Lithium/iodine exchange (77, tBuLi, Et2O, 1 h, −78 °C) followed by addition of 78 resulted in a high-yielding reaction delivering 115 as an inconsequential mixture of diastereomers (2:1) in 75% yield. After oxidation at C6, three additional steps were needed to reach the ring-closing metathesis (RCM) substrate 116. Extensive experimentation revealed that under virtually all conditions attempted a 3:1 ratio of the regioisomeric products 118 and 117 was obtained after oxidation with DMP. The isomer 117 clearly arises from the RCM at the C10 double bond. Nevertheless, 118 could be isolated in a practical 57% yield. Cuprate addition, followed by a challenging multiple deprotection/EF ketal formation, provided 120. Additional functional group manipulations were aimed at the introduction of nitrogen at C1 and the adjustment of the oxidation state at C34. Achieving this goal required seven steps to arrive at the intermediate 122. In the concluding three-step sequence, reduction of the azide with trimethylphosphane, imine formation under mild conditions developed by Kishi and coworkers,[24a] and a crucial ester hydrolysis delivered the natural enantiomer of pinnatoxin A.
Scheme 27.
Completion of the synthesis.
4. Conclusion
As is evident from this microreview, pinnatoxin A, as a prototypical member of the expanding family of spiroimine natural products, has inspired a wealth of synthetic strategies and innovative methods applied in the synthesis of this complex compound. Examples include new insights into the chemistry of aliphatic cyclic imines, Diels–Alder cycloadditions, a variety of ketalization processes, new variants of the Ireland–Claisen rearrangement and other [3,3]-sigma-tropic transpositions, and a number of other transformations with complex, multifunctionalized intermediates.
It is particularly notable that chemical synthesis is currently the only realistic source of the important bioactive natural product, in view of the fact that a producing organism remains elusive. Intriguingly, essentially every report citing the biological activity of pinnatoxin A refers to the marine toxin as a calcium-channel activator, even though the evidence for the mode of action is based on limited phenotypic data provided by the group that reported its isolation.[4] More thorough and convincing studies to elucidate the biological activity of pinnatoxin A are clearly needed, and it is total synthesis that is the most likely source of material for these studies.
Acknowledgments
This work was supported by the National Institutes of Health/National Institute of General Medical Sciences (R01 GM077379), the National Science Foundation (CAREER CHE-0836757), and generous gifts from Eli Lilly (Lilly Grantee Award to A. Z.) and Amgen (Young Investigator Award to A. Z.).
Biographies

Stéphane Beaumont was born in Normandy in 1981. After studying at the IUP of Chemistry and Biology in Nantes and at the University of Paris XI, he received his PhD under the direction of Dr. Robert H. Dodd and Dr. Philippe Dauban at the Institut de Chimie des Substances Naturelles, Gif-sur-Yvette. After postdoctoral studies with Professor Armen Zakarian at the University of California Santa Barbara, he joined GALAPAGOS as a medicinal chemistry scientist in 2010.

Elizabeth A. Ilardi received her B.S. in Chemistry from The College of William and Mary in Virginia in 2006. She is currently working on her Ph.D. in organic chemistry under the supervision of Dr. Armen Zakarian at the University of California Santa Barbara. The focus of her studies includes the development of new methodologies to be applied towards the total syntheses of natural products.

Nicholas D. C. Tappin was born in Manchester in the UK. He attended Queens’ College, University of Cambridge, where he obtained a B.A. and an M.Sc. after studies with flow chemistry in the ITC of the Steven Ley Group. In 2009 he made the leap across the Atlantic to UC Santa Barbara, where he is currently working on the synthesis of natural products.

Armen Zakarian was born in Moscow and completed his undergraduate studies at Moscow State University in 1994. His Diploma research was carried out at the Zelinsky Institute of Organic Chemistry with Dr. Vladimir Borodkin. He received his Ph.D. under the direction of Professor Robert A. Holton at Florida State University in 2001, and then spent two years (2002–2004) in the laboratories of Professor Larry E. Overman (University of California, Irvine) as a postdoctoral research associate. He began his independent academic appointment in August 2004, and he is currently a faculty member at the Department of Chemistry and Biochemistry, University of California Santa Barbara. His research interests include synthetic organic chemistry spanning the total synthesis of natural products, bioorganic chemistry, and the development of synthetic methodology.
References
- 1.a) For a relevant overview of these compounds: Molgó J, Girard E, Benoit E. In: Phycotoxins: Chemistry and Biochemistry. Botana LM, editor. Blackwell Publishing; 2007. pp. 319–355. Kita M, Uemura D. Chem. Lett. 2005;34:454–459. Kuramoto M, Arimoto H, Uemura D. Mar. Drugs. 2004;2:39–54.
- 2.Chou T, Haino T, Kuramoto M, Uemura D. Tetrahedron Lett. 1996;37:4027–4030. [Google Scholar]
- 3.Suthers BD, Jacobs MF, Kitching W. Tetrahedron Lett. 1998;39:2621–2624. [Google Scholar]
- 4.Zheng SZ, Huang FL, Chen SC, Tan XF, Zhuo JB, Peng J, Xie RW. Chin. J. Mar. Drugs. 1990;33:33–35. [Google Scholar]
- 5.Rosewater J. Indo-Pacific Mollusca. 1961;1:175–226. [Google Scholar]
- 6.a) Uemura D, Chuo T, Haino T, Nagatsu A, Fukuzawa S, Zheng S, Chen H. J. Am. Chem. Soc. 1995;117:1155–1156. [Google Scholar]; b) Kita M, Uemura D. Prog. Mol. Subcellular Biol. 2006;43:25–51. doi: 10.1007/978-3-540-30880-5_2. [DOI] [PubMed] [Google Scholar]
- 7.For a review concerning toxicologies: Munday R. In: Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection. 2nd ed. Botana LM, editor. CRC Press; 2008. pp. 581–594.
- 8.Takada N, Umemura N, Suenaga K, Chou T, Nagatsu A, Haino T, Yamada K, Uemura D. Tetrahedron Lett. 2001;42:3491–3494. [Google Scholar]
- 9.Tindall DR, Dickey RW, Carlson RD, Morey-Gaines G. ACS Symposium. Series 262. Washington DC: American Chemical Society; 1984. pp. 225–240. [Google Scholar]
- 10.Selected examples: Martin DF, Padilla GM. In: Bioactive Compounds from the Sea Martin. Humm HJ, Lane CE, editors. vol. 1. New York: Marcel Dekker Inc.; 1974. pp. 151–174. Lin YY, Risk M, Ray SM, Van Engen D, Clardy J, Golik J, James JC, Nakanishi K. J. Am. Chem. Soc. 1981;103:6773–6775. Rein KS, Borrone J. J. Compl. Biochem. Phys. Part B. 1999;124:117. doi: 10.1016/s0305-0491(99)00107-8.
- 11.Anderson DM. In: Physiological Ecology of Harmful Algal Blooms. Anderson DM, Cembella AD, Hallegraeff GM, editors. Springer; 1998. pp. 29–48. [Google Scholar]
- 12.Bivalve Molluscs as Vectors of Marine Biotoxins Involved in Seafood Poisoning, Ciminiello P, Fattorusso E. Prog. Mol. Subcellular Biol. 2006;43:52–82. doi: 10.1007/978-3-540-30880-5_3.
- 13.Cembella AD, Lewis NI, Quilliam MA. Phycologia. 2000;39:67–74. [Google Scholar]
- 14.a) Hu T, Curtis JM, Oshima Y, Quilliam MA, Walter JA, Watson-Wright WM, Wright JLC. J. Chem. Soc., Chem. Commun. 1995:2159–2161. [Google Scholar]; b) Falk M, Burton IW, Hu T, Walter JA, Wright JLC. Tetrahedron. 2001;57:8659–8665. [Google Scholar]
- 15.Hu T, Curtis JM, Walter JA, Wright JLC. Tetrahedron Lett. 1996;37:7671–7674. [Google Scholar]
- 16.a) Seki T, Satake M, Mackenzie L, Kaspar HF, Yasumoto T. Tetrahedron Lett. 1995;36:7093–7096. [Google Scholar]; b) Stewart M, Blunt JW, Munro MHG, Robinson WT, Hannah DT. Tetrahedron Lett. 1997;38:4889–4890. [Google Scholar]
- 17.Munday R, Towers NR, Mackenzie L, Beuzenberg V, Holland PT, Miles CO. Toxicon. 2004;44:173–178. doi: 10.1016/j.toxicon.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 18.a) Gymnodimine B: Miles CO, Wilkins AL, Stirling DJ, MacKenzie AL. J. Agric. Food Chem. 2000;48:1373–1376. doi: 10.1021/jf991031k. b) gymnodimine C: Miles CO, Wilkins AL, Stirling DJ, MacKenzie AL. J. Agric. Food Chem. 2003;51:4838–4840. doi: 10.1021/jf030101r. For a recent total synthesis of (−)-gymnodimine, see: Kong K, Romo D, Lee C. Angew. Chem. Int. Ed. 2009;48:7402–7405. doi: 10.1002/anie.200903432. and studies: Ahn Y, Cardenas GI, Yang J, Romo D. Org. Lett. 2001;3:751–754. doi: 10.1021/ol0155081.
- 19.Lu C, Lee G, Huang R, Chou H. Tetrahedron Lett. 2001;42:1713–1716. [Google Scholar]
- 20.Torigoe K, Murata M, Yasumoto T. J. Am. Chem. Soc. 1988;110:7876–7877. [Google Scholar]
- 21.Hu T, de Freitas ASW, Curtis JM, Oshima Y, Walter JA, Wright JLC. J. Nat. Prod. 1996;59:1010–1014. doi: 10.1021/np960439y. [DOI] [PubMed] [Google Scholar]
- 22.a) Tachibana K, Scheuer FJ. J. Am. Chem. Soc. 1981;103:3469–2471. [Google Scholar]; b) Cembella AD. J. Appl. Phycology 1. 1989:307–310. [Google Scholar]
- 23.Takada N, Uemura N, Suenaga K, Uemura D. Tetrahedron Lett. 2001;42:3495–3497. [Google Scholar]
- 24.a) Matsuura F, Peters R, Anada M, Harried SS, Hao J, Kishi Y. J. Am. Chem. Soc. 2006;128:7463–7465. doi: 10.1021/ja0618954. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hao J, Matsuura F, Kishi Y, Kita M, Uemura D, Asai N, Iwashita T. J. Am. Chem. Soc. 2006;128:7742–7743. doi: 10.1021/ja061893j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.For a review on bis-spiroketal systems, see: Brimble MA, Furkert DP. Curr. Org. Chem. 2003;7:1461–1484.
- 26.MacKinnon SL, Cembella AD, Burton IW, Lewis N, LeBlanc P, Walter JA. J. Org. Chem. 2006;71:8724–8731. doi: 10.1021/jo0608873. [DOI] [PubMed] [Google Scholar]
- 27.Matsuura F, Hao J, Reents R, Kishi Y. Org. Lett. 2006;8:3327–3330. doi: 10.1021/ol0611548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCauley JA, Nagasawa K, Lander PA, Mischke SG, Semones MA, Kishi Y. J. Am. Chem. Soc. 1998;120:7647–7648. [Google Scholar]
- 29.For the BCDEF-ring fragment, see: Sugimoto T, Ishihara J, Murai A. Tetrahedron Lett. 1997;38:7379–7382. Sugimoto T, Ishihara J, Murai A. Synlett. 1999:541–544. Ishihara J, Tojo S, Kamikawa A, Murai A. Chem. Commun. 2001:1392–1393. For the A-ring fragment, see: Ishihara J, Horie M, Shimada Y, Tojo S, Murai A. Synlett. 2002;3:403–406. 1,7,9-Trioxadispiro[5.1.5.2]pentadecane structural studies: Ishihara J, Sugimoto T, Murai A. Synlett. 1998:603–606.
- 30.a) Sinaÿ P, Pougny JR. Tetrahedron Lett. 1976;17:4073–4076. [Google Scholar]; b) Schmidt RR, Michel J. Carbohydr. Chem. 1985;4:141–169. [Google Scholar]; c) Lemieux RU, Ratcliffe RM. Can. J. Chem. 1979;57:1244–1251. [Google Scholar]
- 31.Sakamoto S, Sakazaki H, Hagiwara K, Kamada K, Ishii K, Noda T, Inoue M, Hirama M. Angew. Chem. Int. Ed. 2004;43:6505–6510. doi: 10.1002/anie.200461802. [DOI] [PubMed] [Google Scholar]
- 32.Noda T, Ishiwata A, Uemura S, Sakamoto S, Hirama M. Synlett. 1998:298–300. [Google Scholar]
- 33.Barili PL, Berti G, Catelani G, Cini C, D’Andrea F, Mastrorilli E. Carbohydr. Res. 1995;278:43–57. doi: 10.1016/0008-6215(95)00227-8. [DOI] [PubMed] [Google Scholar]
- 34.The original synthesis utilized NaN(TMS)2 for the intramolecular alkylation starting from a diastereomeric mixture; refer to: Nitta A, Ishiwata A, Noda T, Hirama M. Synlett. 1999;695 and references cited therein.
- 35.Nakamura S, Kikuchi F, Hashimoto S. Angew. Chem. 2008;120:7199–7202. [Google Scholar]
- 36.Nakamura S, Inagaki J, Kudo M, Sugimoto T, Obara K, Nakajima M, Hashimoto S. Tetrahedron. 2002;58:10353–10374. [Google Scholar]
- 37.Nakamura S, Kikuchi S, Hashimoto S. Tetrahedron: Asymmetry. 2008;19:1059–1067. [Google Scholar]
- 38.Stivala CE, Zakarian A. J. Am. Chem. Soc. 2008;130:3774. doi: 10.1021/ja800435j. [DOI] [PubMed] [Google Scholar]
- 39.Pelc M, Zakarian A. Org. Lett. 2005;7:1629–1631. doi: 10.1021/ol050321l. [DOI] [PubMed] [Google Scholar]
- 40.Pelc M, Zakarian A. Tetrahedron Lett. 2006;47:7519–7523. [Google Scholar]
- 41.Unpublished studies by Pelc M. Florida State University; 2005.
- 42.a) Qin Y, Stivala CE, Zakarian A. Angew. Chem. 2007;46:7466. doi: 10.1002/anie.200702142. [DOI] [PubMed] [Google Scholar]; b) Stivala CE, Zakarian A. Tetrahedron Lett. 2007;48:6845–6848. [Google Scholar]; c) Stivala CE, Zakarian A. Org. Lett. 2009;11:839–842. doi: 10.1021/ol8027797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.a) Ireland RE, Mueller RH, Willard AK. J. Am. Chem. Soc. 1976;98:2888. [Google Scholar]; b) Sun X, Collum DB. J. Am. Chem. Soc. 2000;122:2452. [Google Scholar]; c) Romesberg FE, Collum DB. J. Am. Chem. Soc. 1995;117:2166. [Google Scholar]; Zhao P, Lucht NL, Kenkre SL, Collum DB. J. Org. Chem. 2004;69:242. doi: 10.1021/jo030221y. [DOI] [PubMed] [Google Scholar]; d) Cain CM, Cousins RPC, Coumbarides G, Simpkins NS. Tetrahedron. 1990;46:523. [Google Scholar]; e) Shirai R, Tanaka M, Koga K. J. Am. Chem. Soc. 1986;108:543. doi: 10.1021/ja00263a051. [DOI] [PubMed] [Google Scholar]; f) Shirai R, Aoki K, Sato D, Kim H-D, Murakata M, Yasakuta T, Koga K. Chem. Pharm. Bull. 1994;42:690. [Google Scholar]; g) Shirai R, Aoki K, Sato D, Tanaka M, Kawasaki H, Koga K. Tetrahedron. 1997;53:5963. [Google Scholar]
- 44.Lu CD, Zakarian A. Org. Lett. 2007;9:3161–3163. doi: 10.1021/ol071266e. [DOI] [PubMed] [Google Scholar]
- 45.Lu CD, Zakarian A. Org. Synth. 2008;85:158–171. [Google Scholar]
- 46.For application of this process in the synthesis of other natural products, see: Ilardi EA, Isaacman MJ, Qin Y, Shelly SA, Zakarian A. Tetrahedron. 2009;65:3261–3269.
- 47.a) Deslongchamps P. Stereoelectronic Effects in Organic Chemistry. Oxford: Pergamon Press; 1983. [Google Scholar]; b) Juaristi E, Cuevas G. The Anomeric Effect. Boca Raton: CRC Press; 1995. [Google Scholar]