Abstract
Background
The hypothesis that the failing heart may be energy starved is supported, in part, by observations of reduced rates of ATP synthesis through the creatine kinase (CK) reaction, the primary myocardial energy reservoir, in heart failure (HF) patients. Although murine models have been used to probe HF pathophysiology, it has not been possible to non-invasively measure the rate of ATP synthesis through CK in the in vivo mouse heart. The purpose of this work was to exploit non-invasive spatially-localized magnetic resonance spectroscopy (MRS) techniques to measure ATP flux through CK in in vivo mouse hearts and determine the extent of any reductions in murine HF.
Methods and Results
The Triple Repetition Time Saturation Transfer (TRiST) MRS method of measuring ATP kinetics was first validated in skeletal muscle, rendering similar results to conventional saturation transfer MRS. In normal mouse hearts the in vivo CK pseudo-first-order-rate constant, kf, was 0.32±0.03 s−1 (mean±SD) and the rate of ATP synthesis through CK was 3.16±0.47 µmol/g/s. Thoracic aortic constriction (TAC) reduced kf by 31% (0.23±0.03 s−1, p<0.0001) and ATP synthesis through CK by 51% (1.54±0.25 µmol/g/s, p<0.0001), analogous values to those in failing human hearts.
Conclusions
Despite the small size and high murine heart rate, the ATP synthesis rate through CK is similar in vivo in murine and human hearts and comparably reduced in HF. Because murine TAC shares fundamental energetic similarities with human HF, this model and new MRS approach promise a powerful means to non-invasively probe altered energetics in HF.
Keywords: energetics, CK flux, hypertrophy, in vivo mouse hearts, magnetic resonance spectroscopy
Adenosine 5´-triphosphate (ATP) is the source of chemical energy that maintains intracellular homeostasis and fuels myocardial function. Relatively large rates of ATP synthesis are required to maintain normal cardiac contractile function and it has been hypothesized that inadequate ATP supply contributes to the contractile dysfunction in heart failure (HF)1. The observations that pharmacological HF agents that reduce metabolic demand improve outcomes whereas those that increase energetic demands worsen them are consistent with the energy-starvation hypothesis1–2.
Mouse models offer novel and appealing means to investigate the mechanistic link between altered energy metabolism and contractile function because they allow gene-targeted interventions not currently possible in larger mammalian species and humans. In several important ways murine models mimic functional and energetic aspects of normal human cardiac physiology3. Among many murine HF models, thoracic aortic constriction (TAC) is one that results in pressure-overload hypertrophy, progresses to HF, and shares some gross energetic and functional similarities with clinical human HF4. One factor that has limited testing of the energy-starvation hypothesis is the inability to non-invasively quantify energy transfer and, in particular, the rate of ATP synthesis in the in vivo beating heart.
From mice to humans 31P magnetic resonance spectroscopy (MRS) is the only non-invasive, non-destructive means to quantify the concentrations of cardiac high-energy phosphates, ATP and creatine phosphate (PCr), which are rapidly and reversibly converted by the creatine kinase (CK) reaction, the primary energy reserve of the heart. 31P MRS approaches have identified moderate reductions in cardiac PCr/ATP ratio and minimally reduced [ATP] in patients with HF5–7. 31P MRS techniques called saturation transfer (ST) use frequency-selective irradiation pulses and enable measurement of the rate of ATP synthesis through CK in the beating heart. Because conventional ST approaches are extremely time consuming, more rapid ST approaches have been described recently8 that have enabled, for the first time, measurement of the rate of ATP synthesis through CK in the human heart and detection of 50%–70% reductions in the rate of ATP synthesis through CK in HF8–10.
The extremely small size (~0.1g) and rapid rate (~600/min) of the mouse heart complicate metabolic measurements, including those with 31P MRS. Most mouse 31P MRS studies have been conducted in isolated, perfused hearts11–14. Non-invasive, spatially-localized in vivo 31P MRS studies can be performed under physiologic conditions3–4 and permit repeated studies in the same animals as pathology evolves4. Such approaches have largely been limited to measures of cardiac PCr/ATP ratio although more recently in vivo measures of the absolute concentrations of murine cardiac [ATP] and [PCr] were described and validated15. Unlike the human heart, there have been no reported measures of the rate of ATP synthesis through CK in the in vivo mouse heart. The measurements of ATP flux in the mouse heart have been inaccessible because of long times required by conventional ST techniques. In the present study we apply the Triple Repetition Time Saturation Transfer (TRiST) method for rapidly measuring ATP flux through CK in the human heart10 to measure CK reaction kinetics in the in vivo mouse heart. There were two aims for this study. First, we sought to adapt and validate the 31P MR TRiST method for measuring muscle CK reaction kinetics in the in vivo mouse. Once validated, we applied the new 31P MRS saturation transfer approach to test the hypothesis that in vivo myocardial CK reaction kinetics are reduced in the TAC model of murine HF.
Methods
The procedure and protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University. Experiments were carried out on a Bruker Biospec MRI/MRS spectrometer equipped with a 4.7T/40 cm Oxford magnet and a 12cm actively shielded gradient set, using a custom built probe assembly as previously described3–4, 15.
Skeletal muscle studies
Prior to proceeding to experiments on the heart, the TRiST method was validated in a series of non-localized (NL) saturation transfer studies on mouse upper thigh muscle (n=5), where both TRiST and conventional saturation transfer measures could be obtained in the same exam within a reasonable amount of total experimental time. The details of these experiments are as follows: conventional NL ST measurements were performed using a set of nine TR values of 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 6.0, 10.0, and 16.0 s with 128 signal averages (NEX) and 2 dummy scan (DS), except for the two longest TR values, where NEX=64 and DS= 2 were used to keep the experimental time within reasonable limits. Signal amplitudes for these two experiments with NEX= 64 were multiplied by two in order to correct for the reduced number of averages. In addition, two more spectra were obtained with TR=16 s, one with saturating pulse placed equidistant from the PCr signal, but downfield, and the other with no saturation pulse at all. The intensities of the PCr signal were then used to calculate T1’ using the conventional progressive saturation method, as previously explained in reference 10. For the TRiST method, the T1’ was determined with the 2TR method using signal intensity values acquired with TR=1.5 and 16 s from the same data set. The pseudo-first-order rate constant, kf was determined using the following equation as explained in reference 10.
| (1) |
Heart Studies
In vivo MRI/MRS studies were carried out on 11 male control mice (weight 34 ± 5 g) and ten others seven to eight weeks after TAC (weight 36 ± 6 g), as previously described4,15. Thoracic aortic construction (TAC) was performed as previously described16. Anesthesia was induced with ~2% isoflurane in an induction chamber, and maintained at ~1% in 50/50 air/O2 mixture delivered through a nose cone, as previously described3–4,15. The probe set included a 22-mm 1H MRI and 13-mm 31P MRS coils. MR images were obtained with a 1H MRI FLASH sequence [echo time = 1.5ms; TR =12 ms; and NEX =12]. In the TRiST experiment, localized 31P MR spectra were obtained with a one-dimensional chemical shift imaging (1D CSI) sequence (16 mm field of view, 16 phase encoding steps) using modified BIR4 90° adiabatic pulses. Two acquisitions with different untriggered repetition periods (TR=1.5s, NEX=96 and TR=6s, NEX=32) were acquired in the presence of saturating irradiation pulse applied to the exchanging CK moiety, viz., γ-phosphate of ATP at −2.5ppm, relative to PCr, and another acquisition, fully relaxed (TR=10s, NEX=16) in the presence of control irradiation applied at +2.5ppm. The measured signal intensities were normalized for different NEX. In order to measure PCr concentration from the acquisition with control saturation, separate studies were performed (n=4) where two cardiac spectra were obtained with 1D CSI and TR=16 s, one with the saturating pulse placed at + 2.5ppm and the other with no saturation pulse at all. Intensities of the PCr signal from these two cardiac spectra were used to determine the average correction factor (1.1) arising from spill-over effects of the RF pulse in order to measure PCr concentration.
After the mouse exams were completed, external phantom experiments were also performed on the same day with the same coil set under comparable conditions in order to calculate the absolute concentrations of PCr and ATP, as described previously15. 1H MR Images were analyzed with commercial Paravision software and 31P MR spectral peak areas determined with in-house custom software17. Metabolite signal intensities were determined from the integrated peak areas of the PCr and ATP resonances from voxels centered on cardiac muscle as identified from the high-resolution 1H MR images, as described previously3–4. Voxel shifting was performed when necessary to optimize slice alignment with cardiac structures and minimize skeletal muscle contamination of cardiac spectra18. Although both heart and chest are curved and the voxels extend in both directions, the surface coil size and profile limit signal contributions from the lateral dimensions and thus more than 93% of signal in cardiac voxels obtained with this approach are from the mouse heart3. The same slice was selected to measure the inorganic phosphate peak area in the external phosphate phantom. The [PCr], [ATP] and PCr/ATP ratio were determined for control and TAC mouse hearts as previously explained15. T1’ was calculated from the area of the PCr signal with TRshort (1.5 sec) and TRlong (6 sec) according to the 2TR method19. The kf (s−1) was determined for control and TAC mouse hearts using the TRiST method10 and the rate of ATP synthesis through CK (µmol/g/s) calculated as the product of kf and [PCr], as previously described8–9.
To evaluate the degree of hypertrophy and extent of contractile dysfunction induced by TAC in this setting, a separate cine MRI study was performed in some animals using the following protocol. Specifically, left ventricular (LV) function was quantified from multislice FLASH cine MR images (15 frames, TE=1.5ms, TR=7ms, flip angle=45°) by using ECG and respiratory gating, as previous reported4. MR images were analyzed with ImageJ software and end-diastolic (EDV), end-systolic volume (ESV), LV mass, and LV ejection fraction (EF) were calculated as previously described3–4.
CK Activity
After completing the MRS study, mice were sacrificed, the hearts excised and immediately frozen in liquid nitrogen. Ventricular tissue was homogenized in ice-cold potassium phosphate buffer containing 1 mM EDTA and 1 mM β-mercaptoethanol, pH 7.4 (final concentration, 5 mg tissue/mL). An aliquot was removed for protein assay by the method of Lowry et al.20 using bovine serum albumin as the standard. Subsequently, Triton X-100 was added to the homogenate at a final concentration of 0.1% and total CK activity measured spectrophotometrically (Molecular Devices Sunnyvale, CA) (340nm, 37°C)21 using a kit (C184-0A) from Catachem and according to the manufacturer’s instructions. CK activity was expressed as IU/mg protein. Lung and body weights were determined at the time of sacrifice to assess for pulmonary congestion.
Statistics
A paired t-test was performed to compare conventional and TRiST measures of T1’ and kf in the same animals. The PCr/ATP ratio, [PCr], [ATP], T1’, kf, and CK reaction kinetics of normal and TAC mice heart were compared using non-paired student’s t-test. Differences were considered statistically significant at p ≤ 0.05. All results are shown as mean ± SD.
Results
Skeletal Muscle
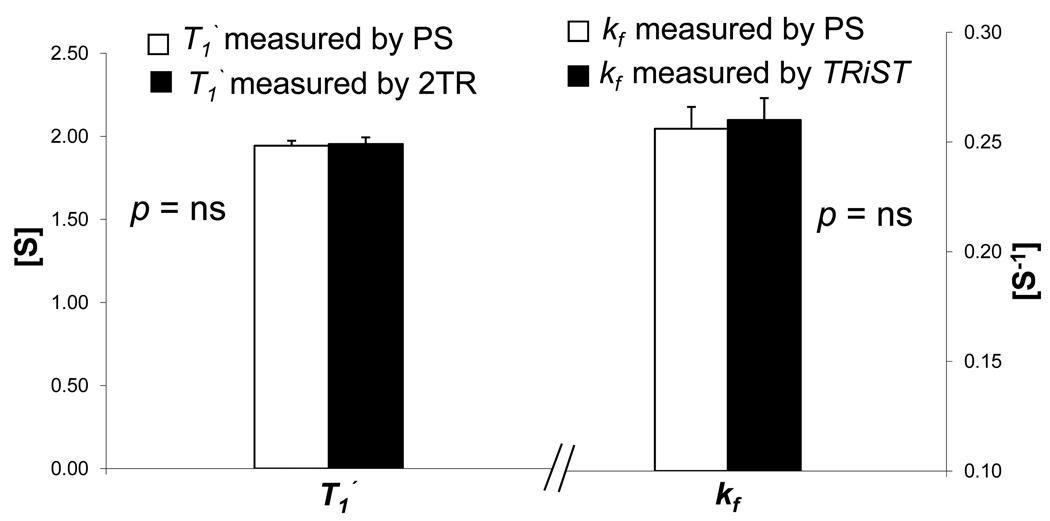
Because a conventional saturation transfer experiment with spatial-localization could not be conducted in the in vivo mouse heart in a time frame that would be tolerated by an anesthetized animal, we validated the faster TRiST approach in the in vivo mouse with non-localized (NL) studies comparing conventional nine point progressive saturation (PS) measurements with those from TRiST in skeletal muscle. A representative high-resolution transverse 1H MR thigh image and corresponding non-localized skeletal muscle 31P MR spectra under different saturating conditions are shown in Figure 1. Figure 2 shows a graph of the PCr peak area versus repetition time of the nine acquisitions and the resulting mono-exponential T1’ fit from one set of in vivo data for the skeletal muscle. The mean in vivo T1’ in the presence of saturating irradiation on γ-phosphate of ATP for conventional PS and TRiST methods were 1.94±0.03 s and 1.95±0.04 s, respectively (Figure 3, n=5, mean difference ~0.5%, p=0.64 by paired comparison). The 9 point PS data acquisition took ~78 min as compared to ~22 min for TRiST, a time saving of ~73% for the same measurement. Figure 3 shows that in these non-localized measurements, the TRiST method renders the same mean in vivo kf as does the conventional PS approach (0.26±0.01s−1 and 0.26±0.01s−1, p=0.56).
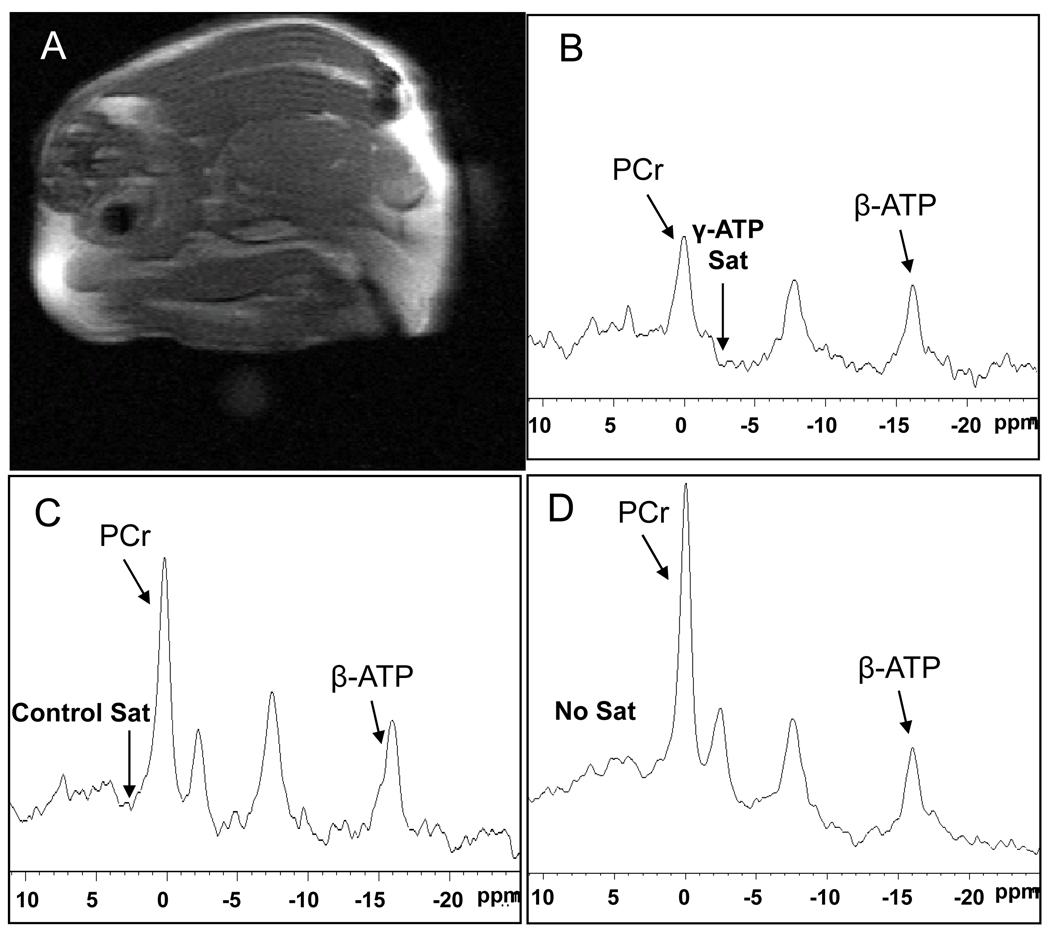
Figure 1.
(A) Typical 1H magnetic resonance image of a mouse at the skeletal muscle with non-localized (B) 31P MR spectrum with γ-phosphate of ATP saturation, (C) control saturation spectrum, (D) no saturation spectrum with TR=16s and NEX=64. PCr; phosphocreatine, β-ATP; β-phosphate of adenosine triphosphate
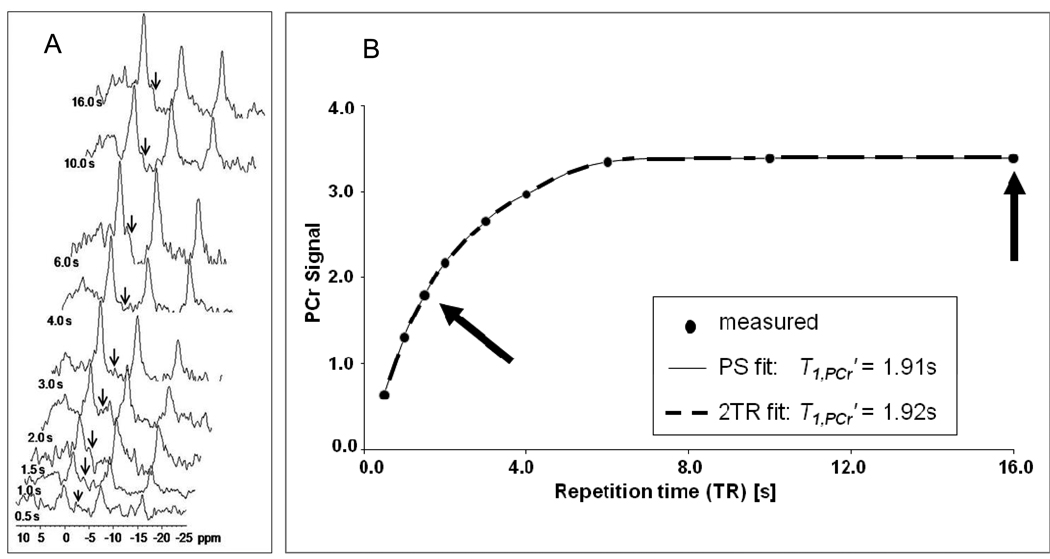
Figure 2.
(A) Stack plot of 31P spectra acquired with γ-phosphate of ATP saturation at different TR (TR values listed to left of each spectrum), (B) PCr signal intensity (a.u.) versus TR (s) during saturation of γ-phosphate of ATP in the skeletal muscle of same representative mouse. T1’ was determined both by conventional saturation transfer analysis fitting all 9 data points (light thin line) and by the 2TR method using only the data points acquired at TR of 1.5s and 16s (dark thick dotted line).
Figure 3.
T1’ and kf measured non-localized in vivo in skeletal muscle (n=5). Conventional PS (white bars) and faster TRiST techniques (black bars) produced identical results within experimental error.
Cardiac Muscle
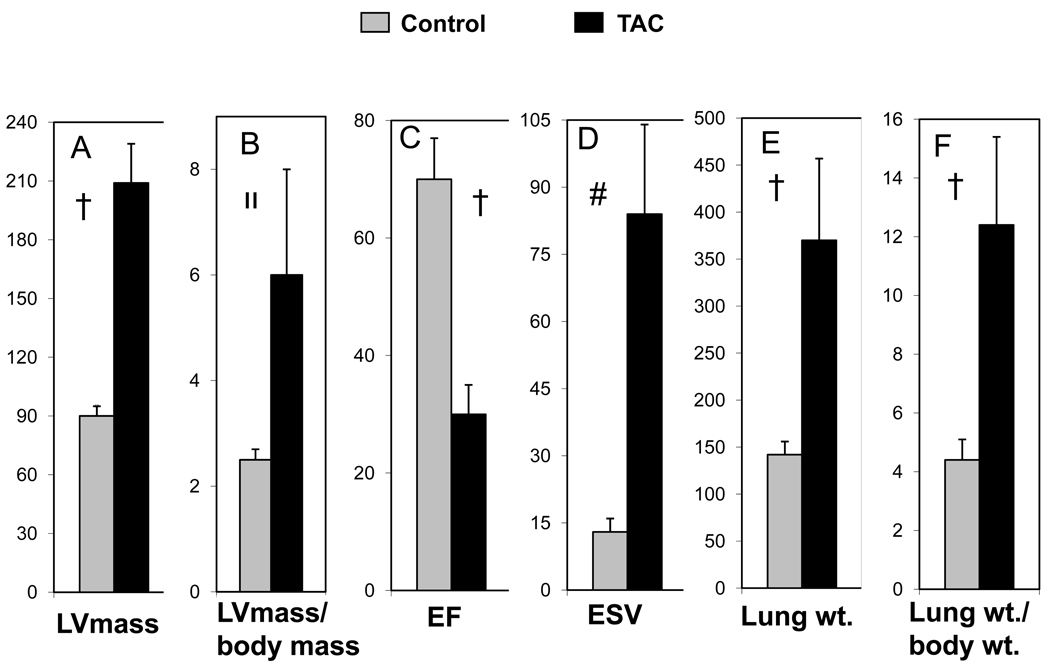
As compared to control animals (n=5), TAC hearts (n=6) exhibited a significant increase in LV mass (90±5 mg vs, 209±27 mg, control vs TAC, respectively, p<0.0001) and the ratio of LV mass (mg)/body mass (g) (2.5±0.2 vs, 6.0±2.0, p<0.005). TAC hearts had significant contractile dysfunction with a reduced LV ejection fraction (70±7% vs, 30±5%, control vs TAC, respectively, p<0.0001) and increased end-systolic volumes (13±3 µl vs, 84±21µl, p<0.0002). This significant degree of contractile dysfunction in TAC hearts was associated with pulmonary congestion as evidenced by an approximate tripling in lung weights (142±14mg vs 370±87mg, control (n=9) vs TAC (n=4), respectively, p<0.0001) and lung/body weight ratios (4.4±0.7 vs 12.4±3.1, p<0.0001), as shown in Figure 4.
Figure 4.
Cardiac functional parameters for control (gray bars) and TAC (black bars) mice: (A) LV mass (mg), (B) LV mass (mg) / body mass (g), (C) Ejection Fraction (%), (D) End Systolic Volume (µl), (E) Lung weight (mg) and (F) Lung wt. (mg) / body wt. (g) ratio.
Control vs TAC mouse †, p <0.0001; װ, p <0.005; #, p <0.0002.
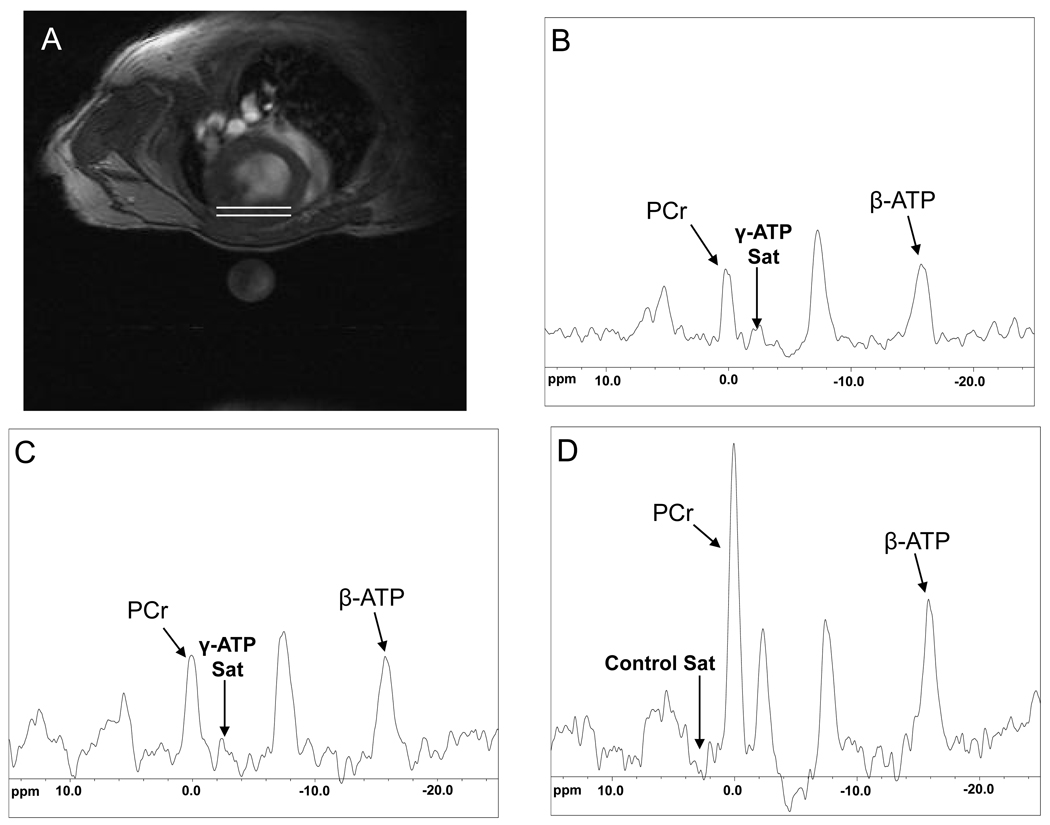
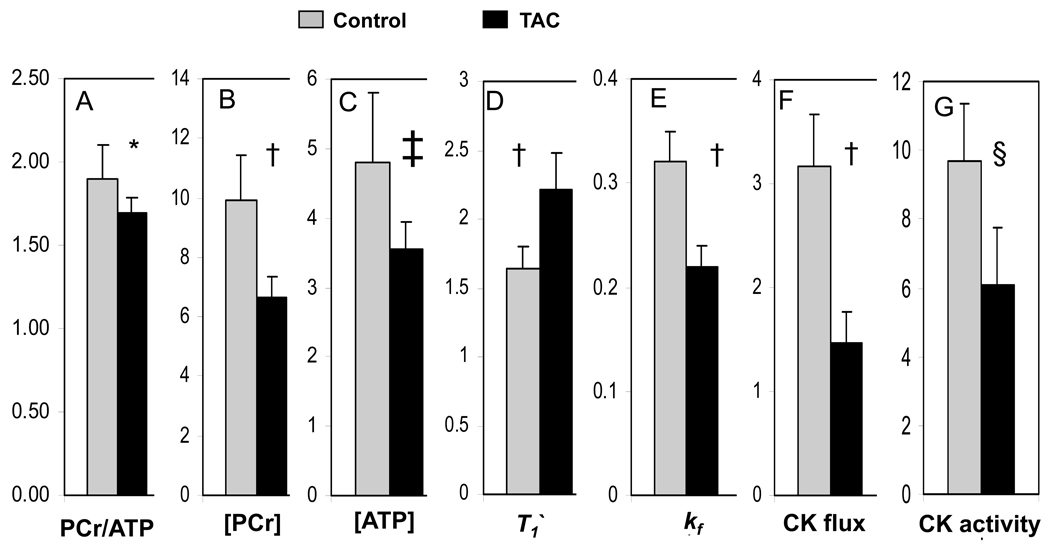
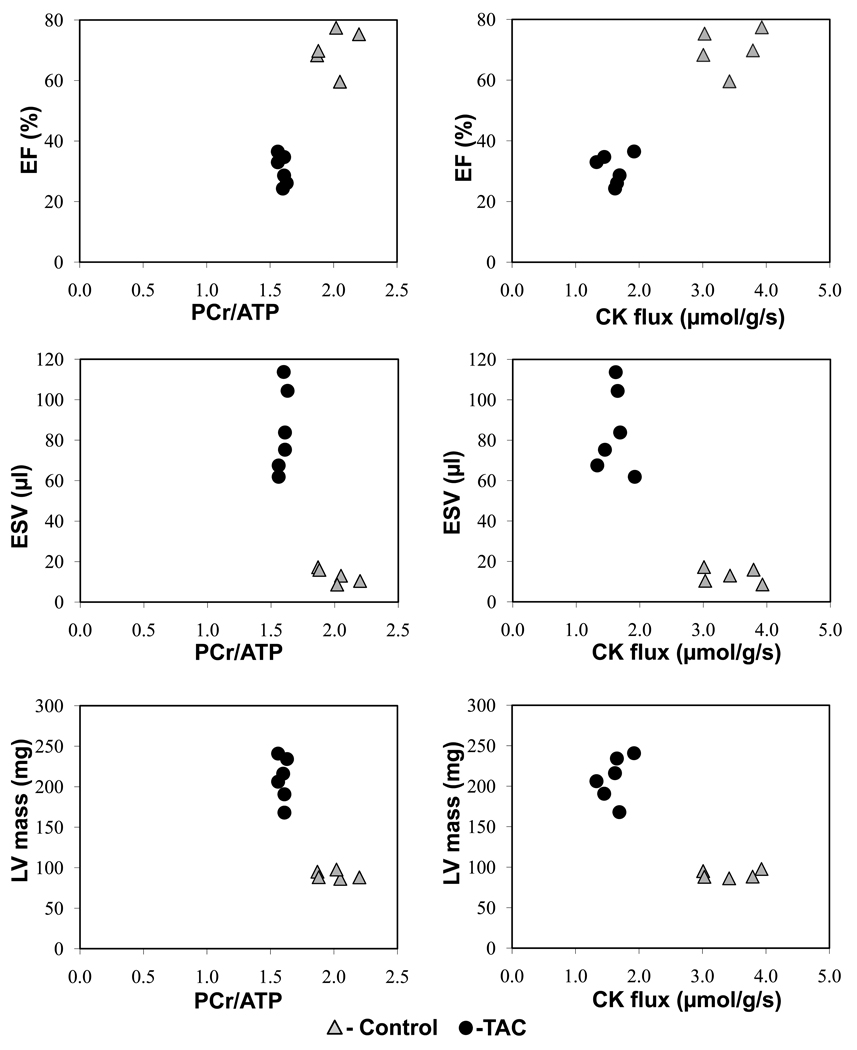
A representative high-resolution transverse 1H MR cardiac image and corresponding spatially-localized cardiac 31P MR spectra under different saturating conditions from the anterior wall of the heart are shown in Figure 5. The decrease in the PCr resonance with selective saturation of γ–ATP is directly proportional to the rate of ATP synthesis through the CK reaction in the heart22. Figure 6 shows summary data for in vivo PCr/ATP ratio, [PCr], [ATP], T1’, kf, CK flux, and in vitro CK activity in control (n=11, heart rate 457±22 min−1) and TAC (n=10, heart rate 451±35 min−1) hearts. The mean in vivo PCr/ATP ratio was significantly reduced in TAC (1.66±0.10) as compared to control mice (1.90±0.17, p<0.001), consistent with prior reports4,15. The mean in vivo [PCr] was reduced by 33% in TAC (6.61±0.59 µmol/g of wet weight) as compared to that of control mice (9.95±1.5 µmol/g of wet weight, p<0.0001). Mean in vivo [ATP] was reduced by 26% in TAC (3.59±0.37 µmol/g of wet weight) as compared to that of control mice (4.81±1.06 µmol/g of wet weight, p<0.003). The [PCr] and [ATP] values and their relative reduction with TAC agree with those previously reported in this model15. In control and TAC mice, the mean in vivo T1’ was 1.64±0.16 s and 2.21±0.27 s, respectively (p<0.0001). Mean in vivo cardiac kf was reduced by 31% in TAC (0.23±0.03 s−1) as compared to that of control mice (0.32±0.03 s−1, p<0.0001) and these values and the relative reduction also agree with those in other species, including humans8–9, 23–24. The mean in vivo forward rate of ATP synthesis through cardiac CK was 3.16±0.47µmol/g/s in control mice and 1.54±0.25 µmol/g/s in TAC mice (p<0.0001). The absolute values and the ~50% reduction in the failing hearts are similar to those observed in normal and failing human studies8–9. In vitro CK activity, measured in the same hearts was also significantly decreased in TAC mice (6.06±1.7 IU/mg protein) as compared to that of controls (9.66 ± 1.8 IU/mg protein, p<0.0006). The relationship between cardiac energetic indices derived from MRS (PCr/ATP and ATP flux through CK) and functional/anatomic indices derived from MRI (EF, ESV, mass) are shown in Figure 7.
Figure 5.
(A) Typical transverse 1H magnetic resonance image of a mouse at the mid left ventricle with the nominal location of 31P MR cardiac voxel denoted between the white lines (B) 31P MR spectrum with γ-phosphate of ATP saturation with TR=1.5s, NEX=96, (C) γ-phosphate ATP saturation with TR=6s, NEX=32, and (D) control saturation spectrum with TR=10s and NEX=16. PCr; phosphocreatine, β-ATP; β-phosphate of adenosine triphosphate
Figure 6.
Cardiac metabolic parameters for control (gray bars) and TAC (black bars) mice: (A) PCr/ATP ratio, (B) PCr concentration ([PCr]) (µmol/g wet weight), (C) ATP concentration ([ATP]) (µmol/g wet weight), (D) T1’ of PCr (s), (E) CK forward pseudo-first-order rate constant, kf (s−1), (F) the rate of ATP synthesis through CK (“CK flux”, µmol/g/s), (G) in vitro CK activity (IU/mg protein).
Control vs TAC mouse *, p <0.001; †, p <0.0001; ‡, p <0.003; §, p <0.0006.
Figure 7.
Cardiac MRS parameters (PCr/ATP ratio, left panel; rate of ATP synthesis through CK “CK flux”, µmol/g/s, right panel) versus cardiac MRI parameters (ejection fraction (EF), %; end systolic volume (ESV), µl; and LV mass, mg for control (triangles) and TAC (filled circle) animals.
Discussion
There are five novel aspects to this work. First, these are the first non-invasive, direct measures of ATP synthesis in the in vivo mouse heart. They were acquired with a non-invasive 31P magnetic resonance spectroscopy ST method, TRiST, initially developed for people, adapted here to mice, and validated here in vivo by comparison with conventional ST measures in skeletal muscle. Second, despite the massive difference in size and resting rate between murine and human hearts, the rate of ATP synthesis (~3 µmol/g/s) through CK, the primary myocardial energy reserve, is remarkably similar per gram between these species. Third, long-standing pressure-overload and heart failure induced by TAC results in a ~30% reduction in kf and a ~50% decline in the rate of ATP synthesis through CK. Fourth, the reduction in ATP flux through CK is much greater than the reduction in PCr/ATP ratio or [ATP] suggesting the previously used indices of myocardial energetics based on metabolite pool sizes underestimate the reduction in myofibrillar ATP delivery in failing hearts. Fifth, the 50% reduction in ATP flux through CK observed here in the in vivo failing TAC mouse heart is similar to the 50% reduction in patients with dilated cardiomyopathy8 and the 65% reduction in patients with pressure-overload hypertrophy and heart failure9, suggesting that this murine model mimics important bioenergetic aspects of human heart failure.
Creatine kinase is the primary energy reserve of the heart, reversibly exchanging a high energy phosphate group between ATP and phosphocreatine (PCr):
This reaction rapidly buffers ADP and ATP in cardiac and skeletal muscle and can be a rapid source of ATP during ischemia and burst activity in skeletal muscle25–29. The CK reaction has also been postulated to shuttle or transfer high energy phosphates from the mitochondria, where ATP is synthesized, to the cytosol, where it is used, and back for rephosphorylation30–31. This spatial buffering function is supported by the smaller size and molecular weight of PCr and creatine, which diffuse more rapidly than ATP and ADP, and by the compartmentalization of separate mitochondrial and cytosolic isoforms of CK, also critical for CK temporal ATP buffering25–26,30. There are several derangements in CK during heart failure that may limit ATP transfer and these include reductions in the pool sizes of CK substrates, reduced CK activity, altered isoform distribution and, most importantly, a reduction in the rate of ATP synthesis through CK1–2, 28,32.
31P MRS is uniquely able to non-invasively and repetitively measure the CK reactants, ATP and PCr, and to quantify the rate of ATP flux through CK in the beating heart33. Saturation transfer is a class of MRS techniques that allows quantification of chemical exchange through specific reactions and such techniques were first used to explore cardiac CK kinetics nearly 30 years ago33. Conventional magnetization transfer experiments are very time consuming, typically requiring seven or more fully-relaxed acquisitions for a single measurement. In addition, spatial-localization techniques, used to obtain 31P signals from the heart in closed-chest animals, are also time-consuming as compared to simple one-pulse experiments used in open-chest or isolated heart studies. Thus, despite the biological appeal of measuring the rate of ATP synthesis non-invasively in the in vivo beating heart under physiologic conditions or as pathology develops, spatially-localized conventional saturation transfer techniques simply require too much time, in some instances many hours, to be easily tolerated by anesthetized animals or by patients. The first measures of ATP flux through CK in the human heart were obtained with a new, more rapid saturation transfer approach that required fewer acquisitions under incompletely relaxed conditions by exploiting acquisitions with precise but different flip angles (four angle saturation transfer, FAST)22. The FAST method required approximately one-seventh the time of conventional saturation transfer and yielded a mean rate of 3.4µmol/g/s for ATP synthesis through CK in the normal human heart8. To overcome power limitations at higher, 3T field strengths for patient studies, another rapid approach was developed that varied the repetition time rather than the flip angle and this method, called TRiST for triple repetition time saturation transfer, gave similar results for CK kinetics in the human heart as prior FAST reports22 and offered a 73% time savings over conventional saturation transfer10. More recently another rapid ST approach was described that uses a strategy of pre-saturation delays numerically optimized for the expected kf34. This method was used to study kinetics in the open-chest dog heart and offered a time savings of up to 82%34. All three approaches were validated by comparison to conventional saturation transfer measurements obtained under non-localized conditions. A similar approach for validation was used here in the in vivo closed-chest mouse.
The mouse is a commonly used, important model for many human diseases because it allows specific gene-targeted interventions of over-expression or knock-out. In the absence of genetic manipulation, the normal mouse heart exhibits some fundamental similarities to normal human heart in high-energy phosphate metabolism and global left ventricular ejection fraction3,15. We adapted TRiST to the mouse heart and validated it by direct comparison with conventional ST measures in skeletal muscle. There was no significant difference between the measured T1’ or kf between the two methods (Figures 1 and 3), although TRiST measures took 73% less time. This time saving allowed us to perform image-guided, spatially-localized TRiST measurements in the mouse heart under in vivo closed-chest conditions despite the small heart weight and rapid heart rates. Although there are large species differences in size and heart rate between mice and humans, the constancy of the cardiac PCr/ATP ratio3, the absolute concentrations of [PCr] and [ATP]15, myocardial oxygen consumption11,35, and, as shown in this study for the first time, the rate of ATP synthesis through CK is striking and speaks to the central importance of the ATP reserve offered by CK across species.
The current findings of a significant ~50% decrease in regeneration of myocardial ATP, through CK, in the murine TAC heart (Figure 6) are consistent with the prior reports in larger animal models of heart failure and clinical studies. In hypertrophied canine hearts one year after aortic banding, the in vivo rate of ATP synthesis through CK was reduced by 50%, while in vitro CK activity was unchanged36. Two months of aortic banding in Yorkshire pigs resulted in severe hypertrophy in all animals, and heart failure in half37. Hypertrophied swine exhibited a 35% mean reduction in ATP flux through CK, while animals in heart failure showed a 57% reduction in ATP flux through in vivo CK and a 48% reduction in in vitro CK activity37. Thus the current findings of a 50% mean reduction in ATP flux through in vivo CK in TAC mouse hearts are consistent with reductions observed in aortic-banded pigs and dogs. Changes in in vitro measures of total CK activity sometimes37 as in this report, but not always36, are similar to changes in the in vivo rate of ATP synthesis through CK. Importantly, the magnitude of the reduction in ATP kinetics observed here in TAC mice is similar to that observed in failing human hearts8–9. In patients with non-ischemic dilated cardiomyopathy the mean rate of ATP synthesis through CK was reduced by 50%8. In patients with pressure-overload hypertrophy, the rate of ATP synthesis through CK was reduced by 30% in those without HF and by 65% in those with HF symptoms9. Thus a significant reduction in the rate of ATP delivery by CK is a common finding in hypertrophied and failing hearts from mice through humans. Because the magnitude of the reduction of in vivo CK ATP capacity is similar in TAC mice and failing human hearts, this model and the non-invasive repetitive aspects of the new technique appear to be appealing, important tools to probe energetic hypotheses of heart failure development and progression.
ATP generated by the CK reaction and that from the ATPase, the latter derived mostly from oxidative metabolism, support contractile energy demands and may be limiting under some conditions, especially high-workloads in large animals38. Murine myocardial energy demands (per gram tissue/unit time) are likely similar to, or only modestly higher, than those of human hearts11,35 because the ATP requirements of the 7–9 fold higher murine heart rates are mostly offset by the 6–7 fold lower wall tension in the dramatically smaller murine LV. Since the rate of ATP synthesis through CK is similar in mice and humans, the ATP reserve provided by CK could, in theory, be inadequate at high-workloads and/or in species with higher energy demands. Because the high-energy phosphate pools are small compared to their high rate of turnover8–9,12 and because high-energy phosphates are consumed on a millisecond time scale during muscle contraction 33,39–41, a 50% reduction in the CK energy reservoir in the failing heart represents a significant deficit in temporal, and possibly spatial, ATP delivery8–9. However, it is still not known whether in fact a reduction in the rate of ATP delivery by CK of the magnitude measured here, or a reduction in that provided by the ATPase, not studied here, is the cause of LV dysfunction in HF. Additional studies determining the direct contractile consequences of independent manipulations of the rate of ATP delivery through such reactions would be needed to answer that question as well as studies at maximal workload conditions.
There are several limitations that merit mention. First, because significant reductions in kf were previously observed in failing hearts but not in compensated, hypertrophied hearts in both pigs37 and patients9, additional, serial studies are needed in mice at earlier times after TAC to determine whether kf can distinguish compensated and failing hypertrophied mouse hearts and predict the development of anatomic remodeling and decreased contractile function in this setting. Second, like other in vivo saturation transfer techniques this 31P MRS TRiST approach applied to the in vivo mouse heart, cannot render CK results for the different sub-cellular compartments42. It does however reveal the net rate of ATP synthesis through CK22 and this parameter is significantly altered in HF patients8–9.
In summary, the first non-invasive, direct, validated measures of ATP synthesis in the in vivo mouse heart are reported. Despite the manifold difference in size and resting rate between mouse and human hearts, the rate of ATP synthesis through CK is remarkably similar per gram between these species. The rate of ATP synthesis through CK is reduced by 50% in the murine TAC heart and this reduction is comparable to that previously reported in canine and ovine pressure-overload models and importantly in human heart failure. The ability to non-invasively measure ATP kinetics in the intact, in vivo mouse heart promises new insights into the role of bioenergetics in contractile dysfunction and ventricular remodeling in this animal model with readily available genetic manipulations and similarities to human physiology and clinical heart failure.
Energy metabolism fuels ongoing myocardial contraction and altered metabolism may contribute mechanistically to several cardiac diseases including heart failure. 31P magnetic resonance spectroscopy (MRS) is the only means to non-invasively quantify the levels of myocardial high-energy phosphates, ATP and creatine phosphate. Magnetization transfer MRS methods were adapted in recent years to measure the rate of ATP turnover in the human heart. Such MRS techniques, when combined with magnetic resonance imaging (MRI) approaches, offer a powerful means to assess cardiac energy metabolism, structure, mass, and contractile function in the same examination. Murine models are increasingly used to probe molecular mechanisms of human disease because of the possibility of creating specific transgenic lines. However, the small size (~0.1g) and high rate (~600/min) of the mouse heart present significant challenges for non-invasive imaging approaches.
In this paper, the first non-invasive in vivo measures of the rate of ATP synthesis through creatine kinase (CK), the major myocardial energy reserve, in the murine heart are reported. They demonstrate that ATP turnover rates in normal and failing mouse hearts are comparable to those reported in human hearts. This validated, non-invasive approach to measuring ATP synthesis rates through CK in the intact mouse can be combined with anatomic and functional measures in the same exam and be repeated over time as pathology develops. The observations demonstrate that murine models recapitulate several fundamental aspects of human myocardial energy metabolism in vivo and support their use to probe specific metabolic contributions to the development and progression of heart failure.
Acknowledgments
Sources of Funding
This work is supported by National Institutes of Health Grants HL-63030 and HL-61912 as well as funds from the Donald W. Reynolds Foundation and the Clarence Doodeman Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Katz AM. Is the failing heart energy depleted? Cardiol Clin. 1998;16:633–644. doi: 10.1016/s0733-8651(05)70040-0. [DOI] [PubMed] [Google Scholar]
- 2.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 3.Chacko VP, Aresta F, Chacko SM, Weiss RG. MRI/MRS assessment of in vivo murine cardiac metabolism, morphology and function at physiological heart rates. Am J Physiol Heart Circ Physiol. 2000;279:H2218–H2224. doi: 10.1152/ajpheart.2000.279.5.H2218. [DOI] [PubMed] [Google Scholar]
- 4.Maslov MY, Chacko VP, Stuber M, Moens AL, Kass DA, Champion HC, Weiss RG. Altered high energy phosphate metabolism predicts contractile dysfunction and subsequent ventricular remodeling in pressure overload hypertrophy mice. Am J Physiol Heart Circ Physiol. 2007;292:H387–H391. doi: 10.1152/ajpheart.00737.2006. [DOI] [PubMed] [Google Scholar]
- 5.Conway MA, Allis J, Ouwerkerk R, Niioka T, Rajagopalan B, Radda GK. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet. 1991;338:973–976. doi: 10.1016/0140-6736(91)91838-l. [DOI] [PubMed] [Google Scholar]
- 6.Hardy CJ, Weiss RG, Bottomley PA, Gerstenblith G. Altered myocardial high-energy phosphate metabolites in patients with dilated cardiomyopathy. Am Heart J. 1991;122:795–801. doi: 10.1016/0002-8703(91)90527-o. [DOI] [PubMed] [Google Scholar]
- 7.Beer M, Seyfarth T, Sandstede J, Landschutz W, Lipke C, Kostler H, Kienlin VM, Harre K, Hahn D, Neubauer S. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol. 2002;40:1267–1274. doi: 10.1016/s0735-1097(02)02160-5. [DOI] [PubMed] [Google Scholar]
- 8.Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci. 2005;102:808–813. doi: 10.1073/pnas.0408962102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, Weiss RG. Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation. 2006;114:1151–1158. doi: 10.1161/CIRCULATIONAHA.106.613646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schär M, El-Sharkawy AM, Weiss RG, Bottomley PA. Triple Repetition Time Saturation Transfer (TRiST) 31P spectroscopy for measuring human creatine kinase reaction kinetics. Mag Reso Med. 2010;63:1493–1501. doi: 10.1002/mrm.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saupe KW, Spindler M, Tian R, Ingwall JS. Impaired cardiac energetics in mice lacking muscle-specific isoenzymes of creatine kinase. Circ Res. 1998;82:898–907. doi: 10.1161/01.res.82.8.898. [DOI] [PubMed] [Google Scholar]
- 12.Saupe WK, Spindler M, Hopkins CAJ, Shen W, Ingwall JS. Kinetic thermodynamic and developmental consequences of deleting creatine kinase isoenzymes from the heart. J Biol Chem. 2000;275:19742–19746. doi: 10.1074/jbc.M001932200. [DOI] [PubMed] [Google Scholar]
- 13.Hove MT, Lygate CA, Fischer A, Schneider JE, Sang AE, Hulbert K, Montefiore LS, Watkins H, Clarke K, Isbrandt D, Wallis J, Neubauer S. Reduced inotropic reserve and increased susceptibility to cardiac ischemia/reperfusion injury in phosphocreatine-deficient guanidinoacetate-N-methyltransferase-knockout mice. Circulation. 2005;111:2477–2485. doi: 10.1161/01.CIR.0000165147.99592.01. [DOI] [PubMed] [Google Scholar]
- 14.Pinz I, Ostroy SE, Hoyer K, Osinska H, Robbins J, Molkentin JD, Ingwall JS. Clacineurin-induced energy wasting in a transgenic mouse model of heart failure. Am J Physiol Heart Circ Physiol. 2008;294:H1459–H1466. doi: 10.1152/ajpheart.00911.2007. [DOI] [PubMed] [Google Scholar]
- 15.Gupta A, Chacko VP, Weiss RG. Abnormal energetics and ATP depletion in pressure-overload mouse hearts: in vivo high-energy phosphate concentration measures by noninvasive magnetic resonance. Am J Physiol Heart Circ Physiol. 2009;297:H59–H64. doi: 10.1152/ajpheart.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguer ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 17.Barker PB, Sibisi S. Non-linear least squares analysis of in vivo 31P NMR data (abstract) Soc Mag Reson Med. 1990;9:1089. [Google Scholar]
- 18.Bottomley PA, Hardy CJ, Roemer PB, Weiss RG. Problems and expediencies in human 31P spectroscopy. The definition of localized volumes, dealing with saturation and technique-dependence of quantification. NMR Biomed. 1989;20:284–289. doi: 10.1002/nbm.1940020518. [DOI] [PubMed] [Google Scholar]
- 19.El-Sharkawy AM, Schär M, Ouwerkerk R, Weiss RG, Bottomley PA. Quantitative cardiac 31P spectroscopy at 3T using adiabatic pulses. Mag Reson Med. 2009;61:785–795. doi: 10.1002/mrm.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Oliver IT. A spectrphotometric method for the determination of creatine phosphokinase and myokinase. Biochem J. 1955;61:116–122. doi: 10.1042/bj0610116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottomley PA, Ouwerkerk R, Lee RF, Weiss RG. Four-Angle Saturation Transfer (FAST) method for measuring creatine kinase rates in vivo. Mag Reso Med. 2002;47:850–863. doi: 10.1002/mrm.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portman MA, Ning XH. Maturational changes in respiratory control through creatine kinase in heart in vivo. Am J Physiol Cell Physiol. 1992;263:C453–C460. doi: 10.1152/ajpcell.1992.263.2.C453. [DOI] [PubMed] [Google Scholar]
- 24.Katz LA, Swain JA, Portman MA. Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am J Physiol Heart Circ Physiol. 1989;256:H265–H274. doi: 10.1152/ajpheart.1989.256.1.H265. [DOI] [PubMed] [Google Scholar]
- 25.Wallimann T, Wyss M, Brdiczka D, Nicolayt K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallimann T. Dissecting the role of creatine kinase. Curr Biol. 1994;4:42–46. doi: 10.1016/s0960-9822(00)00008-7. [DOI] [PubMed] [Google Scholar]
- 27.Ingwall JS, Kramer MF, Fifer MA, Lorell BH, Shemin R, Grossman W, Allen PD. The creatine kinase system in normal and diseased human myocardium. N Engl J Med. 1985;313:1050–1054. doi: 10.1056/NEJM198510243131704. [DOI] [PubMed] [Google Scholar]
- 28.Shen W, Assai K, Uechi M, Mathier MA, Shannon RP, Vatner SF, Ingwall JS. Progressive loss of myocardial ATP due to a loss of total purines during the development of heart failure in dogs. Circulation. 1999;100:2113–2118. doi: 10.1161/01.cir.100.20.2113. [DOI] [PubMed] [Google Scholar]
- 29.Flogel U, Decking UKM, Godecke A, Schrader J. Contribution of NO to ischemia-reperfusion injury in the saline-perfused heart: a study in endothelial NO synthase knockout mice. J Mol Cell Cardiol. 1999;31:827–836. doi: 10.1006/jmcc.1998.0921. [DOI] [PubMed] [Google Scholar]
- 30.Bessman SP, Carpenter CL. The creatine-creatine phosphate energy shuttle. Annu Rev Biochem. 1985;54:831–862. doi: 10.1146/annurev.bi.54.070185.004151. [DOI] [PubMed] [Google Scholar]
- 31.Dzeja PP, Terzic A. Phosphotransfer networks and cellular energetics. J Exp Biol. 2003;206(Pt 12):2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- 32.Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol. 2004;15(555(Pt 1)):1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ingwal JS. Phosphorus nuclear magnetic resonance spectroscopy of cardiac and skeletal muscles. Am J Physiol. 1982;242:H729–H744. doi: 10.1152/ajpheart.1982.242.5.H729. [DOI] [PubMed] [Google Scholar]
- 34.Xiong Q, Li Q, Mansoor A, Jameel MN, Du F, Chen W, Zhang J. Novel strategy for measuring creatine kinase reaction rate in the in vivo heart. Am J Physiol Heart Circ Physiol. 2009;297:H1010–H1019. doi: 10.1152/ajpheart.01195.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson LR, Herrero P, McGill J, Schechtman KB, Ware ZK, Lesniak D, Gropler RJ. Fatty acids and insulin modulate myocardial substrate metabolism in humans with type 1 diabetes. Diabetes. 2008;57:32–40. doi: 10.2337/db07-1199. [DOI] [PubMed] [Google Scholar]
- 36.Ye Y, Wang C, Zhang J, Cho YK, Gong G, Murakami Y, Bache RJ. Myocardial creatine kinase kinetics and isoform expression in hearts with severe LV hypertrophy. Am J Physiol Heart Circ Physiol. 2001;281:H376–H386. doi: 10.1152/ajpheart.2001.281.1.H376. [DOI] [PubMed] [Google Scholar]
- 37.Ye Y, Gong G, Ochiai K, Liu J, Zhang J. High energy phosphate metabolism and creatine kinase in failing hearts: A new porcine model. Circulation. 2001;103:1570–1576. doi: 10.1161/01.cir.103.11.1570. [DOI] [PubMed] [Google Scholar]
- 38.Bache RJ, Zhang J, Murakami Y, Zhang Y, Cho YK, Merkle H, Gong G, From AHL, Ugurbil K. Myocardial oxygenation at high workstates in hearts with left ventricular hypertrophy. Cardiovas Res. 1999;42:616–626. doi: 10.1016/s0008-6363(98)00332-0. [DOI] [PubMed] [Google Scholar]
- 39.Monroe RG. Myocardial oxygen consumption during ventricular concentration and relaxation. Circ Res. 1964;14:294–300. doi: 10.1161/01.res.14.4.294. [DOI] [PubMed] [Google Scholar]
- 40.Fossel ET, Morgan HE, Ingwall JS. Measurement of changes in high-energy phosphates in the cardiac cycle by using gated 31P nuclear magnetic resonance. Proc Natl Acad Sci. 1980;77:3654–3658. doi: 10.1073/pnas.77.6.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung Y, Sharman R, Carlsen R, Unger SW, Larson D, Jue T. Metabolic fluctuation during a muscle contraction cycle. Am J Physiol. 1998;274:C846–C852. doi: 10.1152/ajpcell.1998.274.3.C846. [DOI] [PubMed] [Google Scholar]
- 42.Joubert F, Mazet J-L, Mateo P, Hoerter JA. 31P NMR Detection of subcellular creatine kinase fluxes in the perfused rat heart; contractility modifies energy transfer pathways. J Biol Chem. 2002;277:18469–18476. doi: 10.1074/jbc.M200792200. [DOI] [PubMed] [Google Scholar]