Abstract
The anthrax toxin receptors tumor endothelial marker-8 (TEM-8) and capillary morphogenesis gene-2 (CMG-2) are responsible for allowing entry of anthrax toxin into host cells. However, these receptors were first discovered due to their enhanced expression on endothelial cells undergoing blood vessel growth or angiogenesis in in vitro or in vivo model systems. Targeting and inhibiting angiogenesis is an important strategy for current anti-cancer therapies and treatment of retinal diseases. Structures, tissue expression, and interactions of the TEM-8 and CMG-2 proteins have been documented, and functional roles for these receptors in angiogenesis have recently emerged. TEM-8 appears to regulate endothelial cell migration and tubule formation whereas a role for CMG-2 in endothelial proliferation has been documented. TEM-8 and CMG-2 bind differentially to extracellular matrix proteins including collagen I, collagen IV and laminin and these properties may be responsible for their apparent roles in regulating endothelial cell behavior during angiogenesis. TEM-8-binding moieties have also been suggested to be useful in selectively targeting anti-angiogenic and anti-tumorigenic therapies to tumor endothelium. Additionally, studies of modified forms of lethal toxin (LeTx) have demonstrated that targeted inhibition of MAPKs within tumor vessels may represent an efficacious anti-angiogenic strategy.
Keywords: Endothelial, angiogenesis, anthrax, intracellular signaling, extracellular matrix
2. INTRODUCTION
The growth of new blood vessels or ‘angiogenesis’ is essential for many physiological processes, including embryonic development, wound healing, and tissue regeneration and remodeling. However, uncontrolled or defective angiogenesis also contributes to many pathological processes including tumor growth, metastasis and proliferative retinal diseases. Anti-angiogenic drugs are used as part of the treatment strategies for some types of cancer, and the emergence of anti-vascular endothelial growth factor (VEGF) therapies for age-related macular degeneration has dramatically improved outcomes for patients losing their sight from this disease. Angiogenesis is a multistep process involving degradation of the extracellular matrix (ECM), migration and proliferation of endothelial cells, vessel anastamosis, followed by stabilization of the new vessel network with recruitment of pericytes and establishment of a surrounding basement membrane.
Angiogenesis is controlled on the molecular level by signaling pathways initiated by central angiogenic growth factors such as VEGF through their receptors, resulting in changes in gene expression. While the complex process of angiogenesis has not been fully replicated in cell culture, a number of in vitro assays have been developed to assess the contribution of pro-angiogenic molecules and the effects of anti-angiogenic pharmacological agents on individual aspects of endothelial cell behavior. For example endothelial cell proliferation, migration and tubule formation within collagen gels can all be assessed in vitro. Endothelial cells undergoing tubule formation within 3D collagen gels upregulate numerous genes including genes involved in basement matrix assembly, growth factors, adhesion receptors and capillary morphogenesis gene-2 (CMG-2) (1). The latter protein was later identified as a receptor for anthrax toxin and in that context named anthrax toxin receptor 2 (ANTXR2) (2).
Within tumors, blood vessels are often fragile and leaky with poor pericyte recruitment and defective establishment of basement membranes. Tumor vessels also differentially express many different genes when compared with normal functional blood vessels (3). A panel of ‘tumor endothelial markers’ (TEMs), which are overexpressed in malignant colorectal tumor endothelium compared to normal endothelium, have been described (3). One of these genes, increased in expression in tumor endothelium, TEM-8 was subsequently discovered to be a receptor for anthrax toxin. Thus, both of the known receptors for anthrax toxin, CMG-2 and TEM-8, were first described as genes upregulated in the vasculature during angiogenesis.
3. ANTHRAX TOXIN RECEPTORS - TEM-8 and CMG-2
3.1. TEM-8 and CMG-2 in anthrax toxicity
Bacillus anthracis is a gram-positive rod-shaped spore forming bacterium and the causative agent of anthrax toxicity (4). B. anthracis spores are taken up in macrophages adjacent to the epithelial route of entry, which may be through the skin, gastrointestinal tract or by inhalation in the respiratory tract. Macrophages are then transported to the lymph nodes where germination of the spores occurs. There are two major virulence factors for B. anthracis, the capsule which protects against phagocytosis and a tripartite toxin. Individual subunits of the anthax toxin—lethal factor (LF), edema factor (EF), and protective antigen (PA)—are released from the bacterium and cause pathology in infected human or animal hosts. The three proteins are non-toxic individually, but assemble into toxic complexes on the surface of receptor-expressing host cells. PA is an 83 kDa pore-forming protein that binds to the anthrax receptors on the surface of the target cell, orchestrating entry of the two enzymatic components of the toxin, LF and EF, into the cell cytoplasm.
PA can bind to two cell surface receptors, TEM-8 and CMG-2, also known as ANTXR1 and ANTXR2 respectively (2, 5). Receptor-bound PA is proteolytically cleaved by furin, resulting in the removal of a 20 kDa fragment of PA from the N terminus. The remaining receptor bound 63kDa PA heptamerizes, instigating the formation of a prepore that can bind up to 3 molecules of LF or EF and triggering internalization of the complex. Following internalization, the low pH environment of the endocytic compartment causes a conformational rearrangement of the toxin complex allowing for delivery of LF and EF to the cytosol where they act on their intracellular substrates. LF is a 90-kDA zinc-dependent metalloprotease that cleaves mitogen-activated protein kinase kinases (6, 7) and EF (89kDa) is a calmodulin-dependent adenylate cyclase (8). Endocytosis requires toxin-induced ubiquitination of anthrax receptors by the E3 ligase Cbl (9). Recent studies have also demonstrated that clathrin-mediated endocytosis of the toxin is dependent on the heterotetrameric adaptor protein AP-1 (10).
The intracellular actions of EF and LF impair affected cells and can ultimately lead to death of the host. Treatment with antibiotics can be lifesaving at early stages of the disease, but if sufficient toxin has been delivered the disease is often lethal. It is unclear whether B. anthracis utilizes TEM-8 and CMG-2 merely as portals of entry into the cell or if additional cytoplasmic signaling mechanisms from these receptors further augment anthrax entry and pathogenesis. Mouse mutants lacking the transmembrane regions of each of the receptors have been recently described and are viable (11, 12). Experiments in CMG-−/− and TEM-8−/− mice demonstrated that CMG-2 is the major receptor responsible for mediating anthrax lethality in mice (12).
3.2. Diseases linked to TEM-8 and CMG-2 mutations
Although the precise functions of the anthrax receptors in the absence anthrax toxin are currently unknown, diseases resulting from mutations in the genes for TEM-8 and CMG-2 suggest that these proteins have important physiological functions distinct from their role during anthrax toxin endocytosis. The CMG-2 gene is located on chromosome 4q of the human genome (1). Evidence for an important function for CMG-2 in extracellular matrix interaction comes from mutations in this gene that are linked to both juvenile hyaline fibromatosis (JHF) and infantile systemic hyalinosis (ISH) (13). These systemic hyalinoses syndromes are generalized fibromatoses characterized by accumulation of hyaline in the dermis. Both are believed to be allelic disorders, with ISH having a more severe phenotype. Two of the ISH patient-derived mutations result in deletions of transmembrane or cytosolic regions of the protein (13). The extracellular domain of CMG-2 is known to bind to both Collagen I and laminin (1). Fibroblasts from patients with CMG-2 mutations exhibit altered interactions with laminin, although significant differences in interactions with Collagen I or Collagen IV were not observed with these cells (13).
The human TEM-8 gene is located on chromosome 2p14. A TEM-8 mutation has been described in a patient with infantile haemangioma, a disease characterized by localized and rapidly growing areas of angiogenesis (14). TEM-8 mutants expressed in HDMECs result in reduced VEGFR1 expression and increased expression of phosphorylated VEGFR2 and phosphorylated ERK (14). The consequence of this mutation is increased interactions between TEM-8, VEGFR2 and beta 1 integrin proteins and a reduction in integrin activity (14).
3.3. TEM-8 and CMG-2 protein structures
TEM-8 and CMG-2 are type one transmembrane proteins containing extracellular von Willebrand Factor A (VWA) domains of approximately 200 amino acids, which in turn contain a metal ion dependent adhesion site (MIDAS) motif. The VWA domains of TEM-8 and CMG-2 are the sites for the metal-dependent interaction with PA. VWA domains facilitate protein-protein interactions when found on extracellular matrix proteins such as collagen VI and collagen XIV, and cell adhesion receptors such as the integrins. CMG-2 was originally described as a 386 amino acid protein with an extracellular, transmembrane and cytoplasmic domain (1), with additional isoforms resulting from differential splicing of CMG-2 mRNA also described (see below) (2). Three splice variants of TEM-8 have also been described (see below) (5, 15, 16). Across the genome, the two proteins are most closely related to each other. TEM-8 splice variant 1 and CMG-2489 share 40% amino acid identity throughout their sequence and 60% amino acid identity within their VWA domains (2).
Binding of the PA portion of anthrax toxin to the VWA/1 domain of both TEM-8 or CMG-2 is dependent on the presence of a divalent cation (2, 5). Both receptors bind PA in the presence of Mn2+, Mg2+ or Zn2+, and CMG-2 has been shown to also use Ca2+ as the divalent cation (2, 17). CMG-2/PA binding exhibits an approximately 5-fold preference for Mg2+ rather than Ca2+ (18). The crystal structure of the extracellular VWA domain of CMG-2 has been described and it appears as an open conformation in contrast to the VWA domains of alpha integrins (19). The interaction of PA with the VWA domain of CMG-2 is of a relatively high affinity (~200 pM) when compared to interactions between VWA domains of other proteins and their natural ligands (18). The dissociation rate of the CMG-2/PA complex is also very slow, and this property along with the tight binding of PA suggests the potential therapeutic utility of soluble forms of CMG-2 for anthrax toxicity (18). The affinity of PA for the VWA domains of TEM-8 in the presence of divalent metal cations is significantly lower (Kd in the high nM range) than that for CMG-2 and is comparable to the affinities for reported for integrin-ligand interactions (20).
Four different isoforms of CMG-2, encoded by alternatively spliced mRNA transcripts have been predicted (Figure 1) (2). CMG-2489 has a putative signal peptide, extracellular VWA/I domain and a putative transmembrane region (2). CMG-2488 is almost identical to CMG-2489 apart from 12 alternate amino acids at the cytoplasmic tail (2). CMG-2386 lacks amino acids 213–315 of the full length protein and CMG-2322 is predicted to be a secreted protein, lacking the transmembrane domain (2). The transmembrane domain of CMG-2 is predicted to reside from amino acid residues 318–336 of CMG-2489 and CMG-2488 and residues 215–233 of CMG-2386 (2). The VWA domain is located from residues 44–213 and a putative signal peptide exists from residues 1–33 (1). Within endothelial cells, CMG-2 GFP fusion protein studies have shown expression of the medium length CMG-2386 isoform in the endoplasmic reticulum and in intracellular vesicles, but not in the Golgi apparatus or plasma membrane (1). The CMG-2 protein also contains regions with homology to WASP, a cdc42-binding protein that regulates the actin cytoskeleton (1).
Figure 1.
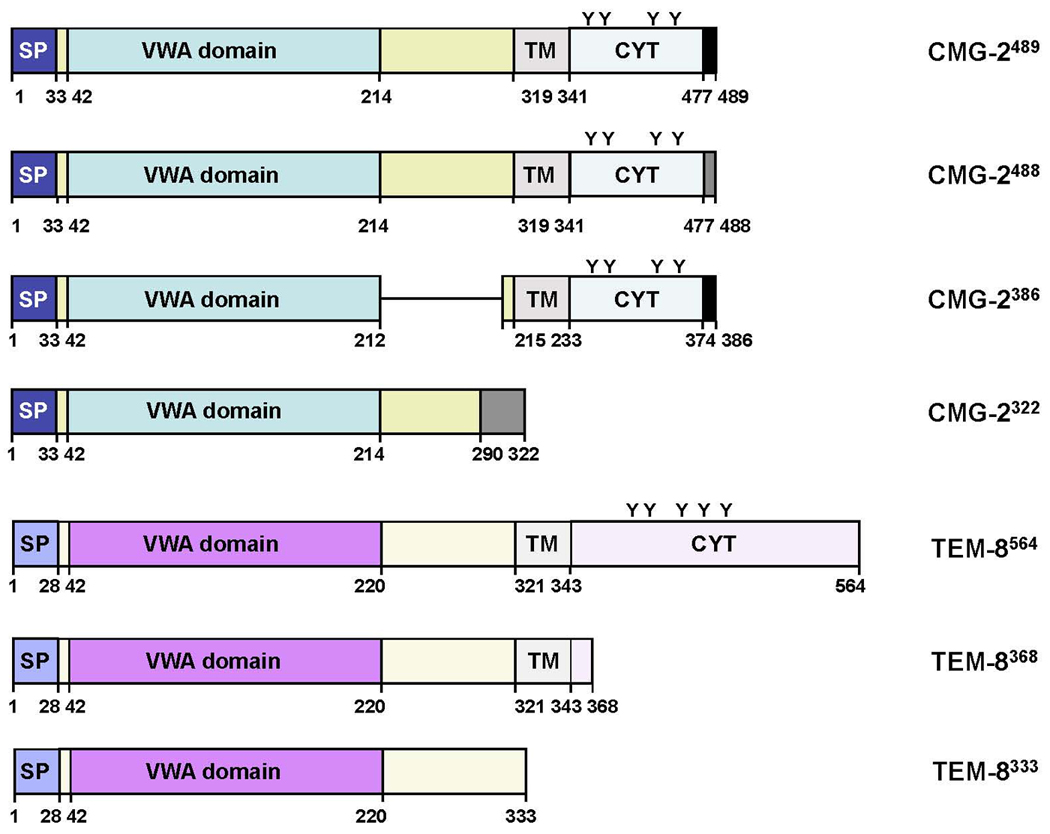
Protein structure of the TEM-8 and CMG-2 splice variants. There are three known splice variants of TEM-8 and four splice variants of CMG-2. Putative signal peptides (SP), Von Willebrand factor type A (VWA) domains, transmembrane domains (TM) and cytoplasmic (CYT) domains are denoted. Tyrosine residues within the cytoplasmic tails of TEM-8 and CMG-2 are also shown (Y).
Three protein variants of TEM-8 result from alternative splicing of the TEM-8 gene (Figure 1). The long isoform is a type I transmembrane protein of 564 amino acids in length, with a long 220-amino acid cytoplasmic tail, 73 amino acids longer than CMG-2 (2, 15). The medium isoform is a 386 amino acid protein with a much shorter cytoplasmic tail (5). The short isoform does not contain a transmembrane region, cannot internalize PA and is predicted to be a secreted protein (16). Both the long and medium isoforms function as receptors for PA and contain VWA/I domains with MIDAS regions to allow binding of PA (15). Truncation studies of the TEM-8 receptor have demonstrated that the extracellular region and transmembrane region of this receptor are essential for PA binding, processing, oligomer formation, and translocation of lethal toxin into the cytosol, whereas the cytoplasmic region is not required for these processes (16). In the absence of anthrax toxin TEM-8 resides in intracellular vesicular compartments as well as in the cell membrane (21). TEM-8 is recycled between recycling endosomes and the cell membrane in a process dependent on Rab11 GTPase but unaffected by anthrax protective antigen (21).
The cytoplasmic tails of TEM-8 and CMG-2 have important roles in regulating the half life of the receptors at the plasma membrane. Palmitoylation of cytoplasmic cysteine residues increase the half-life of the receptors by preventing premature clearance from the cell membrane (9). Both receptors contain tyrosine residues within their cytoplasmic tails, which become phosphorylated following binding of PA (22). It was recently demonstrated that phosphorylation of the receptors is required for efficient toxin uptake (22).
3.4. Expression of TEM-8 and CMG-2
The initial discoveries of TEM-8 and CMG-2 described upregulated expression of these genes during angiogenesis; in endothelial cells lining tumor blood vessels and during tubule formation in vitro respectively (1, 3). However, it has become apparent that both receptors are more widely expressed. Both TEM-8 and CMG-2 proteins are expressed in epithelia lining the organs that constitute anthrax toxins site of entry – skin, lung, and small intestine (23, 24).
Early studies using in situ hybridization analysis of human tissue demonstrated expression of TEM-8 in endothelial cells lining the vessels of colorectal tumors, but not within the surrounding normal tissue (15). Importantly, expression of TEM-8 protein was also shown in the blood vessels of colon cancer tissues with no expression detected in patient-matched normal colonic mucosa (25). Increased expression of TEM-8 was also detected in endothelial cells of many different types of human tumors, including bladder, colon, esophageal, and lung, with no expression detected in the vessels of normal tissues (25). In mouse models of carcinogenesis, expression of TEM-8 mRNA has been observed on the vessels of B16 mouse melanoma and HCT116 colon tumors (15). TEM-8 is expressed on human umbilical vein endothelial cells (HUVEC) both in situ and during early passages when cultured (26). Our laboratory has observed expression of the long, medium and short isoforms of TEM-8 in human microvascular endothelial cells (HMVECs) in culture (unpublished data).
TEM-8 is temporally regulated and induced during chick embryogenesis (27). TEM-8 was identified as being induced from developmental stage HH10, from a screen for FGF inducible genes in the chicken facial mesenchyme. FGF signaling is sufficient, but not necessary to induce TEM-8 expression in the chicken facial mesenchyme (27). In situ hybridization studies have demonstrated expression of TEM-8, in common with TEM-1 and TEM-5, at the mRNA level in endothelial cells of the developing embryonic mouse liver and brain but not in adult mouse liver or brain, further suggesting a possible role for TEM-8 during development (15). However, a more recent study has demonstrated expression of each of the TEM-8 isoforms (long, medium and short) in adult mouse tissues by western blot. The short, secreted isoform of TEM-8 that does not act as a cell-surface receptor for PA was found by western blot to be expressed in adult mouse heart, skin, liver, brain and kidney (24). The medium isoform is expressed in mouse liver, skin, small intestine, and kidney (24). The largest isoform was determined to be the most widely expressed splice variant in the mouse tissues analysed, with protein expression detected by western blot in heart, skin, small intestine, ovary, testis, spleen, liver, lung and kidney (24). Subsequent immunohistochemical analysis localized expression to epithelial cells of the tissues which represent routes of entry for B. anthracis; the skin, lung and intestine (24). However, the cell type expressing TEM-8 in other mouse tissues remains to be determined. Collectively, the studies by Nanda et al and Bonuccelli et al indicate that TEM-8 may be differentially expressed in the epithelium of the normal small and large intestine (24, 25). However, further comparative studies of human tissues will be required to confirm whether this is the case as different antibodies were used and expression of TEM-8 was studied in the mouse but not the human small intestine.
CMG-2 mRNA expression has been documented in many human tissues, including heart, skeletal muscle, colon, spleen, kidney, liver, small intestine, placenta, and lung (2). Immunohistochemical studies have shown expression of CMG-2 in the epithelial cells lining mouse skin, colon, and lung as well as in the vascular endothelium of these tissues (23). In human breast, CMG-2 is expressed in endothelial cells from both normal and malignant tissues, with further expression detected in breast tumor stroma and basement membranes but not cancer cells (23). CMG-2 expression co-localizes with Collagen IV, a putative CMG-2 ligand in both normal and malignant breast tissue (23).
Given the similar expression patterns of CMG-2 and TEM-8 in the epithelium of skin, lung, and intestine, these proteins may be co-expressed in certain tissues (23, 24). It is also possible, although undocumented to date, that the anthrax receptors interact in certain situations and cell types as part of a heteromeric complex.
4. TEM-8 AND CMG-2 EXPRESSION AND FUNCTION WITHIN THE VASCULATURE
The TEM-8 and CMG-2 cell surface receptors mediate entry of anthrax toxin into cells of host organisms, and their function in this pathogenic process has been well described. However, the function of these receptors expressed in epithelial, endothelial and other celltypes in the absence of the toxin remains unclear. Following their initial descriptions as genes upregulated during angiogenesis, CMG-2 and TEM-8 have been proposed as potential targets for anti-angiogenic therapy and putative functions for these proteins of relevance to the process of new blood vessel formation or ‘angiogenesis’ have been described. These functions include binding to extracellular matrix proteins, and regulation of endothelial cell migration, proliferation and tubule formation as summarized in Figure 2. In the context of anthrax toxin endocytosis, it has been reported that the anthrax receptors complex with another transmembrane protein, lipoprotein related protein 6 (LRP6), and that the intracellular domains of the anthrax receptors couple to intracellular signaling molecules, including src (22, 28). Should similar interactions and signaling occur within endothelial cells during angiogenesis, they may provide mechanisms for regulation of extracellular matrix protein interactions and/or signal transduction from cell surface complexes.
Figure 2.
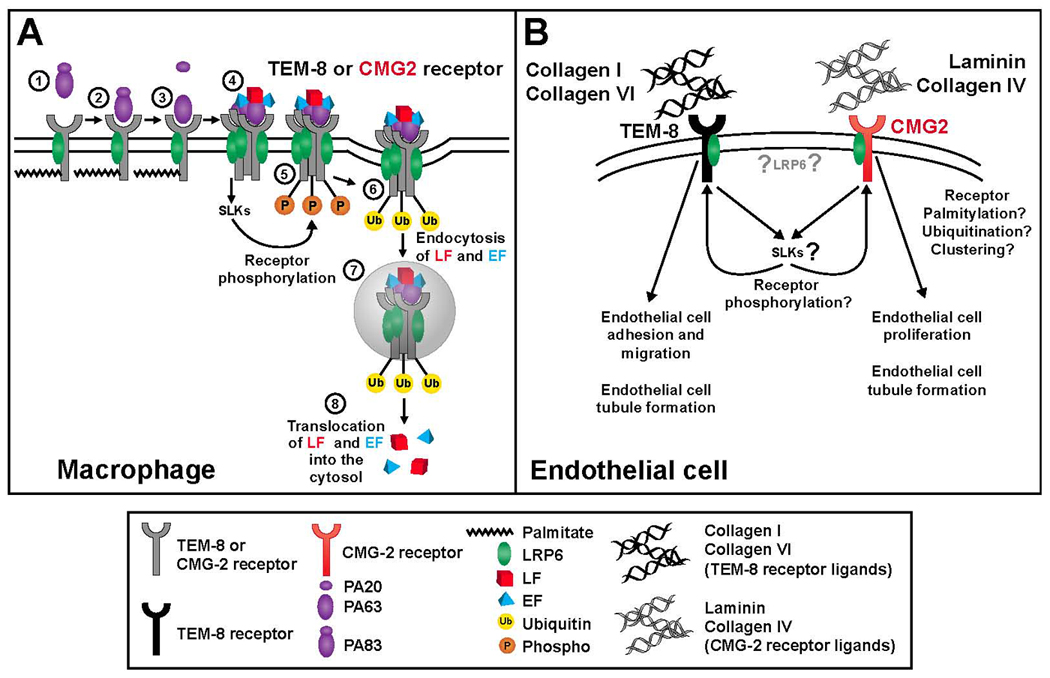
Facilitation of cellular anthrax toxin entry (A) and angiogenesis (B) by the anthrax toxin receptors. A. Entry of anthax toxin into macrophages. 1. PA binds to the palmitoylated and LRP6 bound macrophage cell anthrax receptors (TEM-8 or CMG-2). 2. PA is cleaved by furin like proteases with PA63 remaining bound to the receptor. 3. Clustering of anthrax toxin receptors and forming of a heptameric prepore induces src like kinase (SLK) signaling and binding of lethal toxin (LT) and edema toxin (ET) to the receptors. 4. SLK activation leads to phosphorylation of anthrax receptors. 5. Phosphorylation of anthrax receptors leads to ubiquitination of the receptors. 6. Endocytosis of LT and ET along with the receptors. 7. LT and ET are released into the cytoplasm. B. Putative functions of the anthrax receptors in angiogenesis. TEM-8 is known to bind to collagen I and collagen VI while CMG-2 binds to collagen IV and laminin. TEM-8 is thought to be involved in the regulation of endothelial cell migration and CMG-2 is proposed to regulate both endothelial cell proliferation and tubule formation. The influence of LRP6, SLK signaling, and anthrax receptor phosphorylation, palmitoylation, ubiquitination, and clustering, on these angiogenic functions in endothelial cells remains to be determined.
4.1. Interactions of TEM-8 and CMG-2 with ECM
Extracellular matrix provides an essential scaffold for maintaining the organization of vascular endothelial cells into blood vessels. In addition, many different extracellular matrix proteins have been documented to regulate the process of angiogenesis in a positive or negative manner. Collagen I, collagen IV, fibrinogen, and fibronectin augment angiogenesis by multiple mechanisms including increased endothelial cell proliferation, survival, migration, adhesion and tubule formation and enhancement of the activity of VEGF (29–33). Conversely, thrombospondins 1 and 2, and decorin are examples of extracellular matrix proteins that have anti-angiogenic functions such as inhibition of endothelial cell growth, migration, tube formation and inhibition of VEGF production (34–38). Alternate forms of these extracellular matrix proteins including metalloprotease cleaved proteins, soluble forms, synthetic or natural fragments have differential effects on blood vessel formation, and represent additional mechanisms for the regulation of angiogenesis in disease (39–43).
TEM-8 and CMG-2 are known to bind differentially to extracellular matrix proteins, and these interactions may underlie the functional roles of these receptors in the absence of anthrax toxin (Figure 2B). CMG-2 binds to collagen IV, laminin, and fibronectin but not to osteopontin, another extracellular matrix protein (1). It has been suggested that CMG-2 may be involved in basement membrane matrix assembly as it also colocalizes with Hsp47, a chaperone protein for collagen I and IV, in the endoplasmic reticulum (1).
TEM-8 interacts with collagen I, gelatin, and collagen VI (25, 26, 44). A yeast two-hybrid screen of 3.5 million distinct cDNAs from fetal brain demonstrated that the extracellular domain of TEM-8 binds to the alpha 3 subunit of collagen VI at the COOH-terminal C5 domain (25). Like TEM-8, collagen (alpha 3) VI is also preferentially expressed on tumor endothelium in comparison with normal endothelium (25). TEM-8 and collagen (alpha 3) VI co-localize on the blood vessels of human lung, colon, and esophageal tumors (25). Collagen (alpha 3) VI is upregulated during wound healing, a process involving an angiogenic response and Collagen (alpha 3) VI was also one of the genes found to be highly upregulated in tumor endothelium by St. Croix et al. Further evidence for the importance of TEM-8 in cellular interactions with extracellular matrix proteins comes from knockout mouse studies. Mice lacking TEM-8 are viable, although one group has reported that female mice have reproductive defects which may be related to deposits of extracellular matrix found in the ovaries and uteri of these mice (11, 12). Excessive deposits of extracellular matrix are seen in many other organs of TEM-8−/− mice including the periodontal ligament of the incisors and the skin (11).
4.2. Signaling downstream of the anthrax receptors
The intracellular domains of the anthrax toxin receptors and their binding partners have been far less well studied than the extracellular domains. In fact, until recently it was unclear whether their relatively large cytoplasmic tails served any purpose in the established function of these receptors, cellular uptake of anthrax toxin (Figure 2A). LRP6 is thus far the only protein known to bind to the intracellular regions of anthrax receptor proteins (28, 45). However various investigations have tentatively implicated src-like kinase (SLK) signaling, Wnt signaling, and PI3 kinase signaling from the anthrax receptors during the process of anthrax toxin endocytosis (Figure 2A) (22, 28, 45). The intracellular domains of anthrax toxin receptors lack intrinsic kinase activity and other intrinsic signaling activities have not yet been described for these regions. Thus, recruitment of various transduction proteins would be required to transmit any potential signaling cascade from these receptors.
Recent studies have demonstrated that binding of the PA subunit of anthrax toxin to either TEM-8 or CMG-2 triggers tyrosine phosphorylation of the receptors via the src-like kinases, src and fyn (22). The src family of kinases are involved in multiple signaling pathways and many diverse cellular functions including invasion, proliferation and survival. Tyrosine phosphorylation of the receptor is required for efficient uptake of anthrax toxin as mutant receptors without tyrosine residues exhibit strongly delayed endocytosis (22). Src dependent phosphorylation of the anthrax receptors is required for their subsequent ubiquitination, which in turn is essential for clathrin-mediated endocytosis of the receptors and internalization of anthrax toxin (22).
Lipoprotein related protein 6 (LRP6), a Wnt signaling protein, was identified as being involved in anthrax toxin endocytosis from an expression sequence tagged (EST) screen that silenced chromosomal genes (45). LRP5 and LRP6 are co-receptors for Wnt in the canonical Wnt signaling pathway. Toxin binding to the anthrax receptors triggers tyrosine phosphorylation of LRP6 (28). Protective antigen also induces redistribution of LRP6 into detergent-resistant membranes expressing calveolin-1 and its subsequent endocytosis (28). In contrast to the src kinase activity on anthrax toxin receptors, tyrosine phosphorylation of LRP6 is not required for efficient endocytosis of anthrax toxin (28). While two independent studies have demonstrated that the anthrax receptors bind to LRP6 (28, 45), the importance of this protein during toxin endocytosis remains somewhat controversial (28, 45, 46). The emerging evidence from siRNA studies indicates that LRP6 is not required for endocytosis, but that its presence may accelerate this process (28). Apparent discrepancies with a study by Ryan et al which failed to demonstrate any significant role for LRP6 in toxin endocytosis may relate to the different knockdown efficiencies or alternatively differences in the concentrations of PA and LF used in each of the studies (28, 46). For example, Abrami et al showed that each of three LRP6 siRNAs reduced LRP6 protein expression appreciably, but inhibited PA oligimerization and MEK1 cleavage by varying degrees (28). Ryan and Young used concentrations of PA and LF that were approximately 4-fold greater than those used by Abrami et al, possibly leaving cells less sensitive to the absence of LRP6 in these experiments (28, 46). Mouse fibroblasts derived from LRP6−/− mice are still sensitive to anthrax toxin, further demonstrating that LRP6 is not a requirement for toxin endocytosis (47).
However, recent evidence suggests that intracellular interactions of the anthrax receptors with LRP6 may have an important role in the absence of anthrax toxin. Anthrax receptors can control the levels of LRP6 protein in cells and hence elements of the Wnt signaling cascade (28). Stabilization of β-catenin following stimulation with Wnt is strongly impaired in HeLa cells where either receptor has been knocked down (28). Binding to extracellular matrix proteins may constitute the most important physiological role for the anthrax receptors, and these interactions in turn may play a crucial role in the regulation of intracellular Wnt signaling. Although the role of LRP6 in angiogenesis has not been studied, it is interesting to note that Wnt signaling through the Frizzled receptors is crucial for normal retinal vascular development and retinal angiogenesis (48, 49). Furthermore, in vitro studies have implicated the Wnt/β-catenin pathway in promotion of endothelial survival and proliferation (50–52).
A second gene suggested as being important for anthrax toxicity through the EST screen conducted by Wei et al is ARAP3 (Arf GAP and Rho GAP with ankyrin repeat and pleckstrin homology domains) (45). ARAP3 is a protein which was originally identified in a screen for phosphotidylinositol-(3,4,5)-trisphosphate binding proteins (53). The mechanism by which ARAP3 contributes to toxin endocytosis remains unclear. Interestingly, ARAP3 is known to be involved in cell attachment and cell spreading on extracellular matrix, which may be relevant for the putative roles of the anthrax receptors in angiogenesis in the absence of anthrax toxin (54, 55).
Thus, a number of proteins which interact with the anthrax receptors during anthrax toxicity have been described (Figure 2A). Clustering, ubiquitination, palmitoylation and phosphorylation of the receptors can all regulate anthrax toxin uptake and signaling through src kinases are evoked following toxin binding (Figure 2A). It remains to be determined whether these alterations in signaling and receptor status similarly regulate responses in endothelial cells following extracellular matrix binding, or influence angiogenesis in vivo (Figure 2B).
4.3. TEM-8 and endothelial cell function
In the absence of anthrax toxin, TEM-8 is believed to play a crucial role in the regulation of endothelial cell shape and migration. Endothelial cells overexpressing TEM-8 migrate at three times the rate of regular endothelial cells and exhibit enhanced adhesion to Collagen I (26). Importantly, the extracellular domain of TEM-8 (TEM-8 ED) inhibits endothelial cell migration, presumably by interacting with extracellular matrix substrates or directly with co-receptors of TEM-8, and acting as a dominant negative protein in this assay. Additional evidence for the role of TEM-8 in endothelial cell migration comes from studies where ribozyme transgenes were used to knockdown TEM-8 in immortalized human umbilical cord endothelial cells (HECVs). These cells, with reduced expression of TEM-8, exhibited reduced migration and tubule formation in comparison with control HECVs (56).
The extracellular domain of TEM-8 interacts strongly with gelatin and collagen I in protein assays and it can inhibit the adhesion of endothelial cells to gelatin or collagen I (26). Overexpression of the full length TEM-8 protein in microvascular rat epididymal fat pad endothelial cells (RFPECs) enhances their adhesion to both gelatin and collagen, with more subtle pro-adhesive effects when endothelial cells are plated on fibronectin or vitronectin (26). However, overexpression of the full length TEM-8 protein does not affect the ability of endothelial cells to bind to laminin (26).
The mechanism by which TEM-8 mediates binding to collagen I is cell type specific. For example, blocking the β1 integrin using antibodies inhibited TEM-8 mediated adhesion of HEK293 cells but not rabbit synovial fibroblasts (44). TEM-8 regulates HEK293 cell spreading by coupling extracellular proteins such as collagen I and PA to the actin cytoskeleton (44). Attachment of TEM-8 expressing HEK293 cells to a PA coated surface resulted in dramatic cell spreading and cell body extension. This suggests that engagement of the TEM-8 receptor by PA or other endogenous ligands may regulate endothelial cell adhesion and spreading in vivo. Expression of the extracellular domain of TEM-8 in HEK293 cells is sufficient to mediate adhesion but not sufficient for cell spreading on PA coated surfaces (44). Thus, TEM-8 acts as an adhesion molecule to mediate cell spreading via its cytoplasmic tail. Disruption of the TEM-8 recycling mechanism between endosomes and the cell surface inhibits cell spreading on PA-coated surfaces, without affecting anthrax toxin internalization (21).
TEM-8 interacts directly with the actin cytoskeleton in a similar manner to the integrins, and spreading of HEK293 cells overexpressing TEM-8 can be inhibited by a cytoskeleton-disrupting drug, cytochalasin D (44). TEM-8 and actin co-localize at the base of lamellipodia and along actin filaments extending into the lamellipodia during cell spreading (44). However, the distribution pattern of TEM-8 was not reminiscent of that seen with integrins during cell adhesion, where expression within focal adhesion complexes and co-localization with stress fibres is typical. In the absence of anthrax toxin, the TEM-8 receptor is organized and clustered at the cell surface in an actin-dependent manner not seen for the CMG-2 receptor (21). Furthermore, in contrast to integrins, TEM-8 can function as a single subunit receptor in mediating cell adhesion (44). Interestingly, it has been suggested that binding of actin via the cytoplasmic tail of TEM-8 may regulate the affinity state of the receptor, both in terms of its ability to bind PA and, in the absence of the toxin, extracellular matrix proteins (57). The functions of TEM-8 in cell adhesion and cell spreading may represent a mechanistic framework to understand studies demonstrating increased endothelial cell migration in cells overexpressing TEM-8 (26). Linkage of TEM-8 to the actin cytoskeleton may also be important for a number of other processes in angiogenesis including signal transduction, cell division, and morphogenesis.
TEM-8 protein expression is increased in vitro during tube formation in collagen gels (26). Experiments in CHO cells which do not normally express TEM-8, suggest that the presence of the extracellular VWA domain of TEM-8 is sufficient to induce ‘tubule formation’ of these cells in Matrigel, a basement membrane suspension (56). Although CHO cells are not of endothelial origin, transfection of full length TEM-8, a transmembrane/VWA construct or the VWA domain alone caused these cells to form ‘tubules’ over 24 hours (56). In contrast, the presence of the extracellular domain without the VWA domain, the transmembrane domain alone or the intracellular domain alone did not induce the formation of tubules in these cells (56). These experiments suggest that interactions of TEM-8 with basement membrane proteins, without a requirement for intracellular signaling from the TEM-8 receptor, may be sufficient for the organization of cells into tubule structures. Alternatively, a TEM-8 co-receptor may be involved in the signaling that results in tubule formation in these cells. In any event, interpretation of the relevance of experiments involving TEM-8 expression in CHO cells to the process of angiogenesis should be taken in context, as these cells represent a ‘non-endothelial’ cell-line.
The generation of mice lacking the TEM-8 receptor has provided further indications of important roles for TEM-8 in the regulation of extracellular matrix deposition and tumorigenesis. TEM-8−/− mice have been generated in two independent studies with two different strains of mice, targeting different exons of the TEM-8 gene. The deletion induced by Liu et al results in expression of a secreted gene product without a transmembrane region, whereas Cullen et al deleted the first exon containing the promoter region and start codon and TEM-8 is not expected to be expressed in any form at the protein level (11, 12). Interestingly, although expression of TEM-8 has been documented in the vasculature of developing mouse embryonic brain and liver tissues (15), both studies found that the absence of TEM-8 did not affect survival, normal growth or development, with the exception of excess deposition of extracellular matrix reported by Cullen et al in many tissues and dental dysplasia reported by both groups (11, 12). The excess matrix accumulation could be explained if recycling of TEM-8 between endosomes and the cell surface contributes to clearance of Collagen I and Collagen VI in wild-type mice (21). In addition, although recombinant soluble forms of the TEM-8 receptor have anti-angiogenic effects in vitro (26), defects in physiological angiogenesis were not observed in TEM-8−/− mice (11). Interestingly, Liu et al reported that female TEM-8−/− mice could become pregnant but subsequent embryonic development was impaired (12). In contrast, the TEM-8−/− mice generated by Cullen et al do not have fertility problems (11, 12). This discrepancy may arise as a result of the expression of a secreted mutant TEM-8 protein which could affect fertility by interacting with natural ligands of TEM-8, such as extracellular matrix proteins (12).
Tumor growth is delayed in TEM-8−/− knockouts when B16 melanoma cells were implanted into these mice (11). Thus, the absence of TEM-8 within non-tumor host cells affects tumor growth. Given the documented induction of TEM-8 expression in the endothelium of tumor vessels and the observations of defective cell migration in endothelial cells with reduced expression of TEM-8, it might be expected that reduced tumor vessel growth in TEM-8−/− mice may have been responsible for the effect on tumor growth. Surprisingly, differences in vessel density or pericyte coverage within B16 tumors implanted in wild-type and TEM-8−/− mice were not observed (11). However, while tumor microvessel density is a useful indication of prognosis for some human cancers, it does not always accurately reflect the degree of angiogenic activity within the tumor (58). Thus, the exact mechanisms responsible for the contribution of host derived TEM-8 to tumor growth remain to be determined. Cullen et al also described similar levels of apoptosis, hypoxia, proliferating endothelial cell number, macrophage cell numbers and other myeloid cells within B16 melanoma tumors in wild-type and TEM-8−/− mice (11). Interestingly, the effects on tumor growth appeared to be tumor dependent as Lewis Lung carcinoma growth was not significantly altered in TEM-8 knockout mice.
4.4. CMG-2 and endothelial cell function
Evidence for the role of CMG-2 in endothelial cell function, in the absence of anthrax toxin, has emerged only recently. CMG-2 appears to regulate both endothelial cell proliferation and tubule formation in culture, with little influence on the migration of endothelial cells observed. RNA interference approaches have demonstrated that reduced CMG-2 expression in human umbilical vein endothelial cells (HUVECs) results in reduced proliferation and VEGF-mediated capillary network formation in Collagen I gels in vitro, whereas the migration of these cells in a cellular scratch wound assay remains unaffected (23). Conversely, overexpression of CMG-2 in HUVECs causes increased proliferation and tubule network formation of these cells with no effect on cell migration (23).
Given the relatively high affinity of PA for CMG-2, it is likely that PA can inhibit binding of CMG-2 to its natural ligands during anthrax infection. It remains to be determined whether this assists the process of toxin endocytosis or pathogenesis. In the context of angiogenesis, a recent study from our laboratory demonstrated that blockade of anthrax receptors using a form of PA with three amino acids mutated, PA-SSSR, can inhibit endothelial cell migration and angiogenesis in vivo (59). PA-SSSR is a mutant which is resistant to cleavage by endogenous furin-like proteases, and remains bound to the anthrax toxin receptors for an extended period of time without being internalized compared to native PA (60). PA-SSSR inhibited VEGF and serum-induced human microvascular endothelial cell (HMVEC) migration with no effect on endothelial cell proliferation. PA-SSSR also inhibits angiogenesis in vivo in the corneal micropocket assay and inhibits Lewis Lung carcinoma growth. In these experiments neither Lethal Factor nor Edema Factor were administered along with PA-SSSR, excluding direct effects of the toxins on endothelial or tumor cells as the mechanisms for reduced angiogenesis and tumor growth. In common with PA, PA-SSSR has a higher affinity for CMG-2 than TEM-8.
4.5. Targeting TEM-8 in cancer
Anti-angiogenic and anti-tumorigenic molecules which target TEM-8 may represent tumor-selective agents given the enhanced expression of TEM-8 that has been described in the blood vessels of many tumor types compared to normal tissues (3, 15, 25). The knowledge of preferential expression of TEM-8 in the tumor vasculature has also been manipulated in order to target a number of potential anti-tumorigenic therapeutics to the tumor vasculature in pre-clinical models in the absence of anthrax toxin. Anti-angiogenic TEM-8 targeted therapies include a fusion protein which combines part of an anti-TEM-8 antibody with truncated tissue factor, reducing colorectal tumor volume by localizing at tumor blood vessels and disrupting tumor vasculature by promoting local thrombosis (61). A DNA vaccine against TEM-8 which inhibits tumor growth in mice due to its anti-angiogenic effects has also been developed (62).
TEM-8-Fc is an antibody-like molecule with anti-angiogenic properties that also inhibits metastasis of some tumors (63). Tumors treated with this molecule had reduced density of blood vessels (63). The morphology of the tumor cells was also affected in treated animals, which could be secondary to anti-angiogenic effect, or alternatively could indicate some additional anti-tumor effects of this molecule (63). This therapeutic protein may function to prevent TEM-8 binding to the M2 isozyme of pyruvate kinase (M2-PK) as proteomic studies demonstrated a direct interaction of TEM-8-Fc with this enzyme in HepG2 tumor homogenates (63). M2-PK is an isozyme of PK predominantly found in tumors, also termed tumor PK and is known to have a role in tumor cell growth and metastasis (64, 65). The expression of TEM-8, in common with other TEMs may be increased in immunosuppressive, pro-angiogenic dendritic cells that are found in tumor microenvironments. It has been demonstrated that TEM-8 expression levels in dendritic cell based cancer vaccines are related to clinical outcome (66). Outside of the vasculature, expression of TEM-8 has been documented within tumor cells (67, 68) and within other celltypes (24, 61). Although comparative studies of both mouse and human tissues suggest that the most abundant expression of TEM-8 is seen in within tumor vessels compared to other cells within the tumor milieu or normal tissues (3, 15, 25), effects of TEM-8 targeted therapies on epithelial tissues and other cells expressing TEM-8 at lower levels should be monitored for off-target effects.
5. ANTI-ANGIOGENIC AND TUMORICIDAL STRATEGIES USING BIOLOGICAL ANTHRAX TOXIN MOLECULES
Other strategies involving native and modified anthrax toxin related molecules have been used as anti-angiogenic or direct tumoricidal agents. Lethal toxin (LeTx) is composed of PA and LF, whereas edema toxin (EdTx) is composed of PA and EF. Anti-angiogenic effects of both LeTx and EdTx, have been reported due to their respective effects on MAPK inhibition and cAMP within endothelial cells. In addition, direct tumoricidal effects of LeTx related to MAPK inhibition have been reported and were initially believed to represent the primary mechanism for its inhibitory effects on tumor growth. Subsequent studies have identified direct anti-angiogenic effects of LeTx and suggest that this may be the primary mechanism for the potent anti-tumorigenic effects of this toxin. However, toxicity of LeTx and immune responses generated by administration of PA will need to be rigorously investigated and minimized before LeTx and associated agents can be considered as clinical therapies.
5.1. Tumoricidal effects of LeTx
Protective antigen (PA) has been used in a number of studies to deliver and facilitate the entry of toxins, anthrax derived and others, into tumor cells resulting in tumoricidal activity. Treatment of tumor cells with PA combined with FP59, a B. anthracis lethal toxin/Pseudomonas exotoxin A fusion protein induces tumor cell death. FP59 consists of the first 254 amino acids of lethal toxin, the minimal region required for uptake of the toxin, fused to the ADP ribosylation domain of Pseudomonas exotoxin A (69). A number of mutations to PA increase its selectivity and targeting of tumor cells, bioavailability at the tumor site, and decrease toxicity of the associated toxins. These include mutations where the furin cleavage site is replaced by cleavage sites for proteases known to be overexpressed in tumor cells such as matrix metalloproteinase (MMP) or urokinase plasminogen activator (uPA) (70–73). A combination of mutated PA molecules, PA with uPA or MMP cleavage sites and PA impaired binding sites for LF, has tumoricidal activity in vitro and in mice with reduced animal toxicity compared with administration of individual PA molecules solely mutated to alter cleavage sites (74). In addition, it has been reported that mutants of protective antigen that preferentially bind to TEM-8 rather than CMG-2 achieve even more selective tumoricidal activity when administered along with FP59 (75).
In vitro, the native form of LeTx has strong toxic effects on many different tumor celltypes as a result of inhibitory effects on MAP kinase signaling (76–78). Many tumors exhibit constitutive MAPK signaling rendering these tumor cells more susceptible to LeTx induced toxicity in vitro (79). For example, some tumor cells, including melanoma cells contain a point mutation in B-RAF, resulting in enhanced MAPK activity (79, 80). This mutation results in a constitutively active B-Raf kinase that continuously stimulates downstream components of the signaling pathway even in the absence of growth factors. Surprisingly, MMP-cleavable LeTx can effectively reduce tumor growth in vivo in tumor xenograft models, where the implanted tumor cells were unaffected by LeTx treatment in vitro (73, 81). This intriguing finding has led to detailed studies of the effects of LeTx and the discovery of the role of MAP kinase signaling in angiogenesis and the tumor vasculature as detailed below.
5.2. Anti-angiogenic effects of LeTx
Evidence for anti-angiogenic effects of LeTx have come from in vivo models of anaplastic thyroid carcinoma (ATC) and other tumors and endothelial cell culture experiments. Surprisingly, LeTx was shown to have a similar effect on tumor growth and survival in immunocompromised mice, regardless of whether the orthotopically implanted tumor cells contain the B-RAF mutation which causes enhanced MAPK activity (81). ATC tumors from mice treated with LeTx displayed reduced endothelial cell recruitment and tumor vascularization (81). LeTx has also been shown to have effects on non-tumor vasculature. The developing mouse retinal vasculature is known to be perturbed following intravitreal injection of LeTx, delaying early vascular development and resulting in abnormal tuft formation at later stages (postnatal day 8) of retinal vascular development (82)
Additional experiments in a longer-term xenograft model of well-established ATC, where tumors had been growing for over three weeks suggest that MMP-activated LeTx can inhibit tumorigenesis by alternative mechanisms depending on tumor stage. MMP-activated LeTx induced necrosis of tumor cells just eighteen hours after a single dose of LeTx in this model, without affecting the vasculature of these tumors (81). The mechanism for this dramatic and rapid effect is unclear as of yet, but may involve decreased perfusion of tumor tissue resulting from hemorrhage (83, 84).
In vivo intratumoral injections of LeTx inhibit tumor growth and have also been shown to reduce the number of blood vessels within the tumors, at concentrations that do not cause animal toxicity in immunocompromised mice (76). An MMP-activated form of LeTx has shown potent anti-angiogenic activity, and inhibited tumor growth (73). Importantly, toxicity was also reduced and the immune response to the altered PA protein was approximately 6-fold lower than that generated by native PA (73).
The anti-angiogenic activity of LeTx is likely due to inhibition of p38 mitogen activated protein kinase and c-Jun NH(2)-terminal kinase, leading to decreased endothelial differentiation and invasiveness (85). MMP-activated LeTx reduces expression of proangiogenic MMPs in endothelial cells, thereby reducing their extracellular matrix remodeling potential and providing a potential mechanism for the anti-angiogenic effect of LeTx (85). In cell culture, MMP-activated LeTx reduces endothelial cell invasion, and the ability of endothelial cells to cleave collagen I, collagen IV and gelatin, and form blood-vessel like tubules (85). LeTx also has a direct anti-proliferative effect on human umbilical vein endothelial cells in culture and has also been shown to induce apoptosis of cultured endothelial cells (78, 86).
5.3. Anti-angiogenic effects of EdTx
Anti-endothelial effects of edema toxin (EdTx) on endothelial cells, which are due to increased intracellular cAMP following internalization, have also been described. Anthrax edema toxin is an adenylyl cyclase that induces the generation of cyclic AMP in cells. Anthrax edema toxin has been shown to inhibit endothelial cell chemotaxis, but not proliferation, via a downstream effector of cAMP, Epac (87).
6. PERSPECTIVE
It is now well documented through both cell culture systems and animal models that TEM-8 and CMG-2 play a role in angiogenesis. TEM-8 influences cell migration and tubule formation and CMG-2 promotes endothelial cell proliferation. Binding of these receptors to extracellular matrix proteins is likely to play a key role in these functions. However, despite recent knowledge on signaling from these receptors during toxin endocytosis, the exact molecular and signaling mechanisms by which the anthrax receptors mediate angiogenic responses are less clear.
Wnt or src signaling may be induced following engagement of the receptors with extracellular matrix proteins. Palmitoylation, ubiquitination, phosphorylation and clustering of the anthrax receptors regulate toxin uptake (9, 22), and these events may also influence endothelial cell behavior. The mechanisms and regulation of the anthrax receptors in the context of cell adhesion to extracellular matrix proteins needs to be further delineated. What are the properties of these receptors, for example, that would distinguish them from the integrins in these processes? An important question in the context of angiogenesis is the identity of the natural ligands for these receptors in vivo. Splice variants of the anthrax receptors may provide a further level of regulation of the angiogenic response, with soluble receptors potentially acting as inhibitors of angiogenic signaling, much like the effects of soluble VEGF receptors on VEGFR2 signaling. Indeed a recombinant soluble TEM-8 receptor has demonstrated anti-angiogenic activity in vitro (26). It will be interesting to determine whether the mechanisms of TEM-8 (and potentially CMG-2) receptor recycling between endosomal and cell membrane compartments which have been described in PC12 and Hek293 cells (21), also exist within endothelial cells. The temporal and comparative expression of TEM-8 and CMG-2 during angiogenesis in vivo will also provide important information on their function and regulation.
Investigation of the effects of LeTx and modified forms of PA administered along with lethal factor in pre-clinical cancer models demonstrate that these biological therapeutics have potent anti-angiogenic and anti-tumorigenic effects. These studies highlight both the importance of MAPK pathways in angiogenesis, and the effectiveness of using tumor specific expression of molecules, in this case the MMPs, at tumor sites as a tumor selective approach. Future studies, are likely to focus on adaptation of the strategy of MAPK inhibition for clinical applications for the treatment of solid tumors. Further adaptation of the PA and lethal factor components may be necessary in order to minimize toxicity and unwanted immunogenic responses to these proteins in patients, while maintaining anti-tumor effects.
In summary, while much remains unknown about the molecular mechanisms and interacting proteins of the anthrax receptors during angiogenesis, they represent exciting new targets for anti-angiogenic therapies. Future anti-angiogenic therapies may target blockade of the TEM-8 or CMG-2 receptor, or deliver anti-angiogenic moieties using TEM-8 as a marker of tumor vessels. In addition, the effects of modified forms of LeTx targeting tumor vasculature in preclinical models suggest that MAPK inhibition is a useful strategy for anti-angiogenic therapy.
ACKNOWLEDGEMENTS
Support from the Department of Defense CDMRP BCRP (M.S.R.), and the National Institute of Health (M.S.R) is acknowledged.
Abbreviations
- ANTXR
anthrax receptor
- ATC
anaplastic thyroid carcinoma
- CMG
capillary morphogenesis gene
- ECM
extracellular matrix
- EF
edema factor
- EdTx
edema toxin
- HMVEC
human microvascular endothelial cell
- HUVEC
human umbilical vein endothelial cell
- LeTx
lethal toxin
- LF
lethal factor
- LRP
lipoprotein related protein
- MAPK
mitogen-activated protein kinase
- MIDAS
metal ion dependent adhesion site
- MMP
matrix metalloprotease
- PA
protective antigen
- SLK
src like kinase
- TEM
tumor endothelial marker
- VEGF
Vascular endothelial growth factor
- VWA
von Willebrand Factor A
Footnotes
Publisher's Disclaimer: This is an, un-copyedited, author manuscript that has been accepted for publication in the Frontiers in Bioscience. Cite this article as appearing in the Journal of Frontiers in Bioscience. Full citation can be found by searching the Frontiers in Bioscience (http://bioscience.org/search/authors/htm/search.htm) following publication and at PubMed (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=pubmed) following indexing. This article may not be duplicated or reproduced, other than for personal use or within the rule of "Fair Use of Copyrighted Materials" (section 107, Title 17, U.S. Code) without permission of the copyright holder, the Frontiers in Bioscience. From the time of acceptance following peer review, the full final copy edited article of this manuscript will be made available at http://www.bioscience.org/. The Frontiers in Bioscience disclaims any responsibility or liability for errors or omissions in this version of the un-copyedited manuscript or in any version derived from it by the National Institutes of Health or other parties.
REFERENCES
- 1.Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114:2755–2773. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- 2.Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2003;100:5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 4.Mock M, Fouet A. Anthrax. Annu Rev Microbiol. 2001;55:647–671. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- 5.Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- 6.Klimpel KR, Arora N, Leppla SH. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol Microbiol. 1994;13:1093–1100. doi: 10.1111/j.1365-2958.1994.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 7.Duesbery NS, Webb CP, Leppla SH, Gordon VM, Klimpel KR, Copeland TD, Ahn NG, Oskarsson MK, Fukasawa K, Paull KD, Vande Woude GF. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 8.Robertson DL, Tippetts MT, Leppla SH. Nucleotide sequence of the Bacillus anthracis edema factor gene (cya): a calmodulin-dependent adenylate cyclase. Gene. 1988;73:363–371. doi: 10.1016/0378-1119(88)90501-x. [DOI] [PubMed] [Google Scholar]
- 9.Abrami L, Leppla SH, van der Goot FG. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J Cell Biol. 2006;172:309–320. doi: 10.1083/jcb.200507067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrami L, Bischofberger M, Kunz B, Groux R, van der Goot FG. Endocytosis of the anthrax toxin is mediated by clathrin, actin and unconventional adaptors. PLoS Pathog. 6:e1000792. doi: 10.1371/journal.ppat.1000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cullen M, Seaman S, Chaudhary A, Yang MY, Hilton MB, Logsdon D, Haines DC, Tessarollo L, St Croix B. Host-Derived Tumor Endothelial Marker 8 Promotes the Growth of Melanoma. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Crown D, Miller-Randolph S, Moayeri M, Wang H, Hu H, Morley T, Leppla SH. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0905409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowling O, Difeo A, Ramirez MC, Tukel T, Narla G, Bonafe L, Kayserili H, Yuksel-Apak M, Paller AS, Norton K, Teebi AS, Grum-Tokars V, Martin GS, Davis GE, Glucksman MJ, Martignetti JA. Mutations in capillary morphogenesis gene-2 result in the allelic disorders juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet. 2003;73:957–966. doi: 10.1086/378781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, Bischoff J, Vikkula M, Boye E, Olsen BR. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14:1236–1246. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61:6649–6655. [PubMed] [Google Scholar]
- 16.Liu S, Leppla SH. Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J Biol Chem. 2003;278:5227–5234. doi: 10.1074/jbc.M210321200. [DOI] [PubMed] [Google Scholar]
- 17.Bradley KA, Mogridge J, Jonah G, Rainey A, Batty S, Young JA. Binding of anthrax toxin to its receptor is similar to alpha integrin-ligand interactions. J Biol Chem. 2003;278:49342–49347. doi: 10.1074/jbc.M307900200. [DOI] [PubMed] [Google Scholar]
- 18.Wigelsworth DJ, Krantz BA, Christensen KA, Lacy DB, Juris SJ, Collier RJ. Binding stoichiometry and kinetics of the interaction of a human anthrax toxin receptor, CMG2, with protective antigen. J Biol Chem. 2004;279:23349–23356. doi: 10.1074/jbc.M401292200. [DOI] [PubMed] [Google Scholar]
- 19.Lacy DB, Wigelsworth DJ, Scobie HM, Young JA, Collier RJ. Crystal structure of the von Willebrand factor A domain of human capillary morphogenesis protein 2: an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2004;101:6367–6372. doi: 10.1073/pnas.0401506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scobie HM, Thomas D, Marlett JM, Destito G, Wigelsworth DJ, Collier RJ, Young JA, Manchester M. A soluble receptor decoy protects rats against anthrax lethal toxin challenge. J Infect Dis. 2005;192:1047–1051. doi: 10.1086/432731. [DOI] [PubMed] [Google Scholar]
- 21.Gu J, Faundez V, Werner E. Endosomal recycling regulates anthrax toxin receptor 1/tumor endothelial marker 8-dependent cell spreading. Exp Cell Res. 2010 doi: 10.1016/j.yexcr.2010.03.026. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abrami L, Kunz B, van der Goot FG. Anthrax toxin triggers the activation of src-like kinases to mediate its own uptake. Proc Natl Acad Sci U S A. 2010;107:1020–1024. doi: 10.1073/pnas.0910782107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves CV, Dufraine J, Young JA, Kitajewski J. Anthrax toxin receptor 2 is expressed in murine and tumor vasculature and functions in endothelial proliferation and morphogenesis. Oncogene. 2009 doi: 10.1038/onc.2009.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonuccelli G, Sotgia F, Frank PG, Williams TM, de Almeida CJ, Tanowitz HB, Scherer PE, Hotchkiss KA, Terman BI, Rollman B, Alileche A, Brojatsch J, Lisanti MP. ATR/TEM8 is highly expressed in epithelial cells lining Bacillus anthracis' three sites of entry: implications for the pathogenesis of anthrax infection. Am J Physiol Cell Physiol. 2005;288:C1402–C1410. doi: 10.1152/ajpcell.00582.2004. [DOI] [PubMed] [Google Scholar]
- 25.Nanda A, Carson-Walter EB, Seaman S, Barber TD, Stampfl J, Singh S, Vogelstein B, Kinzler KW, St Croix B. TEM8 interacts with the cleaved C5 domain of collagen alpha 3(VI) Cancer Res. 2004;64:817–820. doi: 10.1158/0008-5472.can-03-2408. [DOI] [PubMed] [Google Scholar]
- 26.Hotchkiss KA, Basile CM, Spring SC, Bonuccelli G, Lisanti MP, Terman BI. TEM8 expression stimulates endothelial cell adhesion and migration by regulating cell-matrix interactions on collagen. Exp Cell Res. 2005;305:133–144. doi: 10.1016/j.yexcr.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Herrmann D, Ferrer-Vaquer A, Lahsnig C, Firnberg N, Leibbrandt A, Neubuser A. Expression and regulation of ANTXR1 in the chick embryo. Dev Dyn. 239:680–687. doi: 10.1002/dvdy.22194. [DOI] [PubMed] [Google Scholar]
- 28.Abrami L, Kunz B, Deuquet J, Bafico A, Davidson G, van der Goot FG. Functional interactions between anthrax toxin receptors and the WNT signalling protein LRP6. Cell Microbiol. 2008;10:2509–2519. doi: 10.1111/j.1462-5822.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- 29.Lohler J, Timpl R, Jaenisch R. Embryonic lethal mutation in mouse collagen I gene causes rupture of blood vessels and is associated with erythropoietic and mesenchymal cell death. Cell. 1984;38:597–607. doi: 10.1016/0092-8674(84)90514-2. [DOI] [PubMed] [Google Scholar]
- 30.Whelan MC, Senger DR. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J Biol Chem. 2003;278:327–334. doi: 10.1074/jbc.M207554200. [DOI] [PubMed] [Google Scholar]
- 31.Herbst TJ, McCarthy JB, Tsilibary EC, Furcht LT. Differential effects of laminin, intact type IV collagen, and specific domains of type IV collagen on endothelial cell adhesion and migration. J Cell Biol. 1988;106:1365–1373. doi: 10.1083/jcb.106.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dejana E, Languino LR, Polentarutti N, Balconi G, Ryckewaert JJ, Larrieu MJ, Donati MB, Mantovani A, Marguerie G. Interaction between fibrinogen and cultured endothelial cells. Induction of migration and specific binding. J Clin Invest. 1985;75:11–18. doi: 10.1172/JCI111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wijelath ES, Rahman S, Namekata M, Murray J, Nishimura T, Mostafavi-Pour Z, Patel Y, Suda Y, Humphries MJ, Sobel M. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ Res. 2006;99:853–860. doi: 10.1161/01.RES.0000246849.17887.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 35.Iruela-Arispe ML, Bornstein P, Sage H. Thrombospondin exerts an antiangiogenic effect on cord formation by endothelial cells in vitro. Proc Natl Acad Sci U S A. 1991;88:5026–5030. doi: 10.1073/pnas.88.11.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, Detmar M. Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci U S A. 1999;96:14888–14893. doi: 10.1073/pnas.96.26.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765–4777. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 38.Davies Cde L, Melder RJ, Munn LL, Mouta-Carreira C, Jain RK, Boucher Y. Decorin inhibits endothelial migration and tube-like structure formation: role of thrombospondin-1. Microvasc Res. 2001;62:26–42. doi: 10.1006/mvre.2001.2311. [DOI] [PubMed] [Google Scholar]
- 39.Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, Torre A, Maeshima Y, Mier JW, Sukhatme VP, Kalluri R. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem. 2000;275:1209–1215. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- 40.Petitclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras MP, Jr, Hudson BG, Brooks PC. New functions for non-collagenous domains of human collagen type IV. Novel integrin ligands inhibiting angiogenesis and tumor growth in vivo. J Biol Chem. 2000;275:8051–8061. doi: 10.1074/jbc.275.11.8051. [DOI] [PubMed] [Google Scholar]
- 41.Staton CA, Brown NJ, Rodgers GR, Corke KP, Tazzyman S, Underwood JC, Lewis CE. Alphastatin, a 24-amino acid fragment of human fibrinogen, is a potent new inhibitor of activated endothelial cells in vitro and in vivo. Blood. 2004;103:601–606. doi: 10.1182/blood-2003-07-2192. [DOI] [PubMed] [Google Scholar]
- 42.Yi M, Ruoslahti E. A fibronectin fragment inhibits tumor growth, angiogenesis, and metastasis. Proc Natl Acad Sci U S A. 2001;98:620–624. doi: 10.1073/pnas.98.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrari do Outeiro-Bernstein MA, Nunes SS, Andrade AC, Alves TR, Legrand C, Morandi: V. A recombinant NH(2)-terminal heparin-binding domain of the adhesive glycoprotein, thrombospondin-1, promotes endothelial tube formation and cell survival: a possible role for syndecan-4 proteoglycan. Matrix Biol. 2002;21:311–324. doi: 10.1016/s0945-053x(02)00010-0. [DOI] [PubMed] [Google Scholar]
- 44.Werner E, Kowalczyk AP, Faundez V. Anthrax toxin receptor 1/tumor endothelium marker 8 mediates cell spreading by coupling extracellular ligands to the actin cytoskeleton. J Biol Chem. 2006;281:23227–23236. doi: 10.1074/jbc.M603676200. [DOI] [PubMed] [Google Scholar]
- 45.Wei W, Lu Q, Chaudry GJ, Leppla SH, Cohen SN. The LDL receptor-related protein LRP6 mediates internalization and lethality of anthrax toxin. Cell. 2006;124:1141–1154. doi: 10.1016/j.cell.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 46.Ryan PL, Young JA. Evidence against a human cell-specific role for LRP6 in anthrax toxin entry. PLoS One. 2008;3:e1817. doi: 10.1371/journal.pone.0001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young JJ, Bromberg-White JL, Zylstra C, Church JT, Boguslawski E, Resau JH, Williams BO, Duesbery NS. LRP5 and LRP6 are not required for protective antigen-mediated internalization or lethality of anthrax lethal toxin. PLoS Pathog. 2007;3:e27. doi: 10.1371/journal.ppat.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 50.Masckauchan TN, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM, Khoo A, Tycko B, Brown AM, Kitajewski J. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell. 2006;17:5163–5172. doi: 10.1091/mbc.E06-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- 52.Wright M, Aikawa M, Szeto W, Papkoff J. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem Biophys Res Commun. 1999;263:384–388. doi: 10.1006/bbrc.1999.1344. [DOI] [PubMed] [Google Scholar]
- 53.Krugmann S, Anderson KE, Ridley SH, Risso N, McGregor A, Coadwell J, Davidson K, Eguinoa A, Ellson CD, Lipp P, Manifava M, Ktistakis N, Painter G, Thuring JW, Cooper MA, Lim ZY, Holmes AB, Dove SK, Michell RH, Grewal A, Nazarian A, Erdjument-Bromage H, Tempst P, Stephens LR, Hawkins PT. Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol Cell. 2002;9:95–108. doi: 10.1016/s1097-2765(02)00434-3. [DOI] [PubMed] [Google Scholar]
- 54.I ST, Nie Z, Stewart A, Najdovska M, Hall NE, He H, Randazzo PA, Lock P. ARAP3 is transiently tyrosine phosphorylated in cells attaching to fibronectin and inhibits cell spreading in a RhoGAP-dependent manner. J Cell Sci. 2004;117:6071–6084. doi: 10.1242/jcs.01526. [DOI] [PubMed] [Google Scholar]
- 55.Krugmann S, Stephens L, Hawkins PT. Purification of ARAP3 and characterization of GAP activities. Methods Enzymol. 2006;406:91–103. doi: 10.1016/S0076-6879(06)06008-3. [DOI] [PubMed] [Google Scholar]
- 56.Rmali KA, Puntis MC, Jiang WG. TEM-8 and tubule formation in endothelial cells, its potential role of its vW/TM domains. Biochem Biophys Res Commun. 2005;334:231–238. doi: 10.1016/j.bbrc.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 57.Go MY, Chow EM, Mogridge J. The cytoplasmic domain of anthrax toxin receptor 1 affects binding of the protective antigen. Infect Immun. 2009;77:52–59. doi: 10.1128/IAI.01073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn't tell us. J Natl Cancer Inst. 2002;94:883–893. doi: 10.1093/jnci/94.12.883. [DOI] [PubMed] [Google Scholar]
- 59.Rogers MS, Christensen KA, Birsner AE, Short SM, Wigelsworth DJ, Collier RJ, D'Amato RJ. Mutant anthrax toxin B moiety (protective antigen) inhibits angiogenesis and tumor growth. Cancer Res. 2007;67:9980–9985. doi: 10.1158/0008-5472.CAN-07-0829. [DOI] [PubMed] [Google Scholar]
- 60.Gordon VM, Klimpel KR, Arora N, Henderson MA, Leppla SH. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect Immun. 1995;63:82–87. doi: 10.1128/iai.63.1.82-87.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernando S, Fletcher BS. Targeting tumor endothelial marker 8 in the tumor vasculature of colorectal carcinomas in mice. Cancer Res. 2009;69:5126–5132. doi: 10.1158/0008-5472.CAN-09-0725. [DOI] [PubMed] [Google Scholar]
- 62.Ruan Z, Yang Z, Wang Y, Wang H, Chen Y, Shang X, Yang C, Guo S, Han J, Liang H, Wu Y. DNA vaccine against tumor endothelial marker 8 inhibits tumor angiogenesis and growth. J Immunother. 2009;32:486–491. doi: 10.1097/CJI.0b013e3181a1d134. [DOI] [PubMed] [Google Scholar]
- 63.Duan HF, Hu XW, Chen JL, Gao LH, Xi YY, Lu Y, Li JF, Zhao SR, Xu JJ, Chen HP, Chen W, Wu CT. Antitumor activities of TEM8-Fc: an engineered antibody-like molecule targeting tumor endothelial marker 8. J Natl Cancer Inst. 2007;99:1551–1555. doi: 10.1093/jnci/djm132. [DOI] [PubMed] [Google Scholar]
- 64.Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 65.Mazurek S, Grimm H, Oehmke M, Weisse G, Teigelkamp S, Eigenbrodt E. Tumor M2-PK and glutaminolytic enzymes in the metabolic shift of tumor cells. Anticancer Res. 2000;20:5151–5154. [PubMed] [Google Scholar]
- 66.Venanzi FM, Petrini M, Fiammenghi L, Bolli E, Granato AM, Ridolfi L, Gabrielli F, Barucca A, Concetti A, Ridolfi R, Riccobon A. Tumor endothelial marker 8 expression levels in dendritic cell-based cancer vaccines are related to clinical outcome. Cancer Immunol Immunother. 2009 doi: 10.1007/s00262-009-0717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rouleau C, Menon K, Boutin P, Guyre C, Yoshida H, Kataoka S, Perricone M, Shankara S, Frankel AE, Duesbery NS, Vande Woude G, Biemann HP, Teicher BA. The systemic administration of lethal toxin achieves a growth delay of human melanoma and neuroblastoma xenografts: assessment of receptor contribution. Int J Oncol. 2008;32:739–748. [PubMed] [Google Scholar]
- 68.Felicetti P, Mennecozzi M, Barucca A, Montgomery S, Orlandi F, Manova K, Houghton AN, Gregor PD, Concetti A, Venanzi FM. Tumor endothelial marker 8 enhances tumor immunity in conjunction with immnization against differentiation Ag. Cytotherapy. 2007;9:23–34. [PubMed] [Google Scholar]
- 69.Arora N, Leppla SH. Residues 1–254 of anthrax toxin lethal factor are sufficient to cause cellular uptake of fused polypeptides. J Biol Chem. 1993;268:3334–3341. [PubMed] [Google Scholar]
- 70.Liu S, Aaronson H, Mitola DJ, Leppla SH, Bugge TH. Potent antitumor activity of a urokinase-activated engineered anthrax toxin. Proc Natl Acad Sci U S A. 2003;100:657–662. doi: 10.1073/pnas.0236849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu S, Bugge TH, Leppla SH. Targeting of tumor cells by cell surface urokinase plasminogen activator-dependent anthrax toxin. J Biol Chem. 2001;276:17976–17984. doi: 10.1074/jbc.M011085200. [DOI] [PubMed] [Google Scholar]
- 72.Liu S, Netzel-Arnett S, Birkedal-Hansen H, Leppla SH. Tumor cell-selective cytotoxicity of matrix metalloproteinase-activated anthrax toxin. Cancer Res. 2000;60:6061–6067. [PubMed] [Google Scholar]
- 73.Liu S, Wang H, Currie BM, Molinolo A, Leung HJ, Moayeri M, Basile JR, Alfano RW, Gutkind JS, Frankel AE, Bugge TH, Leppla SH. Matrix metalloproteinase-activated anthrax lethal toxin demonstrates high potency in targeting tumor vasculature. J Biol Chem. 2008;283:529–540. doi: 10.1074/jbc.M707419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu S, Redeye V, Kuremsky JG, Kuhnen M, Molinolo A, Bugge TH, Leppla SH. Intermolecular complementation achieves high-specificity tumor targeting by anthrax toxin. Nat Biotechnol. 2005;23:725–730. doi: 10.1038/nbt1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen KH, Liu S, Bankston LA, Liddington RC, Leppla SH. Selection of anthrax toxin protective antigen variants that discriminate between the cellular receptors TEM8 and CMG2 and achieve targeting of tumor cells. J Biol Chem. 2007;282:9834–9845. doi: 10.1074/jbc.M611142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duesbery NS, Resau J, Webb CP, Koochekpour S, Koo HM, Leppla SH, Vande Woude GF. Suppression of ras-mediated transformation and inhibition of tumor growth and angiogenesis by anthrax lethal factor, a proteolytic inhibitor of multiple MEK pathways. Proc Natl Acad Sci U S A. 2001;98:4089–4094. doi: 10.1073/pnas.061031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koo HM, VanBrocklin M, McWilliams MJ, Leppla SH, Duesbery NS, Woude GF. Apoptosis and melanogenesis in human melanoma cells induced by anthrax lethal factor inactivation of mitogen-activated protein kinase kinase. Proc Natl Acad Sci U S A. 2002;99:3052–3057. doi: 10.1073/pnas.052707699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang D, Ding Y, Luo WM, Bender S, Qian CN, Kort E, Zhang ZF, VandenBeldt K, Duesbery NS, Resau JH, Teh BT. Inhibition of MAPK kinase signaling pathways suppressed renal cell carcinoma growth and angiogenesis in vivo. Cancer Res. 2008;68:81–88. doi: 10.1158/0008-5472.CAN-07-5311. [DOI] [PubMed] [Google Scholar]
- 79.Abi-Habib RJ, Urieto JO, Liu S, Leppla SH, Duesbery NS, Frankel AE. BRAF status and mitogen-activated protein/extracellular signal-regulated kinase kinase 1/2 activity indicate sensitivity of melanoma cells to anthrax lethal toxin. Mol Cancer Ther. 2005;4:1303–1310. doi: 10.1158/1535-7163.MCT-05-0145. [DOI] [PubMed] [Google Scholar]
- 80.Alfano RW, Leppla SH, Liu S, Bugge TH, Herlyn M, Smalley KS, Bromberg-White JL, Duesbery NS, Frankel AE. Cytotoxicity of the matrix metalloproteinase-activated anthrax lethal toxin is dependent on gelatinase expression and B-RAF status in human melanoma cells. Mol Cancer Ther. 2008;7:1218–1226. doi: 10.1158/1535-7163.MCT-08-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alfano RW, Leppla SH, Liu S, Bugge TH, Ortiz JM, Lairmore TC, Duesbery NS, Mitchell IC, Nwariaku F, Frankel AE. Inhibition of tumor angiogenesis by the matrix metalloproteinase-activated anthrax lethal toxin in an orthotopic model of anaplastic thyroid carcinoma. Mol Cancer Ther. 9:190–201. doi: 10.1158/1535-7163.MCT-09-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bromberg-White JL, Boguslawski E, Duesbery NS. Perturbation of mouse retinal vascular morphogenesis by anthrax lethal toxin. PLoS One. 2009;4:e6956. doi: 10.1371/journal.pone.0006956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ding Y, Boguslawski EA, Berghuis BD, Young JJ, Zhang Z, Hardy K, Furge K, Kort E, Frankel AE, V. Hay R, Resau JH, Duesbery NS. Mitogen-activated protein kinase kinase signaling promotes growth and vascularization of fibrosarcoma. Mol Cancer Ther. 2008;7:648–658. doi: 10.1158/1535-7163.MCT-07-2229. [DOI] [PubMed] [Google Scholar]
- 84.Warfel JM, Steele AD, D'Agnillo F. Anthrax lethal toxin induces endothelial barrier dysfunction. Am J Pathol. 2005;166:1871–1881. doi: 10.1016/S0002-9440(10)62496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alfano RW, Leppla SH, Liu S, Bugge TH, Meininger CJ, Lairmore TC, Mulne AF, Davis SH, Duesbery NS, Frankel AE. Matrix metalloproteinase-activated anthrax lethal toxin inhibits endothelial invasion and neovasculature formation during in vitro morphogenesis. Mol Cancer Res. 2009;7:452–461. doi: 10.1158/1541-7786.MCR-08-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kirby JE. Anthrax lethal toxin induces human endothelial cell apoptosis. Infect Immun. 2004;72:430–439. doi: 10.1128/IAI.72.1.430-439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hong J, Doebele RC, Lingen MW, Quilliam LA, Tang WJ, Rosner MR. Anthrax edema toxin inhibits endothelial cell chemotaxis via Epac and Rap1. J Biol Chem. 2007;282:19781–19787. doi: 10.1074/jbc.M700128200. [DOI] [PubMed] [Google Scholar]


