Abstract
Transcriptional repression by nuclear hormone receptors is thought to result from a unison of targeting chromatin modification and disabling the basal transcriptional machinery. We used Xenopus oocytes to compare silencing effected by the thyroid hormone receptor (TR) and its mutated version, the oncoprotein v-ErbA, on partly and fully chromatinized TR-responsive templates in vivo. Repression by v-ErbA was not as efficient as that mediated by TR, was significantly more sensitive to histone deacetylase (HDAC) inhibitor treatment and, unlike TR, v-ErbA required mature chromatin to effect repression. We find that both v-ErbA and TR can recruit the corepressor N-CoR, but, in contrast to existing models, show a concomitant enrichment for HDAC3 that occurs without an association with Sin3, HDAC1/RPD3, Mi-2 or HDAC5. We propose a requirement for chromatin infrastructure in N-CoR/HDAC3-effected repression and suggest that the inability of v-ErbA to silence on partly chromatinized templates may stem from its impaired capacity to interfere with basal transcriptional machinery function. In support of this notion, we find v-ErbA to be less competent than TR for binding to TFIIB in vitro and in vivo.
Keywords: histone deacetylase (HDAC)3/N-CoR/thyroid hormone receptor/transcriptional repression/v-ErbA
Introduction
Embryogenesis and adult ontogeny in metazoa offer many examples of particular cell types undergoing a regulated transition from proliferation to cell cycle arrest and differentiation. A complex phenomenon, in specific instances this event can be experimentally triggered by a simple signal, e.g. hormonal treatment. Nuclear hormone receptors (NHRs), including those for vitamin D (Haussler et al., 1997), thyroid hormone (T3) (Brown et al., 1995; Tata, 1996) and 9-cis retinoic acid (Sucov and Evans, 1995), provide excellent models for this transition. According to current data, genes required for cell cycle arrest and the differentiated phenotype are silenced by DNA-bound receptors in the absence of hormone and are activated in its presence (Xu et al., 1999). An important and poorly understood aspect of repression by NHRs stems from the necessity for their constitutive function on chromatin templates (Samuels et al., 1982). In proliferating cells, cycles of DNA replication, chromatin assembly and chromosome condensation impose on the unliganded receptor the requirement for continued association with DNA to mediate repression on a variety of chromatin substrates, including those that are in the process of assembly (Adams and Kamakaka, 1999).
Studies of thyroid hormone receptor (TR) have found two pathways likely to be involved in transcriptional repression (Hanna-Rose and Hansen, 1996). In vitro experiments with purified DNA templates (Fondell et al., 1993, 1996; Tong et al., 1995) indicated that unliganded TR binds various components of the basal transcriptional machinery, including TATA-binding protein (TBP) and transcription factor IIB (TFIIB), and suggested that the receptor interferes with an early step of pre-initiation complex formation, possibly by affecting the association between TFIIB and DNA-bound TBP (TFIID).
In separate studies, use of unliganded TR as ‘bait’ in yeast two-hybrid screens (Chen and Evans, 1995; Hoerlein et al., 1995; Seol et al., 1995; Dressel et al., 1999) led to the identification of several large polypeptides, termed ‘corepressors’. In assays based on transient transfection of plasmids carrying synthetic multimerized TR response elements (TREs), corepressors potentiate repression by TR and other members of the nuclear hormone superfamily, and a positive correlation exists between the ability of mutated forms of TR to interact physically with corepressors (as gauged from in vitro assays) and their ability to silence transcription driven by such artificial promoters (Torchia et al., 1998; Xu et al., 1999). Functional aspects of corepressor action were revealed when biochemical approaches identified their physical association with the histone deacetylase (HDAC) RPD3/HDAC1 via the adapter–auxiliary corepressor molecule Sin3 (Heinzel et al., 1997; Nagy et al., 1997). Together with data that HDAC inhibitors such as trichostatin A (TSA) alleviate receptor-mediated silencing, this added TR and other class II NHRs to a growing list of transcriptional repressors in metazoa that exploit HDAC targeting to effect repression, such as Mad/Max (Heinzel et al., 1997; Laherty et al., 1997), Rb (Brehm et al., 1998; Luo et al., 1998; Magnaghi-Jaulin et al., 1998), groucho (Chen et al., 1999), MeCP2 (Jones et al., 1998; Nan et al., 1998) and Ikaros (Koipally et al., 1999).
The relative contribution to receptor-effected silencing made by recruiting corepressors versus that made by direct interaction of the unliganded receptor with the basal transcriptional machinery remains to be determined. It is also unclear whether HDAC function accounts for the entirety of repression mediated by corepressors recruited by unliganded NHRs to their natural targets in a native chromosomal context. Published data (Muscat et al., 1998; Wong and Privalsky, 1998) are consistent with the notion that such corepressors as mSin3a, SMRT and N-CoR exploit both HDAC-dependent and -independent mechanisms for effecting repression; the latter may also involve interactions with the basal transcriptional machinery. In the present study we addressed this problem via the use of two well-studied regulatory factors, the NHR TR/retinoid X receptor (RXR) and the oncoprotein v-ErbA.
The avian erythroblastosis virus (AEV) causes fatal erythroleukemia in chickens (Beug et al., 1994); leukemic transformation requires the action of normal or mutated oncogenic receptor tyrosine kinases in cooperation with a mutated version of chicken TRα, the oncoprotein v-ErbA (Sap et al., 1986; Weinberger et al., 1986). v-ErbA is thought to constitutively silence currently unidentified TR-regulated genes required for proliferation induction, differentiation arrest and/or protection from apoptosis in hematopoietic precursor cells (Damm et al., 1989; Sap et al., 1989; Beug et al., 1996; Bauer et al., 1998; Stunnenberg et al., 1999). This property of v-ErbA is known to derive from nine point mutations in its ligand-binding domain that decrease affinity for thyroid hormone (Munoz et al., 1988; Zenke et al., 1990), as well as the deletion of a conserved C-terminal α-helix that mediates transcriptional activation by various NHRs (Danielian et al., 1992; Saatcioglu et al., 1993; Tone et al., 1994; Moras and Gronemeyer, 1998). Importantly, T3 treatment not only induces cell cycle arrest and differentiation of primary chicken erythroblasts, but also markedly decreases the amount of HDAC activity associated with TR (Bauer et al., 1998). These data provide strong evidence supporting a functional role for HDAC recruitment in silencing by TR.
The interest in and utility of v-ErbA for investigating mechanisms of repression by TR stems from the presumed nature of this oncoprotein as a ‘pure’ transcriptional silencer in conjunction with its function as an oncogene: while TR is competent to engage in functional interactions pertaining to both repression and activation (Baniahmad et al., 1993; Tone et al., 1994; Tong et al., 1995; Lavigne et al., 1999), functional and biochemical data for v-ErbA can, potentially, point to the relevance of such interactions to repression per se. Importantly, in contrast to v-ErbA, TR does not contribute to oncogenesis unless strongly overexpressed and stabilized in a repressive conformation via the withdrawal of TR and RXR ligands (Bauer et al., 1998). Studies as to the mechanism by which v-ErbA represses target genes and causes leukemia have been severely limited by the fact that repression by v-ErbA has not been analyzed in a native chromatin environment. The Xenopus oocyte system is uniquely suited to addressing this issue, since microinjection of purified double-stranded DNA into the oocyte nucleus leads to progressive chromatinization of the injected DNA (Almouzni and Wolffe, 1993b). By generating functional and biochemical data for v-ErbA-mediated gene silencing in such a context, new insights into the relevance of v-ErbA interactions with corepressors versus basal transcription factors to the mechanisms of repression per se can be expected.
We report here our comparative analysis of chicken TRα (c-ErbA) and v-ErbA as transcriptional regulators of the Xenopus TRβA gene promoter. We find that both proteins can repress basal transcription in this system, but repression by v-ErbA is more dependent on template chromatinization than that effected by TR, and is also significantly more sensitive to treatment with the HDAC inhibitor, TSA. We find, however, that v-ErbA is quite efficient in recruiting the corepressor N-CoR and HDAC activity. Suprisingly, no Sin3 or HDAC1, but rather HDAC3, was found enriched in the v-ErbA–N-CoR complex. Similarly, we show that ligand-regulated targeting of N-CoR to TR is accompanied by an enrichment for HDAC3, but not Sin3, HDAC1 or the Mi-2 ATPase. Finally, we show that both unliganded TR and v-ErbA can bind TBP in vitro, but that v-ErbA is less competent than TR for binding to TFIIB in vitro and in vivo. We propose that targeting of the N-CoR–HDAC3 complex represents the chromatin-dependent component of transcriptional repression effected by v-ErbA and by TR.
Results
v-ErbA and unliganded chicken TR silence basal transcription driven by the Xenopus TRβA gene promoter
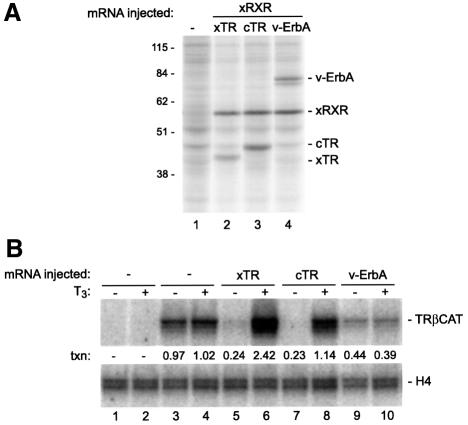
We used mRNA microinjection to establish that chicken TRα and v-ErbA can be synthesized in the Xenopus oocyte (Figure 1A, lanes 3 and 4), and then used gel-shift analysis and genomic DNase I footprinting to show that the resulting proteins are competent for binding to the previously characterized (Shi et al., 1992; Machuca et al., 1995; Wong et al., 1995, 1997) T3- and TR-responsive upstream enhancer of the Xenopus TRβA gene (data not shown). An in vivo transcription assay (Figure 1B) demonstrated that both unliganded Xenopus TRβ and chicken TRα efficiently silenced basal transcription of the TRβA gene (Figure 1B, compare lanes 3 and 4 with 5 and 7), and treatment with T3 led to relief of transcriptional repression (lanes 6 and 8). As expected (Zenke et al., 1990), v-ErbA silenced basal transcription of the TRβA gene in a ligand-insensitive manner (Figure 1B, lanes 9 and 10), albeit not as efficiently as TR (compare lanes 5 and 7 with 9 and 10). We conclude that v-ErbA can moderately repress basal transcription of a Xenopus TR-regulated gene in vivo.
Fig. 1. Chicken TRα (c-ErbA) and v-ErbA can be expressed in the Xenopus oocyte and can repress transcription driven by the TRβA gene promoter. (A) In vivo synthesis of chicken TRα and v-ErbA. Oocytes were left uninjected (lane 1), or injected with 5 ng of Xenopus RXRα mRNA along with 5 ng of mRNA for Xenopus TRβ (lane 2), chicken TRα (lane 3) or v-ErbA (lane 4) mRNA. The oocytes were cultured in the presence of [35S]methionine for 10 h, and oocyte protein extract was prepared as described in Materials and methods. Proteins synthesized in the oocyte during the incubation period were visualized by SDS–PAGE and autoradiography. The positions of the proteins derived from the injected mRNAs are indicated to the right of the autoradiograph; molecular weight marker size is indicated to the left. (B) Transcriptional regulation by chicken TRα and v-ErbA. Groups of 20 oocytes were injected into the cytoplasm with 5 ng of Xenopus RXRα mRNA (lanes 5–10) along with 5 ng of Xenopus TRβ (lanes 5 and 6), chicken TRα (lanes 7 and 8) or v-ErbA (lanes 9 and 10) mRNA; the oocytes in lanes 1–4 were not injected with mRNA. After a 12 h incubation, the oocytes were left uninjected (lanes 1 and 2), or injected into the nucleus with 1 ng (lanes 3–10) of double-stranded reporter plasmid DNA (pTRβA); the oocytes were then cultured for an additional 16 h in the absence (–) or presence (+) of 100 nM thyroid hormone (T3), following which total RNA was extracted and levels of H4 and TRβA mRNA were measured by primer extension as described in Materials and methods. The primer against histone H4 mRNA that is stored in the oocyte was included in all reactions to serve as a loading control. Transcriptional activity (txn) was quantitated by normalizing the signal for TRβA in each sample against that for H4 in that sample, and expressing the result in arbitrary units where 1 unit = transcriptional activity in the absence of injected mRNA (the average of signals from lanes 3 and 4).
HDAC is required for establishing and maintaining transcriptional repression effected by v-ErbA and chicken TR
Targeted modification of chromatin provides a candidate mechanism for transcriptional silencing by NHRs (Wolffe, 1997; Torchia et al., 1998; Xu et al., 1999); work from this and other laboratories pointed to a role for HDAC in mediating repression (Heinzel et al., 1997; Nagy et al., 1997; Wong et al., 1998b). We evaluated the capacity of an HDAC inhibitor, TSA, to interfere with transcriptional repression effected by v-ErbA and by unliganded chicken TR.
Since histone deacetylation is required for chromatin assembly (Adams and Kamakaka, 1999), we used two distinct experimental regimens to distinguish between relief of transcriptional repression via inhibition of HDAC activity targeted by receptor and a failure to establish a repressed state due to the inability to assemble chromatin properly; as shown in Figure 2A, following mRNA injection and receptor synthesis, we either included TSA in the medium immediately after DNA injection (lower half), or allowed chromatin assembly in the absence of TSA, and then monitored its effects on more completely assembled chromatin templates (upper half).
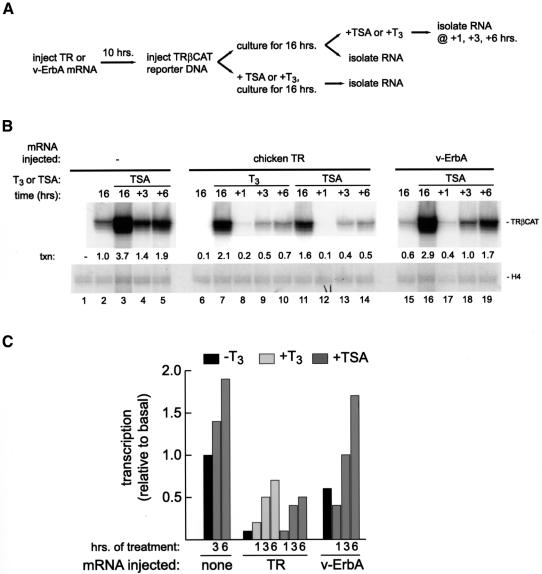
Fig. 2. Analysis of the effects of an HDAC inhibitor (TSA) on the establishment and maintenance of transcriptional silencing by TR and v-ErbA. (A) Schematic representation of the two experimental regimens used. (B) Groups of 20 oocytes were left uninjected (lanes 1–5), injected into the cytoplasm with 1 ng each of Xenopus RXRα and chicken TRα mRNA (lanes 6–14), or 3 ng each of Xenopus RXRα and wild-type v-ErbA mRNA (lanes 15–19). Following incubation for 10 h, 500 pg of pTRβA double-stranded reporter plasmid DNA were injected into the nucleus (all lanes except lane 1). Following injection, oocytes in lanes 2, 6 and 15 were cultured for 16 h and total RNA extracted for analysis (these served to measure basal transcription and the repressed state in the presence of TR or v-ErbA, respectively). Oocytes in lanes 3, 11 and 16 were cultured for the same length of time in the presence of 33 nM TSA prior to total RNA extraction; this regimen revealed the effects on transcriptional activity of an HDAC inhibitor present during chromatin assembly and maturation (the effect of 100 nM T3 is shown in lane 7). To determine the effect of TSA or T3 on transcriptional activity of a mature chromatin template, groups of oocytes were injected with receptor mRNA, cultured for 10 h to allow protein synthesis, injected with reporter plasmid DNA, and cultured for 16 h to allow full chromatin assembly. TSA or T3 (33 or 100 nM, respectively) was then added where indicated, and samples of 20 oocytes were removed for total RNA isolation at 1, 3 and 6 h following addition. Transcriptional activity of the reporter plasmid was analyzed and quantitated as described in the legend to Figure 1B. (C) A graphical representation of the data in (B).
Chromatin was assembled in the absence (Figure 2B, lane 2) or presence of receptor (TR, lane 6; v-ErbA, lane 15) for 16 h. As expected (Figure 1B), both proteins repressed basal transcription, albeit with different efficiencies. The oocytes were then placed into TSA or T3, and the effect of this treatment on transcriptional activity of the TRβA promoter was monitored over the course of the next 6 h. As shown in Figure 2B, lanes 12–14, TSA relieved transcriptional repression established by chicken TR; we noted that from a kinetic standpoint, at early timepoints the addition of ligand (lanes 8–10) had the same effect on transcription from a TR-bound promoter as treatment with the HDAC inhibitor (lanes 12–14), while over the course of 16 h, T3 stimulated transcription to a greater extent than TSA did (compare lanes 7 and 11). TSA efficiently relieved repression established by v-ErbA (Figure 2B, lanes 17–19) and had a reproducibly more significant effect on v-ErbA-repressed chromatin than on TR-repressed chromatin (compare lane 14 with lane 19).
It is informative to compare the effect that TSA has on basal transcription (Figure 2B, lanes 4 and 5) with its effect on receptor-repressed chromatin. When the promoter is bound by TR, TSA treatment reproducibly relieves transcriptional repression to only a fraction of the level seen in the absence of TR (compare lane 5 with lane 14). In contrast, the stimulation of transcription by TSA on a v-ErbA-repressed template reproducibly approached that exerted by TSA in the absence of v-ErbA (Figure 2B, compare lane 5 with lane 19).
These experiments establish that an HDAC inhibitor will prevent both the establishment as well as the maintenance of transcriptional silencing effected by chicken TR or by v-ErbA, and that a chromatin template silenced by v-ErbA is significantly more sensitive to TSA treatment than a template silenced by TR.
Distinct requirements for template chromatinization in repression by v-ErbA and by TR in vivo
The robust relief of v-ErbA-effected repression by an HDAC inhibitor (Figure 2B) suggested to us that the properties of v-ErbA as a transcriptional regulator of the TRβA gene might be affected by the extent to which the DNA target is assembled into chromatin. The Xenopus oocyte system is uniquely suited to addressing this issue experimentally: microinjection of purified double-stranded DNA into the oocyte nucleus leads to gradual chromatinization of the injected DNA over the course of 3–12 h (Almouzni et al., 1990; Almouzni and Wolffe, 1993b); thus, one can examine the transcriptional activity of DNA constructs at various levels of assembly into chromatin.
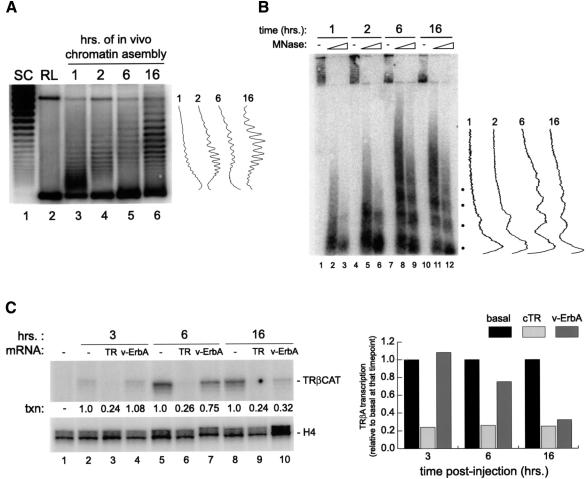
Figure 3A and B shows that at early timepoints following injection of DNA into the oocyte nucleus (Figure 3A, lanes 3 and 4, and B, lanes 2, 3, 5 and 6), nucleosome density over the injected DNA is low and the DNA is highly accessible to micrococcal nuclease. At later timepoints, a more robust nucleosomal ladder is visualized over the injected DNA (Figure 3B, lanes 8, 9, 11 and 12), and topological criteria also indicate that chromatin is more mature (Figure 3A, lanes 5 and 6).
Fig. 3. An analysis of requirements for template chromatinization in transcriptional repression by TR and v-ErbA. (A) Alterations in plasmid topology due to progressive chromatinization were revealed by extracting plasmid DNA from injected oocytes (see below) at the indicated timepoints and analyzing the samples on a chloroquine-containing gel along with a sample of supercoiled (lane 1) and relaxed (lane 2) plasmid. Southern blotting was performed with a 0.8 kb EcoRI–SmaI fragment corresponding to positions –1325 to –515 in the TRβA gene promoter. PhosphorImager-generated scans of data from the indicated timepoints are shown to the right of the gel. (B) A large group of oocytes was injected into the nucleus with 3 ng of pTRβA double-stranded reporter plasmid DNA. At indicated timepoints, 30 oocytes were removed, homogenized as described in Materials and methods, and 1/3 of the sample was left untreated with nuclease (lanes 1, 4, 7 and 10), or treated with 30 or 120 Worthington units of MNase (the first and second lane of each pair, respectively). DNA was extracted, fractionated on a 2% agarose gel and analyzed by Southern blotting as described in Materials and methods. PhosphorImager-generated scans of data from the right lane of each pair at the indicated timepoints are shown to the right of the gel. (C) A large group of oocytes was uninjected (lanes 1, 2, 5 and 8), injected into the cytoplasm with 1 ng each of Xenopus RXRα and chicken TRα mRNA (lanes 3, 6 and 9), or 2 ng each of Xenopus RXRα and wild-type v-ErbA mRNA (lanes 4, 7 and 10). Following incubation for 10 h, 500 pg of pTRβA double-stranded reporter plasmid DNA were injected into the nucleus (all lanes except lane 1). At 3, 6 and 16 h following DNA injection (lanes 2–4, 5–7 and 8–10, respectively), samples of 20 oocytes were removed and total RNA isolated. Transcriptional activity of the reporter was analyzed by primer extension as described in the legend to Figure 1B. For each timepoint the basal level of transcription (lanes 2, 5 and 8, respectively) was set to 1.0. A graphical representation of the data is shown to the right of the autoradiograph.
Figure 3C shows a representative result of one of several experiments in which we injected oocytes with mRNA for TR/RXR or v-ErbA/RXR and, following receptor synthesis, injected double-stranded TRβA reporter DNA into the nucleus of control and receptor-containing oocytes. The transcriptional activity of the TRβA promoter was then monitored over the course of time by isolating total RNA from oocytes at defined timepoints following injection. Figure 3C, lanes 3, 6 and 9, shows that the efficiency of chicken TR as a transcriptional repressor is independent of duration of time allowed for template chromatinization; over multiple experiments, we found that unliganded chicken TRα repressed transcription as soon as basal transcription can be detected in its absence (data not shown)—this agrees with published observations from our laboratory on Xenopus TR (Wong et al., 1997). In contrast, we found that the level of repression effected by v-ErbA was positively correlated with the length of time allowed for assembly of the injected DNA into chromatin: at an early timepoint, v-ErbA reproducibly failed to repress basal transcription (compare lanes 2 and 4 in Figure 3C). At 6 h post-injection, as more histone octamers became associated with the DNA (Figure 3A, lane 5 and B, lanes 8 and 9), v-ErbA exerted a moderate silencing effect on basal transcription (Figure 3C, lanes 5 and 7). Finally, when chromatin assembled on the template became more mature (Figure 3A, lane 6 and B, lanes 11 and 12), repression by v-ErbA reproducibly reached its maximal levels (Figure 3C, lanes 8 and 10).
The experiments described in this section resulted from our observation that chromatin templates repressed by TR and v-ErbA are differentially sensitive to an HDAC inhibitor (Figure 2). In an extension of this observation, the oncoprotein v-ErbA is shown here to have a greater requirement than TR for a chromatin target in effecting transcriptional repression.
v-ErbA and TR associate with the corepressor N-CoR and HDAC in the absence of Sin3, HDAC1/RPD3 or Mi-2
Our data are consistent with the hypothesis that HDAC activity targeted by chromatin-bound v-ErbA is responsible for effecting repression. Previous studies from this laboratory have proven the utility of the oocyte as a model system for biochemical characterization of protein complexes involved in transcriptional repression via histone deacetylation (Jones et al., 1998; Wade et al., 1998); such complexes are abundant in the oocyte, where they form a molecular stockpile in preparation for multiple rounds of early embryonic division. Since the only exogenous protein component in our transcription assays is the DNA-bound repressor, our findings indicate that the Xenopus oocyte contains sufficient quantities of auxiliary components of the repression pathway to allow the complete abolition of transcription driven by a DNA template that is ∼2 orders of magnitude more abundant than the oocyte’s own genome (1 ng of reporter plasmid DNA versus 12 pg of genomic DNA; Figure 1B, lanes 5 and 7).
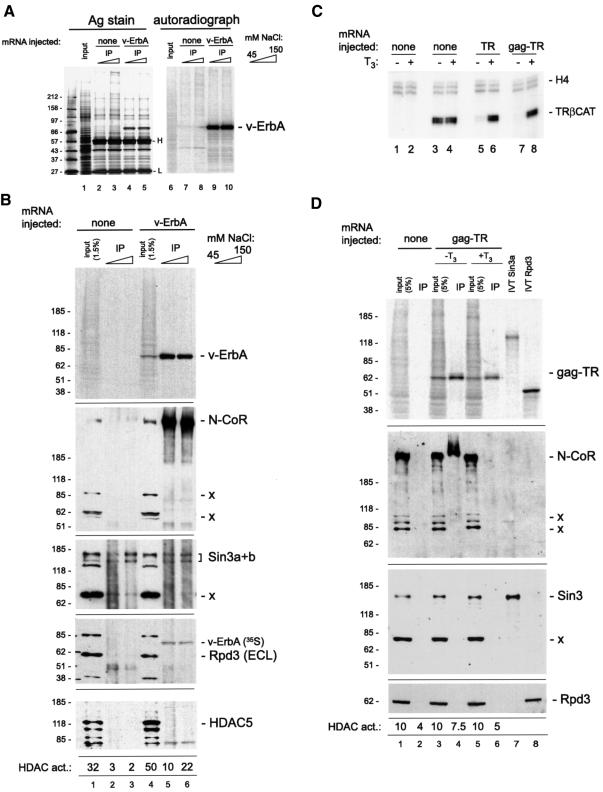
We exploited a unique biochemical property of the v-ErbA oncoprotein—the retroviral gag moiety on its N-terminus—as a tool to investigate its protein partners in effecting TSA-sensitive transcriptional repression. We used mRNA microinjection to express v-ErbA in the oocyte and a monoclonal antibody directed against the gag epitope to demonstrate that a polypeptide of the expected size can be efficiently immunoprecipitated from whole oocyte extract (Figure 4A, lanes 4 and 5) under conditions of low to moderate ionic strength. Importantly for the interpretation of subsequent experiments, silver staining revealed a number of polypeptides that associated with the antibody in extracts from oocytes that were not injected with v-ErbA (Figure 4A, lanes 2 and 3). Proteins synthesized in the oocyte during the course of the experiment were labeled in vivo by [35S]methionine, and autoradiographic analysis of the silver-stained Laemmli SDS–PAGE shown on the left in Figure 4A revealed that the predominant labeled species in the immunoprecipitation corresponds in size to the expected protein product of the v-ErbA mRNA (Figure 4A, lanes 9 and 10), and that no such entity was observed when no mRNA was injected (lanes 7 and 8). We conclude that the v-ErbA oncoprotein can be efficiently immunoprecipitated from whole oocyte extract.
Fig. 4. A biochemical analysis of associations of the v-ErbA oncoprotein in the oocyte. (A) v-ErbA can be immunoprecipitated from whole oocyte extract using a monoclonal antibody against the gag domain. Oocytes (100 per sample) were left uninjected (lanes 1–3 and 6–8) or injected with 10 ng of mRNA for v-ErbA (lanes 4, 5, 9 and 10) and cultured in the presence of [35S]methionine for 16 h; immunoprecipitation with anti-gag monoclonal antibody was performed as described in Materials and methods in 45 mM NaCl-containing buffer, followed by washing in buffer containing the amount of NaCl indicated on the right. One third of the immunoprecipitate was resolved on a 4–12% Laemmli SDS–PAGE followed by silver staining (lanes 1–5) and autoradiographic analysis of the silver-stained gel (lanes 6–10). The ‘input’ lane contains 1/20 of an oocyte equivalent of whole oocyte protein extract; the position of molecular weight markers is indicated to the left of the gel, the v-ErbA-sized polypeptide—to the right of the gel, and of immunoglobulin light (L) and heavy (H) chain—between lanes 5 and 6. (B) Recruitment of N-CoR and HDAC activity to v-ErbA is not accompanied by an enrichment for Sin3, HDAC1 or 5. One third of the immunoprecipitate described in the legend to (A) was resolved on a 4–12% Laemmli SDS–PAGE along with the indicated quantity of input whole oocyte extract, transferred to a nitrocellulose membrane and analyzed by autoradiography (top panel) and sequential western blotting with antibody against Xenopus N-CoR (second panel), Sin3 (third panel), Rpd3p/HDAC1 (fourth panel) and mouse HDAC5 (fifth panel). In all panels, the position of molecular weight markers is indicated to the right, and the position of the antigen targeted by the antibody is to the left of the autoradiograph. The positions of additional polypeptides that cross-react with the antibodies are indicated by –x; longer (∼5–10 min) exposures to X-ray film used in the fourth and fifth panels reveal a band corresponding precisely to the 35S-labeled v-ErbA polypeptide along with the chemiluminescence (ECL)-derived signal from the target antigen. The numbers below the fifth panel were obtained by measuring HDAC activity in the remaining 1/3 of the immunoprecipitate exactly as described (Wade et al., 1999b) and expressing the resulting values in arbitrary units relative to a blank sample. (C) The gag tag does not affect the properties of TR as a transcriptional regulator of the Xenopus TRβA gene. Oocytes were injected with mRNA for Xenopus RXRα (lanes 5–8) along with mRNA chicken TRα (lanes 5 and 6) or chicken TRα tagged with the AEV gag moiety on its N-terminus (lanes 7 and 8), followed by injection of 1 ng of double-stranded TRβA reporter DNA (lanes 3–8) and analysis of transcription by primer extension as described in the legend to Figure 1B. (D) Ligand-regulated recruitment to TR of N-CoR and HDAC activity is not accompanied by an enrichment for Sin3 or HDAC1/RPD3. Sixty oocytes were left uninjected (lanes 1 and 2), or injected with 10 ng of mRNA for gag-tagged chicken TRα (lanes 3–6) and cultured in [35S]methionine-containing medium in the presence or absence of 100 nM T3, as indicated. Immunoprecipitation with antibody against gag was performed as described in (B); binding and washing was performed in 45 mM NaCl and 1 µM T3 where indicated. An aliquot of the immunoprecipitate along with the indicated quantity of input extract and an aliquot of in vitro translated Xenopus Sin3 (lane 7) was resolved on a 4–12% Laemmli SDS–PAGE, transferred to nitrocellulose and analyzed by autoradiography (top panel; quantitation of the gag-TR signal reveals a 2-fold greater amount in lane 4 as compared with lane 6) and by western blotting with antibody against N-CoR (middle panel) and Sin3 (bottom panel). An identical immunoprecipitation was split into two aliquots, and one was analyzed by western blotting as above with an antibody against RPD3 (bottom panel), while the other was assayed for HDAC activity as above.
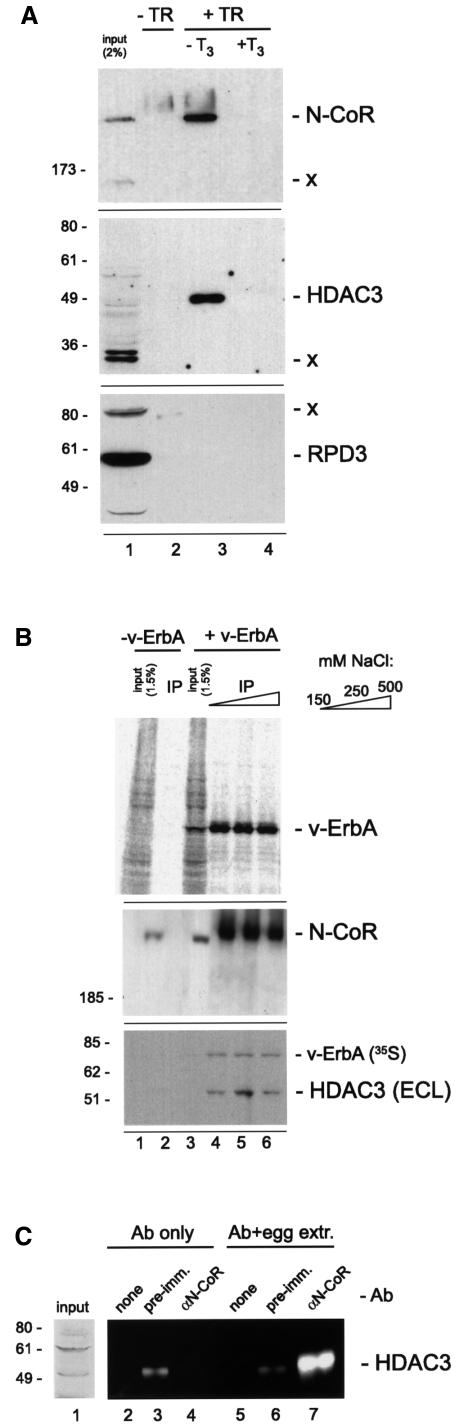
An aliquot of this immunoprecipitate was analyzed by western blotting for the presence of proteins that interacted with the agarose–antibody complex in a v-ErbA-dependent manner. Following SDS–PAGE and transfer to a nitrocellulose membrane, autoradiographic analysis confirmed that the immunoprecipitation leads to a significant enrichment for target antigen in the precipitate (Figure 4B, top panel, lanes 5 and 6). We then used rabbit antisera against an N-terminal portion of Xenopus N-CoR (P.Jones, L.Sachs, N.Rouse and Y.-B.Shi, manuscript submitted) to reveal an extraordinary enrichment for this corepressor in immunoprecipitates from oocytes injected with v-ErbA (Figure 4B, second panel, lanes 5 and 6); we were reproducibly able to show that >30% of all N-CoR contained in the input sample (∼30–60 oocyte equivalents of protein extract) associated with v-ErbA under these conditions. Importantly, <0.5% of N-CoR contained in the input associated with the beads in the absence of v-ErbA (Figure 4B, second panel, compare lane 1 with lanes 2 and 3), which was not an artefactual consequence of selective proteolysis or insufficient antibody used in the control reactions (Figure 4A, compare lanes 2 and 3 with 4 and 5). Further evidence for the specificity of the interaction lies in the reproducible finding that additional, lower molecular weight polypeptides contained in whole oocyte extract that react with the N-CoR antibody (identified by ‘–x’ in the second panel of Figure 4B) fail to associate with v-ErbA (compare lanes 5 and 6 with lane 4 in Figure 4B).
Based on published observations (Heinzel et al., 1997), we assumed that the corepressor associated with v-ErbA in the oocyte targets the enzyme RPD3 (HDAC1) to the oncoprotein via the adapter molecule Sin3; to our surprise, however, no association between Sin3 and v-ErbA was observed. Data in lanes 5 and 6 in the second and third panels of Figure 4B show that the massive v-ErbA-dependent enrichment for N-CoR in the immunoprecipitate is not accompanied by an enrichment for Sin3; background quantities (<1% input) of Sin3 protein associated with the antibody both in the presence and absence of v-ErbA (Figure 4B, compare lanes 2 and 3 with 5 and 6), indicative of a non-specific association that is likely to reflect the abundance of the Sin3 protein in oocytes (Vermaak et al., 1999). We also failed to observe an association between v-ErbA and either RPD3/HDAC1 (Figure 4B, fourth panel) or HDAC5 (Figure 4B, bottom panel). We performed a similar experiment in low ionic strength buffer (5 mM KCl, 1.5 mM MgCl2) and failed to observe an association between v-ErbA and either Sin3, HDAC1, HDAC5 or RbAp48 under conditions of a robust v-ErbA-dependent enrichment for N-CoR (data not shown). HDAC did, however, associate with v-ErbA in our experiments, as shown by assaying the immunoprecipitate for HDAC activity using in vitro hyperacetylated histones as substrate (bottom of Figure 4B). While, as expected, both input samples efficiently released [3H]acetate from core histones (Figure 4B, bottom, lanes 1 and 4), immunoprecipitation of such activity was unequivocally dependent on v-ErbA (compare lanes 2 and 3 with 5 and 6). These data suggested that an entity other than HDAC1 or HDAC5 capable of deacetylating core histones associates with v-ErbA in a manner that is independent of an enrichment for Sin3, and that such binding is accompanied by an interaction with N-CoR.
The utility of the gag moiety in enriching for v-ErbA-dependent repression machinery components prompted us to expand its use to the parent molecule of v-ErbA, TRα. In particular, we aimed to exploit the ligand responsiveness of TR as a transcriptional regulator (Figure 1B, lanes 5, 6, 7 and 8) to gauge the functional relevance of the biochemical interactions we observe with v-ErbA, which does not respond to ligand (Figure 1B). We used chicken TRα tagged with AEV gag on its N-terminus (Zenke et al., 1990) and confirmed that the tag does not in any detectable way alter the properties of TR as a transcriptional regulator in this system (Figure 4C, compare lanes 5 and 6 with 7 and 8). We then used mRNA microinjection coupled with in vivo labeling by [35S]methionine and immunoprecipitation with the anti-gag monoclonal antibody to confirm that gag-TR can be efficiently precipitated from whole oocyte extract (Figure 4D, lanes 3 and 4).
As expected from published observations (Heinzel et al., 1997) and our findings with v-ErbA (Figure 4B), TR-dependent enrichment of N-CoR in the immunoprecipitate was observed under conditions of moderate ionic strength (45 mM NaCl, 5 mM KCl, 1.5 mM MgCl2; Figure 5B, middle panel, compare lanes 2 and 4). In an unexpected contradiction to published data that ligand-driven release of N-CoR from TR cannot be achieved in solution in the absence of DNA (Hoerlein et al., 1995), inclusion of 100 nM T3 in the oocyte culture medium and 1 µM T3 in the binding buffer and the wash solution reaction eliminated detectable N-CoR from the immunoprecipitate. The use of T3 reproducibly decreased by ∼1.5-fold the amount of TR present in oocyte extract; we do not understand the biochemical basis of this effect (it is not due to homodimerization of unliganded TRα, as the same phenomenon was observed with TRβ, which does not homodimerize in the unliganded state; data not shown), and hypothesize that it could result from a shorter half-life of the protein in the oocyte in the presence of ligand, or else a moderately lower extraction efficiency of liganded TR from whole extract. Importantly, however, even though the antigen targeted in the immunoprecipitation is ∼2-fold less abundant in the presence than in the absence of hormone (compare lanes 4 and 6 in Figure 4D, top panel), T3 did not affect N-CoR levels in the oocyte (compare lanes 3 and 5 in Figure 4D, second panel), and while ∼20% of input N-CoR associated with TR when no ligand was present (lane 4), no N-CoR could be detected in its presence (lane 6). We interpret these data as indicative of a robust ligand-regulated release of N-CoR by gag-TR in solution in the absence of a TRE.
Fig. 5. Further characterization of N-CoR/HDAC enrichment by v-ErbA and TR. (A) Immunoprecipitation from 100 uninjected oocytes (lane 2) or oocytes injected with v-ErbA mRNA (lanes 4–6) was performed as described above, except the binding buffer contained 95 mM NaCl (final concentration of monovalent cations in the reaction = 100 mM), and the washing was performed with buffer containing NaCl at the concentration indicated to the right of the gel (100 mM for lane 2). One sixth of the sample was resolved on a 6% Laemmli SDS–PAGE along with the indicated quantity of input whole oocyte extract (lanes 1 and 3) and an aliquot of in vitro translated Xenopus Sin3 (lane 7). Western blotting was performed with antibody against Sin3 (top two panels; these represent two different exposures of the same membrane), followed by stripping and reprobing with an antibody for N-CoR (bottom panel). (B) The gag moiety fails to recruit N-CoR, and ligand fails to eliminate N-CoR from v-ErbA. Oocytes were left uninjected (lanes 1 and 2), injected with an mRNA representing gag-tagged TR with the entire ligand-binding domain deleted (lanes 3 and 4), v-ErbA (lanes 5–8) or gag-TR (lanes 9–12), and cultured in medium containing [35S]methionine. Following immunoprecipitation in 50 mM NaCl in the absence or presence of 1 µM T3 (as indicated) with anti-gag antibody, one half of the immunoprecipitate was resolved on a 4–20% PAGE and autoradiographed (top panel), while the other half was resolved on a 6% PAGE and immunoblotted with antibody against N-CoR (second and third panel; the lower of the two represents a longer exposure of the upper, included to demonstrate N-CoR in all the input samples) and Mi-2 (lower panel). Lanes labeled ‘i’ contain 1% input protein.
In full agreement with our finding that an association between N-CoR and v-ErbA is not accompanied by the recruitment of Sin3 (Figure 4B), we failed (Figure 4D, third panel) to detect levels of Sin3 >0.5% of input in the immunoprecipitates that contained significant quantities of TR and N-CoR (Figure 4D, first and second panel, respectively). In addition, HDAC activity that associated with TR in this experiment (bottom of Figure 4D) was not due to a recruitment of RPD3/HDAC1(Figure 4D, lower panel), which was undetectable in the immunoprecipitate. We interpret these data to indicate that v-ErbA and TR share a corepressor and HDAC recruitment pathway that does not involve an enrichment for the abundant proteins Sin3 or HDAC1.
To obviate concerns that sequential stripping and reprobing of the same membrane prevented the detection of Sin3 in the immunoprecipitate, or that the immunoreactive polypeptides shown in Figure 4B and D do not represent Sin3, we reversed the order of probing. Samples from an immunoprecipitation experiment in which agarose–antibody–protein pellets were washed with increasing salt concentrations were subjected to western analysis with the Sin3 antibody (Figure 5A). Strong cross-reactivity of a polypeptide comigrating precisely with in vitro translated Sin3a (Vermaak et al., 1999) shown in Figure 5A, lane 7 was observed in the input (lanes 1 and 3) but none of the immunoprecipitated samples (lanes 4–6). As confirmed by a longer exposure of the same membrane (Figure 5A, middle panel), the assay was sensitive to as little as 0.5% input Sin3; thus, under conditions of medium to high ionic strength, no meaningful quantity of Sin3 protein associates with v-ErbA. In contrast, robust N-CoR recruitment to v-ErbA was observed when this membrane was stripped and reprobed with an antibody against N-CoR (Figure 5A, lower panel, lanes 4–6).
Since both antigens used in the assay carried a retroviral gag domain on their N-terminus, it remained formally possible that the recruitment of N-CoR we observe was not physiological, but rather due to the gag moiety. To address this issue, we deleted the ligand-binding domain from gag-TR, and demonstrated by mRNA microinjection that the resulting gag-TRDBD fusion could be expressed in the oocyte and immunoprecipitated as efficiently as gag-TR (Figure 5B, top panel, lanes 3 and 4). Western blotting of the immunoprecipitate revealed a robust enrichment for N-CoR in the presence of v-ErbA (Figure 5B, second panel, lane 6) or gag-TR (lane 10), but not gag-TRDBD. Thus, as expected, we show that the gag moiety does not contribute to corepressor recruitment. In addition, we sought to confirm that the unexpected ligand-driven release of corepressor from TR in solution (Figure 4D) was not an artefact; we immunoprecipitated v-ErbA in the presence of T3 (Figure 5B, top panel, lanes 7 and 8) and showed its continued association with N-CoR (middle panel, lanes 7 and 8) under conditions where no detectable N-CoR associated with liganded TR (middle panel, lanes 11 and 12). Finally, we used immunoblotting against the Mi-2 protein (Wade et al., 1998) to demonstrate that no detectable quantities of this exceedingly abundant ATPase associated with TR or v-ErbA (bottom panel) under conditions of robust N-CoR recruitment (second panel); we note that in this assay, as little as 0.1% input Mi-2 could be detected, and none of it bound to TR.
We interpret these data to indicate that the ligand-binding domain of TR and v-ErbA is competent for the targeting of corepressor and HDAC activity, and that such targeting—regulated by ligand in the case of TR but not in the case of v-ErbA—is not accompanied by recruitment of RPD3/HDAC1 in any biochemical form, either free or associated with the adapter molecule Sin3 (Heinzel et al., 1997) or the Mi-2 ATPase (Xue et al., 1998).
v-ErbA and TR associate with the corepressor N-CoR and HDAC3
Since HDAC activity, but no HDAC1 or HDAC5 could be detected in the immunoprecipitates of v-ErbA and TR (Figure 4), we sought to identify this HDAC using commercially available antibodies against other HDACs. To that end, we used an antibody against HDAC3 (Yang et al., 1997; Dangond et al., 1998; Emiliani et al., 1998), a class I HDAC related to, but distinct from, HDAC1/RPD3, to assay for its potential targeting. As shown in Figure 6A, enrichment for N-CoR occurred only in the presence of TR and in the absence of ligand (top panel, lane 3). This interaction was accompanied by an extraordinary enrichment for HDAC3 (Figure 6A, middle panel, compare lanes 1 and 3); it was dependent on TR (lane 2) and exceptionally sensitive to ligand (compare lanes 3 and 4). In full agreement with previous observations (Figure 4D), no detectable HDAC1/RPD3 associated with TR (bottom panel).
Fig. 6. TR and v-ErbA recruit HDAC3. (A) TR enriches for N-CoR and HDAC3, but not RPD3. A large aliquot of Xenopus egg extract was adjusted to 50 mM NaCl and 0.1% NP-40 and incubated with a preparation of bead-immobilized GST–TR (lanes 3 and 4) or GST (lane 2). Where indicated, 2.5 µM T3 was included in the reaction. Following washing as described in Figure 4D, proteins associated with the beads were analyzed by immunoblotting against N-CoR (top panel), HDAC3 (middle panel) and RPD3 (bottom panel). (B) v-ErbA-dependent enrichment for N-CoR in the immunoprecipitate is accompanied by an enrichment for HDAC3. One half of the immunoprecipitate described in the legend to Figure 5A was resolved on a 4–12% Laemmli SDS–PAGE, and analyzed by autoradiography (top panel) and western blotting with antibody against N-CoR (middle panel) and human HDAC3 (bottom panel). As in Figure 4B, the longer (10 min) exposure used in the bottom panel reveals both the 35S-labeled antigen directly targeted in the immunoprecipitation as well as chemiluminescence-derived signal from the antibody used in the western blot. Low efficiency of the anti-HDAC3 antibody prevents the visualization of HDAC3 in the 0.5 oocyte equivalents of extract used in the input lanes (lanes 1 and 3). (C) N-CoR and HDAC3 co-immunoprecipitate. A preparation of bead-immobilized rabbit antiserum against N-CoR (lanes 4 and 7), the cognate pre-immunization serum (lanes 3 and 6) or the beads with no antibody (lanes 2 and 5) was incubated in Xenopus egg extract adjusted to 50 mM NaCl and 0.1% NP-40. The proteins associating with the antibody were eluted with glycine and analyzed for the presence of HDAC3 by immunoblotting with rabbit-derived anti-HDAC3 serum and extended duration ECL reagents, followed by visualization and quantitation on a CCD camera-equipped ChemiImager-4400 low light imaging system. The small amount of rabbit anti-N-CoR antibody released from the beads (lanes 2–4) yields Ig heavy chain signal at 50 kDa (lanes 2–4), i.e. at the molecular weight of HDAC3; quantitation indicates a 60-fold greater signal in lane 7 (anti–N-CoR antibody + egg extract) than in lane 4 (anti–N-CoR antibody only). Lane 1 contains an aliquot of egg extract analyzed on the same gel and probed with conventional ECL reagents, followed by autoradiography.
To substantiate our discovery of HDAC3 targeting to TR, we sought to extend this observation to v-ErbA. As shown in Figure 6B (middle panel), v-ErbA-dependent enrichment for N-CoR from whole oocyte extract occurred even in 0.5 M NaCl (lane 6), and this recruitment was accompanied by a v-ErbA-dependent enrichment for HDAC3 (bottom panel, compare lane 2 with lanes 4–6) that was equally salt resistant (lane 6).
We hypothesized that the corepressor acts as an adapter molecule that bridges TR/v-ErbA with HDAC3. If that were the case, one would expect N-CoR and HDAC3 to associate biochemically. To investigate this issue, we performed immunoprecipitations from egg extract with an antibody against N-CoR and showed a significant enrichment for HDAC3 that was unambiguously dependent on N-CoR-reactive idiotypes in the serum (Figure 6C, compare lanes 6 and 7), since pre-immunization serum failed to enrich for HDAC3 (compare lanes 3 and 6).
These data suggest that a major contribution to the HDAC activity found enriched by TR and v-ErbA can be attributed to the recruitment of HDAC3, and, further, that the corepressor N-CoR and HDAC3 can associate with the v-ErbA oncoprotein under stringent conditions. Finally, the association between N-CoR and HDAC3 suggests that the corepressor acts to bridge unliganded receptor and HDAC.
Distinct pattern of interactions by TR and v-ErbA with components of the basal transcriptional machinery
While corepressor-mediated HDAC recruitment by TR and v-ErbA is likely to contribute to receptor-effected transcriptional repression, both proteins appear to be equally competent for that recruitment (Figures 4B and D, and 6A and B). We hypothesized, therefore, that the distinct requirements for template chromatin assembly in effecting repression (Figure 3C) might be the consequence of an impairment of the v-ErbA protein in exploiting a pathway for repression auxiliary to N-CoR and HDAC targeting.
Studies of silencing by TR in a biochemically defined in vitro transcription system with purified DNA templates implicated binding by TR to components of the basal machinery such as TBP and TFIIB in mediating repression (Fondell et al., 1993, 1996). In addition, however, more recent data (Muscat et al., 1998; Wong and Privalsky, 1998) have suggested that a functional interaction between the corepressors N-CoR/SMRT and TFIIB might play a similar role. We asked whether the distinct transcription silencing properties exhibited by v-ErbA as compared with TR—the dependence on chromatin for silencing and a greater sensitivity to HDAC inhibitors—can be correlated with a hypothetical deficiency in v-ErbA interactions with the basal transcriptional machinery.
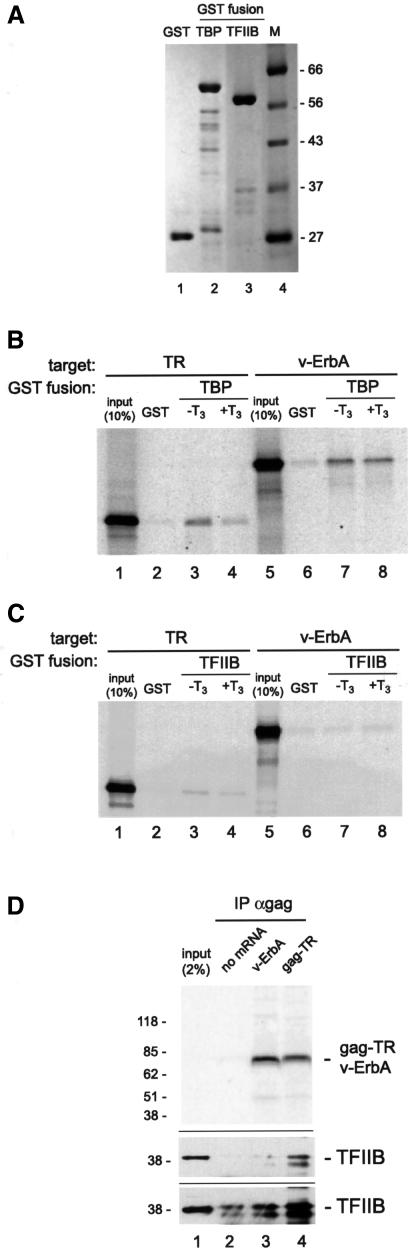
We used recombinant TBP and TFIIB fused to glutathione S-transferase (GST) (Figure 7A) in an in vitro assay for their ability to interact with chicken TR and v-ErbA. As shown in Figure 7B and C, lanes 1–4, chicken TR interacted with both proteins; in precise agreement with published observations (Tone et al., 1994; Fondell et al., 1996), the TR–TBP interaction was adversely affected by addition of ligand (Figure 7B, lane 4), whereas TR and TFIIB interacted ligand independently (Figure 7C, lane 4).
Fig. 7. In vitro and in vivo assays for the ability of TR and v-ErbA to interact with components of the basal transcription machinery. (A) A preparation of glutathione–Sepharose beads attached to GST (lane 1), GST–TBP (lane 2) or GST–TFIIB (lane 3) was boiled in Laemmli SDS–PAGE loading buffer, electrophoresed, and the recombinant proteins visualized by Coomassie Brilliant Blue staining. Lane ‘M’ contains molecular weight markers the sizes of which are indicated to the right of the gel. (B) Equimolar quantities of reticulocyte-lysate-translated TR (lanes 1–4) or v-ErbA (lanes 5–8) were mixed with beads fused to GST (lanes 2 and 6) or GST–TBP (lanes 3, 4, 7 and 8). T3 was included at 1 µM where indicated. Following incubation and washing in buffer containing 0.5 M NaCl and 0.1% NP-40, the proteins bound to the beads were eluted and analyzed by SDS–PAGE and autoradiography. The ‘input’ lanes contain 1/10 of the total protein used in each reaction. (C) The ability of TR (lanes 1–4) and v-ErbA (lanes 5–8) to bind GST–TFIIB was assayed exactly as described in the legend to (B). (D) v-ErbA exhibits a moderate impairment in binding to TFIIB in vivo. Oocytes were left uninjected (lane 2) or injected with mRNA for v-ErbA (lane 3) or gag-TR (lane 4); the injected oocytes were cultured in [35S]methionine-containing medium. Immunoprecipitation with antibody against gag was performed as described in Figure 4B, except the binding and washing buffers contained 5 mM NaCl and 5 mM KCl. The entire immunoprecipitate was resolved on a 4–12% Laemmli SDS–PAGE along with the indicated quantity of input extract and analyzed by autoradiography (top panel) and western blotting against TFIIB (the middle and lower panels represent different exposures of the same membrane).
Figure 7B, lanes 5–8 shows that v-ErbA bound to GST–TBP; we note that this interaction was resistant to 0.5 M NaCl and 0.1% NP-40, as well as treatment with ligand. In contrast, as shown in Figure 7C, lanes 5–8, under identical conditions, v-ErbA failed to bind to GST–TFIIB at levels above background binding to GST (lanes 6–8). In additional experiments we found that GST–TFIIB was susceptible to proteolysis in bacterial cells used for its generation (data not shown), and that preparations of GST–TFIIB proteolysed to a greater extent than the one shown in Figure 7A, lane 3, exhibited moderate binding to v-ErbA (data not shown)—most likely a consequence of the biochemical ‘stickiness’ of partial-length GST–TFIIB fragments.
To resolve conclusively the matter of v-ErbA/TR–TFIIB interaction, we investigated this issue in vivo. We injected v-ErbA and gag-TR mRNA into the oocyte and used immunoprecipitation under conditions of low stringency (5 mM NaCl, 5 mM KCl, 1.5 mM MgCl2, 0.05% NP-40) to assay for potential TFIIB enrichment. We were able to reveal reproducibly a greater amount of TFIIB targeted to TR (Figure 7D, middle panel, lane 4) than to the antibody alone (lane 2) or to v-ErbA (lane 3). This preference for TR over v-ErbA was not a consequence of insufficient TFIIB in the oocyte, as experiments carried out under conditions of ∼5-fold overexpression of TFIIB yielded the same result (data not shown). We note, however, that only a small fraction (∼2%) of input TFIIB is bound by TR and v-ErbA under conditions of extraordinary enrichment for N-CoR by both proteins (Figure 4B and D), suggesting that direct binding of TR to TFIIB, rather than interactions via corepressor (Muscat et al., 1998; Wong and Privalsky, 1998), may occur in vivo.
These experiments aimed to reveal a biochemical correlate to our observations that v-ErbA, but not TR, fails to silence transcription on partly chromatinized DNA. We find that while v-ErbA can bind to TBP as efficiently as TR (Figure 7B), the former protein is moderately impaired in its ability to bind TFIIB in vitro (Figure 7C) and in vivo (Figure 7D).
Discussion
A pathway of repression by targeting N-CoR–HDAC3 to v-ErbA and TR
Unliganded TR is a remarkably efficient repressor of basal transcription in Xenopus (Wong et al., 1995), comparable in potency only to the extraordinarily rapid methylation-coupled transcriptional repression pathways also operating in the oocyte (Kass et al., 1997). Earlier work had suggested that histone deacetylation may contribute to repression by TR (Wong et al., 1997), and that auxiliary pathways may exist. The present study begins their functional dissection using a mutated version of TR, the oncoprotein v-ErbA.
Our data are consistent with previous models implicating HDAC-associated corepressors in repression by unliganded TR (Heinzel et al., 1997; Nagy et al., 1997). Evidence for a functional relationship between N-CoR recruitment and TR-effected repression in this system comes from the precise correlation between effects of ligand on transcription in the presence of TR (Figure 1B) and on association with N-CoR (Figures 4D and 6A) and HDAC3 (Figure 6A). A previous study (Hoerlein et al., 1995) indicated that T3 fails to release N-CoR from TR unless the latter is bound to DNA. In contrast, we report here the robust disruptive effect of ligand on the interaction between N-CoR endogenous to the oocyte and ectopically introduced TR in the absence of a TRE (Figures 4D, 5B and 6A). It is possible that the more extensive regimen of treatment with T3 in the present study, as well as our reliance on endogenous N-CoR rather than overexpressed N-CoR as used by Hoerlein et al. (1995) contributed to this difference.
Our biochemical analysis yielded the unexpected observation that the large quantity of endogenous N-CoR recruited to v-ErbA (Figure 4B) and to TR (Figure 4D) is not associated with Sin3. In addition, we show that an enrichment for HDAC activity and HDAC3 via N-CoR occurs without an association with HDAC1/RPD3 or Sin3 (Figure 4). Since TR and v-ErbA exhibit robust repression function in the oocyte (Figure 1C) and associate with N-CoR without engaging Sin3 or RPD3 (Figures 4 and 5), we suggest that a pathway for repression via an N-CoR/SMRT–Sin3–HDAC1 complex (Heinzel et al., 1997; Nagy et al., 1997) is not exploited by TR or by v-ErbA in Xenopus.
It is important to note that in Xenopus a Sin3-containing complex targets HDAC to methylated DNA via the transcriptional repressor MeCP2 to effect methylation-dependent transcriptional silencing (Jones et al., 1998), and that the HDAC RPD3 is contained in an exceedingly abundant complex with the Mi-2 ATPase (Wade et al., 1998). Both Sin3 and RPD3, therefore, are present in sufficient quantities to accommodate an interaction with N-CoR, and, in fact, our data do not address the possibility of the existence of such a complex in the oocyte, but do argue against the possibility that such a complex mediates transcriptional repression by v-ErbA and by unliganded chicken TR. We also note that while a major form of HDAC in the oocyte is represented by the six-subunit Mi-2 complex (Wade et al., 1998), the lack of targeting of its three core components, HDAC1, Mi-2 or RbAp48, to unliganded TR (Figures 4D and 5B), which occurs while significant amounts of N-CoR and HDAC3 are bound by TR, strongly argues against a previously hypothesized (Xue et al., 1998) role for the Mi-2 complex in TR-effected repression.
Figure 4 shows that the recruitment of HDAC activity to v-ErbA is accompanied by an enrichment for the class I deacetylase HDAC3, the third ortholog in metazoa of the budding yeast HDAC Rpd3p (Yang et al., 1997; Dangond et al., 1998; Emiliani et al., 1998). Its identification in association with v-ErbA and N-CoR in the absence of Sin3 and HDAC1 is consistent with its lack of interaction with Sin3 in vitro (Emiliani et al., 1998) and the failure to reveal the corepressors N-CoR and SMRT in Sin3-containing complexes (Hassig et al., 1997; Zhang et al., 1997). Our data do not exclude the possibility that additional HDACs other than HDAC1 or -5 associate with v-ErbA–N-CoR. Recent studies revealed that a novel HDAC associates with the corepressor SMRT (Kao et al., 2000), and that ∼2% of endogenous N-CoR in 293T cells associates with HDAC4 (Huang et al., 2000), one of several recently identified metazoan orthologs of the yeast HDAC Hda1p. Neither of these two studies, however, addressed the issue of whether these associations contribute to transcriptional repression by NHRs. The observation that the corepressors N-CoR and SMRT interact with HDAC5 in yeast two-hybrid assays (Huang et al., 2000; Kao et al., 2000) prompted us to investigate whether such a complex might contribute to repression by v-ErbA in Xenopus, but we failed to observe an association of HDAC5 with v-ErbA-targeted N-CoR (Figure 4B).
The functional utility to the cell of multiple enzymes that perform the same reaction of histone tail deacetylation remains unclear. In this regard, it is curious that when assayed in the context of a hyperacetylated nucleosome, HDAC3 was reported to be more robust than HDACs 1 or 2 in deacetylating histone H4 (Emiliani et al., 1998). Diacetylated histone H4 is deposited onto DNA during de novo chromatin assembly in S phase, with deacetylation occurring over ∼30 subsequent minutes (Shimamura and Worcel, 1989; Ura et al., 1997; Adams and Kamakaka, 1999). In Xenopus, inhibition of HDAC activity during chromatin assembly greatly stimulates transcription of the TRβA promoter (Figure 2), as does overexpression of Sin3 in the oocyte (Vermaak et al., 1999), presumably via cytoplasmic sequestration of a Sin3-associated HDAC. Taken together, these observations support the earlier hypothesis that HDAC1/RPD3 mediates assembly-coupled deacetylation of histone H4 (Rundlett et al., 1996) and open the possibility that during chromatin assembly, HDAC3 targeted by v-ErbA (Figure 4) or TR (Figure 6) to specific loci might accelerate this deacetylation relative to other sites in the genome and promote the generation of a repressive chromatin structure. Indeed, we have previously reported that unliganded TR is most effective in repressing transcription during replicative chromatin assembly (Wong et al., 1995, 1998b). The Xenopus oocyte system is well suited to investigating the possibility of accelerated deacetylation being targeted by unliganded TR and v-ErbA.
A requirement for chromatin infrastructure in repression by v-ErbA–N-CoR–HDAC3 and auxiliary pathways in repression by TR
The present study revealed two unexpected differences between TR and v-ErbA as transcriptional regulators of the TRβA gene: first, the former protein can repress basal transcription at all states of target template chromatinization, while the efficiency of the latter as a transcriptional repressor is positively correlated with the extent of the assembly of its target into chromatin (Figure 3); secondly, the HDAC inhibitor TSA stimulates a promoter repressed by v-ErbA to a much greater extent than a promoter repressed by TR. These observations shed new light on (i) the previous finding that the Xenopus HDAC RPD3 requires a greater extent of template chromatinization than does unliganded TR to effect repression (Wong et al., 1997) and (ii) on our observation that TR and v-ErbA both complex with N-CoR–HDAC3 (Figure 6). These findings are fully consistent with the hypothesis that some feature of a chromatin template—perhaps overall nucleosome density, or some other structural parameter acquired during in vivo chromatin maturation—is required to allow both targeted or philandering HDAC to effect repression. Thus, we propose that recruitment of the N-CoR–HDAC3 corepressor complex represents the chromatin-dependent component of repression effected by unliganded TR, and that v-ErbA relies on such recruitment to effect repression to a greater extent than TR. This latter suggestion stems from three observations: (i) the capacity of TSA to relieve most of the repression effected by v-ErbA (compare lanes 3 and 16 in Figure 2B); (ii) the nearly identical requirements that overexpressed HDAC and v-ErbA have for template chromatinization in effecting repression (present study and Wong et al., 1997); and (iii) the finding that a v-ErbA mutant known to be impaired in corepressor recruitment (Chen and Evans, 1995) is also impaired in silencing in the oocyte system (J.Yee, F.D.Urnov and A.P.Wolffe, unpublished), while TR carrying the cognate mutation is competent for repression (T.N.Collingwood and A.P.Wolffe, unpublished).
What evidence, then, can be provided for the existence of auxiliary pathways in repression by TR, and how can one explain the impairment exhibited by v-ErbA in exploiting these pathways? As shown in Figure 2B, global inhibition of HDAC activity via TSA treatment during chromatin assembly strongly activates basal transcription driven by the TRβA promoter (compare lanes 2 and 3). When the promoter is bound by unliganded TR, however, TSA relieves repression to only a fraction of the level seen in the absence of receptor (Figure 2B, compare lanes 3 and 11). The same phenomenon is observed if chromatin is allowed to mature in the absence of an HDAC inhibitor: the capacity of TSA to activate transcription from a mature chromatin template is markedly reduced by TR bound to the promoter (Figure 2B, compare lanes 4 and 5 with 13 and 14). These findings are inconsistent with the notion that HDAC targeting accounts for the entirety of transcriptional repression effected by TR.
It is instructive to compare our data with observations from studies of other HDAC-recruiting transcriptional repressors. For instance, TSA only partly relieves repression mediated by the Drosophila repressor groucho (Chen et al., 1999), and genetic data suggest that pathways additional to HDAC1/Rpd3p recruitment are exploited by this repressor (Mannervik and Levine, 1999). Similarly, repression by the retinoblastoma protein-associated corepressor RBP1 is thought to be effected via both HDAC-dependent and -independent mechanisms, and, accordingly, TSA fails to alleviate fully repression mediated by RBP1 (Luo et al., 1998; Lai et al., 1999). While there is no in vivo functional evidence regarding the mechanisms responsible for the operation of such auxiliary pathways, data from Roeder and coworkers obtained using in vitro assays (Fondell et al., 1993, 1996) led to the hypothesis of a correlation between the capacity of TR to interfere with transcription pre-initiation complex formation and its ability to bind TBP and TFIIB. As shown in Figure 7, while both TR and v-ErbA can bind to TBP, v-ErbA is moderately impaired in direct binding to TFIIB in an in vitro (Figure 6B) and an in vivo assay (Figure 7C). It is possible that v-ErbA is deficient in an interaction with an additional component of the transcriptional apparatus or some regulatory macromolecular complex known to interact with NHRs (Lee et al., 1995; Rachez et al., 1998). Biochemical analysis revealed the unexpected capacity of v-ErbA to associate with several currently unidentified polypeptides in the oocyte that do not bind to unliganded TR (F.D.Urnov, unpublished observations). It is possible that such interactions represent mutation-induced de novo acquisitions relative to TR and obstruct surfaces on the LBD of v-ErbA that are used by TR to effect repression; future studies will exploit the biochemical approach described here to study such differences between v-ErbA and unliganded gag-tagged TR in repression-related protein–protein interactions.
In a previous study with an in vitro system, excess TBP was used to saturate repression-related interaction interfaces on the surface of TR and abrogate transcriptional repression (Fondell et al., 1996). In the oocyte, however, overexpression of TBP failed to relieve TR-driven repression on both TATA-less (TRβA) and TATA-containing (HSV-tk) promoters (F.D.Urnov, unpublished observations); this was surprising, considering that the oocyte contains very little endogenous TBP (Veenstra et al., 1999), and that a highly sensitive luciferase assay was used that could detect even marginal effects. Thus, solid evidence regarding the existence and relevance of a basal machinery-directed repression pathway by TR in vivo remains to be obtained for any system, and other pathways for repression, e.g. the relocalization of TR-bound DNA to a transcriptionally incompetent nuclear domain (Dernburg et al., 1996), deserve investigation.
Whatever the precise molecular underpinnings of non-HDAC-based repression pathways in TR function, it is useful to consider that unliganded TR maintains many cell types in metazoa in the proliferative state (Brown et al., 1995; Beug et al., 1996), where loci repressed by TR undergo repeated cycles of chromatin disassembly and reassembly (Adams and Kamakaka, 1999). On immature chromatin templates, pathways for repression auxiliary to HDAC targeting may provide a useful means for the maintenance of transcriptional silencing, while the N-CoR–HDAC3 pathway described here may accelerate the maturation of chromatin in a locus-specific fashion.
Materials and methods
Plasmid constructs for transcription analysis and in vitro mRNA synthesis
Transcription assays were performed using pTRβA (Wong et al., 1995) as a reporter construct; this plasmid contains a fragment of the Xenopus laevis TRβA gene (Shi et al., 1992) corresponding to positions (–1335)–(+313) relative to the major transcription start site (Wong et al., 1998a) fused to a 275 bp fragment of the CAT gene and cloned into pBluescript II KS(–) (Stratagene, La Jolla, CA). The coding portions of the various v-ErbA alleles of Gallus gallus TRα (NR1A1; Committee, 1999) and of the gag-TRα fusion (Zenke et al., 1990) were obtained from cognate clones in pSFCV-LE (Fuerstenberg et al., 1990) by PCR and cloned into pT7-TS (Zorn and Krieg, 1997) between an X.laevis β-globin 5′ and 3′ untranslated region. The clone for X.laevis TRβ (NR1A2) in pSP64(A) (Promega, Madison, WI) was a kind gift from Dr D.Brown (Carnegie Institute); the clone of X.laevis RXRα (NR2B1) was described previously (Wong et al., 1995). All plasmids were linearized with EcoRI and used as templates in capped mRNA synthesis reactions using mMessage mMachine in vitro transcription kits (Ambion, Austin, TX) driven by SP6 (Xenopus TRβ and RXRα) or T7 (all other constructs) polymerase.
Preparation, microinjection and maintenance of oocytes
Stage VI X.laevis oocytes were prepared as described previously (Almouzni and Wolffe, 1993a). For each experimental sample, 25–30 healthy oocytes were injected into the cytoplasm with 0.25–10 ng of mRNA (see specific figure legends for amount) in 32.2–41.4 nl of diethyl pyrocarbonate (DEPC)-treated water and cultured in modified Barth’s solution (MBSH) buffer (Gurdon, 1968) for 8–12 h at 18°C; where indicated, 10 µCi/ml of in vivo cell labeling grade l-[35S]methionine (Amersham Pharmacia Biotech, Arlington Heights, IL) were added to the medium. Following translation of the injected mRNA, 0.5–1.0 ng of pTRβA DNA purified from E.coli by gravity flow on anion-exchange resin (Qiagen, Valencia, CA) was injected into the nucleus in 13.8 nl of DEPC-treated water, and the oocytes were cultured as above for 14–16 h, or for the length of time stated in the figure legends. Where indicated, 100 nM 3,3′,5-triiodo-l-thyronine (Sigma Chemical, St Louis, MO) or 33 nM TSA (Wako Chemicals, Richmond, VA) was included in the culture medium.
Protein isolation
Following mRNA microinjection and subsequent culture (see above), 10 healthy oocytes were homogenized in 50–100 µl of 20 mM Tris–Cl pH 7.5, 50 mM KCl, 1 mM dithiothreitol (DTT), 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 8% glycerol. Debris and yolk were pelleted in a microcentrifuge for 15 min at 4°C, and the cleared supernatant was stored at –70°C until use. In vitro translated proteins were synthesized in rabbit reticulocyte lysate by using 1 µg of supercoiled plasmid DNA template in a TnT Coupled (Xenopus RXRα) or Quick Coupled (all other proteins) transcription/translation reaction (Promega). For in vivo labeling analysis, extract from 1 oocyte equivalent per experimental sample was fractionated on a 4% stacking/10% resolving SDS–PAGE (Laemmli, 1970); the gel was dried and autoradiographed.
RNA isolation and transcript analysis by primer extension
Total RNA was extracted from 18–20 oocytes per experimental sample as previously described (Wong et al., 1995), with modifications: the oocytes were rinsed with MBSH buffer, homogenized in 200 µl of 0.25 M Tris–Cl pH 7.5, and mixed with 500 µl of RNAzol B (TelTest, Friendswood, TX). After addition of 50 µl of chloroform, a 15 min incubation on ice and microcentrifugation for 15 min, the aqueous phase was removed and RNA precipitated by addition of an equal volume of isopropanol, incubation on ice for 15 min, and microcentrifugation. The pellet was rinsed with 70% ethanol, resuspended in TE buffer pH 7.5, and the RNA was precipitated with LiCl. The pellet was rinsed with 70% ethanol and resuspended in DEPC-treated water to one oocyte equivalent/µl. Three to five oocyte equivalents of RNA were annealed in 0.6× First Strand reverse transcriptase buffer (30 mM Tris–Cl pH 8.3, 45 mM KCl, 1.8 mM MgCl2, 3 mM DTT final; Life Technologies, Gaithersburg, MD) to 0.2 pmol each of end-labeled primer H4 (Wong et al., 1998b) and end-labeled primer I (Wong et al., 1995); the former anneals to histone H4 mRNA stored in the oocyte, whereas the latter anneals at positions (+80)–(+109) to the mRNA transcribed from the TRβA gene. The annealing regimen was as follows: 10 min at 65°C, 30 min at 55°C, 20 min at 37°C and 5 min at room temperature. The primer extension was performed with 100 U of SuperScript II RNase H– reverse transcriptase, 2 U of RNase inhibitor (both from Life Technologies), and 75 µM each dNTP for 1 h at 42°C. Reaction products were precipitated with ethanol, resolved on a 6% urea–PAGE in 1× TBE, and analyzed by exposure to Molecular Dynamics PhosphorImager screens or Kodak X-OMAT/AR X-ray film at room temperature.
Analysis of chromatin assembly in vivo
A large group of oocytes was injected with 3 ng of TRβA DNA. For topological analysis, at defined timepoints following injection, 10 healthy oocytes were homogenized in 0.25 M Tris–Cl pH 7.5, followed by addition of an equal volume of stop buffer (30 mM EDTA pH 8.0, 20 mM Tris–Cl pH 7.5, 1% SDS), and DNA purification via proteinase K treatment and phenol extraction as described above for DNase I analysis. DNA from 0.5 oocyte equivalents was fractionated on a 1% agarose gel in 1× TPE buffer containing 90 µg/ml chloroquine as described (Clark and Wolffe, 1991; Clark, 1998). For nucleosome density measurements, 30 healthy oocytes removed at each timepoint were homogenized in 300 µl of buffer C (15 mM Tris pH 7.5, 60 mM KCl, 15 mM NaCl, 10 mM NaHSO3, 0.34 M sucrose, 0.15 mM β-mercaptoethanol; Kornberg et al., 1989); the homogenate was split into three aliquots of 100 µl, to one of which an equal volume of stop buffer was immediately added and DNA extracted as above. To the remaining two aliquots, CaCl2 was added to a final concentration of 3 mM, the samples were immediately warmed to 37°C for 1 min, and treated with 30 or 120 Worthington units of MNase (NFCP grade, Worthington) for 2 min at 37°C. The reactions were stopped and the DNA extracted as described above. One oocyte equivalent from each sample was fractionated on a 2% high-resolution blend agarose (Amresco) gel in 1× Tris-acetate–EDTA. Southern blotting with random-primed probes was performed as described above.
Immunoprecipitations from whole oocyte and egg extract
Oocytes were injected with the indicated amounts of mRNA and cultured for 12–24 h at 18°C in MBSH medium containing [35S]methionine. The oocytes were rinsed with MBSH and homogenized in 5–10 µl/oocyte of extraction buffer (Jones et al., 1998): 20 mM HEPES pH 7.5, 5 mM KCl, 1.5 mM MgCl2, 1 mM EGTA, 10 mM β-glycerophosphate, 0.05% NP-40, 0.5 mM DTT, 0.2 mM PMSF, 1 µg/ml leupeptin, 2 µg/ml pepstatin, 3 µg/ml aprotinin. NaCl was added to the buffer to 5–95 mM where indicated. All subsequent manipulations were performed in the cold room. The homogenate was centrifuged at 14 000 r.p.m. in a benchtop mictrocentrifuge and the clear middle layer was removed for experimentation. Ascites fluid containing mouse monoclonal antibody against the AEV gag protein (Bauer et al., 1998) was used at a dilution of 1:500; the oocyte extract was mixed with the antibody for 30 min prior to addition of 5 µl bed volume of protein G–Sepharose beads (Amersham Pharmacia Biotech) that were blocked by rotating in extraction buffer containing 200 µg/ml bovine serum albumin (BSA) for 1 h. The protein extract and the beads were mixed by rotation for 2–6 h, pelleted for 15 s at 3000 r.p.m. and washed three times with 400 µl of extraction buffer containing, where indicated, additional NaCl and NP-40 (see figure legends). For analysis of gag-TR association, a small volume of concentrated T3 in 100 mM NaOH was added to a final concentration of 1–2.5 µM to the homogenization/binding and wash solutions, and an identical volume of 100 mM NaOH was added to these solutions for other samples. The beads were resuspended into extraction buffer and aliquoted for analysis.
For experiments with egg extract prepared as described (Wade et al., 1999b), 5–10 µl of glutathione–Sepharose (Amersham Pharmacia) matrix with immobilized GST or GST–TR (residues 169–456 of human TRβ; Tone et al., 1994) or CnBr-activated Sepharose (Amersham Pharmacia) with serum crosslinked and treated with dimethylpimelimidate as described (Harlow and Lane, 1999) were blocked with 0.2–0.5 mg/ml BSA and added to 200–400 µl of egg extract adjusted with NaCl and NP-40 to the concentration indicated. Following binding in the cold room, the matrix was washed three times with 400 µl of immunoprecipitation buffer, and the bound protein was eluted by boiling in SDS–PAGE sample buffer or by 0.2 M glycine pH 2.5.
Western blotting
Bead and input samples were boiled in SDS–PAGE loading buffer, fractionated on conventional 6 or 10% Laemmli SDS–PAGE or on 4–12 or 4–20% PAGE (Novex), and then transferred to Hybond ECL nitrocellulose membranes (Amersham Pharmacia Biotech). The membranes were blocked for 1 h at room temperature or overnight at 4°C in a 5% solution of non-fat dry milk in 50 mM Tris–Cl pH 7.5, 150 mM NaCl, 0.05% Tween 20 (Sigma, St. Louis, MO) and probed with the following antibodies, listed as ‘protein, species of target antigen, dilution, reference’: N-CoR, Xenopus, 1:500 (P.Jones, L.Sachs and Y.-B.Shi, manuscript submitted); Sin3A/B, Xenopus, 1:1000 (Jones et al., 1998); HDAC1/RPD3, Xenopus, 1:2500 (Vermaak et al., 1999); Mi-2, Xenopus, 1:1000 (Wade et al., 1999a); HDAC5, Mus musculus, 1:1000 (generous gift of Saadi Khochbin); HDAC3, Homo sapiens, 1:500 or 1:250 (Upstate Biotechnology, Lake Placid, NY); TFIIB, H.sapiens, 1:500 (C18; Santa Cruz Biotechnology). Detection with secondary antibody conjugated to HRP (Amersham Pharmacia Biotech) and ECL reagents (Pierce) was performed according to the manufacturers’ instructions.
GST pulldown assay
Human TBP (Ge and Roeder, 1994) and human TFIIB fused to GST (gift of Dr Hui Ge) were expressed in E.coli and purified by affinity chromatography on glutathione–Sepharose 4B (Amersham Pharmacia Biotech) as recommended by the manufacturer; the fusion protein was not eluted from the matrix. Chicken TRα and v-ErbA were synthesized in vitro using rabbit reticulocyte lysate (TnT Quick Coupled System; Promega) and evaluated for their ability to bind either protein in a GST pulldown assay as described (Tone et al., 1994), with modifications: non-specific binding sites on the matrix were saturated by rotation in the presence of 0.5 mg/ml BSA for 30 min at room temperature in pulldown buffer (Tone et al., 1994), and, following incubation with the in vitro synthesized protein, the matrix was washed twice with 250 µl of phosphate-buffered saline (PBS) containing an additional 350 mM NaCl and 0.1% NP-40. T3 was added in 1 mM NaOH to a final concentration of 1 µM where indicated, and an equal volume of 1 mM NaOH was added to the other samples.
Acknowledgments
Acknowledgements
We thank Saadi Khochbin for the antibody against HDAC5, Hui Ge for the TBP and TFIIB fusion constructs, and Paul Wade for egg extract and hyperacetylated tritiated histones. F.D.U. expresses his sincere gratitude to Paul Wade, Peter Jones, GertJan Veenstra and Yoshiaki Azuma for their invaluable advice and stimulating discussions throughout the course of this work.
Note added in proof
After the present work was submitted for publication, Guenther et al. (2000) reported the biochemical charcterization of a complex between the corepressor SMRT, HDAC3 and several additional polypeptides. In further agreement with our observations, this complex is reported not to contain HDAC1/RPD3 or Sin3.
References
- Adams C.R. and Kamakaka,R.T. (1999) Chromatin assembly: biochemical identities and genetic redundancy. Curr. Opin. Genet. Dev., 9, 185–190. [DOI] [PubMed] [Google Scholar]
- Almouzni G. and Wolffe,A.P. (1993a) Nuclear assembly, structure, and function: the use of Xenopus in vitro systems. Exp. Cell Res., 205, 1–15. [DOI] [PubMed] [Google Scholar]
- Almouzni G. and Wolffe,A.P. (1993b) Replication-coupled chromatin assembly is required for the repression of basal transcription in vivo. Genes Dev., 7, 2033–2047. [DOI] [PubMed] [Google Scholar]
- Almouzni G., Mechali,M. and Wolffe,A.P. (1990) Competition between transcription complex assembly and chromatin assembly on replicating DNA. EMBO J., 9, 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad A., Ha,I., Reinberg,D., Tsai,S., Tsai,M.J. and O’Malley,B.W. (1993) Interaction of human thyroid hormone receptor β with transcription factor TFIIB may mediate target gene derepression and activation by thyroid hormone. Proc. Natl Acad. Sci. USA, 90, 8832–8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A., Mikulits,W., Lagger,G., Stengl,G., Brosch,G. and Beug,H. (1998) The thyroid hormone receptor functions as a ligand-operated developmental switch between proliferation and differentiation of erythroid progenitors. EMBO J., 17, 4291–4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Mullner,E.W. and Hayman,M.J. (1994) Insights into erythroid differentiation obtained from studies on avian erythroblastosis virus. Curr. Opin. Cell Biol., 6, 816–824. [DOI] [PubMed] [Google Scholar]
- Beug H., Bauer,A., Dolznig,H., von Lindern,M., Lobmayer,L., Mellitzer,G., Steinlein,P., Wessely,O. and Mullner,E. (1996) Avian erythropoiesis and erythroleukemia: towards understanding the role of the biomolecules involved. Biochim. Biophys. Acta, 1288, M35–M47. [DOI] [PubMed] [Google Scholar]
- Brehm A., Miska,E.A., McCance,D.J., Reid,J.L., Bannister,A.J. and Kouzarides,T. (1998) Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature, 391, 597–601. [DOI] [PubMed] [Google Scholar]
- Brown D.D., Wang,Z., Kanamori,A., Eliceiri,B., Furlow,J.D. and Schwartzman,R. (1995) Amphibian metamorphosis: a complex program of gene expression changes controlled by the thyroid hormone. Recent Prog. Horm. Res., 50, 309–315. [DOI] [PubMed] [Google Scholar]
- Chen G., Fernandez,J., Mische,S. and Courey,A.J. (1999) A functional interaction between the histone deacetylase rpd3 and the corepressor groucho in Drosophila development. Genes Dev., 13, 2218–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.D. and Evans,R.M. (1995) A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature, 377, 454–457. [DOI] [PubMed] [Google Scholar]
- Clark D.J. (1998) Counting nucleosome cores on circular DNA using topoisomerase I. In Gould,H. (ed.), Chromatin. IRL Practical Approach Series. Oxford University Press, Oxford, UK, pp. 139–152. [Google Scholar]
- Clark D.J. and Wolffe,A.P. (1991) Superhelical stress and nucleosome-mediated repression of 5S RNA gene transcription in vitro. EMBO J., 10, 3419–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee (1999) A unified nomenclature system for the nuclear receptor superfamily. Cell, 97, 161–163. [DOI] [PubMed] [Google Scholar]
- Damm K., Thompson,C.C. and Evans,R.M. (1989) Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature, 339, 593–597. [DOI] [PubMed] [Google Scholar]
- Dangond F., Hafler,D.A., Tong,J.K., Randall,J., Kojima,R., Utku,N. and Gullans,S.R. (1998) Differential display cloning of a novel human histone deacetylase (HDAC3) cDNA from PHA-activated immune cells. Biochem. Biophys. Res. Commun., 242, 648–652. [DOI] [PubMed] [Google Scholar]
- Danielian P.S., White,R., Lees,J.A. and Parker,M.G. (1992) Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J., 11, 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg A.F., Broman,K.W., Fung,J.C., Marshall,W.F., Philips,J., Agard,D.A. and Sedat,J.W. (1996) Perturbation of nuclear architecture by long-distance chromosome interactions. Cell, 85, 745–759. [DOI] [PubMed] [Google Scholar]
- Dressel U., Thormeyer,D., Altincicek,B., Paululat,A., Eggert,M., Schneider,S., Tenbaum,S.P., Renkawitz,R. and Baniahmad,A. (1999) Alien, a highly conserved protein with characteristics of a corepressor for members of the nuclear hormone receptor superfamily. Mol. Cell. Biol., 19, 3383–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emiliani S., Fischle,W., Van Lint,C., Al-Abed,Y. and Verdin,E. (1998) Characterization of a human RPD3 ortholog, HDAC3. Proc. Natl Acad. Sci. USA, 95, 2795–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondell J.D., Roy,A.L. and Roeder,R.G. (1993) Unliganded thyroid hormone receptor inhibits formation of a functional preinitiation complex: implications for active repression. Genes Dev., 7, 1400–1410. [DOI] [PubMed] [Google Scholar]
- Fondell J.D., Brunel,F., Hisatake,K. and Roeder,R.G. (1996) Unliganded thyroid hormone receptor α can target TATA-binding protein for transcriptional repression. Mol. Cell. Biol., 16, 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerstenberg S. et al. (1990) Ectopic expression of the erythrocyte band 3 anion exchange protein, using a new avian retrovirus vector. J. Virol., 64, 5891–5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H. and Roeder,R.G. (1994) The high mobility group protein HMG1 can reversibly inhibit class II gene transcription by interaction with the TATA-binding protein. J. Biol. Chem., 269, 17136–17140. [PubMed] [Google Scholar]
- Guenther M.G., Lane,W.S., Fischle,W., Verdin,E., Lazar,M.A. and Shiekhattar,R. (2000) A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev., 14, 1048–1057. [PMC free article] [PubMed] [Google Scholar]
- Gurdon J.B. (1968) Changes in somatic cell nuclei inserted into growing and maturing amphibian oocytes. J. Embryol. Exp. Morphol., 20, 401–414. [PubMed] [Google Scholar]
- Hanna-Rose W. and Hansen,U. (1996) Active repression mechanisms of eukaryotic transcription repressors. Trends Genet., 12, 229–234. [DOI] [PubMed] [Google Scholar]
- Harlow E. and Lane,D. (1999) Using Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Hassig C.A., Fleischer,T.C., Billin,A.N., Schreiber,S.L. and Ayer,D.E. (1997) Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell, 89, 341–347. [DOI] [PubMed] [Google Scholar]
- Haussler M.R., Haussler,C.A., Jurutka,P.W., Thompson,P.D., Hsieh,J.C., Remus,L.S., Selznick,S.H. and Whitfield,G.K. (1997) The vitamin D hormone and its nuclear receptor: molecular actions and disease states. J. Endocrinol., 154, S57–S73. [PubMed] [Google Scholar]
- Heinzel T. et al. (1997) A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature, 387, 43–48. [DOI] [PubMed] [Google Scholar]
- Hoerlein A.J. et al. (1995) Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature, 377, 397–404. [DOI] [PubMed] [Google Scholar]
- Huang E.Y., Zhang,J., Miska,E.A., Guenther,M.G., Kouzarides,T. and Lazar,M.A. (2000) Nuclear receptor corepressors partner with class II histone deacetylases in a Sin3-independent repression pathway. Genes Dev., 14, 45–54. [PMC free article] [PubMed] [Google Scholar]
- Jones P.L., Veenstra,G.J., Wade,P.A., Vermaak,D., Kass,S.U., Landsberger,N., Strouboulis,J. and Wolffe,A.P. (1998) Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature Genet., 19, 187–191. [DOI] [PubMed] [Google Scholar]
- Kao H.Y., Downes,M., Ordentlich,P. and Evans,R.M. (2000) Isolation of a novel histone deacetylase reveals that class I and class II deacetylases promote SMRT-mediated repression. Genes Dev., 14, 55–66. [PMC free article] [PubMed] [Google Scholar]
- Kass S.U., Landsberger,N. and Wolffe,A.P. (1997) DNA methylation directs a time-dependent repression of transcription initiation. Curr. Biol., 7, 157–165. [DOI] [PubMed] [Google Scholar]
- Koipally J., Renold,A., Kim,J. and Georgopoulos,K. (1999) Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO J., 18, 3090–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R.D., LaPointe,J.W. and Lorch,Y. (1989) Preparation of nucleosomes and chromatin. Methods Enzymol., 170, 3–14. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Laherty C.D., Yang,W.M., Sun,J.M., Davie,J.R., Seto,E. and Eisenman,R.N. (1997) Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell, 89, 349–356. [DOI] [PubMed] [Google Scholar]
- Lai A., Lee,J.M., Yang,W.-M., DeCaprio,J.A., Kaelin,W.G., Seto,E. and Branton,P.E. (1999) RBP1 recruits both histone deacetylase-dependent and -independent repression activities to retinoblastoma family proteins. Mol. Cell. Biol., 19, 6632–6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne A.C., Mengus,G., Gangloff,Y.G., Wurtz,J.M. and Davidson,I. (1999) Human TAFII55 interacts with the vitamin D3 and thyroid hormone receptors and with derivatives of the retinoid X receptor that have altered transactivation properties. Mol. Cell. Biol., 19, 5486–5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.W., Choi,H.S., Gyuris,J., Brent,R. and Moore,D.D. (1995) Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol. Endocrinol., 9, 243–254. [DOI] [PubMed] [Google Scholar]
- Luo R.X., Postigo,A.A. and Dean,D.C. (1998) Rb interacts with histone deacetylase to repress transcription. Cell, 92, 463–473. [DOI] [PubMed] [Google Scholar]
- Machuca I., Esslemont,G., Fairclough,L. and Tata,J.R. (1995) Analysis of structure and expression of the Xenopus thyroid hormone receptor β gene to explain its autoinduction. Mol. Endocrinol., 9, 96–107. [DOI] [PubMed] [Google Scholar]
- Magnaghi-Jaulin L., Groisman,R., Naguibneva,I., Robin,P., Lorain,S., Le Villain,J.P., Troalen,F., Trouche,D. and Harel-Bellan,A. (1998) Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature, 391, 601–605. [DOI] [PubMed] [Google Scholar]
- Mannervik M. and Levine,M. (1999) The Rpd3 histone deacetylase is required for segmentation of the Drosophila embryo. Proc. Natl Acad. Sci. USA, 96, 6797–6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moras D. and Gronemeyer,H. (1998) The nuclear receptor ligand-binding domain: structure and function. Curr. Opin. Cell Biol., 10, 384–391. [DOI] [PubMed] [Google Scholar]
- Munoz A., Zenke,M., Gehring,U., Sap,J., Beug,H. and Vennstrom,B. (1988) Characterization of the hormone-binding domain of the chicken c-erbA/thyroid hormone receptor protein. EMBO J., 7, 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat G.E., Burke,L.J. and Downes,M. (1998) The corepressor N-CoR and its variants RIP13a and RIP13Δ1 directly interact with the basal transcription factors TFIIB, TAFII32 and TAFII70. Nucleic Acids Res., 26, 2899–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L., Kao,H.Y., Chakravarti,D., Lin,R.J., Hassig,C.A., Ayer,D.E., Schreiber,S.L. and Evans,R.M. (1997) Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell, 89, 373–380. [DOI] [PubMed] [Google Scholar]
- Nan X., Ng,H.H., Johnson,C.A., Laherty,C.D., Turner,B.M., Eisenman,R.N. and Bird,A. (1998) Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature, 393, 386–389. [DOI] [PubMed] [Google Scholar]
- Rachez C., Suldan,Z., Ward,J., Chang,C.P., Burakov,D., Erdjument-Bromage,H., Tempst,P. and Freedman,L.P. (1998) A novel protein complex that interacts with the vitamin D3 receptor in a ligand-dependent manner and enhances VDR transactivation in a cell-free system. Genes Dev., 12, 1787–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundlett S.E., Carmen,A.A., Kobayashi,R., Bavykin,S., Turner,B.M. and Grunstein,M. (1996) HDA1 and RPD3 are members of distinct yeast histone deacetylase complexes that regulate silencing and transcription. Proc. Natl Acad. Sci. USA, 93, 14503–14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatcioglu F., Bartunek,P., Deng,T., Zenke,M. and Karin,M. (1993) A conserved C-terminal sequence that is deleted in v-ErbA is essential for the biological activities of c-ErbA (the thyroid hormone receptor). Mol. Cell. Biol., 13, 3675–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H.H., Perlman,A.J., Raaka,B.M. and Stanley,F. (1982) Organization of the thyroid hormone receptor in chromatin. Recent Prog. Horm. Res., 38, 557–599. [DOI] [PubMed] [Google Scholar]
- Sap J., Munoz,A., Damm,K., Goldberg,Y., Ghysdael,J., Leutz,A., Beug,H. and Vennstrom,B. (1986) The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature, 324, 635–640. [DOI] [PubMed] [Google Scholar]
- Sap J., Munoz,A., Schmitt,J., Stunnenberg,H. and Vennstrom,B. (1989) Repression of transcription mediated at a thyroid hormone response element by the v-erb-A oncogene product. Nature, 340, 242–244. [DOI] [PubMed] [Google Scholar]
- Seol W., Choi,H.S. and Moore,D.D. (1995) Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol. Endocrinol., 9, 72–85. [DOI] [PubMed] [Google Scholar]
- Shi Y.B., Yaoita,Y. and Brown,D.D. (1992) Genomic organization and alternative promoter usage of the two thyroid hormone receptor β genes in Xenopus laevis. J. Biol. Chem., 267, 733–738. [PubMed] [Google Scholar]
- Shimamura A. and Worcel,A. (1989) The assembly of regularly spaced nucleosomes in the Xenopus oocyte S-150 extract is accompanied by deacetylation of histone H4. J. Biol. Chem., 264, 14524–14530. [PubMed] [Google Scholar]
- Stunnenberg H.G., Garcia-Jimenez,C. and Betz,J.L. (1999) Leukemia: the sophisticated subversion of hematopoiesis by nuclear receptor oncoproteins. Biochim. Biophys. Acta, 1423, F15–F33. [DOI] [PubMed] [Google Scholar]
- Sucov H.M. and Evans,R.M. (1995) Retinoic acid and retinoic acid receptors in development. Mol. Neurobiol., 10, 169–184. [DOI] [PubMed] [Google Scholar]
- Tata J.R. (1996) Metamorphosis: an exquisite model for hormonal regulation of post-embryonic development. Biochem. Soc. Symp., 62, 123–136. [PubMed] [Google Scholar]
- Tone Y., Collingwood,T.N., Adams,M. and Chatterjee,V.K. (1994) Functional analysis of a transactivation domain in the thyroid hormone β receptor. J. Biol. Chem., 269, 31157–31161. [PubMed] [Google Scholar]
- Tong G.X., Tanen,M.R. and Bagchi,M.K. (1995) Ligand modulates the interaction of thyroid hormone receptor β with the basal transcription machinery. J. Biol. Chem., 270, 10601–10611. [DOI] [PubMed] [Google Scholar]
- Torchia J., Glass,C. and Rosenfeld,M.G. (1998) Co-activators and co-repressors in the integration of transcriptional responses. Curr. Opin. Cell Biol., 10, 373–383. [DOI] [PubMed] [Google Scholar]
- Ura K., Kurumizaka,H., Dimitrov,S., Almouzni,G. and Wolffe,A.P. (1997) Histone acetylation: influence on transcription, nucleosome mobility and positioning, and linker histone-dependent transcriptional repression. EMBO J., 16, 2096–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra G.J., Destree,O.H. and Wolffe,A.P. (1999) Translation of maternal TATA-binding protein mRNA potentiates basal but not activated transcription in Xenopus embryos at the midblastula transition. Mol. Cell. Biol., 19, 7972–7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermaak D., Wade,P.A., Jones,P.L., Shi,Y.B. and Wolffe,A.P. (1999) Functional analysis of the SIN3-histone deacetylase RPD3-RbAp48-histone H4 connection in the Xenopus oocyte. Mol. Cell. Biol., 19, 5847–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade P.A., Jones,P.L., Vermaak,D. and Wolffe,A.P. (1998) A multiple subunit Mi-2 histone deacetylase from Xenopus laevis cofractionates with an associated Snf2 superfamily ATPase. Curr. Biol., 8, 843–846. [DOI] [PubMed] [Google Scholar]
- Wade P.A., Gegonne,A., Jones,P.L., Ballestar,E., Aubry,F. and Wolffe,A.P. (1999a) Mi-2 complex couples DNA methylation to chromatin remodelling and histone deacetylation. Nature Genet., 23, 62–66. [DOI] [PubMed] [Google Scholar]
- Wade P.A., Jones,P.L., Vermaak,D. and Wolffe,A.P. (1999b) Purification of a histone deacetylase complex from Xenopus laevis: preparation of substrates and assay procedures. Methods Enzymol., 304, 715–725. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson,C.C., Ong,E.S., Lebo,R., Gruol,D.J. and Evans,R.M. (1986) The c-erb-A gene encodes a thyroid hormone receptor. Nature, 324, 641–646. [DOI] [PubMed] [Google Scholar]
- Wolffe A.P. (1997) Transcriptional control. Sinful repression. Nature, 387, 16–17. [DOI] [PubMed] [Google Scholar]
- Wong C.W. and Privalsky,M.L. (1998) Transcriptional repression by the SMRT-mSin3 corepressor: multiple interactions, multiple mechanisms, and a potential role for TFIIB. Mol. Cell. Biol., 18, 5500–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J., Shi,Y.B. and Wolffe,A.P. (1995) A role for nucleosome assembly in both silencing and activation of the Xenopus TRβ A gene by the thyroid hormone receptor. Genes Dev., 9, 2696–2711. [DOI] [PubMed] [Google Scholar]
- Wong J., Shi,Y.B. and Wolffe,A.P. (1997) Determinants of chromatin disruption and transcriptional regulation instigated by the thyroid hormone receptor: hormone-regulated chromatin disruption is not sufficient for transcriptional activation. EMBO J., 16, 3158–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J., Liang,V.C., Sachs,L.M. and Shi,Y.B. (1998a) Transcription from the thyroid hormone-dependent promoter of the Xenopus laevis thyroid hormone receptor βA gene requires a novel upstream element and the initiator, but not a TATA box. J. Biol. Chem., 273, 14186–14193. [DOI] [PubMed] [Google Scholar]
- Wong J., Patterton,D., Imhof,A., Guschin,D., Shi,Y.B. and Wolffe,A.P. (1998b) Distinct requirements for chromatin assembly in transcriptional repression by thyroid hormone receptor and histone deacetylase. EMBO J., 17, 520–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Glass,C.K. and Rosenfeld,M.G. (1999) Coactivator and corepressor complexes in nuclear receptor function. Curr. Opin. Genet. Dev., 9, 140–147. [DOI] [PubMed] [Google Scholar]
- Xue Y., Wong,J., Moreno,G.T., Young,M.K., Cote,J. and Wang,W. (1998) NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol. Cell, 2, 851–861. [DOI] [PubMed] [Google Scholar]
- Yang W.M., Yao,Y.L., Sun,J.M., Davie,J.R. and Seto,E. (1997) Isolation and characterization of cDNAs corresponding to an additional member of the human histone deacetylase gene family. J. Biol. Chem., 272, 28001–28007. [DOI] [PubMed] [Google Scholar]
- Zenke M., Munoz,A., Sap,J., Vennstrom,B. and Beug,H. (1990) v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell, 61, 1035–1049. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Iratni,R., Erdjument-Bromage,H., Tempst,P. and Reinberg,D. (1997) Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell, 89, 357–364. [DOI] [PubMed] [Google Scholar]
- Zorn A.M. and Krieg,P.A. (1997) The KH domain protein encoded by quaking functions as a dimer and is essential for notochord development in Xenopus embryos. Genes Dev., 11, 2176–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]