Abstract
CD147 is a broadly expressed plasma membrane glycoprotein containing two immunoglobulin-like domains and a single charge-containing transmembrane domain. Here we use co-immunoprecipitation and chemical cross-linking to demonstrate that CD147 specifically interacts with MCT1 and MCT4, two members of the proton-linked monocarboxylate (lactate) transporter family that play a fundamental role in metabolism, but not with MCT2. Studies with a CD2–CD147 chimera implicate the transmembrane and cytoplasmic domains of CD147 in this interaction. In heart cells, CD147 and MCT1 co-localize, concentrating at the t-tubular and intercalated disk regions. In mammalian cell lines, expression is uniform but cross-linking with anti-CD147 antibodies caused MCT1, MCT4 and CD147, but not GLUT1 or MCT2, to redistribute together into ‘caps’. In MCT-transfected cells, expressed protein accumulated in a perinuclear compartment, whereas co-transfection with CD147 enabled expression of active MCT1 or MCT4, but not MCT2, in the plasma membrane. We conclude that CD147 facilitates proper expression of MCT1 and MCT4 at the cell surface, where they remain tightly bound to each other. This association may also be important in determining their activity and location.
Keywords: CD147/chaperone/lactate transport/monocarboxylate transporter/OX-47
Introduction
The cell surface glycoprotein CD147 (also known as OX-47, EMMPRIN, HT7 and basigin) is broadly distributed, and expressed at particularly high levels at the retinal pigment epithelium and neonatal blood–brain barrier (Seulberger et al., 1992), on tumour cells (Biswas et al., 1995) and on activated T cells (Paterson et al., 1987; Kasinrerk et al., 1992). It is a member of a small family of closely related proteins (Miyauchi et al., 1991), including GP70 and SDR1 (Shirozu et al., 1996). These proteins are predicted to have common structural features including immunoglobulin-like domains in the extracellular region, a short cytoplasmic tail and a single transmembrane domain (Fossum et al., 1991). The transmembrane regions show a very high degree of cross-species conservation, particularly in the case of CD147 (Kasinrerk et al., 1992) where the sequences in the rabbit and chicken homologues are identical. Such a high degree of conservation suggests that this transmembrane region of CD147 may have an important functional role beyond anchoring the protein in the membrane.
Another shared feature of the transmembrane regions of members of the CD147 family is a centrally positioned glutamic acid residue (Fossum et al., 1991). A charged amino acid deep within the transmembrane region is a common feature of proteins with multiple transmembrane domains (Saier, 1994), but unusual for a protein with a single transmembrane domain (Green, 1991). Other single-pass proteins with a transmembrane charge include FcεRIα, FcγRIII (CD16) and the natural killer cell killer activatory receptors. Each of these has been shown to engage in a functionally important lateral association with another transmembrane protein (Lanier et al., 1989, 1998). An analogous interaction would seem possible for CD147.
We have previously used covalent cross-linking to demonstrate a close physical association between GP70, a protein related to CD147, and the erythrocyte proton-linked lactate transporter MCT1 (Poole and Halestrap, 1997). MCT1 is a 55 kDa protein containing 12 membrane-spanning regions (Poole et al., 1996) that runs at ∼45 kDa on SDS–PAGE. It is a member of the proton-linked monocarboxylate transporter (MCT) family, which is known to contain at least eight mammalian members (Halestrap and Price, 1999). Of these, only four isoforms (MCT1–MCT4) have been functionally characterized, and in each case they have been shown to catalyse the proton-linked transport of short-chain substituted monocarboxylates such as lactate, pyruvate, acetoacetate and β-hydroxybutyrate. Of these, lactic acid is metabolically the most important. As the end product of glycolysis, and a substrate for oxidative metabolism and gluconeogenesis, it must be transported very rapidly across the plasma membrane of most cells (Poole and Halestrap, 1993; Halestrap and Price, 1999).
In this paper we show that CD147 is specifically associated with the MCT transporter isoforms MCT1 and MCT4, and propose that it may act as a chaperone, facilitating their cell surface expression and/or appropriate location.
Results
MCT1 and MCT4 co-immunoprecipitate with CD147
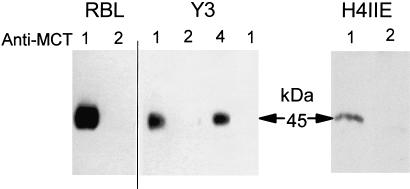
Figure 1 shows that the three cell lines Y3, RBL and H4IIE all contain CD147, but different complements of MCT isoforms. Both Y3 and RBL cells contain MCT1 and MCT4, whilst H4IIE cells contain MCT1 and MCT2. All the cell lines contained both GP70 and CD147, with the latter showing several bands characteristic of multiple glycosylation states that are known to vary between cell types (Kanekura et al., 1991; Nehme et al., 1995). A polyclonal antipeptide antibody against the C-terminus of CD147 was used for the western blotting experiments reported in Figure 1 since this detected CD147 on blots much more effectively than the CD147 monoclonal antibody (mAb) that was raised against a crude lymphocyte membrane preparation. However, this polyclonal antibody was not able to immunoprecipitate CD147 from detergent-solubilized cells whereas the mAb was effective for this purpose, although it preferentially immunoprecipitated the 43 kDa form of the protein (see for example Figure 4B). Using this mAb, CD147 was immunoprecipitated from each cell line and the co-precipitation of MCT isoforms detected by western blotting (Figure 2). Where present, MCT1 and MCT4 were co-precipitated with CD147, but MCT2 was not. In contrast, immunoprecipitation using the OX-34 antibody did not reveal co-precipitation of MCT1 from Y3 cells (Figure 2) or MCT4 (data not shown).

Fig. 1. Expression of MCT1, MCT2, MCT4, GP70 and CD147 in mammalian cell lines and Xenopus oocytes. Crude membrane fractions were prepared from Y3, RBL and H4IIE cells and Xenopus oocytes, proteins separated by SDS–PAGE and western blotting performed with antibodies against MCT1, MCT2, MCT4, GP70 and CD147 as indicated.
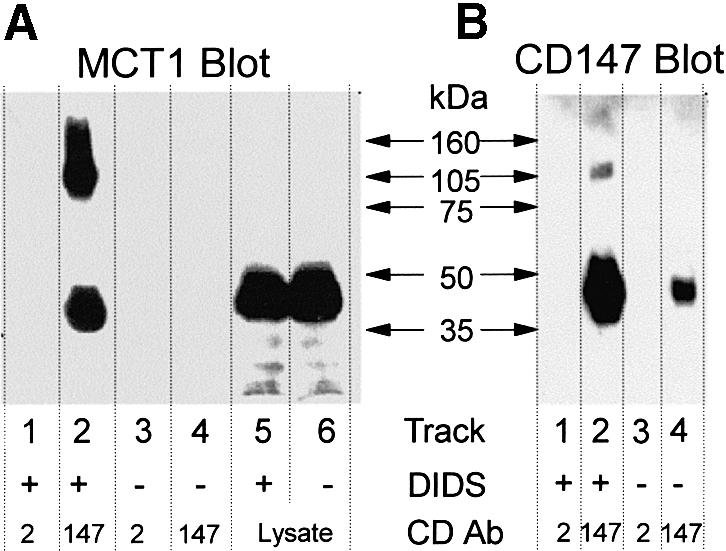
Fig. 4. DIDS cross-links MCT1 to CD147 but not CD2 in RBL cells. RBL cells transfected with CD2 were treated with DIDS or left untreated as indicated. Crude lysate and CD147 or CD2 immunoprecipitates were separated by SDS–PAGE and western blots performed with anti-MCT1 (A) and anti-CD147 (B) antibodies. Longer exposure did reveal the presence of MCT1 in the CD147 immunoprecipitate from untreated cells, but not in either of the CD2 immunoprecipitates (not shown).
Fig. 2. Immunoprecipitation of CD147 from Y3 and H4IIE cells co-precipitates MCT1 and MCT4 but not MCT2. CD147 was immunoprecipitated from Y3, RBL and H4IIE cells, proteins separated by SDS–PAGE and western blotted with antibodies against MCT1, MCT2 and MCT4 as shown. Control (OX-34) precipitate from Y3 cells did not cause co-precipitation of MCT1 (final lane) or MCT4 (data not shown).
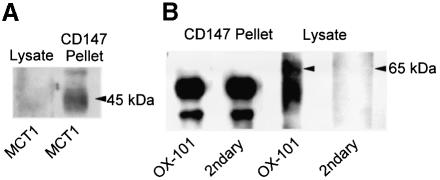
In order to confirm that the observed co-immunoprecipitation of CD147 and MCT isoforms was due to specific binding, additional control experiments were performed. The relative enrichment of MCT isoforms in the immunoprecipitate was compared with that of another cell surface protein containing multiple transmembrane regions: CD47 (subsequently referred to as OX-101 for clarity). Data are presented in Figure 3. In Figure 3A, we show that the amount of MCT1 in the cell lysate was too low to detect, whereas it was readily detected in the CD147 immunoprecipitate. In contrast, the data in Figure 3B show that OX-101 is detected strongly in the lysate, but not in the immunoprecipitate. It should be noted that the two bands detected in the immunoprecipitate (Figure 3B, lanes 1 and 2) are not due to OX-101, but to immunoglobulin from the beads, which is detected by the secondary antibody alone.
Fig. 3. Immunoprecipitation of CD147 from RBL cells co-precipitates MCT1 but not OX-101. CD147 was immunoprecipitated from RBL cells, and the precipitate and lysate separated by SDS–PAGE then western blotted for MCT1 [(A) left lane, lysate; right lane, immunoprecipitate) and the unrelated transmembrane protein OX-101 [(B) lanes 1 and 2, precipitate; lanes 3 and 4, lysate; lanes 2 and 4 were probed with secondary antibody only, to reveal non-specific bands].
Our data suggest a specific interaction between CD147 and MCT1, and this was confirmed by the use of 4,4′-diisothiocyanostilbene-2,2′-disulfonate (DIDS) to cross-link the two proteins (Figure 4). DIDS contains two isothiocyano groups that can cross-link two lysine groups in close proximity. It has been used previously to demonstrate the interaction between MCT1 and GP70 in rat erythrocytes (Poole and Halestrap, 1997). RBL cells transfected with CD2 were treated with DIDS, and the presence of MCT1–CD147 or MCT1–CD2 conjugates investigated by immunoprecipitation using antibodies against either CD2 or CD147. Western blotting of the CD2 immunoprecipitate with anti-MCT1 antibodies revealed the presence of neither MCT1 nor a conjugate protein (Figure 4A, lane 1) whereas in the CD147 precipitate two anti-MCT1 reactive bands of 45 and 95 kDa were detected (Figure 4A, lane 2). The 95 kDa band is of the expected size for an MCT1–CD147 conjugate and a band of similar size could be detected with an anti-CD147 antibody (Figure 4B, lane 2). The putative cross-linked conjugate was not present in sufficient quantity to be detected in the crude lysate (Figure 4B, lanes 5 and 6) and thus its presence in the CD147 immunoprecipitate implies enrichment by direct immunoprecipitation, rather than co-precipitation. In western blots of immunoprecipitate from control cells (not treated with DIDS) at the same protein loading, neither MCT1 nor the CD147–MCT1 conjugate were detected (Figure 4B, lanes 3 and 4), although longer exposure did reveal the presence of MCT1 in the CD147 immunoprecipitate, but not the CD2 immunoprecipitate, as expected (data not shown). Surprisingly, the amount of CD147 detected in the immunoprecipitate of the non-treated cells was also less than in the treated cells, suggesting that DIDS-labelled CD147 may immunoprecipitate more readily than native CD147.
The observation that MCT1 can interact with CD147 but not CD2 provides the means to establish whether it is the transmembrane domain or the extracellular domain of CD147 that is involved in this interaction. A hybrid protein containing the extracellular domain of CD2 and the transmembrane and cytoplasmic domains of CD147 was expressed in RBL cells, and immunoprecipitated using an anti-CD2 antibody. As shown in Figure 5, the immunoprecipitate contained MCT1 but not MCT2, implying that the transmembrane and cytoplasmic domains of CD147 are sufficient to mediate the interaction with MCT1.

Fig. 5. The transmembrane and cytoplasmic regions of CD147 are sufficient to mediate the interaction with MCT1. The CD2–CD147 hybrid protein was immunoprecipitated from transfected RBL cells using anti-CD2 antibody, and western blotted for MCT1 (lane 1) and MCT2 (lane 2). MCT1 is clearly detected, contrasting with Figure 2, in which MCT1 is not detected in CD2 immunoprecipitate from CD2-transfected RBL cells. The bars represent 50 μm.
Co-localization of MCT1 and MCT4 with CD147
If MCT1 and MCT4 interact specifically with CD147, they would be predicted to co-localize in the cell. This was shown to be the case in isolated rat cardiac myocytes by confocal microscopy (Figure 6). In these cells, MCT1 has a very characteristic distribution with greater expression at t-tubules and particularly strong expression in the intercalated disk region (Garcia et al., 1994; Halestrap et al., 1997; Halestrap and Price, 1999). The distribution of CD147 exactly matches this, as illustrated in the overlay (orange/yellow) of the green fluorescein isothiocyanate (FITC) fluorescence of the anti-CD147 antibody and the red tetramethylrhodamine isothiocyanate (TRITC) fluorescence of the anti-MCT1 antibody. Neither MCT2 nor MCT4 could be detected in rat cardiac myocytes (see Halestrap and Price, 1999).
Fig. 6. Co-localization of MCT1 and CD147 in isolated rat cardiac myocytes. Dual excitation confocal microscopy was performed on fixed isolated rat heart cells treated with antibodies against MCT1 (TRITC–secondary) and CD147 (FITC–secondary). The bars represent 50 μm.
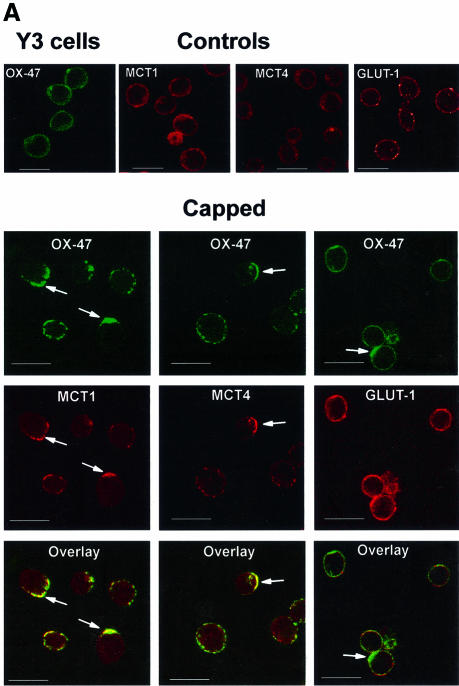
In order to confirm that the co-localization reflected a physical interaction between the two proteins, rather than confinement to a shared membrane compartment, we treated cultured Y3 cells with antibodies to the extracellular region of CD147, and a secondary antibody that induced cross-linking. This caused CD147 to aggregate, often leading to a ‘cap’ of CD147 at the pole of the cell. If MCT1 and MCT4 interact with CD147 in situ, they too should be capped under the same conditions and the data presented in Figure 7 demonstrate that this is indeed the case. Figure 7A shows diffuse staining of MCT1, MCT4 and CD147 on untreated cells, whilst after antibody treatment all three proteins co-localized into concentrated regions (‘caps’) on the cell surface. In contrast, GLUT1 remained diffuse. Similar experiments performed in H4IIE cells, which contain both MCT1 and MCT2, again showed that CD147 and MCT1 were concentrated into caps in some cells, whilst MCT2 remained diffusely distributed (Figure 7B).

Fig. 7. Relocalization of CD147 by antibody treatment causes parallel relocalization of MCT1 and MCT4 but not GLUT1 or MCT2. In (A), Y3 cells were ‘capped’ (arrows) by CD147 primary and FITC–secondary antibody, fixed and treated with antibodies against MCT1, MCT4 (TRITC–secondary) or GLUT1 (CY3–secondary). Uncapped cells (top row) were treated as in Figure 6. Confocal fluorescence microscopy revealed the incorporation of MCT1 and MCT4 into the CD147 caps as confirmed by the orange/yellow in the overlay. (B) H4IIE cells, which contain MCT1 and MCT2 but not MCT4, were subjected to the same capping procedure as the Y3 cells although the capping (arrows) was less profound. The bars represent 20 µm.
MCT1 and MCT4 expression at the cell surface requires co-expression of CD147
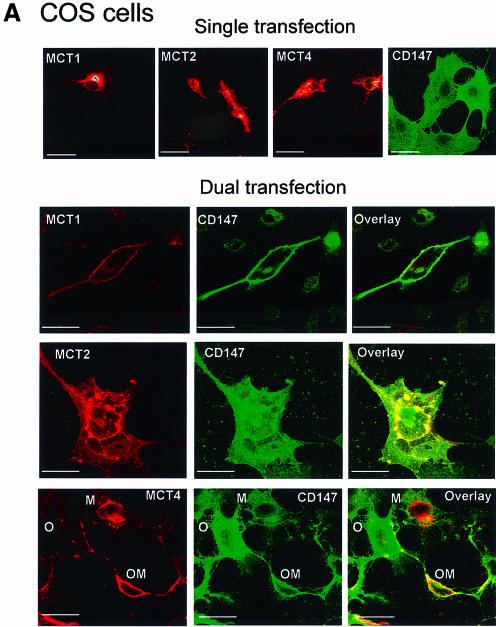
We have transfected a range of mammalian cell lines with various MCT isoforms, and in all cases found that little of the expressed protein reached the plasma membrane. Rather, as shown in Figure 8 for both COS and HeLa cells, most remained associated with a perinuclear compartment that probably reflects retention of the protein in the endoplasmic reticulum and Golgi apparatus. In contrast, co-expression of CD147 and MCT1 or MCT4 in transiently transfected COS cells led to substantial expression of both proteins at the cell surface, rather than their retention in the perinuclear region (Figure 8A). However, when MCT2 was co-expressed with CD147, both proteins remained within intracellular compartments, consistent with the data from the immunoprecipitation experiments, which imply that MCT2 does not interact with CD147. Figure 8B shows similar data for HeLa cells, in which CD147 was co-expressed with either MCT1 or MCT4. Thus, in both COS and HeLa cells co-expression of CD147 with MCT1 or MCT4 enabled both proteins to be properly targeted to the plasma membrane.
Fig. 8. Co-transfection with CD147 enables expression of MCT1 and MCT4, but not MCT2, at the plasma membrane. In (A), COS cells were transiently transfected with both CD147 and MCT1, MCT2 or MCT4 in the pCI-neo vector and the expression and location of CD147 (FITC–secondary) and MCT1, MCT2 or MCT4 (TRITC–secondary) were detected by dual excitation confocal microscopy. Within the field of cells shown for the MCT4–CD147 transfection, one cell overexpressed primarily MCT4 (M), another CD147 (O) and a third both proteins (OM). Only in the latter case were both proteins properly targeted to the plasma membrane and such cells frequently appeared more rounded. In (B), HeLa cells were transiently transfected with both CD147 and MCT1 or MCT4 in the pTRE vector. Appropriate levels of expression were achieved by addition of tetracycline (10 pg/ml). Dual excitation confocal microscopy was used to the reveal the expression and location of CD147, MCT1 and MCT4 as above. The bars represent 20 µm.
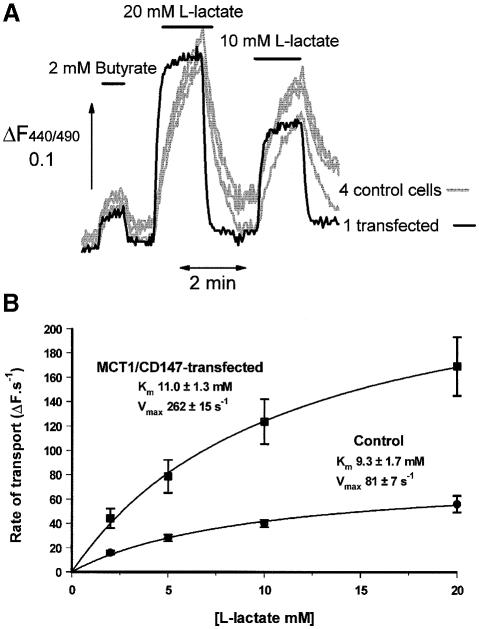
In order to confirm that the surface expression of MCT1 in cells co-expressing MCT1 and CD147 correlated with substantially enhanced rates of lactate transport, transiently transfected cells were loaded with 2′,7′-bis (carboxyethyl)-5(6)-carboxyfluorescein (BCECF) and the rate of intracellular acidification following the addition of lactate determined for a large number of cells. Illustrative data are shown in Figure 9. In non-transfected cells, rates of transport showed relatively little variation; the fastest rates were never more than twice the slowest rate in any group of cells. In contrast, in those cells that had been subjected to transfection with both CD147 and MCT1, ∼3% of cells were found to demonstrate rates of transport 3–4 times greater than control cells, as illustrated by a single trace in Figure 9A. These cells almost certainly represent those that had been successfully transfected with both constructs since in several experiments it was demonstrated by immunofluorescence microscopy that only ∼2–5% of cells overexpressed both proteins. Kinetic analysis of the transport into non-transfected and transfected cells was performed using four separate lactate concentrations and mean results from four cells are shown in Figure 9B. As predicted, the increased rate of transport in the transfected cells reflected a 3- to 4-fold increase in Vmax rather than a change in Km.
Fig. 9. Co-transfection of COS cells with CD147 and MCT1 leads to a large stimulation of lactate transport. In (A), COS cells that had been transiently transfected with both CD147 and MCT1 were loaded with BCECF and rates of transport by five individual cells determined from the rate of change of the 440/490 nm fluorescence ratio upon superfusion with the concentrations of l-lactate or butyrate indicated. Butyrate was included to confirm that transfection did not change the plasma membrane pH gradient (Wang et al., 1994). The four cells labelled control showed similar rates of transport to non-transfected cells determined in a parallel experiment, and were therefore assumed to be untransfected. In (B), mean kinetic data are provided for four control cells and four transfected cells with elevated rates of transport similar to those shown in (A). Cells transiently transfected with MCT1 alone did not show rates of transport significantly different from non-transfected cells (data not shown and see the text for discussion).
When cells were transiently transfected with MCT1 or MCT4 alone, measurement of transport rates did reveal some cells with increased rates of transport, but the increase was never greater than ∼2-fold (data not shown). This was not sufficiently different from the natural variation in rates we observed in control cells to allow transfected cells to be distinguished unequivocally from their non-transfected neighbours. Such data are consistent with the poor expression of the transporters at the plasma membrane in singly transfected cells observed by confocal microscopy (Figure 8), leading to only a modest increase in rates of lactate transport. However, this problem could be overcome by making stable transfectants as shown in Figure 10. In such cells, although much of the expressed MCT1 and MCT4 remained in intracellular compartments, some expression at the plasma membrane could be observed and this was accompanied by about a 2-fold increase in transport rates. Attempts to make stable transfectants containing both CD147 and either MCT1 or MCT4 were unsuccessful.
Fig. 10. Stable transfection of COS cells with MCT1 or MCT4 leads to a modest stimulation of lactate transport. Non-transfected COS cells or stable transfectants overexpressing either MCT1 or MCT4 were loaded with BCECF and rates of lactate transport by a field of cells determined from the rate of change of the 440/490 nm fluorescence ratio upon superfusion with 5 mM l-lactate as indicated. The expression of MCT1 and MCT4 in the transfected cells was visualized using immunofluorescence microscopy. The bars represent 20 µm.
Discussion
We have demonstrated by co-immunoprecipitation and chemical cross-linking that there is a specific and strong interaction between CD147 and two members of the proton-coupled monocarboxylate transporter family, MCT1 and MCT4. Similar cross-linking studies in rat erythrocytes demonstrated that, in these cells, MCT1 interacts with GP70 (embigin), another member of the highly conserved basigin family (Poole and Halestrap, 1997). The demonstration that MCT1 also associates tightly with a hybrid protein containing the extracellular domain of CD2 but the transmembrane and intracellular domains of CD147 suggests that it is the transmembrane and cytoplasmic regions of CD147 that are important in the interaction. These domains are strongly conserved in members of the basigin family, consistent with the ability of GP70 to interact with MCT1. The presence of a conserved glutamate residue in the transmembrane domain of CD147 (Fossum et al., 1991) and a conserved arginine in the eighth transmembrane domain of all MCT isoforms except MCT5 (Halestrap and Price, 1999) may be important in this interaction. However, other factors are also likely to be important since MCT2, which also possess the conserved arginine, does not appear to interact with CD147. It would seem likely that another ancillary protein may be required for the correct expression of MCT2, but this has yet to be identified.
Evidence for a close physical association between CD147 and specific monocarboxylate transporters is also provided by their striking co-localization at the cell surface (Figure 6), which is maintained even after extreme perturbation of CD147 distribution with mAbs (Figure 7). Furthermore, the expression pattern of CD147 in different tissues is compatible with it playing a role in lactate transport. In highly glycolytic cells such as white muscle cells, activated lymphocytes and tumour cells, an excess of lactic acid is produced, which must be exported from the cell to avoid a drop in intracellular pH (Poole and Halestrap, 1993; Haji-Michael et al., 1999; Halestrap and Price, 1999). In these cells the predominant monocarboxylate transporter is usually MCT4 (Wilson et al., 1998; Juel and Halestrap, 1999). Conversely, cells with a high oxidative capacity, such as heart and red skeletal muscle, are rich in mitochondria and may utilize lactate as an energy source. In these cells there is a net influx of lactic acid and MCT1 predominates. CD147 expression is high in both these cell types (Paterson et al., 1987; Muraoka et al., 1993; Caudroy et al., 1999). Especially high levels of both MCT1 (Gerhart et al., 1999) and CD147 (Finnemann et al., 1997) are found in the retinal pigment epithelium, the barrier between the retina, a net producer of lactate, and the choroidal blood supply. In the retinal pigment epithelium, CD147 and MCT1 are both restricted to the apical membrane. Furthermore, expression of both CD147 and MCT1 is increased in response to stimulation of metabolic activity. For example, it has been demonstrated that CD147 is upregulated on activated splenocytes (Paterson et al., 1987) and hepatocytes (Nehme et al., 1995), whilst MCT1 expression is increased in metabolically active skeletal muscle (Halestrap and Price, 1999; Juel and Halestrap, 1999) and activated macrophages (Hahn et al., 2000).
Our data suggest that the interaction between CD147 and MCT1 or MCT4 may be required for translocation and/or correct localization of the transporters to the plasma membrane. Thus, co-transfection experiments showed that only in the presence of overexpressed CD147 were MCT1 and MCT4 correctly localized to the plasma membrane (Figure 8). In contrast, Xenopus oocytes, which have very little endogenous MCT activity, readily express MCT1 and MCT4 when injected with the appropriate cRNA, whilst MCT2 is less well expressed (Bröer et al., 1998, 1999; Halestrap and Price, 1999). A plausible explanation for this would be the presence of significant quantities of an endogenous CD147 homologue in Xenopus oocytes that can assist MCT1 and MCT4 expression at the plasma membrane. In support of this possibility, using western blotting with a polyclonal antibody against CD147 we have confirmed that a crude oocyte membrane preparation does contain such a protein (see Figure 1).
The association reported here, between a single-pass transmembrane protein and a multi-pass transporter, fits an emerging trend of ancillary proteins acting as chaperones in the expression of membrane transporters. Translocation of the AE1 anion exchanger (band 3) to the plasma membrane of Xenopus oocytes is greatly enhanced by co-expression of glycophorin (Bruce et al., 1994), whilst the 4F2 heavy chain (CD98) interacts with and regulates expression of a family of amino acid transporters (Kanai et al., 1998; Mastroberardino et al., 1998; Pfeiffer et al., 1999; Torrents et al., 1999). Similarly, the single-pass glycoprotein CD36 and a 12-pass protein have been implicated in long-chain fatty acid transport across the plasma membrane (Abumrad et al., 1999). In addition to their chaperone role, such interactions may also modify the activity of transporters (Bruce et al., 1994) and the ligand specificity of receptors (McLatchie et al., 1998). CD147 may play a similar role in modifying the properties of MCTs, but the large amount of CD147 present in Xenopus oocytes has prevented us testing this hypothesis directly. The presence of immunoglobulin-like domains predicts an intercellular recognition function for the extracellular region of CD147 (Barclay et al., 1997). Ligand binding by a member of the immunoglobulin superfamily can have profound effects on an associated membrane transporter or channel. For example, when the neurotrophin receptor TrkB, a member of the immunoglobulin superfamily with a single membrane-spanning region, binds its ligand, a sodium channel undergoes a conformational change resulting in a rapid sodium influx (Kafitz et al., 1999). However, in our attempts to mimic CD147 ligation by antibody-induced cross-linking we have been unable to demonstrate changes in lactate transport kinetics (data not shown).
Alternatively, binding to a cell-surface ligand could affect the subcellular distribution of CD147. The three-domain isoform of SDR1 has recently been shown to be capable of homophilic adhesion through its N-terminal domain (Smalla et al., 2000). The presence of an exon in the CD147 gene encoding a potential additional N-terminal domain with homology to the homophilic binding domain of SDR1 (Langnaese et al., 1997) raises the possibility that an alternatively spliced form of CD147 could engage in a similar interaction. This would offer an explanation for the localization of CD147 to sites of cell–cell contact (Schlosshauer et al., 1995). As redistribution of CD147 results in a corresponding redistribution of MCT1 (Figure 7), this provides a mechanism for the localization of MCT1 to sites of close contact between adjacent cells observed in the retina (Bergersen et al., 1999). Interactions of the extracellular region of CD147 may therefore organize MCT distribution on adjacent cells into a lactate exchange interface, allowing efficient transport between lactate-producing and lactate-utilizing cells. Organization of intercellular transport in this manner has been described for a neuronal sodium channel, the β-subunit of which contains an immunoglobulin-like domain that interacts in a homophilic fashion (Malhotra et al., 2000).
Other basigin family members may bind different ligands through the extracellular immunoglobulin-like domains, which are less similar than the transmembrane and cytoplasmic regions. GP70, which shares the ability of CD147 to interact with MCT1, is strongly and broadly expressed during early stages of embryogenesis (Fan et al., 1998), but has a much more restricted distribution in adults, being found at high levels only during pregnancy in the ovary and uterus in mice (Huang et al., 1990). It remains to be seen whether the other CD147 subfamily members SDR1 (gp55) and ZOV3 interact with MCTs. Like CD147, SDR1 has a broad distribution, but is expressed at particularly high levels in neurons (Langnaese et al., 1997), where it is enriched in the synaptic membrane (Hill et al., 1988). Similarly, the avian protein ZOV3, for which no mammalian homologue has been found, is expressed at detectable levels only in hormone-secreting cells of the ovarian follicle (Kunita et al., 1997). These different distributions may reflect differences in monocarboxylate transport requirements in specialized tissues, or an association between certain CD147 family members and other members of the MCT family.
Materials and methods
Antibodies
Polyclonal antibodies were raised in rabbit against the C-terminus of rat MCT1 and MCT2 and human MCT1 and MCT4, and purified by affinity purification using the immobilized peptide as described previously (Poole et al., 1996; Jackson et al., 1997; Wilson et al., 1998). A polyclonal antibody against a C-terminal peptide of CD147 was kindly provided by Dr Will Mawby (Department of Biochemistry, University of Bristol, Bristol, UK). Polyclonal antibodies against the cytoplasmic tail of rat embigen (GP70) were a generous gift of Dr Sean Guenette (John Wayne Cancer Institute, Santa Monica, CA). The mouse mAb RET.PE-2, recognizing rat CD147 (Finnemann et al., 1997), was kindly provided by Dr Colin Barnstable (Yale, CT). The mouse mAbs OX-21, OX-34 and OX-47 recognize human Factor I, rat CD2 and CD147, respectively (all referenced in the European Collection of Animal Cell Cultures at www.camr.org.uk/ecacc.html). The mouse mAb OX-101 is specific for rat CD47 (our unpublished data). For detection of GLUT1, a goat polyclonal antibody was obtained from Santa Cruz Biotechnology. All secondary antibodies for immunofluorescence microscopy were from Sigma.
Constructs
The coding regions of rat MCT1, MCT2 and human MCT4 were subcloned into the EcoRI site of the pCI-neo mammalian expression vector (Promega) and the EcoRI site of pTRE response plasmid used in the Tet-Off gene expression system (Clontech). The coding region of rat CD147 was subcloned into the EcoRI and XbaI sites of the pCIneo vector and the EcoRI and XbaI sites of the pTRE vector. The resulting constructs were checked by sequencing and expressed in COS cells by a liposome-mediated transfection procedure as detailed below. Full-length rat CD2 was cloned into pKG5 (He et al., 1988), a modified form of pSVtk neo (Gould et al., 1987). To construct the CD2–CD147 hybrid protein, the extracellular domains of rat CD2 and the transmembrane and intracellular regions of CD147 were joined by overlapping PCR (junctional sequence GLP/RVR) and spliced into pKG5 at the BamHI and HindIII sites (Garnett, 1993). The resulting constructs were expressed in RBL.2H3 cells.
Cells
Isolated rat heart cells were kindly provided by Dr Elinor Griffiths (Bristol Heart Institute). COS cells and HeLa cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM from Gibco-BRL) supplemented with 10% fetal bovine serum, 2 mM l-glutamine (Gibco-BRL), 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma). For HeLa cells with regulator plasmid TET-Off, 100 µg/ml geneticin (Gibco-BRL) was added. The rat lymphoblastic cell line Y3 and the rat basophilic leukaemia cell line RBL.2H3 were maintained in RPMI 1640 medium (Gibco-BRL) supplemented as for DMEM. For transfected RBL cells, 500 µg/ml geneticin was added.
Transfection
For immunofluorescence studies, coverslips were placed in six-well tissue culture plates and seeded with 1–3 × 105 cells/well in 2 ml of complete medium, whilst for studies of lactate transport, 10 cm plates were seeded with 2–5 × 105 cells in 10 ml of complete medium. Cells were incubated at 37°C in a CO2 incubator until they were 60–70% confluent and then transfection was carried out as follows. The appropriate quantity of the required plasmid (1.26 µg of DNA/well or 8.3 µg of DNA/10 cm plate for single transfections; 0.63 or 4.15 µg of each DNA for double transfections) was diluted into 200 µl of serum-free medium (OPTI-MEM reduced serum medium; Gibco-BRL) containing 8.2 or 54 µl of LipofectAmine reagent (Gibco-BRL) and incubated at room temperature for 45 min to allow DNA–liposome complexes to form. Cells were washed once with serum-free medium, then 0.8 ml/2.8 ml of serum-free medium was added to the DNA–liposome complexes, mixed gently and overlaid onto the cells. The cells were incubated for 5 h at 37°C in a CO2 incubator, after which the transfection mixture was removed and replaced with complete medium. Cells were fixed and stained for the confocal microscope or used for transport experiments 48 h after transfection. RBL.2H3 cells were transfected with pKG5 constructs by electroporation (Chu et al., 1987), using the Gene Pulser (Bio-Rad), and transfected cells selected in medium containing 1 mg/ml geneticin.
Cross-linking
Cross-linking was carried out using the bifunctional stilbene disulfonate DIDS (ICN Biomedicals Inc.), as described previously (Poole and Halestrap, 1997). Cells were suspended in citrate buffer at ∼2 × 107 cells/ml with 100 µM DIDS, and incubated at 37°C for 1 h. The cells were then washed three times in cold phosphate-buffered saline (PBS) prior to lysis and immunoprecipitation.
Immunoprecipitation
Cells were harvested with PBS/EDTA, washed twice in cold PBS, then suspended in cold lysis buffer [1% Brij 97 (Sigma), 10 mM Tris pH 7.4, 140 mM NaCl, 1 mM EDTA, 2.5 mM iodoacetamide, 1 mM phenylmethylsulfonyl fluoride] at ∼2 × 108 cells/ml. The insoluble fraction was removed by centrifugation, and the soluble fraction pre-cleared over a column of OX-21-coupled CNBr-activated Sepharose (Amersham Pharmacia Biotech). The pre-cleared lysate was loaded onto a column of antibody-coated Sepharose (OX-34 or RET.PE-2), the columns were washed with 50 column volumes of low-salt buffer (25 mM Tris, 140 mM NaCl, 0.02% NaN3 pH 7.3, 0.1% Brij 97), pulsed with 5 column volumes of high-salt buffer (25 mM Tris, 500 mM NaCl, 0.02% NaN3 pH 7.3, 0.1% Brij 97), then 5 column volumes of low-salt buffer, followed by a brief pulse of low-salt buffer without detergent. The immunoprecipitate was then eluted in 0.05 M diethylamine pH 11.5, 0.05% deoxycholate, and concentrated using Amicon Centriprep and Microcon (Millipore), before mixing with an equal volume of 2× sample buffer for SDS–PAGE. It was confirmed that this procedure did not elute the primary antibody from the beads and thus, when necessary, it was possible to develop western blots with the same antibody as used for immunoprecipitation. Alternatively (for H4IIE data of Figure 2 and for Figure 3), pre-cleared lysate was incubated with a small aliquot of antibody-coated Sepharose, and the immunoprecipitated material retrieved after washing by solubilizing in SDS–PAGE sample buffer.
Western blotting
Western blotting was performed using polyclonal anti-MCT1, -MCT2, -MCT4 and -GP70 antibodies at 1 in 1000 and anti-CD147 at 1 in 100. mAbs against CD147 and CD2 were used at 1 in 5. Blots were developed using appropriate secondary antibodies and ECL/ECL+ detection (Amersham Pharmacia Biotech).
Immunofluorescence microscopy
Freshly isolated heart cells were allowed to settle on poly-l-lysine-coated cover slips for 5 min whilst cultured cells were grown on coverslips as described above. After one wash with PBS, cells were fixed with 4% paraformaldehyde for 20 min then permeabilzed with 0.3% (v/v) Triton X-100 (Sigma) for 45 s. After two washes with PBS, non-specific binding of antibody was reduced by blocking with 10% (v/v) porcine normal serum for 60 min. Incubation of two primary antibodies, anti-MCT1 (1 in 100) and anti-CD147 (1 in 10), supplemented with 1% bovine serum albumin was carried out for 45 min simultaneously. Coverslips were washed three times with PBS then incubated for 45 min at room temperature with secondary antibody: TRITC-conjugated anti-rabbit IgG for MCT (1 in 200; Sigma) or FITC-conjugated anti-mouse IgG for CD147 (Sigma; 1 in 200). After washing, samples were mounted in Mowiol with DABCO and examined with a Leica TCS-NT confocal scanning microscope (63× 1.32 Na oil immersion objective).
Capping
Y3 or H4IIE cells were detached from the cell culture flask by gentle agitation and centrifuged for 5 min at 150 g. Cells were washed twice in ice-cold PBS, resuspended in 1 ml of tissue culture supernatant containing the mAbs OX-47 and PE-2 (the use of two primary antibodies gives greater frequency of cap formation; data not shown) and incubated on ice for 30 min. Cells were then centrifuged and washed twice in ice-cold PBS and the pellet resuspended in secondary antibody, FITC-conjugated anti-mouse IgG (Jackson ImmunoResearch; 1 in 1000). Cells were resuspended every 15 min for a total of 1 h. Aliquots of cells were taken and treated as described for immunofluorescence microscopy, with second primary antibody (1 in 100) against MCT1, MCT2, MCT4 or GLUT1, and then secondary antibody: TRITC-conjugated anti-rabbit IgG for MCTs (1 in 200; Sigma) or CY3 conjugated anti-goat IgG for GLUT1 (Jackson ImmunoResearch; 1 in 200). It was confirmed that apparent co-localization of fluorophores was not the result of ‘bleed-through’ of one signal into the other by using the two excitation wavelengths of the laser independently and by using each fluorescent secondary antibody in the absence of the other. Images were processed using Photoshop (version 5, Adobe).
Transport
Initial rates of l-lactate transport into control and transfected cells were determined at 25°C from the rate of intracellular acidification measured using the pH-sensitive fluorescent dye BCECF as described previously (Wilson et al., 1998), but with the following modification. Rather than use a photomultiplier to detect the fluorescent light, an intensified CCD camera was used with filter and camera control as well as data analysis controlled by Axon Imaging Workbench software (Axon Instruments, Inc., 1101 Chess Drive, Foster City, CA 94404-1102). This enabled the selection of 10–15 cells whose rates of transport could be determined simultaneously. Excitation was at 440 and 490 nm and emission at >530 nm. Rates of intracellular acidification are reflected by an increase in the 440/490 nm fluorescence ratio.
Acknowledgments
Acknowledgements
We thank Dr Alexandre Carmo for providing us with the CD2 transfected RBL2H3 cells and Dr Mark Jepson for his assistance in the confocal microscopy. This work was supported by project grants from The Wellcome Trust (UK), the British Heart Foundation and the Medical Research Council. We also thank the Medical Research Council (UK) for providing an Infrastructure Award (G4500006) to establish the School of Medical Sciences Imaging Facility at the University of Bristol.
References
- Abumrad N., Coburn,C. and Ibrahimi,A. (1999) Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells: CD36, FATP and FABPm. Biochim. Biophys. Acta, 1441, 4–13. [DOI] [PubMed] [Google Scholar]
- Barclay A.N., Brown,M.H., Law,S.K., McKnight,A.J., Tomlinson,M. and van der Merwe,P.A. (1997) The Leucocyte Antigen Factsbook, 2nd edn. Academic Press, London, UK. [Google Scholar]
- Bergersen L., Johannsson,E., Veruki,M.L., Nagelhus,E.A., Halestrap,A., Sejersted,O.M. and Ottersen,O.P. (1999) Cellular and subcellular expression of monocarboxylate transporters in the pigment epithelium and retina of the rat. Neuroscience, 90, 319–331. [DOI] [PubMed] [Google Scholar]
- Biswas C., Zhang,Y., DeCastro,R., Guo,H., Nakamura,T., Kataoka,H. and Nabeshima,K. (1995) The human tumour cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res., 55, 434–439. [PubMed] [Google Scholar]
- Bröer S., Schneider,H.P., Bröer,A., Rahman,B., Hamprecht,B. and Deitmer,J.W. (1998) Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem. J., 333, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer S., Bröer,A., Schneider,H.P., Stegen,C., Halestrap,A.P. and Deitmer,J.W. (1999) Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem. J., 341, 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce L.J., Groves,J.D., Okubo,Y., Thilaganathan,B. and Tanner,M.J. (1994) Altered band 3 structure and function in glycophorin A- and B-deficient (MkMk) red blood cells. Blood, 84, 916–922. [PubMed] [Google Scholar]
- Caudroy S., Polette,M., Tournier,J.M., Burlet,H., Toole,B., Zucker,S. and Birembaut,P. (1999) Expression of the extracellular matrix metalloproteinase inducer (EMMPRIN) and the matrix metalloproteinase-2 in bronchopulmonary and breast lesions. J. Histochem. Cytochem., 47, 1575–1580. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa,H. and Berg,P. (1987) Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res., 15, 1311–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q.W., Kadomatsu,K., Uchimura,K. and Muramatsu,T. (1998) Embigin/basigin subgroup of the immunoglobulin superfamily: different modes of expression during mouse embryogenesis and correlated expression with carbohydrate antigenic markers. Dev. Growth Differ., 40, 277–286. [DOI] [PubMed] [Google Scholar]
- Finnemann S.C., Marmorstein,A.D., Neill,J.M. and Rodriguez-Boulan,E. (1997) Identification of the retinal pigment epithelium protein RET-PE2 as CE-9/OX-47, a member of the immunoglobulin superfamily. Invest. Ophthalmol. Vis. Sci., 38, 2366–2374. [PubMed] [Google Scholar]
- Fossum S., Mallett,S. and Barclay,A.N. (1991) The MRC OX-47 antigen is a member of the immunoglobulin superfamily with an unusual transmembrane sequence. Eur. J. Immunol., 21, 671–679. [DOI] [PubMed] [Google Scholar]
- Garcia C.K., Goldstein,J.L., Pathak,R.K., Anderson,R.G.W. and Brown,M.S. (1994) Molecular characterization of a membrane transporter for lactate, pyruvate and other monocarboxylates—implications for the Cori cycle. Cell, 76, 865–873. [DOI] [PubMed] [Google Scholar]
- Garnett D. (1993) Characterization of leucocyte glycoproteins with reference to their mode of membrane attachment. DPhil thesis, University of Oxford, Oxford, UK. [Google Scholar]
- Gerhart D.Z., Leino,R.L. and Drewes,L.R. (1999) Distribution of monocarboxylate transporters MCT1 and MCT2 in rat retina. Neuroscience, 92, 367–375. [DOI] [PubMed] [Google Scholar]
- Gould K.G., Scotney,H., Townsend,A.R., Bastin,J. and Brownlee,G.G. (1987) Mouse H-2k-restricted cytotoxic T cells recognize antigenic determinants in both the HA1 and HA2 subunits of the influenza A/PR/8/34 hemagglutinin. J. Exp. Med., 166, 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N.M. (1991) Biological membranes. The semiotics of charge. Nature, 351, 349–350. [DOI] [PubMed] [Google Scholar]
- Hahn E.L., Halestrap,A.P. and Gamielli,R.L. (2000) Expression of the lactate transporter MCT1 in macrophages. Shock, 13, 253–260. [DOI] [PubMed] [Google Scholar]
- Haji-Michael P.G., Ladriere,L., Sener,A., Vincent,J.L. and Malaisse,W.J. (1999) Leukocyte glycolysis and lactate output in animal sepsis and ex vivo human blood. Metabolism, 48, 779–785. [DOI] [PubMed] [Google Scholar]
- Halestrap A.P. and Price,N.T. (1999) The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem. J., 343, 281–299. [PMC free article] [PubMed] [Google Scholar]
- Halestrap A.P., Wang,X.M., Poole,R.C., Jackson,V.N. and Price,N.T. (1997) Lactate transport in heart in relation to myocardial ischemia. Am. J. Cardiol., 80, 17A–25A. [DOI] [PubMed] [Google Scholar]
- He Q., Beyers,A.D., Barclay,A.N. and Williams,A.F. (1988) A role in transmembrane signaling for the cytoplasmic domain of the CD2 T lymphocyte surface antigen. Cell, 54, 979–984. [DOI] [PubMed] [Google Scholar]
- Hill I.E., Selkirk,C.P., Hawkes,R.B. and Beesley,P.W. (1988) Characterization of novel glycoprotein components of synaptic membranes and postsynaptic densities, gp65 and gp55, with a monoclonal antibody. Brain Res., 461, 27–43. [DOI] [PubMed] [Google Scholar]
- Huang R.P., Ozawa,M., Kadomatsu,K. and Muramatsu,T. (1990) Developmentally regulated expression of embigin, a member of the immunoglobulin superfamily found in embryonal carcinoma cells. Differentiation, 45, 76–83. [DOI] [PubMed] [Google Scholar]
- Jackson V.N., Price,N.T., Carpenter,L. and Halestrap,A.P. (1997) Cloning of the monocarboxylate transporter isoform MCT2 from rat testis provides evidence that expression in tissues is species-specific and may involve post-transcriptional regulation. Biochem. J., 324, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C. and Halestrap,A.P. (1999) Lactate transport in skeletal muscle—role and regulation of the monocarboxylate transporter. J. Physiol. (Lond.), 517, 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafitz K.W., Rose,C.R., Thoenen,H. and Konnerth,A. (1999) Neurotrophin-evoked rapid excitation through TrkB receptors. Nature, 401, 918–921. [DOI] [PubMed] [Google Scholar]
- Kanai Y., Segawa,H., Miyamoto,K., Uchino,H., Takeda,E. and Endou,H. (1998) Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem., 273, 23629–23632. [DOI] [PubMed] [Google Scholar]
- Kanekura T., Miyauchi,T., Tashiro,M. and Muramatsu,T. (1991) Basigin, a new member of the immunoglobulin superfamily: genes in different mammalian species, glycosylation changes in the molecule from adult organs and possible variation in the N-terminal sequences. Cell Struct. Funct., 16, 23–30. [DOI] [PubMed] [Google Scholar]
- Kasinrerk W., Fiebiger,E., Stefanova,I., Baumruker,T., Knapp,W. and Stockinger,H. (1992) Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin and chicken HT7 molecule. J. Immunol., 149, 847–854. [PubMed] [Google Scholar]
- Kunita R., Nakabayashi,O., Kikuchi,T. and Mizuno,S. (1997) Predominant expression of a Z-chromosome-linked immunoglobulin superfamily gene, ZOV3, in steroidogenic cells of ovarian follicles and in embryonic gonads of chickens. Differentiation, 62, 63–70. [DOI] [PubMed] [Google Scholar]
- Langnaese K., Beesley,P.W. and Gundelfinger,E.D. (1997) Synaptic membrane glycoproteins gp65 and gp55 are new members of the immunoglobulin superfamily. J. Biol. Chem., 272, 821–827. [DOI] [PubMed] [Google Scholar]
- Lanier L.L., Yu,G. and Phillips,J.H. (1989) Co-association of CD3 ζ with a receptor (CD16) for IgG Fc on human natural killer cells. Nature, 342, 803–805. [DOI] [PubMed] [Google Scholar]
- Lanier L.L., Corliss,B., Wu,J. and Phillips,J.H. (1998) Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity, 8, 693–701. [DOI] [PubMed] [Google Scholar]
- Malhotra J.D., Kazen-Gillespie,K., Hortsch,M. and Isom,L.L. (2000) Sodium channel β subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell–cell contact. J. Biol. Chem., 275, 11383–11388. [DOI] [PubMed] [Google Scholar]
- Mastroberardino L., Spindler,B., Pfeiffer,R., Skelly,P.J., Loffing,J., Shoemaker,C.B. and Verrey,F. (1998) Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature, 395, 288–291. [DOI] [PubMed] [Google Scholar]
- McLatchie L.M., Fraser,N.J., Main,M.J., Wise,A., Brown,J., Thompson,N., Solari,R., Lee,M.G. and Foord,S.M. (1998) RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature, 393, 333–339. [DOI] [PubMed] [Google Scholar]
- Miyauchi T., Masuzawa,Y. and Muramatsu,T. (1991) The basigin group of the immunoglobulin superfamily: complete conservation of a segment in and around transmembrane domains of human and mouse basigin and chicken HT7 antigen. J. Biochem. (Tokyo), 110, 770–774. [DOI] [PubMed] [Google Scholar]
- Muraoka K., Nabeshima,K., Murayama,T., Biswas,C. and Koono,M. (1993) Enhanced expression of a tumour-cell-derived collagenase-stimulatory factor in urothelial carcinoma: its usefulness as a tumour marker for bladder cancers. Int. J. Cancer, 55, 19–26. [DOI] [PubMed] [Google Scholar]
- Nehme C.L., Fayos,B.E. and Bartles,J.R. (1995) Distribution of the integral plasma membrane glycoprotein CE9 (MRC OX-47) among rat tissues and its induction by diverse stimuli of metabolic activation. Biochem. J., 310, 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson D.J., Jefferies,W.A., Green,J.R., Brandon,M.R., Corthesy,P., Puklavec,M. and Williams,A.F. (1987) Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol. Immunol., 24, 1281–1290. [DOI] [PubMed] [Google Scholar]
- Pfeiffer R., Rossier,G., Spindler,B., Meier,C., Kuhn,L. and Verrey,F. (1999) Amino acid transport of y+L-type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family. EMBO J., 18, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R.C. and Halestrap,A.P. (1993) Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am. J. Physiol., 264, C761–C782. [DOI] [PubMed] [Google Scholar]
- Poole R.C. and Halestrap,A.P. (1997) Interaction of the erythrocyte lactate transporter (monocarboxylate transporter 1) with an integral 70-kDa membrane glycoprotein of the immunoglobulin superfamily. J. Biol. Chem., 272, 14624–14628. [DOI] [PubMed] [Google Scholar]
- Poole R.C., Sansom,C.E. and Halestrap,A.P. (1996) Studies of the membrane topology of the rat erythrocyte H+/lactate cotransporter (MCT1). Biochem. J., 320, 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M.H. Jr (1994) Computer-aided analyses of transport protein sequences: gleaning evidence concerning function, structure, biogenesis and evolution. Microbiol. Rev., 58, 71–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosshauer B., Bauch,H. and Frank,R. (1995) Neurothelin: amino acid sequence, cell surface dynamics and actin colocalization. Eur. J. Cell Biol., 68, 159–166. [PubMed] [Google Scholar]
- Seulberger H., Unger,C.M. and Risau,W. (1992) HT7, neurothelin, basigin, gp42 and OX-47—many names for one developmentally regulated immuno-globulin-like surface glycoprotein on blood–brain barrier endothelium, epithelial tissue barriers and neurons. Neurosci. Lett., 140, 93–97. [DOI] [PubMed] [Google Scholar]
- Shirozu M. et al. (1996) Characterization of novel secreted and membrane proteins isolated by the signal sequence trap method. Genomics, 37, 273–280. [DOI] [PubMed] [Google Scholar]
- Smalla K.H. et al. (2000) The synaptic glycoprotein neuroplastin is involved in long-term potentiation at hippocampal CA1 synapses. Proc. Natl Acad. Sci. USA, 97, 4327–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrents D. et al. (1999) Identification of SLC7A7, encoding y+LAT-1, as the lysinuric protein intolerance gene. Nature Genet., 21, 293–296. [DOI] [PubMed] [Google Scholar]
- Vernon-Wilson E.F., Kee,W.-J., Willis,A.C., Barclay,A.N., Simmons,D.L. and Brown,M.H. (2000) CD47 (integrin associated protein) is a ligand for rat macrophage membrane signal regulatory protein SIRP (OX41) and human SIRPa1. Eur. J. Immunol., in press. [DOI] [PubMed] [Google Scholar]
- Wang X.M., Levi,A.J. and Halestrap,A.P. (1994) Kinetics of the sarcolemmal lactate carrier in single heart cells using BCECF to measure pH(i). Am. J. Physiol, 267, H1759–H1769. [DOI] [PubMed] [Google Scholar]
- Wilson M.C. et al. (1998) Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J. Biol. Chem., 273, 15920–15926. [DOI] [PubMed] [Google Scholar]