Abstract
We have examined the localization of DNA replication of the Bacillus subtilis phage φ29 by immunofluorescence. To determine where phage replication was localized within infected cells, we examined the distribution of phage replication proteins and the sites of incorporation of nucleotide analogues into phage DNA. On initiation of replication, the phage DNA localized to a single focus within the cell, nearly always towards one end of the host cell nucleoid. At later stages of the infection cycle, phage replication was found to have redistributed to multiple sites around the periphery of the nucleoid, just under the cell membrane. Towards the end of the cycle, phage DNA was once again redistributed to become located within the bulk of the nucleoid. Efficient redistribution of replicating phage DNA from the initial replication site to various sites surrounding the nucleoid was found to be dependent on the phage protein p16.7.
Keywords: Bacillus subtilis/bacteriophage φ29/immunofluorescence/in vivo DNA replication
Introduction
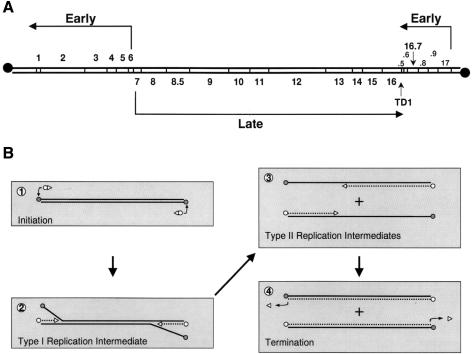
The Bacillus subtilis phage φ29 is one of the best characterized phages and serves as a paradigm for the study of double-stranded DNA viruses in Gram-positive bacteria (Salas, 1991; Salas and Rojo, 1993; Salas et al., 1996). The genome of φ29 is a linear double-stranded DNA molecule of 19 285 bp, whose sequence is completely known (Yoshikawa and Ito, 1982; Garvey et al., 1985; Vlcek and Paces, 1986). Regulation of φ29 DNA transcription, divided into an early and a late stage, has been studied extensively in vivo as well as in vitro (for reviews see Salas and Rojo, 1993; Rojo et al., 1998). The late expressed genes, all transcribed from a single operon present in the central part of the phage genome, encode the phage structural proteins, proteins involved in phage morphogenesis and those required for lysis of the infected cell (Figure 1A). The early expressed genes are present in two operons that flank the late operon (Figure 1A). The operon located at the right side of the φ29 genome encodes, in addition to protein p17 (Crucitti et al., 1998) and p16.7 (W.J.J.Meijer, A.Serna-Rico and M.Salas, submitted), four proteins of unknown function. The early operon located at the left side of the φ29 genome encodes the transcriptional regulator protein p4 and various proteins that are directly involved in phage DNA replication, such as the DNA polymerase, terminal protein (TP), single-stranded DNA-binding protein (SSB), double-stranded DNA-binding protein (DBP) and protein p1 (see Discussion).
Fig. 1. Schematic representation of the genetic and transcriptional organization of the φ29 genome and its in vitro DNA replication mechanism. (A) Genetic and transcription map of the phage φ29 genome. The direction of transcription and length of the transcripts are indicated by arrows. The positions of the various genes are indicated between the two DNA strands. The positions of the open reading frames 16.9, 16.8, 16.6 and 16.5, located at the right side of the φ29 genome, are indicated with the numbers .9, .8, .6 and .5, respectively. TD1 is the position of a bidirectional transcriptional terminator. Filled circles represent the covalently linked φ29 terminal protein. The map is adapted from Mellado et al. (1976a). (B) Mechanism of in vitro φ29 DNA replication (see text for details). Circles and triangles represent TP and DNA polymerase, respectively. Synthesized DNA strands are indicated with broken lines.
A schematic overview of the in vitro φ29 DNA replication mechanism is shown in Figure 1B. Initiation of φ29 DNA replication occurs via a so-called protein-primed mechanism (reviewed in Salas, 1991; Salas et al., 1996). The linear φ29 genome contains a TP molecule covalently linked at each of its 5′ DNA ends, which constitute the origins of replication. Initiation of DNA replication starts by recognition of the origin by a heterodimer formed by the φ29 DNA polymerase and the primer TP. The DNA polymerase then catalyses the addition of the first dAMP (Blanco and Salas, 1984) to the primer TP (Hermoso et al., 1985). The TP and the DNA polymerase remain associated until a short DNA primer of nine nucleotides has been formed. After this so-called transition step, these two proteins dissociate and the DNA polymerase continues processive elongation until replication of the nascent DNA strand is completed (Méndez et al., 1997). Replication, which starts at both DNA ends, is coupled to strand displacement. This results in the generation of so-called type I replication intermediates consisting of full-length double-stranded φ29 DNA molecules with one or more single-stranded DNA branches of varying lengths. When the two converging DNA polymerases merge, a type I replication intermediate becomes physically separated into two type II replication intermediates. Each of these consists of a full-length φ29 DNA molecule in which a portion of the DNA, starting from one end, is double-stranded and the portion spanning to the other end is single-stranded (Inciarte et al., 1980; Gutiérrez et al., 1991).
The subcellular sites of chromosomal DNA replication in B.subtilis have been reported recently (Lemon and Grossman, 1998). Replication proteins localized to fixed positions, probably associated with the membrane, at mid-cell during a single round of replication, and at additional sites at one quarter and three quarters of the cell during dichotomous replication. Thus, it seems that DNA feeds through a fixed replication factory, rather than DNA polymerase moving around a relatively static chromosome. We wished to determine whether phage φ29 DNA replication was associated with these host cell replication factories, as they could be sites where nucleotide precursors are particularly concentrated, or if it occurred in a completely separate region(s) of the cell. Evidence has been obtained that phage φ29 DNA replication is associated with the cell membrane (Ivarie and Pène, 1973; McGuire et al., 1974, 1977; Bravo and Salas, 1997, 1998; W.J.J.Meijer, A.Serna-Rico and M.Salas, submitted), as is host cell DNA replication.
Here we show that phage φ29 DNA replication occurs at sites different from the host cell replication factories, and undergoes several redistributions at different stages of the infection cycle. At the onset of replication, φ29 DNA nearly always localized as a single focus towards one end of the host cell nucleoid. Later on, the DNA was redistributed to multiple sites on the host cell chromosome–membrane interface, so that phage replication appears to be restricted to a zone surrounding the nucleoid. At late infection times, phage genomes undergo a further redistribution to the region of the cell occupied by the host cell chromosome.
The phage-encoded gene 16.7, which is expressed early after infection, encodes a membrane-localized DNA-binding protein. Redistribution of phage DNA replication surrounding the nucleoid was severely delayed in cells infected with a sus16.7 mutant. The possible role of protein p16.7 in φ29 DNA replication is discussed.
Results
To simplify the interpretation of the results, we have divided the 45 min φ29 infection cycle into three separate stages. During the early stage (0–15 min after infection), transcription of early genes occurs (Figure 1A; Monsalve et al., 1995), proteins are synthesized in preparation for DNA replication, and the first rounds of DNA replication are initiated (Ivarie and Pène, 1973; Jiménez et al., 1977). The middle stage (15–30 min after infection) involves the rapid amplification of the φ29 genome (Ivarie and Pène, 1973; Jiménez et al., 1977). In addition, the (near) complete repression of the early operon located at the right side of the phage genome and the beginning of late gene transcription also occur (Monsalve et al., 1995). The late stage (30–45 min) involves maximal late gene transcription and late protein synthesis, phage packaging and, ultimately, cell lysis (Monsalve et al., 1995).
Unless stated otherwise, the mutant phage φ29 sus14(1242) (Jiménez et al., 1977) was used. The DNA of this phage contains a suppressor-sensitive mutation in gene 14 that encodes the holin protein (Steiner et al., 1993). As a consequence, cell lysis is delayed, which allowed us to examine protein and DNA localization at late infection times. The mutation has no effect on phage DNA replication or phage morphogenesis.
Visualization of total DNA in φ29-infected cells
Prior to lysis, an infected cell contains ∼600–800 copies of the φ29 genome (Schachtele et al., 1970; Jiménez et al., 1977), which corresponds to ∼3–4 B.subtilis genome equivalents. This substantial amount of DNA should be readily detectable within infected cells using the fluorescent DNA stain 4′,6-diamidino-2-phenylindole (DAPI). Therefore, if φ29 DNA replication is compartmentalized within the host cell, we might expect to observe the phage DNA upon DAPI staining.
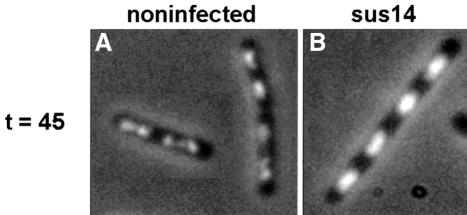
A logarithmically growing B.subtilis 110NA (Materials and methods) culture was divided into two aliquots, one of which was infected with φ29 and the other served as negative control. Samples were withdrawn at various times, fixed, stained with DAPI and analysed by fluorescence microscopy. Comparison of infected and non-infected cells did not reveal any readily observable differences in DAPI staining patterns for samples analysed at the early stage of infection (results not shown). However, the total DNA content and morphology of middle stage and especially those of late stage cells were strikingly different from those of non-infected cells. Figure 2 shows an overlay of phase contrast and DAPI images of non-infected (Figure 2A) and late infection stage cells (Figure 2B). The intensity of the DAPI stain was considerably higher in the infected cells, consistent with the amplified phage genomes contributing the majority of DNA at this stage of the infection cycle. To confirm that the stronger DAPI signals observed in the infected cells were due to accumulated φ29 DNA, this experiment was repeated using a low multiplicity of infection (m.o.i.; <1 phage per cell) so that a mixture of infected and non-infected cells was present in the same sample. As expected, a mixture of cells with either normal or high DNA contents, reflecting non-infected and infected cells, respectively, was observed (data not shown).
Fig. 2. Localization of DNA in non-infected (A) and φ29 sus14(1242) infected (B) B.subtilis cells using DAPI staining. At t = 0, a logarithmically growing B.subtilis culture was split into two aliquots. One of these was infected with phage φ29 sus14(1242) and the other one served as mock-infected control. Samples of both cultures, withdrawn at t = 45 min, were fixed, treated with lysozyme, and the cellular positions of the DNA were determined by fluorescence microscopy in the presence of DAPI. Overlays of phase contrast and DAPI-captured images of non-infected (A) and infected (B) cells are shown.
In addition to a considerable increase in DNA content in late infected cells, a striking difference in DNA morphology was also observed. The DNA of many of the non-infected cells had the characteristic ‘dumb-bell’ appearance of exponentially growing cells (Figure 2A; Hauser and Errington, 1995; Sharpe et al., 1998). However, whilst the distribution of DNA stain appeared normal in the late infected cells, the DNA had a more regular morphology, and was rectangular rather than dumb-bell shaped (Figure 2B). These results suggest that φ29 DNA replication occurs at the region of the cell occupied by the nucleoid rather than throughout the cell cytoplasm. If this is the case, it explains why no additional DAPI signals were observed in early stage infected cell samples, because the low amounts of φ29-related DAPI signal at these infection times would be masked by the relatively strong DAPI signal from the host cell chromosome.
Immunolocalization of TP in infected cells
Since it was not possible to identify the sites of phage DNA replication using DAPI, we examined the localization of the phage-encoded TP. Linear φ29 DNA molecules contain a TP molecule covalently linked at each DNA 5′ end (Ito, 1978; Salas et al., 1978; Yehle, 1978). There fore, the localization of phage DNA can be determined indirectly by studying the cellular distribution of TP throughout the infection cycle.
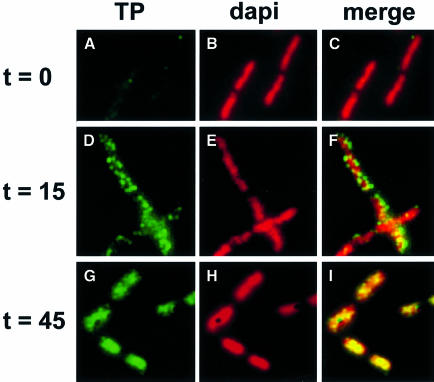
Bacillus subtilis 110NA cells were infected with φ29 and, at different times after infection, samples were withdrawn, fixed and analysed by immunofluorescence (IF) using affinity-purified polyclonal antibodies raised against TP. Representative images of immunostained cells are shown in Figure 3. In non-infected cells, hardly any fluorescent signal derived from the TP-specific antibodies was obtained (Figure 3A and C), showing a high specificity of the purified antibodies against TP. It was not possible to detect TP in cells at the very early stages of infection, presumably due to the very low levels of TP in cells prior to the amplification of DNA during the replication cycle. However, during the middle stage of infection, after the onset of φ29 DNA replication (see below; Ivarie and Pène, 1973; McGuire et al., 1974), a punctate fluorescent signal was observed that appeared to localize around the host cell chromosome (Figure 3D and F). By the late infection stage, the TP-specific signals appeared to fill the volume occupied by the DNA (Figure 3G and I). These results suggest that TP–DNA complexes are localized around the periphery of the host cell chromosome during the middle stage of infection and come to occupy the central part of the cell at late infection times.
Fig. 3. Localization of TP during the φ29 infection cycle. At the indicated times after infection of B.subtilis 110NA cells with phage φ29 sus14(1242), aliquots were harvested, fixed and analysed by immunofluorescence using affinity-purified antibodies against TP. FITC immunofluorescence images (A, D and G) are shown in the left frames, corresponding DAPI images are shown in the middle frames (B, E and H) and combined images of both signals are shown in the right frames (C, F and I).
Redistribution of φ29 DNA replication between the early and middle stages of infection
Immunolocalization of TP suggested that φ29 DNA replication takes place at sites peripheral to the bacterial nucleoid. However, IF would not distinguish between TP molecules covalently linked to the φ29 DNA ends and free TP and/or heterodimers of TP and φ29 DNA polymerase that will be present in infected cells during φ29 DNA replication. Neither would it allow us to determine where the very first rounds of phage DNA replication occur. Sites of DNA replication can be detected directly by IF using incorporation of the thymine analogue 5-bromodeoxyuridine (BrdU) (Lewis and Errington, 1997). To prevent incorporation of BrdU into the host cell chromosome, these experiments were carried out in the presence of 6-(p-hydroxyphenylazo)-uracil (HpUra), a selective inhibitor of DNA polymerase III holoenzyme in Gram-positive bacteria (Brown, 1970). Incorporation of BrdU into the host cell chromosome was abolished when 75 µM HpUra was added to cultures at least 2 min before BrdU (results not shown).
To visualize the BrdU incorporated into double-stranded DNA, an acid denaturation procedure is required (Materials and methods). This treatment also prevents visualization of DNA with DAPI. However, we found that after processing samples for microscopy, the nucleoids could be observed as dark structures within cells in phase contrast images (black arrows; Figure 4A). Therefore, images of the BrdU signals were combined with the corresponding phase contrast images to determine the subcellular localization of phage DNA replication.
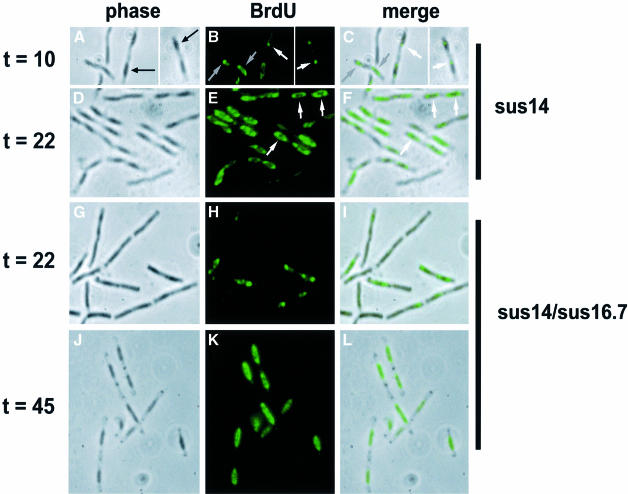
Fig. 4. Localization of φ29 DNA in B.subtilis cells infected with sus14(1242) (A–F) or sus14(1242)/sus16.7(48) (G–L) phage. HpUra was administered to logarithmically growing B.subtilis 110NA cultures 2 min before BrdU addition. Cells were harvested 10 min (A–C), 22 min (D–I) or 45 min (J–L) after infection. After fixation and acid treatment (15 min with 4 M HCl), samples were analysed by immunofluorescence using monoclonal antibodies against BrdU. Phase contrast images (left frames; A, D, G and J), corresponding FITC immunofluorescence images (middle frames; B, E, H and K) and overlays of both images (right frames; C, F, I and L) are shown. The black arrows in (A) indicate a nucleoid observed as a dark structure in the phase contrast image. Single BrdU foci per nucleoid in early stage infected cells in (B) and (C) are indicated by white arrows. Grey arrows in (B) and (C) indicate additional phage DNA sometimes observed in early stage infected cells. White arrows in (E) and (F) point to the ring-like BrdU signals seen in middle stage infected cells.
Cells at the early stage of infection, when the first rounds of phage DNA replication have just initiated, are shown in Figure 4A–C. Interestingly, the BrdU signal corresponding to incorporation into phage DNA was generally concentrated into a single focus per nucleoid (Figure 4B). These foci appeared to lie predominantly towards one end of the host cell nucleoid (white arrows; Figure 4B and C). In addition to the single bright focus, a faint smear of BrdU signal could also sometimes be seen over the rest of the host cell nucleoid, which may correspond to newly replicated copies of the phage DNA diffusing away from their sites of synthesis (grey arrows; Figure 4B and C). BrdU label incorporated into φ29 single-stranded DNA replication intermediates can be visualized without acid denaturation. Also under these conditions, the first BrdU signals detected in cells at the early stage of infection localized in >90% of the cases at the distal end of the bacterial nucleoid (results not shown). Together, these results strongly suggest that the first rounds of φ29 DNA replication occur at the distal end of the nucleoid.
During the middle stage of infection when DNA replication is at its maximum, the BrdU distribution in cells was strikingly different (Figure 4D–F). At this stage, BrdU signals were observed as ring-like structures surrounding the nucleoids (white arrows; Figure 4E and F). Thus, phage DNA replication appears to initiate at a specific location within the host cell and then become distributed to a zone that surrounds the host cell nucleoid. This is strikingly different from the localization of host cell DNA replication factories, suggesting that phage DNA replication is not dependent on host cell auxiliary proteins that may be associated with replication factories.
The results presented above indicated that in vivo the majority of φ29 DNA replication occurs in a zone surrounding the host cell nucleoid. Results obtained in several studies have indicated that φ29 DNA replication occurs at the membrane of infected cells (Ivarie and Pène, 1973; McGuire et al., 1977; Bravo and Salas, 1997, 1998). It has been proposed that the 15.2 kDa φ29-encoded protein p16.7 may be involved in the membrane localization of φ29 DNA replication (W.J.J.Meijer, A.Serna-Rico and M.Salas, submitted). Protein p16.7 is synthesized during the early stage of infection and so is present at the onset of φ29 DNA replication. It is an integral membrane protein containing an N-terminally located transmembrane domain that is required for membrane localization. In addition, purified p16.7 binds DNA in vitro. To test the proposed involvement of p16.7 in membrane localization of φ29 DNA replication, we analysed the distribution of replicating φ29 DNA molecules in B.subtilis cells that were infected with mutant phage φ29 containing a nonsense mutation in codon 48 of gene 16.7. Figure 4G–I shows the localization of BrdU incorporation into sus16.7 phage DNA at the middle stage of infection when ring-like BrdU patterns would be expected (see Figure 4D–F). Rather, BrdU foci, mostly located at the distal end of the nucleoid, were observed (Figure 4H and I), which bore a striking similarity to the pattern of phage DNA localization during the early stages of infection with wild-type phage (see Figure 4A–C). Ring-like BrdU patterns were observed eventually with the mutant, but not until later times after infection (∼45 min; Figure 4J–L).
Localization of p16.7 protein
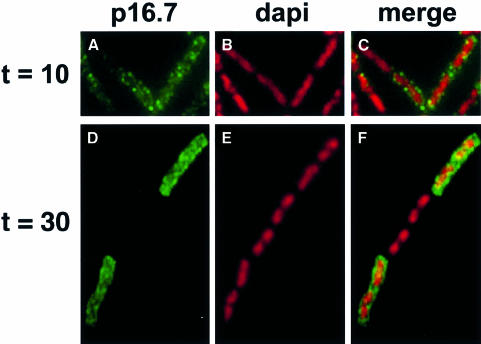
The delay in migration of DNA replication to the edge of the host cell chromosomes in the absence of p16.7 suggested it could have a role in spatial organization of phage replication. Although it has been shown that p16.7 is an integral membrane protein (W.J.J.Meijer, A.Serna-Rico and M.Salas, submitted), its distribution in infected cells was not known. Therefore, the localization of p16.7 in infected cells throughout the infection cycle was determined using affinity-purified polyclonal antibodies raised against p16.7. No fluorescent signal derived from the p16.7-specific antibodies could be detected in mock-infected cells (not shown). Protein p16.7 could be detected by western blot analysis during the early stage of infection (W.J.J.Meijer, unpublished results) and so samples were analysed from this point onwards through the infection cycle. During the early stage of infection, p16.7 localized in a punctate pattern around the periphery of infected cells (Figure 5A–C). Whilst this supported the idea that p16.7 is a membrane-associated protein, the localization was not coincident with that of phage DNA replication at this stage of the infection cycle (see above). During the middle stage of infection, much stronger signals for p16.7 were obtained around the edge of the cell (Figure 5D–F), consistent with the localization of the sites of phage DNA replication at this time point.
Fig. 5. Localization of protein p16.7 during the φ29 infection cycle. Aliquots of B.subtilis 110NA cells, harvested 10 min (A–C) or 30 min (D–F) after infection with sus14(1242) phage, were fixed and analysed by immunofluorescence using affinity-purified antibodies against p16.7. FITC immunofluorescence images (A and D) are shown in the left frames, corresponding DAPI images are shown in the middle frames (B and E) and combined images of both signals are shown in the right frames (C and F). Note that in the image shown at 30 min, the middle cell of the chain was probably not infected with φ29, so no p16.7 signal was detected.
Discussion
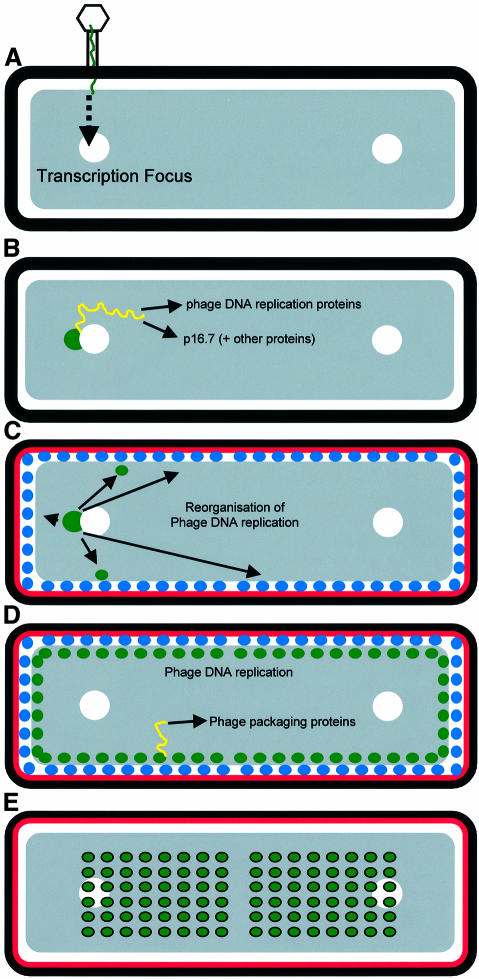
A schematic view of the cell biology of the φ29 DNA replication cycle, based on the results described above, is shown in Figure 6. Following infection (Figure 6A), the φ29 genome becomes localized at one end of the host cell nucleoid where the first rounds of φ29 DNA replication occur, following transcription of the early genes (Figure 6B). At the moment, we cannot say whether the incoming phage DNA is sequestered to a specific site but, if it is, there are two candidates for organized regions of the nucleoid into which phage molecules could be recruited. First, phage DNA could be recruited to SpoOJ–oriC nucleoprotein complexes. SpoOJ binds to several specific DNA sequences, which are clustered in a region of the chromosome that also contains the origin of replication (oriC; Lin and Grossman, 1998). During the cell cycle, this nucleoprotein complex, located at the distal end of the bacterial nucleoid, moves actively in the direction of the cell pole (Ireton et al., 1994; Glaser et al., 1997; Lewis and Errington, 1997; Lin et al., 1997; Webb et al., 1997). Interestingly, the SpoOJ-binding sequence is present five times in the φ29 genome (Murthy et al., 1998). In rapidly growing cells, there can be multiple ongoing rounds of chromosome replication. It seems possible that the SpoOJ system could be exploited in cells infected with more than one phage genome to segregate φ29 DNA molecules into daughter cells following cell division, which would result in greater phage proliferation. A second potential target site for incoming φ29 molecules would be the recently described transcription foci (TF) (Lewis et al., 2000), comprising sites where RNA polymerase (RNAP) becomes highly concentrated to subregions of the chromosome, where rRNA synthesis occurs. TF also co-localize, or at least overlap with SpoOJ–oriC foci, so another reason why φ29 DNA contains multiple SpoOJ-binding sites may be to recruit it to the sites of high RNAP concentration where early operon transcription could proceed most efficiently.
Fig. 6. Schematic representation of φ29 DNA during its infection cycle. See text for details. Transcription foci are indicated with white circles. Phage DNA and phage transcripts are indicated in green and yellow, respectively. p16.7 is indicated in red. Phage DNA replication sites are indicated with blue circles.
Once φ29 DNA replication is initiated at one end of the nucleoid, phage DNA replication spreads out to occupy multiple sites around the nucleoid periphery (Figure 6C and D). This may again be related to the spatial organization of the host cell because these sites have a relatively low RNAP concentration (compared with TF), which could minimize the potential for premature transcription of the late gene operons and also the risk of collisions between DNA polymerase and RNAP. The spreading of DNA replication sites was strongly delayed in the absence of p16.7 protein, implicating this protein in the spatial redistribution of replication sites. The dispersed membrane distribution of p16.7 and its capacity to bind DNA (W.J.J.Meijer, A.Serna-Rico and M.Salas, submitted) are consistent with it playing such a role. Although under the conditions tested p16.7 is not essential for phage viability, two lines of evidence suggest that p16.7 enhances the efficiency of phage replication. First, the level of BrdU signal in cells infected with sus16.7 mutant phage was lower compared with wild-type phage at early infection times. Secondly, the accumulation of φ29 DNA molecules was strongly delayed in cells infected with sus16.7 mutant phage compared with the wild-type phage (W.J.J.Meijer, A.Serna-Rico and M.Salas, submitted).
The delayed time at which sus16.7 DNA came to occupy multiple sites around the nucleoid periphery coincided approximately with the time at which the efficiency of mutant φ29 DNA replication became similar to that of the wild-type situation (W.J.J.Meijer, A.Serna-Rico and M.Salas, submitted). Whereas this may support further the view that p16.7 is involved in the spatial redistribution of phage DNA replication sites, it also suggests that its role is either partially dispensable or partially redundant. Genes located close to 16.7 in the early operon on the right side (Figure 1A) encode a number of proteins of unknown function. It is possible that one or more of these genes encode proteins that contribute to the organization of phage DNA replication. Another protein that may be involved in this process is the φ29-encoded protein p1. Gene 1 is located in the early expressed operon at the left side of the φ29 genome (see Figure 1A), and the majority of protein p1 is associated with the membranes of infected cells (Bravo and Salas, 1997). In addition, purified p1 protein is able to form protofilament sheets in vitro (Bravo and Salas, 1998). Interestingly, the rate of in vivo φ29 DNA synthesis is affected by p1 in a temperature-dependent way (Bravo and Salas, 1997, 1998). Based on these results, it was proposed that p1 is a component of a membrane-associated viral-encoded structure that could provide a scaffold for the assembly of the φ29 DNA replication machinery.
During the later stages of the replication cycle, phage DNA molecules began to accumulate within the bulk of the host cell nucleoid (Figure 6E), suggesting that (most of) the φ29 DNA molecules are released from the membrane during this stage of the infection cycle. Most probably, the nucleoid zone is where φ29 DNA molecules are packaged into virus particles, since the bulk of phage DNA late in infection coincided with the host nucleoid. Such a view would be in agreement with previous studies in which thin sections of infected cells, analysed by electron microscopy, showed mature phage particles mainly located centrally in infected cells, co-localizing with the nucleoid (Hagen et al., 1976; Jiménez et al., 1977).
At present, we do not know how many phage DNA replication sites are present in each cell. However, since phage DNA is amplified ∼800-fold during a 30–45 min period of the infection cycle, we believe that there are many sites, and that more than one round of replication may be ongoing at each site. Further support for this view is the observation that the amount of φ29 DNA polymerase increases from ∼100 to 1000 molecules during the infection cycle (Bravo and Salas, 1997). This situation would therefore be fundamentally different from replication of the host chromosome, which replicates very few distinct sites (Lemon and Grossman, 1998). Since each replication fork has two DNA polymerases, one for polymerization of the leading and the other polymerization of the lagging strand, only a few DNA polymerases would suffice for efficient replication of the B.subtilis chromosome. Indeed, a B.subtilis cell contains ∼40 PolC molecules (Kornberg and Baker, 1992).
In conclusion, phage φ29 DNA and associated components of its replication machinery undergo dynamic changes in distribution during the infection cycle, which may reflect the changing requirements for transcription factors (early), replication factors (middle) and packaging factors (late) during the infection cycle. Further application of cytological methods to phage-infected cells may provide important insights into hitherto unexplored aspects of the virus–host cell interaction.
Materials and methods
Bacterial strains, bacteriophages and growth conditions
Bacillus subtilis 110NA (trpC2, spoOA3, su–; Moreno et al., 1974) and MO-101-P (thr–, spoA–, su+44; Mellado et al., 1976b) were used as non-suppressor and suppressor strains, respectively. Cells were grown at 37°C in LB medium supplemented with 5 mM MgSO4. Logarithmically growing cells (OD600 between 0.4 and 0.5) were infected with phage φ29 at a m.o.i. of 2. Mutant φ29 phages sus14(1242) (Jiménez et al., 1977) or sus14(1242)/sus16.7(48) (W.J.J.Meijer, A.Serna-Rico and M.Salas, submitted) were used for infection. Phage stocks were prepared as described previously (Bravo and Salas, 1998) and phage titres were determined by plating on the B.subtilis suppressor strain. In labelling experiments, the thymine analogue BrdU (Sigma) was added to the growth medium at a final concentration of 300 µM. Incorporation of BrdU into chromosomal DNA of B.subtilis was inhibited by adding HpUra (Brown, 1970) to the growth medium, at a final concentration of 75 µM, 2 min before BrdU addition.
Immunofluorescence microscopy
Samples of cells were fixed and processed essentially as described (Lewis and Errington, 1997) with the following modifications. Samples were filtered as described (Lemon and Grossman, 1998). Blocking buffer contained, in addition to 2% (w/v) bovine serum albumin (BSA), 0.5% (w/v) casein (Sigma Chemical Co.). Affinity-purified rabbit polyclonal antibodies against TP and p16.7 were used at 1:200 dilutions and incubations were carried out either for 1 h at room temperature or overnight at 4°C. Polyclonal antibodies were centrifuged for 10 min at 14 000 g at 4°C before use to precipitate possible antibody aggregates. Monoclonal antibodies against BrdU (Boehringer Mannheim) were used at 1:1000 dilution and incubated for 1 h at room temperature. Fluorescein isothiocyanate (FITC)-conjugated sheep anti-rabbit antibodies were used at a dilution of 3:1000 and incubation carried out for 1 h at room temperature in the dark. All subsequent steps were performed with minimal exposure of the samples to light. For the immunodetection of BrdU, cells were incubated with 4 M HCl for 15 min at room temperature, then washed six times with phosphate-buffered saline (PBS) prior to incubation with anti-BrdU antibodies. All samples were mounted for epifluorescence microscopy in Vectashield antifade (Vector Laboratories, Burlingame, CA) supplemented with 0.2 µg/ml DAPI.
Microscopy, image acquisition and image analysis
Epifluorescence microscopy was performed as described by Lewis and Errington (1997). Image processing was carried out using IPLab Spectrum V3.1.1 (Signal Analytics, Vienna, VA). Final images were assembled in Adobe Photoshop V4.0 for printing.
Acknowledgments
Acknowledgements
We thank Alicia Bravo for critical reading of the manuscript. Work in the Errington laboratory was supported by grants from the Biotechnology and Biological Sciences Research Council (BBSRC). J.E. is the recipient of a Senior Research Fellowship of the BBSRC. Work in the Salas laboratory was supported by research grants 5R01 GM27242-20 from the National Institutes of Health, ERBFMX CT97 0125 from the European Economic Community, PB98-0645 from the Dirección General de Investigación Científica y Técnica, and an Institutional grant from Fundación Ramón Areces. W.J.J.M was supported by the Netherlands Organization for Scientific Research (NWO).
References
- Blanco L. and Salas,M. (1984) Characterization and purification of a phage φ29-encoded DNA polymerase required for the initiation of replication. Proc. Natl Acad. Sci. USA, 81, 5325–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo A. and Salas,M. (1997) Initiation of bacteriophage φ29 DNA replication in vivo: assembly of a membrane-associated multiprotein complex. J. Mol. Biol., 269, 102–112. [DOI] [PubMed] [Google Scholar]
- Bravo A. and Salas,M. (1998) Polymerization of bacteriophage φ29 replication protein p1 into protofilament sheets. EMBO J., 17, 6096–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N.C. (1970) 6-(p-Hydroxyphenylazo)-uracil: a selective inhibitor of host DNA replication in phage-infected Bacillus subtilis. Proc. Natl Acad. Sci. USA, 67, 1454–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crucitti P., Lázaro,J.M., Benes,V. and Salas,M. (1998) Bacteriophage φ29 early protein p17 is conditionally required for the first rounds of viral DNA replication. Gene, 223, 135–142. [DOI] [PubMed] [Google Scholar]
- Garvey K.J., Yoshikawa,H. and Ito,J. (1985) The complete sequence of the Bacillus phage φ29 right early region. Gene, 40, 301–309. [DOI] [PubMed] [Google Scholar]
- Glaser P., Sharpe,M.E., Raether,B., Perego,M., Ohlsen,K. and Errington,J. (1997) Dynamic, mitotic-like behavior of a bacterial protein required for accurate chromosome partitioning. Genes Dev., 11, 1160–1168. [DOI] [PubMed] [Google Scholar]
- Gutiérrez C., Sogo,J.M. and Salas,M. (1991) Analysis of replicative intermediates produced during bacteriophage φ29 DNA replication in vitro. J. Mol. Biol., 222, 983–994. [DOI] [PubMed] [Google Scholar]
- Hagen E.W., Reilly,B.E., Tosi,M.E. and Anderson,D.L. (1976) Analysis of gene function of bacteriophage φ29 of Bacillus subtilis: identification of cistrons essential for viral assembly. J. Virol., 19, 501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser P.M. and Errington,J. (1995) Characterization of cell cycle events during the onset of sporulation in Bacillus subtilis. J. Bacteriol., 177, 3923–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoso J.M., Méndez,E., Soriano,F. and Salas,M. (1985) Location of the serine residue involved in the linkage between the terminal protein and the DNA of φ29. Nucleic Acids Res., 13, 7715–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciarte M.R., Salas,M. and Sogo,J.M. (1980) The structure of replicating DNA molecules of Bacillus subtilis phage φ29. J. Virol., 34, 187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K., Gunther,N.W. and Grossman,A.D. (1994) spoOJ is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J. Bacteriol., 176, 5320–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J. (1978) Bacteriophage φ29 terminal protein: its association with the 5′ termini of the φ29 genome. J. Virol., 28, 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarie R.D. and Pène,J.J. (1973) DNA replication in bacteriophage φ29: the requirement of a viral-specific product for association of φ29 DNA with the cell membrane of Bacillus amyloliquefaciens. Virology, 52, 351–362. [DOI] [PubMed] [Google Scholar]
- Jiménez F., Camacho,A., de la Torre,J., Viñuela,E. and Salas,M. (1977) Assembly of Bacillus subtilis phage φ29. 2. Mutants in the cistrons coding for the non-structural proteins. Eur. J. Biochem., 73, 57–72. [DOI] [PubMed] [Google Scholar]
- Kornberg A. and Baker,T.A. (1992) DNA Replication. W.H.Freeman and Co., New York. [Google Scholar]
- Lemon K.P. and Grossman,A.D. (1998) Localization of bacterial DNA polymerase: evidence for a factory model of replication. Science, 282, 1516–1519. [DOI] [PubMed] [Google Scholar]
- Lewis P.J. and Errington,J. (1997) Direct evidence for active segregation of oriC regions of the Bacillus subtilis chromosome and co-localization with the SpoOJ partitioning protein. Mol. Microbiol., 25, 945–954. [DOI] [PubMed] [Google Scholar]
- Lewis P.J., Thaker,S.D. and Errington,J. (2000) Compartmentalization of transcription and translation in Bacillus subtilis. EMBO J., 19, 710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D.C.H. and Grossman,A.D. (1998) Identification and character ization of a bacterial chromosome partitioning site. Cell, 92, 675–685. [DOI] [PubMed] [Google Scholar]
- Lin D.C.H., Levin,P.A. and Grossman,A.D. (1997) Bipolar localization of a chromosome partition protein in Bacillus subtilis. Proc. Natl Acad. Sci. USA, 94, 4721–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J.C., Pène,J.J. and Barrow-Carraway,J. (1974) Gene expression during the development of bacteriophage φ29. III. Analysis of viral-specific protein synthesis with suppressible mutants. J. Virol., 13, 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J.C., Gilpatrick,M.W. and Pène,J.J. (1977) DNA replication of bacteriophage φ29: effect of two viral genes on the association of phage chromosomes with the host cell membrane. Virology, 78, 234–240. [DOI] [PubMed] [Google Scholar]
- Mellado R.P., Moreno,F., Viñuela,E., Salas,M., Reilly,B.E. and Anderson,D.L. (1976a) Genetic analysis of bacteriophage φ29 of Bacillus subtilis: integration and mapping of reference mutants of two collections. J. Virol., 19, 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado R.P., Viñuela,E. and Salas,M. (1976b) Isolation of a strong suppressor of nonsense mutations in Bacillus subtilis. Eur. J. Biochem., 65, 213–223. [DOI] [PubMed] [Google Scholar]
- Méndez J., Blanco,L. and Salas,M. (1997) Protein-primed DNA replication: a transition between two modes of priming by a unique DNA polymerase. EMBO J., 16, 2519–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsalve M., Mencía,M., Rojo,F. and Salas,M. (1995) Transcription regulation in Bacillus subtilis phage φ29: expression of the viral promoters throughout the infection cycle. Virology, 207, 23–31. [DOI] [PubMed] [Google Scholar]
- Moreno F., Camacho,A., Viñuela,E. and Salas,M. (1974) Suppressor-sensitive mutants and genetic map of Bacillus subtilis bacteriophage φ29. Virology, 62, 1–16. [DOI] [PubMed] [Google Scholar]
- Murthy V., Meijer,W.J.J., Blanco,L. and Salas,M. (1998) DNA polymerase template switching at specific sites on the φ29 genome causes the in vivo accumulation of subgenomic φ29 DNA molecules. Mol. Microbiol., 29, 787–798. [DOI] [PubMed] [Google Scholar]
- Rojo F., Mencía,M., Monsalve,M. and Salas,M. (1998) Transcription activation and repression by interaction of a regulator with the α subunit of RNA polymerase: the model of phage φ29 protein p4. Prog. Nucleic Acid Res. Mol. Biol., 60, 29–46. [DOI] [PubMed] [Google Scholar]
- Salas M. (1991) Protein-priming of DNA replication. Annu. Rev. Biochem., 60, 39–71. [DOI] [PubMed] [Google Scholar]
- Salas M. and Rojo,F. (1993) Replication and transcription of bacteriophage φ29. In Sonenshein,A.L., Hoch,J.A. and Losick,R. (eds), Bacillus subtilis and Other Gram-positive Bacteria: Biochemistry, Physiology, and Molecular Genetics. American Society for Microbiology, Washington, DC, pp. 843–858. [Google Scholar]
- Salas M., Mellado,R.P., Viñuela,E. and Sogo,J.M. (1978) Characterization of a protein covalently linked to the 5′ termini of the DNA of Bacillus subtilis phage φ29. J. Mol. Biol., 119, 269–291. [DOI] [PubMed] [Google Scholar]
- Salas M., Miller,J.T., Leis,J. and DePamphilis,M.L. (1996) Mechanisms for priming DNA synthesis. In DePamphilis,M.L. (ed.), DNA Replication in Eukaryotic Cells. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 131–176. [Google Scholar]
- Schachtele C.F., Orman,R.W. and Anderson,D.L. (1970) Effect of elevated temperature on deoxyribonucleic acid synthesis in bacteriophage φ29-infected Bacillus amyloliquefaciens. J. Virol., 6, 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe M.E., Hauser,P.M., Sharpe,R.G. and Errington,J. (1998) Bacillus subtilis cell cycle as studied by fluorescence microscopy: constancy of cell length at initiation of DNA replication and evidence for active nucleoid partitioning. J. Bacteriol., 180, 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M., Lubitz,W. and Bläsi,U. (1993) The missing link in phage lysis of Gram-positive bacteria: gene 14 of Bacillus subtilis phage φ29 encodes the functional homolog of lambda S protein. J. Bacteriol., 175, 1038–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlcek C. and Paces,V. (1986) Nucleotide sequence of the late region of Bacillus phage φ29 completes the 19285-bp sequence of φ29. Comparison with the homologous sequence of phage PZA. Gene, 46, 215–225. [DOI] [PubMed] [Google Scholar]
- Webb C.D., Teleman,A., Gordon,S., Straight,A., Belmont,A., Lin,D.C.H., Grossman,A.D., Wright,A. and Losick,R. (1997) Bipolar localization of the replication origin regions of chromosomes in vegetative and sporulating cells of B.subtilis. Cell, 88, 667–674. [DOI] [PubMed] [Google Scholar]
- Yehle C.O. (1978) Genome-linked protein associated with the 5′ termini of bacteriophage φ29 DNA. J. Virol., 27, 776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H. and Ito,J. (1982) Nucleotide sequence of the major early region of bacteriophage φ29. Gene, 17, 323–335. [DOI] [PubMed] [Google Scholar]