Summary
Scleroderma is a multisystem disease characterized by a severe inflammatory process and exuberant fibrosis. Lung involvement is a frequent complication and a leading cause of morbidity and mortality in this syndrome. Two major pulmonary syndromes are associated with scleroderma; a pulmonary vascular disorder evolving over time into relatively isolated pulmonary arterial hypertension (PAH), and interstitial lung disease (ILD). Each syndrome, when present, is a cause of morbidity and significantly reduces survival of scleroderma patients when compared to patients free of lung complication. When pulmonary hypertension and ILD are combined, survival is further reduced. Current therapy appears to have no meaningful effect on either condition and, thus, there is a need for better understanding of underlying pathogenic mechanisms. This review focuses on clinical, diagnostic, and therapeutic features of PAH and ILD as well as other frequent but less debilitating lung complications of scleroderma.
Scleroderma or systemic sclerosis (SSc) is a heterogeneous disorder characterized by endothelial dysfunction, dysregulation of fibroblasts resulting in excessive production of collagen, and profound abnormalities of the immune system1. These changes cause progressive fibrosis of the skin and internal organs, system failure and death. While the etiology of SSc is generally unknown, genetic and environmental factors are thought to contribute to host susceptibility2. SSc, whether presenting in the limited or diffuse form, is a systemic disease with the potential for multiple organ system involvement including the gastrointestinal, cardiac, renal, and pulmonary systems3. Pulmonary manifestations of SSc include, but are not limited to, pulmonary vascular diseases such as pulmonary arterial hypertension (PAH) and pulmonary veno-occlusive disease (PVOD), interstitial lung disease (ILD), and increased susceptibility to lung neoplasms. The emphasis of this review will be on ILD and PAH since these two syndromes are by far the most common lung manifestations and leading causes of death in SSc.
1 Incidence and Prevalence of Systemic Sclerosis
Estimates of incidence and prevalence of systemic sclerosis have varied widely by the period of observation, disease definition, and population under study4. Although the incidence of SSc seemed to be increasing in the middle part of the last century, the rate of occurrence has stabilized after the widespread use of a standard classification system5. However, there continues to be marked geographic variation in the occurrence of the disease, supporting a role for environmental factors in disease pathogenesis. Prevalence of SSc ranges from 30-70 cases per million in Europe and Japan6-8 to ~240 cases per million in the United States (US) 5. Incidence varies similarly by geographic area, with the highest rates found in the US (~19 persons per million per year)4.
2 Pulmonary Vascular Involvement
2.1 Scleroderma-Associated Pulmonary Arterial Hypertension
Pulmonary hypertension, defined as a mean pulmonary arterial pressure greater than 25 mm Hg, is a cause of significant morbidity and mortality9-11 and can be isolated in SSc, occurring as PAH (SSc-associated PAH or SSc-PAH), or in combination with ILD [pulmonary hypertension (PH)-ILD]. It has become clearer over the past few years that these two entities (SSc-PAH and PH-ILD) carry a very different prognosis in patients with SSc and will, therefore, be reviewed separately.
PAH, hemodynamically defined as mean pulmonary arterial pressure greater than 25 mmHg with a pulmonary capillary wedge pressure equal or less than 15 mmHg12, is characterized by increased pulmonary vascular resistance due to remodeling and occlusion of the pulmonary arterioles. Left untreated, PAH leads irremediably to right ventricular (RV) hypertrophy, pressure overload and dilation resulting in death, generally from RV failure, within 2-3 years of the initial diagnosis9. It has been increasingly appreciated that the integrity of the RV function, rather than the degree of pulmonary vascular injury, is the major determinant of symptoms and mortality in patients with PAH9, 13. In fact, RV dysfunction at time of presentation, as reflected by an elevation in right atrial pressure, the presence of pericardial effusion or depressed cardiac output, is by far the best prognosticator of death9.
2.1.1 Prevalence of Scleroderma-Associated Pulmonary Arterial Hypertension
Estimates of the prevalence of PAH in patients with SSc have varied widely based on the definition of pulmonary hypertension and the method of obtaining the measurements (i.e., echocardiography or cardiac catheterization). Using echocardiography as the diagnostic tool, prevalence of pulmonary hypertension in SSc have been overestimated, ranging between 35-49%14, 15. For example, in a study of 709 consecutive patients who underwent echocardiography, the prevalence of pulmonary hypertension (defined as RV systolic pressure > 35 mm Hg) was 38%16. However, relying on cardiac catheterization as gold standard for diagnosis, more recent prospective studies have shown that the prevalence of pulmonary hypertension is indeed between 8 and 12%17, 18, a more conservative and accurate figure regarding this complication. In all patients with SSc, PAH significantly worsens survival and is, after ILD, the leading cause of mortality in these patients5, 19, 20. Assuming a conservative 10% estimate of PAH prevalence among patients with SSc in the United States, SSc-PAH may affect as many as 24 individuals per million and thus be more common than other forms of PAH in the World Health Organization (WHO) group 1, including IPAH10. Yet in a large French PAH registry, connective tissue disease ( mainly represented by SSc) accounts for only 15% of PAH cases (as opposed to 40% for IPAH) referred to pulmonary vascular centers21, suggesting that patients with SSc-PAH may be underdiagnosed or referral to specialized centers for diagnosis and treatment may be limited. In a prospective multicenter study, also from France, in which SSc patients with no evidence of PAH at baseline were followed for a mean duration of 41 ± 5 months, the incidence of PAH was estimated at 0.61 cases per 100-patient-years18.
2.1.2 Clinical features
Risk factors for the development of PAH in SSc patients have traditionally included late-onset disease16 although a recent study suggests that early-onset PAH occurs in approximately half of SSc patients22. Additional risk factors include an isolated reduction in Diffusing lung capacity to carbon monoxide (DLCO), an Forced vital capacity (FVC)/DLCO ratio greater than 1.623, 24, or a combined decreased DLCO/alveolar volume with elevation of serum N-terminal pro-brain natriuretic peptide (N-TproBNP) levels25, and female gender23 with post-menopausal onset of disease26 (Box 1). Other factors include the severity24 and duration27 of Raynaud phenomenon, digital ulceration24, 28, multiple telangiectasiae28, 29, and reduced nailfold capillary density28, 30.
Box 1: Risk Factors for Development of Pulmonary Arterial Hypertension in Systemic Sclerosis.
Typically, patients with SSc-PAH are predominantly women, have limited SSc, are older and have seemingly less severe hemodynamic impairment compared to IPAH patients31. Like in IPAH, clinical symptoms are non specific, including dyspnea, and functional limitation which may be more severe than in IPAH due not only to older age but also to frequent involvement of the musculoskeletal system in these patients. SSc-PAH patients also tend to have other organ involvement such as renal dysfunction and intrinsic heart disease. Indeed patients with SSc (even in the absence of PAH) tend to have depressed RV function32, 33 and left ventricular systolic as well as diastolic dysfunction34. Like IPAH patients, SSc-PAH patients have severe RV dysfunction at time of presentation but have more severely depressed RV contractility compared to IPAH patients35. In addition, SSc-PAH patients tend to have more commonly LV diastolic dysfunction and a high prevalence of pericardial effusion (34% compared to 13% for IPAH)31. In both groups, pericardial effusion portends a particularly poor prognosis31. SSc-PAH patients also tend to have more severe hormonal and metabolic dysfunction such as high levels of N-TproBNP36, 37 and hyponatremia38. Both N-TproBNP and hyponatremia have been shown at baseline36-38, and with serial changes (for N-TproBNP36), to correlate with survival in PAH.
2.1.3 Early Detection
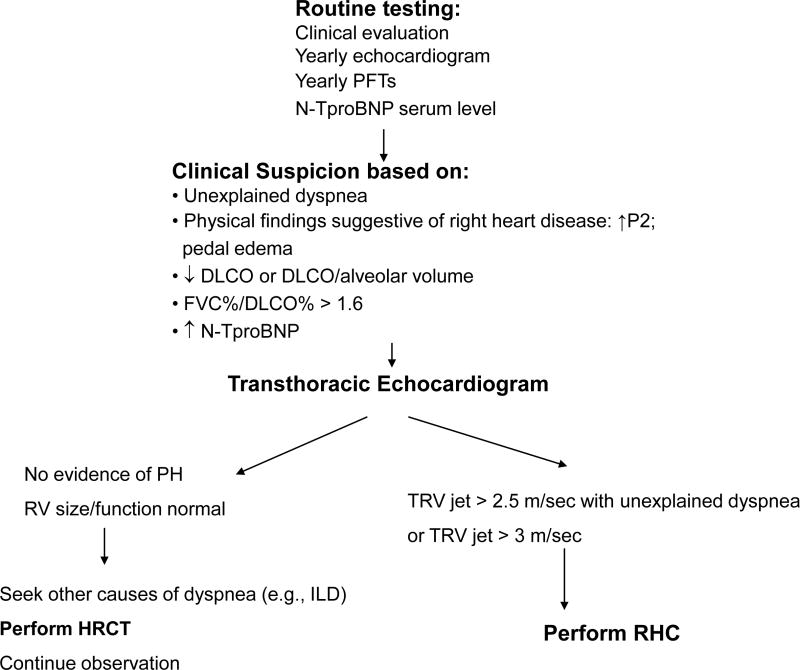
An algorithm for detection of PAH in patients with SSc may be helpful if based on a combination of symptoms and screening echocardiography (Figure 1). In a large French study, patients with SSc with tricuspid regurgitation velocity (TRV) jet by transthoracic echocardiography greater than 3 m/sec, or between 2.5 and 3 m/sec if accompanied by unexplained dyspnea, were systematically referred for right heart catheterization18. This approach allowed to detect incident cases of SSc-PAH with less severe disease (as judged on hemodynamic data) compared to patients with known disease. Therefore, unexplained dyspnea should prompt a search for PAH in these patients, in particular in the setting of a low single breath DLCO or declining DLCO over time24, echocardiographic findings suggestive of the disease (elevated TRV jet or dilated RV or atrium), or elevated levels of N-TproBNP which can reflect cardiac dysfunction and have been found to predict the presence of SSc-PAH36. Thus, systematic screening might allow detection of early disease and prompt therapy which is theoretically beneficial from a prognostic standpoint39.
Figure 1. Algorithm for Detection of PAH in Patients with Scleroderma.
Proposed algorithm for performance of routine clinical tests in patients with scleroderma which may allow early detection of pulmonary arterial hypertension or other causes of functional impairment such as cardiac dysfunction (e.g., left ventricular dysfunction) or parenchymal disease (e.g., IPF).
(DLCO: single breath diffusing capacity to carbon monoxide; FVC: forced vital capacity; HRCT-high resolution computed tomography; ILD: Interstitial lung disease; PFTs: Pulmonary function tests;;; RHC: right heart catheterization; RV: right ventricle; TRV: tricuspid regurgitation jet; ).
2.1.4 The Problem of Pulmonary Veno-Occlusive Disease in SSc-PAH
Pulmonary venoocclusive disease (PVOD) is a syndrome characterized by intimal proliferation and fibrosis of the intrapulmonary veins and venules in addition to some involvement of the arteriolar bed40. A definite diagnosis is usually obtained by biopsy of the lung41, which is however risky in PAH. Alternatively, the diagnosis can be suggested clinically. PVOD appears to be an underrecognized entity in SSc-PAH41, which poses a conundrum considering the known risk of pulmonary edema with the use of vasodilators in this condition41. Compared with PAH, PVOD affects more male than female patients, and is characterized by higher prevalence of tobacco abuse, lower DLCO, lower arterial oxygen tension at rest and more pronounced oxygen desaturation with exercise. High-resolution computed tomography of the chest (HRCT) typically demonstrates centrilobular groundglass opacities, septal lines, and lymph node enlargement41. Two recent histological studies have underlined the more frequent involvement of pulmonary veins than previously recognized in SSc-PAH, perhaps explaining in part why these patients are often refractory to specific PAH treatment as compared with IPAH patients40, 42 and inclined to develop pulmonary edema in response to vasodilators. However, a recent retrospective analysis indicates that epoprostenol, if given at small incremental levels along with high dose diuretics, can improve clinical (e.g., functional class) and hemodynamic (e.g., cardiac index and pulmonary vascular resistance) parameters and might be a reasonable bridge to urgent lung transplantation for PVOD patients43. Ultimately, lung transplantation remains the most suitable alternative for PVOD and should be promptly considered once the diagnosis is firmly established.
2.1.5 Therapy for SSc-PAH
Recognizing that one-year survival rates for SSc-PAH patients range from 50-87%17, 19, 31, 44-46, which are considerably lower than the 88% one year survival for IPAH patients47, the current challenge for the PAH community is to develop improved therapies specifically targeted for these patients.
Evidence of chronically impaired endothelial function48-50, affecting vascular tone and remodeling, has been the basis for current therapy of PAH. Vasodilator therapy using high dose calcium channel blockers is an effective long-term therapy51, but only for a minority of patients (e.g., less than 7%52 of IPAH patients) who demonstrate acute vasodilation (e.g., to NO or adenosine) during hemodynamic testing, and an even smaller number of patients with SSc-PAH. Indeed, the vast majority of SSc-PAH patients fail to show a vasodilator response to acute testing21. Therefore, high dose calcium channel therapy is usually not indicated for patients with SSc-PAH although most patients often receive these drugs at low dosage, typically for Raynaud’s syndrome.
2.1.5.1 Anti-inflammatory drugs
It has been increasingly recognized that inflammation may play a significant role in various types of pulmonary hypertension53, including IPAH and PAH associated with connective tissue diseases (CTD, including SSc) and human immunodeficiency virus infection. Interestingly, occasional patients with severe PAH associated with some forms of CTD (such as systemic lupus erythematosus, primary Sjögren syndrome, and mixed CTD) have had dramatic improvement of their pulmonary vascular disease with corticosteroids and/or immunosuppressive therapy54, emphasizing the relevance of inflammation in these subsets of patients. However, this type of dramatic response is generally not observed in patients with SSc-PAH whose disease is usually quite refractory to immunosuppressive drugs54.
2.1.5.2 Prostaglandins
Prostacyclin (e.g., epoprostenol) has potent pulmonary vasodilator but also anti-platelet aggregating and antiproliferative properties55, and has proven effective in improving the exercise capacity, cardiopulmonary hemodynamics, WHO functional class (WHO FC), symptoms, as well as survival in patients with PAH when given by continuous infusion56-58. Although there have been no randomized trials using this agent to assess long-term effect on survival, analysis of cohorts of patients on continuous intravenous epoprostenol compared with historical control groups (a questionable comparison for many obvious reasons) demonstrated clear benefits in survival in patients with WHO FC III and IV47, 59. Initially proposed as a bridge to lung transplantation, intravenous epoprostenol is now considered as first-line therapy in severe PAH (WHO FC IV) and in some patients an alternative to lung transplantation60.
Treprostinil, a prostacyclin analogue suitable for continuous subcutaneous administration, has been shown to have modest effects on symptoms and hemodynamics in PAH61. In a small study of 16 patients (among whom 6 had CTD related PAH), intravenous treprostinil was shown to improve hemodynamics 6 minute walk distance (6MWD), FC, and hemodynamics after 12 weeks of therapy62. Although the safety profile of this drug is similar to IV epoprostenol, required maintenance doses are usually twice as high compared to epoprostenol. However, for patients with SSc-PAH, the lack of requirement for ice packing and less frequent mixing of the drug offer significant advantages considering these patients’ sensitivity to cold exposure. In summary, both epoprostenol and treprostinil are FDA approved for PAH, but are cumbersome therapies requiring continuous parenteral administration with potential numerous adverse and severe side effects (e.g., infection and possibility of pump failure63), which make these drugs less than ideal.
In SSc-PAH, continuous intravenous epoprostenol improves exercise capacity and hemodynamics64, compared to conventional therapy, however there has been no demonstrable effect on survival. Badesch et al recently demonstrated long-term survival (47% survival rate at 3 years) for SSc-PAH patients treated with intravenous epoprostenol65 suggesting that this is an adequate form of therapy for these patients, although the results are not significantly different form oral medical therapy for these patients44, 66. In addition, several reports of pulmonary edema in SSc-PAH patients treated with prostaglandin derivatives, both in acute and chronic settings, have raised the suspicion of increased prevalence of PVOD in these patients40, 67, 68, and concern about usefulness of these drugs for this entity. Furthermore, considering the frequent digital problems and disabilities that these patients often experience, this form of therapy can be quite challenging and may increase the already heavy burden of disease. Nevertheless, intravenous prostaglandin therapy remains a valuable therapeutic option for patients with SSc-PAH with WHO FC IV, and in FC III patients who demonstrate no improvement on oral therapy.
2.1.5.3 Endothelin receptor antagonists
Randomized, placebo-controlled trials of 12-16 weeks duration demonstrated a beneficial effect of bosentan therapy on functional class, 6MWD, time to clinical worsening and hemodynamics in PAH69, 70. In these studies, roughly one fifth of the population consisted of SSc-PAH patients while a large majority had a diagnosis of IPAH. A subgroup analysis, performed by Rubin et al, reported a non-significant trend towards a positive treatment effect on 6MWD among the SSc-PAH patients treated with bosentan compared to placebo70. At most, bosentan therapy prevented deterioration in these patients (as assessed by an increase of 3 m in the 6MWD in the treated group compared to a decrease of 40 m in the placebo group). This less than optimal effect of therapy in patients with SSc-PAH is unclear but may be related to the severity of PAH at time of presentation, as well as other factors such as, hypothetically, more severe RV and pulmonary vascular dysfunction, as compared to patients with other forms of PAH (e.g., IPAH).
In a recent analysis of patients with PAH associated with CTD (e.g., patients with lupus, overlap syndrome, and other rheumatological disorders) included in randomized clinical trials of bosentan, there was a trend toward improvement in 6MWD and improved survival compared to historical cohorts71. Our experience suggests that long-tem outcome of first-line bosentan monotherapy is inferior in SSc-PAH compared to IPAH patients, with no change in functional class and worse survival in the former group72.
In an effort to target the vasoconstrictive effects of endothelin while preserving its vasodilatory action, selective endothelin-A receptor antagonists have been developed. Sitaxsentan, which is only approved in Europe for treatment of PAH, improved exercise capacity (i.e., change in peak VO2 at week 12, which was the main end-point of the study)73. Elevation in liver enzymes were noted in 10% of patients at the higher dose tested (300 mgs orally once daily). Patients with PAH associated with CTD represented 24% of the study group. A post-hoc analysis of 42 patients (33 patients who received the drug and 9 patients who received placebo) with CTD-PAH demonstrated improved exercise capacity, quality of life, and hemodynamics with sitaxsentan although elevated liver enzymes were reported in 2 patients74. A large pacebo-controlled, randomized trial of ambrisentan, the only currently FDA-approved selective endothelin receptor antagonist, improved 6MWD in PAH patients at week 12 of treatment, however, the effect was larger in patients with IPAH compared to patients with CTD-PAH (range of 50-60 meters versus 15-23 meters, respectively)75. Ambrisentan is generally well tolerated although peripheral edema (in up to 20% of patients75) and congestive heart failure have been reported.
2.1.5.4 Phosphodiesterase inhibitors
Sildenafil, a phosphodiesterase type V inhibitor that reduces the catabolism of cGMP, thereby enhancing the cellular effects mediated by nitric oxide, has become a widely used and highly efficacious therapy for PAH. The SUPER trial showed that sildenafil therapy led to an improvement in the 6 MWD in patients with IPAH and PAH related to CTD or repaired congenital heart disease (patients were predominantly functional class II or III) at all three doses tested (20, 40, and 80 mgs, given three times a day). Since there were no significant differences in clinical effects and time to clinical worsening at week 12 between the doses, the FDA recommended a dose of 20 mgs three times a day. In a post-hoc subgroup analysis of 84 patients with PAH related to CTD (forty-five percent of whom had SSc-PAH), data from the SUPER study suggest that sildenafil at a dose of 20 mgs improved exercise capacity (6MWD), hemodynamic measures and functional class after 12 weeks of therapy76. However, for reasons that remain unclear (but in part related to the limitations of that study such as post-hoc subgroup analysis), there was no effect for the dose of 80 mgs three times a day on hemodynamics in this subgroup of patients with CTD-related PAH. For this reason and because of the potential of increased side-effects (such as bleeding from arterio-venous malformations common in these patients) at high doses, we have opted at our center for sildenafil dosage of 20 mgs three times a day for our SSc-PAH patients as standard therapy. Higher doses are occasionally attempted in case of limited response. At this time, because of its favorable safety profile, oral sildenafil is our drug of choice for first line oral therapy for SSc-PAH patients with FC II or III. The impact of long-term sildenafil therapy on survival in patients with SSc-PAH remains to be determined. Finally, tadalafil, another phosphodiesterase inhibitor, has now been shown to be effective for PAH77 although subgroup analysis has not been performed yet and thus its effects on SSc-PAH remain unclear. Tadalafil has the advantage over sildenafil of single daily dosage.
2.1.5.5 Tyrosine kinase inhibitors
The finding that there is pathologically aberrant proliferation of endothelial and smooth muscle cells in PAH78, as well as increased expression of secreted growth factors such as VEGF and bFGF, has caused a shift in paradigm in treatment strategies for this disease. Some investigators have likened this condition to a neoplastic process reminiscent of advanced solid tumors79. As a result, anti-neoplastic drugs have been tested in experimental models80, 81 and some occasional patient case reports82-84. Two strategies are currently tested for treatment of PAH: disruption of PDGF and VEGF signaling pathways. STI-571/imatinib (Gleevec®/Glivec®), originally developed specifically to inhibit the Bcr-Abl kinase, is the prototypical PDGFR signaling inhibitor currently under investigation85. This and sorafenib (Nexavar®), the second drug currently being tested in PAH, are FDA-approved for other conditions (such as gastrointestinal malignancies and renal and hepatocellular carcinomas) and their efficacy is attributed to their dual inhibition of VEGF and PDGF signaling pathways. Based on evidence that PDGF signaling is an important process in the pathophysiology of PAH86, imatinib has been tested and shown to be effective in experimental models of PAH80. The results of a phase II multicenter trial to evaluate the safety, tolerability, and efficacy of this drug in patients with PAH have recently published and indicate that imitanib is well tolerated in PAH patients. While there was no significant change in 6 minute walk distance (primary end-point) there was a significant decrease in pulmonary vascular resistance and an increase in cardiac output in imatinib-treated patients versus placebo87. Whether these new anti-neoplastic drugs with anti-tyrosine kinase activity will have a role in SSc (where there is evidence for both dysregulated proliferation and increased expression of growth factors such as VEGF88) or in IPAH remains to be determined. Interestingly, a single case report suggests significant improvement of right ventricular function with imatinib treatment in a SSc-PAH patient. Also of note is that imatinib is being investigated for SSc-related interstitial lung disease89.
2.1.5.6 Combined therapy
It is now common practice in various pulmonary hypertension centers to add drugs when patients fail to improve on monotherapy. Adding inhaled iloprost to patients receiving bosentan has been shown to be beneficial in a small, randomized trial90. Combining inhaled iloprost with sildenafil is mechanistically appealing and anecdotally efficacious as these drugs target separate, potentially synergistic pathways90, 91. Several multicenter trials are now exploring the efficacy of various combinations of two oral drugs or one oral and one inhaled drug. The results of the PACES trial demonstrate that adding sildenafil (at a dose of 80 mgs three times a day) to intravenous epoprostenol improves exercise capacity, hemodynamic measurements, time to clinical worsening, and quality of life92. About 21% of these patients had CTD, including 11% with SSc-PAH. Although no specific subgroup analysis is provided, improvement was apparently mainly in patients with IPAH and limited to patients who had relatively better exercise function at baseline. We reported our experience of adding sildenafil to patients with IPAH or SSc-PAH after they failed initial monotherapy with bosentan93. While the combination improved the 6MWD and FC in IPAH patients, the outcome in patients with SSc-PAH was less favorable, but may have halted clinical deterioration. In addition, there were more side-effects reported in the SSc-PAH compared to the IPAH patients, including hepatotoxicity that developed after addition of sildenafil to bosentan monotherapy93. Sildenafil and bosentan are substrates of the CYP 3A4 cytochrome, and combination therapy leads to significant increases in bosentan serum levels, and significant decreases in sildenafil concentration (since bosentan induces CYP 3A4)94. The clinical significance of these findings is unclear at this time, and these two drugs are often used in combination in clinical practice without obvious untoward effects.
2.1.5.7 Anticoagulation
The rationale for the use of anticoagulation in severe PAH is based on pathologic evidence of pulmonary thromboembolic arterial disease and thrombosis in situ in patients with IPAH95, and two clinical studies (a retrospective analysis96 and a small, non-randomized prospective study51) demonstrating a significant beneficial effect of anticoagulation on survival in IPAH. In the former study, survival was significantly prolonged for patients who received long-term on anticoagulation, as compared with those who were not treated with warfarin96. In a study primarily aimed at assessing the effects of high dose calcium channel blockers on IPAH, improved survival with oral anticoagulation was demonstrated a posteriori irrespective of vasodilator use51. Based essentially on the findings of these two studies, anticoagulation is routinely recommended in the treatment of IPAH patients. The role of anticoagulation in other forms of PAH, in particular in SSc-PAH or other forms of CTD is less clear. Theoretically, there is potential for increased bleeding in patients with CTD, particularly with SSc where gastro-intestinal telangiectasias may be common. Our experience with anticoagulation in over 100 patients with SSc-PAH suggests that less than 50% of these patients remain on long-term anticoagulation therapy. The reason for discontinuing anticoagulation is often related to occult bleeding in the gastrointestinal tract from a source often difficult to diagnose in our experience.
2.1.5.8 Lung transplantation
Lung transplantation (LT) is typically offered as a last resort to patients with PAH who fail medical therapy. Although SSc is not an absolute contraindication to lung transplantation, patients with SSc often have associated morbidity and organ dysfunction other than the lung that place them at a specifically high risk for LT. The involvement of the esophagus with severe motility disorder and gastroesophageal reflux in patients with SSc is an example of specific risk that discourages surgeons from considering these patients because of the enhanced post-operative potential of aspiration and damage to the recipient lung. For these reasons, patients with SSc-PAH are often denied the LT option. However, if properly screened and approved for LT, patients with SSc experience similar rates of survival 2 years after the procedure compared with patients who receive LT for pulmonary fibrosis or IPAH97. A recent retrospective study suggests that 1-year survival rate is no different for SSc patients (transplanted for respiratory failure related to PAH or interstitial lung disease) compared to patients with IPF although acute rejection appears to be more common for the former group98.
2.1.6 Survival of patients with SSc-PAH
While there has been a recent significant increase in the number of drugs targeting specific pathways in PAH99, survival of patients with SSc-PAH on modern therapy remains unacceptably low despite recent claims of improvement compared to similar historical controls39. The reason for a discrepancy in survival between IPAH and SSc-PAH patients remains poorly understood and may involve complex pathogenic alterations affecting not only the proximal and distal pulmonary vessels but also the heart (such as inflammatory myocarditis). A recent study focused on SSc-PAH suggests that specific components of right ventricular dysfunction, as well as renal impairment (probably as a mere consequence of severe cardiac dysfunction and neuroendocrine activation), contribute significantly to increased mortality in SSc-PAH patients44. Thus, a better understanding of the underlying pathophysiology of pulmonary vascular and cardiac remodeling, as well as RV-pulmonary vascular coupling in this disease, is needed for better targeted therapy100. Whether specific anti-inflammatory agents or drugs targeting tyrosine kinase activity hold any promise of enhanced response is unclear at this time but needs to be further explored.
2.1.7 Specific Challenges Related to SSc-PAH
Several issues have substantially limited progress in the diagnosis and management of SSc-PAH. First, currently available outcome measures, including the 6-minute walk test and invasive or non-invasive cardiopulmonary hemodynamics, provide global assessments of function. They do not allow dissection or consideration of the components of the cardiovascular response, in particular RV function, proximal and distal vascular remodeling, and the interaction between the pulmonary vasculature and the RV, which may be particularly important in patients with SSc-PAH100. Furthermore, while some progress has been made in understanding the genetics of IPAH with the discovery of specific mutations in the bone morphogenesis protein receptor (BMPR2)101, 102, little is known about genetic and/or phenotypic characteristics that might predict the development of PAH, RV-PV dysfunction, response to therapy, and survival in patients with SSc-PAH. Finally, delay in diagnosis and referral to specialized centers for treatment of PAH may be a contributing factor to poor prognosis in these patients.
In summary, SSc-PAH is a common cause of pulmonary hypertension but has significantly worse outcome compared to other diseases, such as IPAH, within group 1 of the WHO classification. In addition, modern therapy for PAH appears to be of limited value in SSc-PAH. Similarly, currently available markers of disease severity or response to therapy in SSc-PAH are either limited or lacking. Therefore, there is an urgent need to identify potential genetic causes and novel physiologic and imaging biomarkers that will allow a better understanding of the underlying pathogenesis and serve as reliable tools to monitor therapy in this devastating syndrome.
3 Lung Parenchymal Involvement
Most patients with SSc have some degree of lung parenchymal involvement in the form of interstitial thickening and fibrosis which, when extensive may be clinically significant. Interstitial lung disease (ILD) is the most common pulmonary manifestation of SSc, affecting about 40% of patients, and by far the leading cause of morbidity and mortality20. ILD most often complicates the diffuse cutaneous form of SSc103-105 but can also be associated with the limited form of the disease or with SSc without cutaneous involvement (SSc sine scleroderma)106. In addition, there is no correlation between the extent and severity of the skin disease and pulmonary parenchymal involvement107. ILD was found to be associated with African-American ethnicity, high skin score, elevated serum CPK, hypothyroidism, and cardiac involvement in a large cohort of SSc patients108.
3.1 Diagnosis
The development of ILD in patients with SSc may be slow and progressive. The patients may remain asymptomatic despite the presence of physical findings such as crackles on auscultation or interstitial thickening on chest radiography. The symptoms are non specific and most commonly include dyspnea on exertion, a dry cough or fatigue. Chest discomfort or pain and hemoptysis are uncommon. Physical examination will most typically reveal bilateral inspiratory and expiratory crackles (so called “Velcro” crackles) on auscultation of the lung bases. Serum serology may reveal the presence of antitopoisomerase I/Scl-70, anti-U3RNP, Th/To, and antihistone autoantibodies at presentation and thus may identify SSc patients at high risk for developing ILD109-113. However, anti-topoisomerase/Scl-70 has generally limited sensitivity for diagnosis of ILD in SSc patients114. Anticentromere antibodies are unusual in this syndrome115.
Alterations in pulmonary function tests often precede symptoms or changes in chest radiography. However, early ILD cannot be excluded by normal spirometry. A reduction in single breath DLCO appears to be one of the earliest detectable functional correlates of lung disease in SSc-ILD105 and is present in over 70 % of patients. A decrease in DLCO correlates with the severity of ILD detected by high resolution computed tomography (HRCT) and predicts poor outcome116. On the other hand, plain chest radiography is rather insensitive for detecting early ILD. HRCT changes suggestive of ILD include ill-defined, subpleural infiltrates or densities in the posterior segments of the lower lobes, interstitial reticular infiltrates and subpleural honeycombing changes. Traction bronchiectasis and large cystic changes develop with progressive disease114, 117. The extent of pulmonary fibrosis as assessed on HRCT is negatively correlated with FVC on spirometry and is a powerful predictor of survival118. Since SSc-ILD is predominantly subpleural, histological changes are more likely to be found in peripheral lung biopsy compared to other lung locations105. Histological findings correlate relatively well with HRCT findings119.
However, the diagnosis of SSc-ILD is frequently suggested by the constellation of symptoms, physical findings, pulmonary function and HRCT abnormalities. Thus lung biopsies (e.g., video-assisted thoracoscopic biopsies) are seldom needed except for ruling out other parenchymal processes116-118, 120, 121.
In a large study of patients with early symptomatic SSc-ILD, almost half of the patients presented with ground-glass opacification122, which correlated with increased numbers of inflammatory cells in the bronchoalveolar lavage (BAL) fluid. On the other hand, a reticular HRCT pattern is more often associated with changes consistent with a histological diagnosis of usual interstitial pneumonitis (UIP)114, 119, 123, 124. The severity of lung involvement (defined by HRCT) also correlates with the type of inflammatory cells obtained by BAL: excess lymphocytes may be present when radiological changes are minimal while excess eosinophils are associated with early disease and neutrophils with more extensive disease125, supporting the notion that specific inflammatory cells are involved at different stages of SSc-ILD. While patients with SSc-ILD typically have elevated numbers of neutrophils, eosinophils, and lymphocytes, and occasionally mast cells126, performance of BAL for diagnosis is not routinely mandated. However, the presence of greater than 3 percent neutrophils and/or greater than 1 percent eosinophils in BAL fluid is usually indicative of active alveolitis, and may prompt initiation of immunosuppressive therapy127 although recent data indicate that such treatment may not offer any specific advantage (as assessed by changes in FVC) when comparing patients with and without evidence of alveolitis128.
3.2 The impact of gastroesophageal reflux
Impaired esophageal motility and gastroesophageal reflux are common in SSc and may cause recurrent aspiration of gastric content129. Investigators have analyzed the correlation between esophageal dysmotility and the presence of ILD as assessed by pulmonary function testing and HRCT130-132 (124-126). Presence of severe esophageal motor impairment was found to be associated with significantly decreased values of DLCO and evidence of ILD by HRCT. Furthermore, a 2-year follow-up suggested that patients with severe esophageal dysmotility had greater deterioration in DLCO and increased frequency of ILD by HRCT130. The association between esophageal dysmotility and ILD, however, has not always been corroborated132 and, thus, the exact role of esophageal reflux in the pathogenesis of SSc-ILD remains unclear.
3.3 Prognosis
SSc-ILD portends a poor prognosis with increased mortality116, 133-135. In a retrospective study of 953 SSc patients, SSc-ILD patients with severe ILD had a 30% survival rate at 9 years, versus 72% survival rate in patients without major organ involvement135. The most rapid decline in FVC occurred within the initial three years of disease onset, suggesting that lung injury and fibrosis may be relatively early complications.
The prognostic value of BAL is of unclear value116, 126, 136, 137, with one retrospective study suggesting that increased eosinophils in the BAL fluid is associated with a poor prognosis while a larger prospective cohort study of 141 patients with SSc-ILD demonstrating that BAL eosinophils did not correlate with mortality, rate of functional deterioration, or progression-free survival116, 136. Increased neutrophils in BAL fluid was associated with more extensive lung disease on HRCT, a greater reduction in DLCO, and early mortality (HR 8.40, 95% CI 1.91-36.95), but did not predict the rate of functional deterioration or progression-free survival136. As mentioned above, a prospective clinical trial of 158 patients with early-stage SSc and symptomatic lung involvement found that the presence or absence of neutrophils in BAL fluid had no bearing on the rate of worsening or response to therapy138.
Similarly, the prognostic value of lung biopsy in patients with SSc-associated ILD is of limited value whereas assessment of clinical severity and lung function impairment are better predictors of outcome. In a retrospective histopathological evaluation of 80 patients with biopsy proven SSc-ILD, 76 % had non-specific interstitial pneumonitis (NSIP) and 11 % had UIP116, with fairly similar five-year survival rates of 82 and 91 %, respectively. Markers of a poor outcome in this study included low DLCO and a rapid decline of DLCO over three years116.
3.4 Treatment
It is thought that SSc-ILD, like other forms of ILD, results from an initial parenchymal lung injury of yet undefined mechanism, and characterized by significant inflammation followed by dysfunctional repair and exuberant fibrosis. Thus targeting inflammation is appealing since the initial insult likely remains unrecognized and the fibrotic stage is irreversible. However, the potential (and currently small) benefits of therapy must be weighed against the risks of potentially toxic drugs. Therapy may be initiated in patients who have early respiratory symptoms in the context of pulmonary function impairment and radiographic changes in particular if the latter are suggestive of early and active disease such as ground glass infiltrates or recent progression of infiltrates on HRCT.
3.4.1 Immunosuppressive agents
With multiple uncontrolled case series reporting clinical139, 140, functional139-147, and radiographic139, 142, 143 improvement in SSc-ILD patients treated with immunosuppressive agents, two prospective studies were recently completed. In a double-blind multicenter trial (Scleroderma Lung Study) of 158 patients with early SSc-ILD, dyspnea, and evidence of active alveolitis randomly assigned to receiving oral cyclophosphamide (≤2 mg/kg) or placebo daily for one year138, oral daily cyclophosphamide had modest clinical efficacy (a small but significant difference in FVC percent predicted between treatment and placebo) which raised optimism but prompted cautionary advice regarding the use of this toxic drug for these patients148. This is particularly relevant in light of a follow-up study reporting that the initial improvement in FVC138 persisted for 6 month after discontinuing cyclophosphamide therapy but was found insignificant at 24 month follow-up128 although improvement in respiratory symptoms and skin changes persisted. In a second prospective trial comparing monthly infusion of cyclophosphamide combined with prednisolone (20 mg on alternate days) followed by azathioprine versus placebo149, there was only an insignificant trend toward improvement in FVC (primary outcome) in the treatment group. There was, however, no significant improvement in DLCO or dyspnea. The treatment was felt to be well tolerated (no significant side-effects) and, at best, to stabilize pulmonary function tests. Since many studies evaluating the efficacy of cyclophosphamide in SSc-ILD have used low dose glucocorticoid therapy138, 145, 149, and since there is a high risk of SSc renal crisis in these patients, low dose glucocorticoid (~ 10 mg/day of prednisone) is usually recommended with cyclophosphamide.
Azathioprine has also been used for the treatment of SSc-ILD although with less success compared to cyclophosphamide150, as suggested by a non blinded trial comparing these two drugs given in addition to low dose corticosteroids. FVC and DLCO seemed to remain stable in the cyclophosphamide group while these values declined in the azathioprine group. Adverse effects such as leukopenia were more frequent in the cyclophosphamide group. Preliminary data suggest that azathioprine may have a role as maintenance therapy in patients who have completed a course of cyclophosphamide. A retrospective series of 20 patients with SSc-ILD demonstrated stabilization or improvement in pulmonary function tests after a combination of six months of monthly intravenous cyclophosphamide followed by 18 months of azathioprine151.
More recent experience with the use of mycophenolate mofetil, an inhibitor of lymphocyte proliferation, suggests potential effectiveness for this drug in treatment of SSc-ILD152-154, with either improvement or stabilization of pulmonary function155, 156. The results of larger, randomized and controlled trials are needed before any conclusions and recommendations regarding this form of therapy can be drawn.
Imatinib mesylate, a tyrosine kinase inhibitor which has revolutionized the treatment of chronic myelogenous leukemia and gastrointestinal stromal tumors, may be a highly promising therapy for various forms of fibrotic diseases157, 158 including complications of SSc such as SSc-ILD and SSc-PAH (vide supra). Results with this and other more selective tyrosine kinase inhibitors are anxiously awaited.
3.4.2 Hematopoietic stem cell transplantation
Immunoablative therapy followed by autologous hematopoietic stem cell transplantation is being increasingly advocated for the treatment of severe SSc. The rationale is to reset the dysregulated immune system by eradicating exuberantly active auto-immune effector T and B cells and to induce T regulatory response 159, 160. While experience so far indicates significant and sustained improvement of skin thickening and stabilization of major organ dysfunction161, it is unclear at this time whether this therapy will have any impact on lethal SSc complications such as SSc-ILD and SSc-PAH. Hematopoietic cell transplantation should be currently viewed as an experimental form of therapy with sound mechanistic basis.
3.4.3 Lung transplantation
Lung transplantation (LT) is an option for patients with severe SSc-ILD who are not responsive to pharmacologic interventions. Carefully selected SSc patients undergoing lung transplantation have acceptable morbidity and mortality comparable to patients undergoing LT for idiopathic pulmonary fibrosis as suggested by recent studies97, 98, 162. A retrospective survey of 47 patients with SSc (mean age 46 years) who underwent lung transplantation determined that single lung transplantation was more common than double lung transplantation (57 versus 43 percent)163. Fifteen percent of the patients died within 30 days following transplant, and 32 % died later. Late mortality was most commonly due to infection; other causes included respiratory failure, malignancy, and pulmonary hypertension. The one- and three-year survival rates were 68 and 46 %, respectively, which did not differ from patients who received lung transplants for other conditions. One-year survival rate after lung transplantation is similar for SSc compared to IPF patients although acute rejection is more common98.
4 Combined Pulmonary Vascular and Parenchymal Disease in Systemic Sclerosis
Typically, patients with isolated PAH have limited SSc disease and develop PAH after a duration of 10-15 years24. In contrast, patients with diffuse SSc are at greater risk of developing ILD, usually in the first 5 years after diagnosis of SSc when the most rapid rate of decline in FVC is observed164. However, patients with SSc, with limited or diffuse disease, may present at any stage in the course of their disease with pulmonary hypertension, whether associated or not with ILD165. From a phenotypic standpoint, it is important to note that patients with isolated PAH (SSc-PAH, i.e., no ILD) belong to Group 1 (PAH), while patients with pulmonary hypertension and ILD (SSc-ILD-PH) fall into Group 3, of the pulmonary hypertension classification166.
SSc patients with ILD alone have a median survival of 5-8 years167; however, the impact of combined pulmonary hypertension and ILD has not been well characterized. A study by Trad et al in which pulmonary hypertension was assessed by RHC suggests an increased mortality for patients with SSc-ILD-PH compared to ILD alone168. In a recent study by Mathai et al, where consecutive SSc patients with RHC confirmed pulmonary hypertension with or without ILD were analyzed169, survival was significantly worse in SSc-ILD-PH compared to patients with SSc-PAH (1-,2-, and 3-year survival of 82%, 46%, and 39% vs. 87%, 79%, and 64% respectively, log-rank p<0.01). In multivariable analysis, ILD was associated with a 5-fold increased risk of death169. In another study by Condliffe et al of patients with CTD, 3 year survival rate was 28% for SSc-ILD-PH patients, thus significantly worse compared to 47% survival rate in the group of patients with SSc-PAH66. Of note, only 3 patients with SSc-ILD-PH (the group with the worse prognosis) underwent transplantation, probably because of the perceived heightened risk for transplantation in this group. Since standard medical therapy fails to demonstrate any significant benefit for these patients, the authors wisely suggest that prompt referral for transplant assessment of suitable patients should be considered more aggressively in this group66. In summary, very little is known, aside from anecdotal reports170, regarding the efficacy of medical therapy for SSc-PH-ILD. This subset of patients is routinely excluded (and wisely so because of their particularly poor prognosis) from clinical trials of PAH therapies as well as trials investigating therapies for ILD. However, these patients clearly deserve more attention and more focused therapy trials considering their very grim natural history.
5 Other Lung Complications in Systemic Sclerosis
Aside from the common complications of pulmonary vasculopathy and ILD reviewed above, other less frequent pulmonary complications have been reported in SSc. An occasional obstructive ventilator defect has been observed in non smoker CTD (including SSc) patients171. Pleural effusions, perhaps related to serositis172, 173, and spontaneous pneumothorax174 have been reported, the latter often in association with subpleural blebs in the context of ILD.
Several large studies have found a high prevalence of lung cancer in SSc patients175, 176. These patients are typically female with underlying ILD. The histological types of malignancy include bronchoalveolar carcinomas and adenocarcinomas177, tumors less likely to be associated with a history of cigarette smoking178. However, a recent large population-based cohort study has questioned the link between SSc and lung cancer179.
Finally myopathy and myositis in the context of an overlap syndrome are not uncommon complications of SSc and may cause respiratory symptoms through respiratory muscle dysfunction180.
6 Conclusion
Pulmonary complications are common in SSc and, in the case of SSc-ILD and SSc-PAH, the leading causes of death. However, dyspnea may have many causes in patients with SSc and its assessment requires a systematic approach including a thorough history, physical examination, and additional screening testing such as echocardiography and chest imaging. Treatment of the various pulmonary complications remains disappointing although many promising therapies are emerging. A better understanding of the pathophysiology of the disease, whether related to the vasculopathy or parenchymal involvement, is greatly needed and will allow more effective targeted therapy.
Acknowledgments
Supported by NIH/NHLBI HL084946
Glossary
- 6MWT
6 minute walk test
- 6MWD
6 minute walk distance
- CTD
Connective tissue disease
- DLCO
Diffusing lung capacity to carbon monoxide
- FC
Functional class
- FGF
Fibroblast growth factor
- FVC
Forced vital capacity
- HRCT
High resolution computed tomography
- ILD
Interstitial lung disease
- IPAH
Idiopathic pulmonary arterial hypertension
- IPF
Idiopathic pulmonary fibrosis
- LT
Lung transplantation
- N-TproBNP
N-terminal brain natriuretic peptide
- PAH
Pulmonary arterial hypertension
- PH
Pulmonary hypertension
- PVOD
Pulmonary veno-occlusive disease
- RV
Right ventricle
- SSc
Scleroderma
- SSc-PAH
Scleroderma-related PAH
- TRV
Tricuspid regurgitation velocity
- VEGF
Vascular endothelial growth factor
- WHO
World Health Organization
Footnotes
Conflict of interests: none for this article.Otherwise author’s conflicts of interests, in general, are the following: Novartis Advisory Board ; Research Funding from Actelion/United Therapeutics (for REVEAL Registry)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004;140:37–50. [PubMed] [Google Scholar]
- 2.Tan FK. Systemic sclerosis: the susceptible host (genetics and environment) Rheum Dis Clin North Am. 2003;29:211–37. doi: 10.1016/s0889-857x(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 3.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 4.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–55. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 5.Mayes MD. Scleroderma epidemiology. Rheum Dis Clin North Am. 2003;29:239–54. doi: 10.1016/s0889-857x(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 6.Silman A, Jannini S, Symmons D, Bacon P. An epidemiological study of scleroderma in the West Midlands. Br J Rheumatol. 1988;27:286–90. doi: 10.1093/rheumatology/27.4.286. [DOI] [PubMed] [Google Scholar]
- 7.Tamaki T, Mori S, Takehara K. Epidemiological study of patients with systemic sclerosis in Tokyo. Arch Dermatol Res. 1991;283:366–71. doi: 10.1007/BF00371817. [DOI] [PubMed] [Google Scholar]
- 8.Allcock RJ, Forrest I, Corris PA, Crook PR, Griffiths ID. A study of the prevalence of systemic sclerosis in northeast England. Rheumatology (Oxford) 2004;43:596–602. doi: 10.1093/rheumatology/keh124. [DOI] [PubMed] [Google Scholar]
- 9.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 10.Gaine SP, Rubin LJ. Primary pulmonary hypertension. Lancet. 1998;352:719–25. doi: 10.1016/S0140-6736(98)02111-4. [DOI] [PubMed] [Google Scholar]
- 11.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216–23. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 12.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Chin KM, Kim NH, Rubin LJ. The right ventricle in pulmonary hypertension. Coron Artery Dis. 2005;16:13–8. doi: 10.1097/00019501-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Murata I, Kihara H, Shinohara S, Ito K. Echocardiographic evaluation of pulmonary arterial hypertension in patients with progressive systemic sclerosis and related syndromes. Jpn Circ J. 1992;56:983–91. doi: 10.1253/jcj.56.983. [DOI] [PubMed] [Google Scholar]
- 15.Battle RW, Davitt MA, Cooper SM, Buckley LM, Leib ES, Beglin PA, et al. Prevalence of pulmonary hypertension in limited and diffuse scleroderma. Chest. 1996;110:1515–9. doi: 10.1378/chest.110.6.1515. [DOI] [PubMed] [Google Scholar]
- 16.Schachna L, Wigley FM, Chang B, White B, Wise RA, Gelber AC. Age and risk of pulmonary arterial hypertension in scleroderma. Chest. 2003;124:2098–104. doi: 10.1378/chest.124.6.2098. [DOI] [PubMed] [Google Scholar]
- 17.Mukerjee D, St George D, Coleiro B, Knight C, Denton CP, Davar J, et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis. 2003;62:1088–93. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hachulla E, Gressin V, Guillevin L, Carpentier P, Diot E, Sibilia J, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum. 2005;52:3792–800. doi: 10.1002/art.21433. [DOI] [PubMed] [Google Scholar]
- 19.Koh ET, Lee P, Gladman DD, Abu-Shakra M. Pulmonary hypertension in systemic sclerosis: an analysis of 17 patients. Br J Rheumatol. 1996;35:989–93. doi: 10.1093/rheumatology/35.10.989. [DOI] [PubMed] [Google Scholar]
- 20.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66:940–4. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–30. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 22.Hachulla E, Launay D, Mouthon L, Sitbon O, Berezne A, Guillevin L, et al. Is pulmonary arterial hypertension really a late complication of systemic sclerosis? Chest. 2009;136:1211–9. doi: 10.1378/chest.08-3042. [DOI] [PubMed] [Google Scholar]
- 23.Chang B, Schachna L, White B, Wigley FM, Wise RA. Natural history of mild-moderate pulmonary hypertension and the risk factors for severe pulmonary hypertension in scleroderma. J Rheumatol. 2006;33:269–74. [PubMed] [Google Scholar]
- 24.Steen V, Medsger TA., Jr Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum. 2003;48:516–22. doi: 10.1002/art.10775. [DOI] [PubMed] [Google Scholar]
- 25.Allanore Y, Borderie D, Avouac J, Zerkak D, Meune C, Hachulla E, et al. High N-terminal pro-brain natriuretic peptide levels and low diffusing capacity for carbon monoxide as independent predictors of the occurrence of precapillary pulmonary arterial hypertension in patients with systemic sclerosis. Arthritis Rheum. 2008;58:284–91. doi: 10.1002/art.23187. [DOI] [PubMed] [Google Scholar]
- 26.Scorza R, Caronni M, Bazzi S, Nador F, Beretta L, Antonioli R, et al. Post-menopause is the main risk factor for developing isolated pulmonary hypertension in systemic sclerosis. Ann N Y Acad Sci. 2002;966:238–46. doi: 10.1111/j.1749-6632.2002.tb04221.x. [DOI] [PubMed] [Google Scholar]
- 27.Plastiras SC, Karadimitrakis SP, Kampolis C, Moutsopoulos HM, Tzelepis GE. Determinants of pulmonary arterial hypertension in scleroderma. Semin Arthritis Rheum. 2007;36:392–6. doi: 10.1016/j.semarthrit.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Cox SR, Walker JG, Coleman M, Rischmueller M, Proudman S, Smith MD, et al. Isolated pulmonary hypertension in scleroderma. Intern Med J. 2005;35:28–33. doi: 10.1111/j.1445-5994.2004.00646.x. [DOI] [PubMed] [Google Scholar]
- 29.Shah AA, Wigley FM, Hummers LK. Telangiectases in scleroderma: a potential clinical marker of pulmonary arterial hypertension. J Rheumatol. 2010;37:98–104. doi: 10.3899/jrheum.090697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong YY, Nikoloutsopoulos T, Bond CP, Smith MD, Ahern MJ, Roberts-Thomson PJ. Decreased nailfold capillary density in limited scleroderma with pulmonary hypertension. Asian Pac J Allergy Immunol. 1998;16:81–6. [PubMed] [Google Scholar]
- 31.Fisher MR, Mathai SC, Champion HC, Girgis RE, Housten-Harris T, Hummers L, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006;54:3043–50. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 32.Hsiao SH, Lee CY, Chang SM, Lin SK, Liu CP. Right heart function in scleroderma: insights from myocardial Doppler tissue imaging. J Am Soc Echocardiogr. 2006;19:507–14. doi: 10.1016/j.echo.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Lee CY, Chang SM, Hsiao SH, Tseng JC, Lin SK, Liu CP. Right heart function and scleroderma: insights from tricuspid annular plane systolic excursion. Echocardiography. 2007;24:118–25. doi: 10.1111/j.1540-8175.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 34.Meune C, Avouac J, Wahbi K, Cabanes L, Wipff J, Mouthon L, et al. Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: A controlled study of 100 consecutive patients. Arthritis Rheum. 2008;58:1803–9. doi: 10.1002/art.23463. [DOI] [PubMed] [Google Scholar]
- 35.Overbeek MJ, Lankhaar JW, Westerhof N, Voskuyl AE, Boonstra A, Bronzwaer JG, et al. Right ventricular contractility in systemic sclerosis-associated and idiopathic pulmonary arterial hypertension. Eur Respir J. 2008;31:1160–6. doi: 10.1183/09031936.00135407. [DOI] [PubMed] [Google Scholar]
- 36.Williams MH, Handler CE, Akram R, Smith CJ, Das C, Smee J, et al. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2006;27:1485–94. doi: 10.1093/eurheartj/ehi891. [DOI] [PubMed] [Google Scholar]
- 37.Mathai SC, Bueso M, Hummers LK, Boyce D, Lechtzin N, Le Pavec J, et al. Disproportionate elevation of N-terminal pro-brain natriuretic peptide in scleroderma-related pulmonary hypertension. Eur Respir J. 2010;35:95–104. doi: 10.1183/09031936.00074309. [DOI] [PubMed] [Google Scholar]
- 38.Forfia PR, Mathai SC, Fisher MR, Housten-Harris T, Hemnes AR, Champion HC, et al. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:1364–9. doi: 10.1164/rccm.200712-1876OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams MH, Das C, Handler CE, Akram MR, Davar J, Denton CP, et al. Systemic sclerosis associated pulmonary hypertension: improved survival in the current era. Heart. 2006;92:926–32. doi: 10.1136/hrt.2005.069484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorfmuller P, Humbert M, Perros F, Sanchez O, Simonneau G, Müller KM, et al. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol. 2007;38:893–902. doi: 10.1016/j.humpath.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 41.Montani D, Price LC, Dorfmuller P, Achouh L, Jaïs X, Yaïci A, et al. Pulmonary veno-occlusive disease. Eur Respir J. 2009;33:189–200. doi: 10.1183/09031936.00090608. [DOI] [PubMed] [Google Scholar]
- 42.Overbeek MJ, Vonk MC, Boonstra A, Voskuyl AE, Vonk-Noordegraaf A, Smit EF, et al. Pulmonary arterial hypertension in limited cutaneous systemic sclerosis: a distinctive vasculopathy. Eur Respir J. 2009;34:371–9. doi: 10.1183/09031936.00106008. [DOI] [PubMed] [Google Scholar]
- 43.Montani D, Jais X, Price LC, Achouh L, Degano B, Mercier O, et al. Cautious epoprostenol therapy is a safe bridge to lung transplantation in pulmonary veno-occlusive disease. Eur Respir J. 2009;34:1348–56. doi: 10.1183/09031936.00017809. [DOI] [PubMed] [Google Scholar]
- 44.Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, et al. Hemodynamic Predictors of Survival in Scleroderma-related Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2010;182:252–60. doi: 10.1164/rccm.200912-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawut SM, Taichman DB, Archer-Chicko CL, Palevsky HI, Kimmel SE. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest. 2003;123:344–50. doi: 10.1378/chest.123.2.344. [DOI] [PubMed] [Google Scholar]
- 46.MacGregor AJ, Canavan R, Knight C, Denton CP, Davar J, Coghlan J, et al. Pulmonary hypertension in systemic sclerosis: risk factors for progression and consequences for survival. Rheumatology (Oxford) 2001;40:453–9. doi: 10.1093/rheumatology/40.4.453. [DOI] [PubMed] [Google Scholar]
- 47.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106:1477–82. doi: 10.1161/01.cir.0000029100.82385.58. [DOI] [PubMed] [Google Scholar]
- 48.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–21. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 49.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925–32. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 50.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–9. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 51.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 52.Sitbon O, Humbert M, Jais X, Ioos V, Hamid AM, Provencher S, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–11. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 53.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–63. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 54.Sanchez O, Sitbon O, Jais X, Simonneau G, Humbert M. Immunosuppressive therapy in connective tissue diseases-associated pulmonary arterial hypertension. Chest. 2006;130:182–9. doi: 10.1378/chest.130.1.182. [DOI] [PubMed] [Google Scholar]
- 55.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 56.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 57.McLaughlin VV, Genthner DE, Panella MM, Rich S. Reduction in pulmonary vascular resistance with long-term epoprostenol (prostacyclin) therapy in primary pulmonary hypertension. N Engl J Med. 1998;338:273–7. doi: 10.1056/NEJM199801293380501. [DOI] [PubMed] [Google Scholar]
- 58.Rubin LJ, Mendoza J, Hood M, McGoon M, Barst R, Williams WB, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med. 1990;112:485–91. doi: 10.7326/0003-4819-112-7-485. [DOI] [PubMed] [Google Scholar]
- 59.Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Hervé P, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–8. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 60.Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336:111–7. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 61.Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–4. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 62.Tapson VF, Gomberg-Maitland M, McLaughlin VV, Benza RL, Widlitz AC, Krichman A, et al. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: a prospective, multicenter, open-label, 12-week trial. Chest. 2006;129:683–8. doi: 10.1378/chest.129.3.683. [DOI] [PubMed] [Google Scholar]
- 63.Galie N, Manes A, Branzi A. Emerging medical therapies for pulmonary arterial hypertension. Prog Cardiovasc Dis. 2002;45:213–24. doi: 10.1053/pcad.2002.130160. [DOI] [PubMed] [Google Scholar]
- 64.Badesch DB, Tapson VF, McGoon MD, Brundage BH, Rubin LJ, Wigley FM, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med. 2000;132:425–34. doi: 10.7326/0003-4819-132-6-200003210-00002. [DOI] [PubMed] [Google Scholar]
- 65.Badesch DB, McGoon MD, Barst RJ, Tapson VF, Rubin LJ, Wigley FM, et al. Longterm survival among patients with scleroderma-associated pulmonary arterial hypertension treated with intravenous epoprostenol. J Rheumatol. 2009;36:2244–9. doi: 10.3899/jrheum.081277. [DOI] [PubMed] [Google Scholar]
- 66.Condliffe R, Kiely DG, Peacock AJ, Corris PA, Gibbs JS, Vrapi F, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med. 2009;179:151–7. doi: 10.1164/rccm.200806-953OC. [DOI] [PubMed] [Google Scholar]
- 67.Farber HW, Graven KK, Kokolski G, Korn JH. Pulmonary edema during acute infusion of epoprostenol in a patient with pulmonary hypertension and limited scleroderma. J Rheumatol. 1999;26:1195–6. [PubMed] [Google Scholar]
- 68.Palmer SM, Robinson LJ, Wang A, Gossage JR, Bashore T, Tapson VF. Massive pulmonary edema and death after prostacyclin infusion in a patient with pulmonary veno-occlusive disease. Chest. 1998;113:237–40. doi: 10.1378/chest.113.1.237. [DOI] [PubMed] [Google Scholar]
- 69.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–23. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 70.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 71.Denton CP, Humbert M, Rubin L, Black CM. Bosentan treatment for pulmonary arterial hypertension related to connective tissue disease: a subgroup analysis of the pivotal clinical trials and their open-label extensions. Ann Rheum Dis. 2006;65:1336–40. doi: 10.1136/ard.2005.048967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Girgis RE, Mathai SC, Krishnan JA, Wigley FM, Hassoun PM. Long-term outcome of bosentan treatment in idiopathic pulmonary arterial hypertension and pulmonary arterial hypertension associated with the scleroderma spectrum of diseases. J Heart Lung Transplant. 2005;24:1626–31. doi: 10.1016/j.healun.2004.12.113. [DOI] [PubMed] [Google Scholar]
- 73.Barst RJ, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169:441–7. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 74.Girgis RE, Frost AE, Hill NS, Horn EM, Langleben D, McLaughlin VV, et al. Selective endothelin A receptor antagonism with sitaxsentan for pulmonary arterial hypertension associated with connective tissue disease. Ann Rheum Dis. 2007;66:1467–72. doi: 10.1136/ard.2007.069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galie N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–9. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 76.Badesch DB, Hill NS, Burgess G, Rubin LJ, Barst RJ, Galiè N, Simonneau G, et al. Sildenafil for pulmonary arterial hypertension associated with connective tissue disease. J Rheumatol. 2007;34:2417–22. [PubMed] [Google Scholar]
- 77.Galie N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 78.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–65. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 79.Adnot S. Lessons learned from cancer may help in the treatment of pulmonary hypertension. J Clin Invest. 2005;115:1461–3. doi: 10.1172/JCI25399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–21. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moreno-Vinasco L, Gomberg-Maitland M, Maitland ML, Desai AA, Singleton PA, Sammani S, et al. Genomic assessment of a multikinase inhibitor, sorafenib, in a rodent model of pulmonary hypertension. Physiol Genomics. 2008;33:278–91. doi: 10.1152/physiolgenomics.00169.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med. 2005;353:1412–3. doi: 10.1056/NEJMc051946. [DOI] [PubMed] [Google Scholar]
- 83.Patterson KC, Weissmann A, Ahmadi T, Farber HW. Imatinib mesylate in the treatment of refractory idiopathic pulmonary arterial hypertension. Ann Intern Med. 2006;145:152–3. doi: 10.7326/0003-4819-145-2-200607180-00020. [DOI] [PubMed] [Google Scholar]
- 84.Souza R, Sitbon O, Parent F, Simonneau G, Humbert M. Long term imatinib treatment in pulmonary arterial hypertension. Thorax. 2006;61:736. doi: 10.1136/thx.2006.064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carroll M, Ohno-Jones S, Tamura S, Buchdunger E, Zimmermann J, Lydon NB, et al. CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL, and TEL-PDGFR fusion proteins. Blood. 1997;90:4947–52. [PubMed] [Google Scholar]
- 86.Eddahibi S, Humbert M, Sediame S, Chouaid C, Partovian C, Maître B, et al. Imbalance between platelet vascular endothelial growth factor and platelet-derived growth factor in pulmonary hypertension. Effect of prostacyclin therapy. Am J Respir Crit Care Med. 2000;162:1493–9. doi: 10.1164/ajrccm.162.4.2003124. [DOI] [PubMed] [Google Scholar]
- 87.Ghofrani HA, Morrell NW, Hoeper MM, Olschewski H, Peacock AJ, Barst RJ, et al. Imatinib in Pulmonary Arterial Hypertension Patients with Inadequate Response to Established Therapy. Am J Respir Crit Care Med. 2010 doi: 10.1164/rccm.201001-0123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grigoryev DN, Mathai SC, Fisher MR, Girgis RE, Zaiman AL, Housten-Harris T, et al. Identification of candidate genes in scleroderma-related pulmonary arterial hypertension. Transl Res. 2008;151:197–207. doi: 10.1016/j.trsl.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.ten Freyhaus H, Dumitrescu D, Bovenschulte H, Erdmann E, Rosenkranz S. Significant improvement of right ventricular function by imatinib mesylate in scleroderma-associated pulmonary arterial hypertension. Clin Res Cardiol. 2009;98:265–7. doi: 10.1007/s00392-009-0752-3. [DOI] [PubMed] [Google Scholar]
- 90.McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174:1257–63. doi: 10.1164/rccm.200603-358OC. [DOI] [PubMed] [Google Scholar]
- 91.Hoeper MM, Leuchte H, Halank M, Wilkens H, Meyer FJ, Seyfarth HJ, et al. Combining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2006;28:691–4. doi: 10.1183/09031936.06.00057906. [DOI] [PubMed] [Google Scholar]
- 92.Simonneau G, Rubin LJ, Galie N, Barst RJ, Fleming TR, Frost AE, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008;149:521–30. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 93.Mathai SC, Girgis RE, Fisher MR, Champion HC, Housten-Harris T, Zaiman A, et al. Addition of sildenafil to bosentan monotherapy in pulmonary arterial hypertension. Eur Respir J. 2007;29:469–75. doi: 10.1183/09031936.00081706. [DOI] [PubMed] [Google Scholar]
- 94.Hoeper MM, Faulenbach C, Golpon H, Winkler J, Welte T, Niedermeyer J. Combination therapy with bosentan and sildenafil in idiopathic pulmonary arterial hypertension. Eur Respir J. 2004;24:1007–10. doi: 10.1183/09031936.04.00051104. [DOI] [PubMed] [Google Scholar]
- 95.Pietra GG, Edwards WD, Kay JM, Rich S, Kernis J, Schloo B, et al. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation. 1989;80:1198–206. doi: 10.1161/01.cir.80.5.1198. [DOI] [PubMed] [Google Scholar]
- 96.Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation. 1984;70:580–7. doi: 10.1161/01.cir.70.4.580. [DOI] [PubMed] [Google Scholar]
- 97.Schachna L, Medsger TA, Jr, Dauber JH, Wigley FM, Braunstein NA, White B, et al. Lung transplantation in scleroderma compared with idiopathic pulmonary fibrosis and idiopathic pulmonary arterial hypertension. Arthritis Rheum. 2006;54:3954–61. doi: 10.1002/art.22264. [DOI] [PubMed] [Google Scholar]
- 98.Saggar R, Khanna D, Furst DE, Belperio JA, Park GS, Weigt SS, et al. Systemic sclerosis and bilateral lung transplantation: a single center experience. Eur Respir J. 2010;36:893–900. doi: 10.1183/09031936.00139809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–36. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 100.Le Pavec J, Humbert M, Mouthon L, Hassoun PM. Systemic sclerosis-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;181:1285–93. doi: 10.1164/rccm.200909-1331PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–44. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, et al. The International PPH Consortium. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–4. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 103.Diot E, Boissinot E, Asquier E, Guilmot JL, Lemarié E, Valat C, et al. Relationship between abnormalities on high-resolution CT and pulmonary function in systemic sclerosis. Chest. 1998;114:1623–9. doi: 10.1378/chest.114.6.1623. [DOI] [PubMed] [Google Scholar]
- 104.Ostojic P, Damjanov N. Different clinical features in patients with limited and diffuse cutaneous systemic sclerosis. Clin Rheumatol. 2006;25:453–7. doi: 10.1007/s10067-005-0041-0. [DOI] [PubMed] [Google Scholar]
- 105.Wells AU, Rubens MB, du Bois RM, Hansell DM. Serial CT in fibrosing alveolitis: prognostic significance of the initial pattern. AJR Am J Roentgenol. 1993;161:1159–65. doi: 10.2214/ajr.161.6.8249719. [DOI] [PubMed] [Google Scholar]
- 106.Toya SP, Tzelepis GE. The many faces of scleroderma sine scleroderma: a literature review focusing on cardiopulmonary complications. Rheumatol Int. 2009;29:861–8. doi: 10.1007/s00296-009-0878-7. [DOI] [PubMed] [Google Scholar]
- 107.Morelli S, Barbieri C, Sgreccia A, Ferrante L, Pittoni V, Conti F, et al. Relationship between cutaneous and pulmonary involvement in systemic sclerosis. J Rheumatol. 1997;24:81–5. [PubMed] [Google Scholar]
- 108.McNearney TA, Reveille JD, Fischbach M, Friedman AW, Lisse JR, Goel N, et al. Pulmonary involvement in systemic sclerosis: associations with genetic, serologic, sociodemographic, and behavioral factors. Arthritis Rheum. 2007;57:318–26. doi: 10.1002/art.22532. [DOI] [PubMed] [Google Scholar]
- 109.Greidinger EL, Flaherty KT, White B, Rosen A, Wigley FM, Wise RA. African-American race and antibodies to topoisomerase I are associated with increased severity of scleroderma lung disease. Chest. 1998;114:801–7. doi: 10.1378/chest.114.3.801. [DOI] [PubMed] [Google Scholar]
- 110.Okano Y, Steen VD, Medsger TA., Jr Autoantibody to U3 nucleolar ribonucleoprotein (fibrillarin) in patients with systemic sclerosis. Arthritis Rheum. 1992;35:95–100. doi: 10.1002/art.1780350114. [DOI] [PubMed] [Google Scholar]
- 111.Sacks DG, Okano Y, Steen VD, Curtiss E, Shapiro LS, Medsger TA., Jr Isolated pulmonary hypertension in systemic sclerosis with diffuse cutaneous involvement: association with serum anti-U3RNP antibody. J Rheumatol. 1996;23:639–42. [PubMed] [Google Scholar]
- 112.Steen VD, Powell DL, Medsger TA., Jr Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum. 1988;31:196–203. doi: 10.1002/art.1780310207. [DOI] [PubMed] [Google Scholar]
- 113.Wallace DJ, Lin HC, Shen GQ, Peter JB. Antibodies to histone (H2A-H2B)-DNA complexes in the absence of antibodies to double-stranded DNA or to (H2A-H2B) complexes are more sensitive and specific for scleroderma-related disorders than for lupus. Arthritis Rheum. 1994;37:1795–7. doi: 10.1002/art.1780371213. [DOI] [PubMed] [Google Scholar]
- 114.Fischer A, Swigris JJ, Groshong SD, Cool CD, Sahin H, Lynch DA, et al. Clinically significant interstitial lung disease in limited scleroderma: histopathology, clinical features, and survival. Chest. 2008;134:601–5. doi: 10.1378/chest.08-0053. [DOI] [PubMed] [Google Scholar]
- 115.Kane GC, Varga J, Conant EF, Spirn PW, Jimenez S, Fish JE. Lung involvement in systemic sclerosis (scleroderma): relation to classification based on extent of skin involvement or autoantibody status. Respir Med. 1996;90:223–30. doi: 10.1016/s0954-6111(96)90291-7. [DOI] [PubMed] [Google Scholar]
- 116.Bouros D, Wells AU, Nicholson AG, Colby TV, Polychronopoulos V, Pantelidis P, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med. 2002;165:1581–6. doi: 10.1164/rccm.2106012. [DOI] [PubMed] [Google Scholar]
- 117.Kim EA, Lee KS, Johkoh T, Kim TS, Suh GY, Kwon OJ, et al. Interstitial lung diseases associated with collagen vascular diseases: radiologic and histopathologic findings. Radiographics. 2002;22:S151–65. doi: 10.1148/radiographics.22.suppl_1.g02oc04s151. [DOI] [PubMed] [Google Scholar]
- 118.Nakamura Y, Chida K, Suda T, Hayakawa H, Iwata M, Imokawa S, et al. Nonspecific interstitial pneumonia in collagen vascular diseases: comparison of the clinical characteristics and prognostic significance with usual interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:235–41. [PubMed] [Google Scholar]
- 119.Wells AU, Hansell DM, Rubens MB, Cullinan P, Black CM, du Bois RM. The predictive value of appearances on thin-section computed tomography in fibrosing alveolitis. Am Rev Respir Dis. 1993;148:1076–82. doi: 10.1164/ajrccm/148.4_Pt_1.1076. [DOI] [PubMed] [Google Scholar]
- 120.Fujita J, Yoshinouchi T, Ohtsuki Y, Tokuda M, Yang Y, Yamadori I, et al. Non-specific interstitial pneumonia as pulmonary involvement of systemic sclerosis. Ann Rheum Dis. 2001;60:281–3. doi: 10.1136/ard.60.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tansey D, Wells AU, Colby TV, Ip S, Nikolakoupolou A, du Bois RM, et al. Variations in histological patterns of interstitial pneumonia between connective tissue disorders and their relationship to prognosis. Histopathology. 2004;44:585–96. doi: 10.1111/j.1365-2559.2004.01896.x. [DOI] [PubMed] [Google Scholar]
- 122.Goldin JG, Lynch DA, Strollo DC, Suh RD, Schraufnagel DE, Clements PJ, et al. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest. 2008;134:358–67. doi: 10.1378/chest.07-2444. [DOI] [PubMed] [Google Scholar]
- 123.Muller NL, Miller RR. Computed tomography of chronic diffuse infiltrative lung disease. Part 2. Am Rev Respir Dis. 1990;142:1440–8. doi: 10.1164/ajrccm/142.6_Pt_1.1440. [DOI] [PubMed] [Google Scholar]
- 124.Muller NL, Miller RR. Computed tomography of chronic diffuse infiltrative lung disease. Part 1. Am Rev Respir Dis. 1990;142:1206–15. doi: 10.1164/ajrccm/142.5.1206. [DOI] [PubMed] [Google Scholar]
- 125.Wells AU, Hansell DM, Rubens MB, Cullinan P, Haslam PL, Black CM, et al. Fibrosing alveolitis in systemic sclerosis. Bronchoalveolar lavage findings in relation to computed tomographic appearance. Am J Respir Crit Care Med. 1994;150:462–8. doi: 10.1164/ajrccm.150.2.8049830. [DOI] [PubMed] [Google Scholar]
- 126.Harrison NK, Glanville AR, Strickland B, Haslam PL, Corrin B, Addis BJ, et al. Pulmonary involvement in systemic sclerosis: the detection of early changes by thin section CT scan, bronchoalveolar lavage and 99mTc-DTPA clearance. Respir Med. 1989;83:403–14. doi: 10.1016/s0954-6111(89)80072-1. [DOI] [PubMed] [Google Scholar]
- 127.The BAL Cooperative Group Steering Committee. Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. Am Rev Respir Dis. 1990;141:S169–202. doi: 10.1164/ajrccm/141.5_Pt_2.S169. [DOI] [PubMed] [Google Scholar]
- 128.Tashkin DP, Elashoff R, Clements PJ, Roth MD, Furst DE, Silver RM, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176:1026–34. doi: 10.1164/rccm.200702-326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ebert EC. Esophageal disease in scleroderma. J Clin Gastroenterol. 2006;40:769–75. doi: 10.1097/01.mcg.0000225549.19127.90. [DOI] [PubMed] [Google Scholar]
- 130.Marie I, Dominique S, Levesque H, Ducrotté P, Denis P, Hellot MF, et al. Esophageal involvement and pulmonary manifestations in systemic sclerosis. Arthritis Rheum. 2001;45:346–54. doi: 10.1002/1529-0131(200108)45:4<346::AID-ART347>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 131.Lock G, Pfeifer M, Straub RH, et al. Association of esophageal dysfunction and pulmonary function impairment in systemic sclerosis. Am J Gastroenterol. 1998;93(3):341–5. doi: 10.1111/j.1572-0241.1998.00341.x. [DOI] [PubMed] [Google Scholar]
- 132.Ing AJ. Interstitial lung disease and gastroesophageal reflux. Am J Med. 2001;111(Suppl 8A):41S–4S. doi: 10.1016/s0002-9343(01)00847-6. [DOI] [PubMed] [Google Scholar]
- 133.Al-Dhaher FF, Pope JE, Ouimet JM. Determinants of morbidity and mortality of systemic sclerosis in Canada. Semin Arthritis Rheum. 39(4):269–77. doi: 10.1016/j.semarthrit.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 134.Highland KB, Silver RM. New developments in scleroderma interstitial lung disease. Curr Opin Rheumatol. 2005;17(6):737–45. doi: 10.1097/01.bor.0000181534.67685.5a. [DOI] [PubMed] [Google Scholar]
- 135.Steen VD, Medsger TA., Jr Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43(11):2437–44. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 136.Goh NS, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177(11):1248–54. doi: 10.1164/rccm.200706-877OC. [DOI] [PubMed] [Google Scholar]
- 137.Strange C, Bolster MB, Roth MD, et al. Bronchoalveolar lavage and response to cyclophosphamide in scleroderma interstitial lung disease. Am J Respir Crit Care Med. 2008;177(1):91–8. doi: 10.1164/rccm.200705-655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 139.White B, Moore WC, Wigley FM, Xiao HQ, Wise RA. Cyclophosphamide is associated with pulmonary function and survival benefit in patients with scleroderma and alveolitis. Ann Intern Med. 2000;132(12):947–54. doi: 10.7326/0003-4819-132-12-200006200-00004. [DOI] [PubMed] [Google Scholar]
- 140.Akesson A, Scheja A, Lundin A, Wollheim FA. Improved pulmonary function in systemic sclerosis after treatment with cyclophosphamide. Arthritis Rheum. 1994;37(5):729–35. doi: 10.1002/art.1780370518. [DOI] [PubMed] [Google Scholar]
- 141.Airo P, Danieli E, Parrinello G, et al. Intravenous cyclophosphamide therapy for systemic sclerosis. A single-center experience and review of the literature with pooled analysis of lung function test results. Clin Exp Rheumatol. 2004;22(5):573–8. [PubMed] [Google Scholar]
- 142.Griffiths B, Miles S, Moss H, Robertson R, Veale D, Emery P. Systemic sclerosis and interstitial lung disease: a pilot study using pulse intravenous methylprednisolone and cyclophosphamide to assess the effect on high resolution computed tomography scan and lung function. J Rheumatol. 2002;29(11):2371–8. [PubMed] [Google Scholar]
- 143.Pakas I, Ioannidis JP, Malagari K, Skopouli FN, Moutsopoulos HM, Vlachoyiannopoulos PG. Cyclophosphamide with low or high dose prednisolone for systemic sclerosis lung disease. J Rheumatol. 2002;29(2):298–304. [PubMed] [Google Scholar]
- 144.Silver RM, Warrick JH, Kinsella MB, Staudt LS, Baumann MH, Strange C. Cyclophosphamide and low-dose prednisone therapy in patients with systemic sclerosis (scleroderma) with interstitial lung disease. J Rheumatol. 1993;20(5):838–44. [PubMed] [Google Scholar]
- 145.Steen VD, Lanz JK, Jr, Conte C, Owens GR, Medsger TA., Jr Therapy for severe interstitial lung disease in systemic sclerosis. A retrospective study. Arthritis Rheum. 1994;37(9):1290–6. doi: 10.1002/art.1780370904. [DOI] [PubMed] [Google Scholar]
- 146.Apras S, Ertenli I, Ozbalkan Z, et al. Effects of oral cyclophosphamide and prednisolone therapy on the endothelial functions and clinical findings in patients with early diffuse systemic sclerosis. Arthritis Rheum. 2003;48(8):2256–61. doi: 10.1002/art.11081. [DOI] [PubMed] [Google Scholar]
- 147.Davas EM, Peppas C, Maragou M, Alvanou E, Hondros D, Dantis PC. Intravenous cyclophosphamide pulse therapy for the treatment of lung disease associated with scleroderma. Clin Rheumatol. 1999;18(6):455–61. doi: 10.1007/s100670050138. [DOI] [PubMed] [Google Scholar]
- 148.Martinez FJ, McCune WJ. Cyclophosphamide for scleroderma lung disease. N Engl J Med. 2006;354(25):2707–9. doi: 10.1056/NEJMe068095. [DOI] [PubMed] [Google Scholar]
- 149.Hoyles RK, Ellis RW, Wellsbury J, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54(12):3962–70. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 150.Nadashkevich O, Davis P, Fritzler M, Kovalenko W. A randomized unblinded trial of cyclophosphamide versus azathioprine in the treatment of systemic sclerosis. Clin Rheumatol. 2006;25(2):205–12. doi: 10.1007/s10067-005-1157-y. [DOI] [PubMed] [Google Scholar]
- 151.Berezne A, Ranque B, Valeyre D, et al. Therapeutic strategy combining intravenous cyclophosphamide followed by oral azathioprine to treat worsening interstitial lung disease associated with systemic sclerosis: a retrospective multicenter open-label study. J Rheumatol. 2008;35(6):1064–72. [PubMed] [Google Scholar]
- 152.Stratton RJ, Wilson H, Black CM. Pilot study of anti-thymocyte globulin plus mycophenolate mofetil in recent-onset diffuse scleroderma. Rheumatology (Oxford) 2001;40(1):84–8. doi: 10.1093/rheumatology/40.1.84. [DOI] [PubMed] [Google Scholar]
- 153.Swigris JJ, Olson AL, Fischer A, et al. Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissue disease-related interstitial lung disease. Chest. 2006;130(1):30–6. doi: 10.1378/chest.130.1.30. [DOI] [PubMed] [Google Scholar]
- 154.Liossis SN, Bounas A, Andonopoulos AP. Mycophenolate mofetil as first-line treatment improves clinically evident early scleroderma lung disease. Rheumatology (Oxford) 2006;45(8):1005–8. doi: 10.1093/rheumatology/kei211. [DOI] [PubMed] [Google Scholar]
- 155.Zamora AC, Wolters PJ, Collard HR, et al. Use of mycophenolate mofetil to treat scleroderma-associated interstitial lung disease. Respir Med. 2008;102(1):150–5. doi: 10.1016/j.rmed.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 156.Gerbino AJ, Goss CH, Molitor JA. Effect of mycophenolate mofetil on pulmonary function in scleroderma-associated interstitial lung disease. Chest. 2008;133(2):455–60. doi: 10.1378/chest.06-2861. [DOI] [PubMed] [Google Scholar]
- 157.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15(4):255–73. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 158.Rosenbloom J, Jimenez SA. Molecular ablation of transforming growth factor beta signaling pathways by tyrosine kinase inhibition: the coming of a promising new era in the treatment of tissue fibrosis. Arthritis Rheum. 2008;58(8):2219–24. doi: 10.1002/art.23634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Binks M, Passweg JR, Furst D, et al. Phase I/II trial of autologous stem cell transplantation in systemic sclerosis: procedure related mortality and impact on skin disease. Ann Rheum Dis. 2001;60(6):577–84. doi: 10.1136/ard.60.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rabusin M, Andolina M, Maximova N, et al. Immunoablation followed by autologous hematopoietic stem cell infusion for the treatment of severe autoimmune disease. Haematologica. 2000;85(11 Suppl):81–5. [PubMed] [Google Scholar]
- 161.Vonk MC, Marjanovic Z, van den Hoogen FH, et al. Long-term follow-up results after autologous haematopoietic stem cell transplantation for severe systemic sclerosis. Ann Rheum Dis. 2008;67(1):98–104. doi: 10.1136/ard.2007.071464. [DOI] [PubMed] [Google Scholar]
- 162.Shitrit D, Amital A, Peled N, et al. Lung transplantation in patients with scleroderma: case series, review of the literature, and criteria for transplantation. Clin Transplant. 2009;23(2):178–83. doi: 10.1111/j.1399-0012.2009.00958.x. [DOI] [PubMed] [Google Scholar]
- 163.Massad MG, Powell CR, Kpodonu J, et al. Outcomes of lung transplantation in patients with scleroderma. World J Surg. 2005;29(11):1510–5. doi: 10.1007/s00268-005-0017-x. [DOI] [PubMed] [Google Scholar]
- 164.Steen V. Predictors of end stage lung disease in systemic sclerosis. Ann Rheum Dis. 2003;62(2):97–9. doi: 10.1136/ard.62.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chang B, Wigley FM, White B, Wise RA. Scleroderma patients with combined pulmonary hypertension and interstitial lung disease. J Rheumatol. 2003;30(11):2398–405. [PubMed] [Google Scholar]
- 166.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 167.Altman RD, Medsger TA, Jr, Bloch DA, Michel BA. Predictors of survival in systemic sclerosis (scleroderma) Arthritis Rheum. 1991;34(4):403–13. doi: 10.1002/art.1780340405. [DOI] [PubMed] [Google Scholar]
- 168.Trad S, Amoura Z, Beigelman C, et al. Pulmonary arterial hypertension is a major mortality factor in diffuse systemic sclerosis, independent of interstitial lung disease. Arthritis Rheum. 2006;54(1):184–91. doi: 10.1002/art.21538. [DOI] [PubMed] [Google Scholar]
- 169.Mathai SC, Hummers LK, Champion HC, et al. Survival in pulmonary hypertension associated with the scleroderma spectrum of diseases: impact of interstitial lung disease. Arthritis Rheum. 2009;60(2):569–77. doi: 10.1002/art.24267. [DOI] [PubMed] [Google Scholar]
- 170.Olschewski H, Ghofrani HA, Walmrath D, et al. Inhaled prostacyclin and iloprost in severe pulmonary hypertension secondary to lung fibrosis. Am J Respir Crit Care Med. 1999;160(2):600–7. doi: 10.1164/ajrccm.160.2.9810008. [DOI] [PubMed] [Google Scholar]
- 171.Vitali C, Viegi G, Tassoni S, et al. Lung function abnormalities in different connective tissue diseases. Clin Rheumatol. 1986;5(2):181–8. doi: 10.1007/BF02032355. [DOI] [PubMed] [Google Scholar]
- 172.Thompson AE, Pope JE. A study of the frequency of pericardial and pleural effusions in scleroderma. Br J Rheumatol. 1998;37(12):1320–3. doi: 10.1093/rheumatology/37.12.1320. [DOI] [PubMed] [Google Scholar]
- 173.Taormina VJ, Miller WT, Gefter WB, Epstein DM. Progressive systemic sclerosis subgroups: variable pulmonary features. AJR Am J Roentgenol. 1981;137(2):277–85. doi: 10.2214/ajr.137.2.277. [DOI] [PubMed] [Google Scholar]
- 174.Lang B, Ortlieb H, Meske S, Hauke G, Peter HH. Progressive systemic sclerosis presenting with spontaneous pneumothorax. J Rheumatol. 1989;16(2):254–6. [PubMed] [Google Scholar]
- 175.Hill CL, Nguyen AM, Roder D, Roberts-Thomson P. Risk of cancer in patients with scleroderma: a population based cohort study. Ann Rheum Dis. 2003;62(8):728–31. doi: 10.1136/ard.62.8.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rosenthal AK, McLaughlin JK, Linet MS, Persson I. Scleroderma and malignancy: an epidemiological study. Ann Rheum Dis. 1993;52(7):531–3. doi: 10.1136/ard.52.7.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang Y, Fujita J, Tokuda M, Bandoh S, Ishida T. Lung cancer associated with several connective tissue diseases: with a review of literature. Rheumatol Int. 2001;21(3):106–11. doi: 10.1007/s00296-001-0141-3. [DOI] [PubMed] [Google Scholar]
- 178.Roumm AD, Medsger TA., Jr Cancer and systemic sclerosis. An epidemiologic study. Arthritis Rheum. 1985;28(12):1336–40. doi: 10.1002/art.1780281204. [DOI] [PubMed] [Google Scholar]
- 179.Chatterjee S, Dombi GW, Severson RK, Mayes MD. Risk of malignancy in scleroderma: a population-based cohort study. Arthritis Rheum. 2005;52(8):2415–24. doi: 10.1002/art.21225. [DOI] [PubMed] [Google Scholar]
- 180.Olsen NJ, King LE, Jr, Park JH. Muscle abnormalities in scleroderma. Rheum Dis Clin North Am. 1996;22(4):783–96. doi: 10.1016/s0889-857x(05)70301-x. [DOI] [PubMed] [Google Scholar]