Abstract
Recent evidence suggest that cyclooxygenases, COX1 and COX2, differentially affect brain immunity. Limited data exist about their expressional changes in neurodegenerative diseases like in neuro-AIDS. Here, we analyzed the regulation of non-neuronal COX1/2 expression in rhesus macaque brain during infection with SIVδ670 and antiretroviral treatment. COX1 was constitutively expressed in microglia and endothelial cells, and was not changed in early SIV infection. Late stage of disease was characterized by increased COX1 expression in globally activated microglia, macrophage nodules, infiltrates and multinucleated giant cells. Endothelial COX1 expression was unaltered. In contrast, COX2 was not expressed in non-neuronal cells in the brain of uninfected and asymptomatically SIV-infected monkeys, but was induced in nodule and syncitium forming macrophages and in endothelial cells in areas with infiltrates and SIV in monkeys with AIDS. Antiretroviral treatment of AIDS-diseased monkeys with 6-chloro-2',3'-dideoxyguanosine markedly reduced SIV burden, appearance of COX1-positive macrophage nodules, giant cells and infiltrates, and COX2 induction in the brain. But the number of COX1-positive diffuse microglia was still increased in antiretrovirally treated animals as compared to uninfected or asymptomatic SIV-infected monkeys. Our data implicate that both COX isoforms are differentially regulated, and may distinctly modulate local immune responses in the brain during lenitiviral disease.
Keywords: AIDS, Antiretroviral treatment, Encephalitis, Microglia, Prostaglandins
Introduction
The simian immunodeficiency virus (SIV) macaque model resembles infection of individuals with human immunodeficiency virus (HIV) in terms of disease progression and acquired immunodeficiency syndrome (AIDS). Additionally, SIV-infected macaques can develop motor and cognitive impairments as occurs in human neuro-AIDS (Murray et al., 1992; Williams et al., 2008), while Weed et al. (2004) note that behavioral impairment produced by SIV and HIV on homologous primate test batteries, such as the CANTAB, are quite similar. SIV-induced encephalitis is characterized by general gliosis, nodule and giant cell formation, inflammatory cell infiltrates, myelin pallor and vessel leakage (Budka, 1986; Weihe et al., 1993; Lane et al., 1996; Luabeya et al., 2000). Loss of synapses, dendrites and neurons also occurs in SIV-disease (Li et al., 1992; Luthert et al., 1995; Bissel et al., 2002). Neurodegenerative damage could be caused by virus-derived as well as host-derived neurotoxic products (Li et al., 1992), and is thought to be related to SIV replication and the number of inflammatory cells infiltrating and becoming activated in the central nervous system (Glass et al., 1995; Bissel et al., 2002).
Cyclooxygenases (COX) catalyze the rate-limiting step in the conversion of arachidonic acid to prostaglandins and thromboxanes, lipid mediators involved in several physiological and pathological processes in the brain (Smith et al., 2000; Bosetti, 2007). Inflammation, associated with an increased expression of COX and elevated levels of prostaglandins, has been implicated in a variety of acute and chronic neurologic and neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis and HIV-infection (Griffin et al., 1994; Maihöfner et al., 2003; Teismann et al., 2003; Hoozemans et al., 2008). COX-derived prostaglandins promote migration of monocyte-derived cells (Legler et al., 2006; Tajima et al., 2008) as well as breakdown of the blood-brain barrier (BBB) (Schmidley et al., 1992). The two distinct COX isoforms, COX1 and COX2, share 60% homology in their amino acids sequence and have comparable kinetics (Smith et al., 2000). However, the two isoforms differ in regulatory mechanisms, preferential coupling to upstream and downstream enzymes (Murakami and Kudo, 2004), and in modulating inflammatory response in the brain (Aid et al., 2008; Choi et al., 2008).
Data on the role of COX1 and COX2 are controversial and complicated by the use of different animal models. COX1 has been classically considered as the isoform primarily responsible for homeostatic prostaglandin synthesis (Phillis et al., 2006). By contrast, COX2 is mainly induced in response to inflammatory stimuli, which led to the concept that selective inhibition of COX2 can reduce inflammation without affecting the physiological functions of COX1-derived prostaglandins and to the massive development and marketing of Non-Steroidal Anti-Inflammatory Drugs that selectively inhibit COX2 over COX1. Thus, it is not surprising that in several experimental models COX2 has been linked to antiinflammatory and neuroprotective properties, raising some concern about the potential adverse effects of selective COX2 inhibitors. Recent studies also have indicated a previously unrecognized proinflammatory role of COX1 in the pathophysiology of acute and chronic neurological disorders (Schwab et al., 2002; Pepicelli et al., 2005; Candelario-Jalil et al., 2007; Choi et al., 2008).
Identifying the cellular sources of COX1 and COX2 synthesis is of primary importance in the understanding of their differential modulation of local innate immune responses in the central nervous system. In the present study we addressed this issue. We analyzed comprehensively the cellular expression of COX1 and COX2 in control rhesus monkey brain, their individual as well as joint regulation in early and late stage of SIV infection, and the effect of antiretroviral treatment with the lipophilic 6-chloro-2',3'-dideoxyguanosin (6-Cl-ddG).
Methods
Virus stock and inoculation procedures
Procedures performed on rhesus macaques have been described previously (Depboylu et al., 2004). Experiments involving the use of rhesus macaques were approved by the Animal Care and Use Committee of Bioqual, Inc., an NIH-approved and Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited research facility. All experiments were carried out under the ethical guidelines promulgated in the NIH Guide for the Care and Use of Laboratory Animals. Healthy juvenile rhesus macaques were inoculated intravenously with 10 rhesus infectious doses of cell-free SIVB670. Following inoculation, animals were monitored and examined for clinical evidence of disease. In short intervals blood and cerebrospinal fluid samples were obtained from the animals. Duration of infection lasted between 2.5 and 22 months. AIDS-defining criteria included one or more of the following: loss of body weight over 10%, intractable diarrhoea/dehydration requiring fluid replacement, oral lesions/thrush and other opportunistic infections (Depboylu et al., 2004). At time of euthanasia 8 macaques exhibited clinical signs of AIDS and 5 not. As controls 4 age-matched non-infected macaques were used.
Antiretroviral treatment
Additionally, 8 SIV-infected monkeys were analyzed which were treated with 6-Cl-ddG subcutaneously when their viral load was found to be >100,000 virions/mL in plasma and >100 virions/mL in cerebrospinal fluid in more than 2 consecutive examinations (Depboylu et al., 2004). Only, 1 monkey received 6-Cl-ddG (200 mg/kg/day) for 3 weeks. The other 7 monkeys received 10 mg/kg/day 2',3'-dideoxyinosine (ddI) for 3 weeks for clinical stabilization and then 75 mg/kg/day of 6-Cl-ddG for 6 weeks. The vehicle for ddI administration was phosphate-buffered saline (PBS) and for 6-Cl-ddG administration 70% propylene glycol/30% PBS.
Brain tissue preparation
At sacrifice anesthetized rhesus monkeys were perfused transcardially with PBS and formalin/PBS. Tissue specimens were obtained during necropsy and immersionfixed overnight. Tissue blocks were postfixed in Bouin-Hollande solution and processed for paraffin embedding (Depboylu et al., 2004).
Single and double immunohistochemistry
Immunohistochemistry (IHC) was carried out on deparaffinized brain tissue sections (7 µm) and incubated for 15 min at 92–95°C in citrate buffer (pH 6.0) in a pressure cooker for antigen retrieval, as previously described (Depboylu et al., 2004). Visualization of bound primary antibodies was performed using biotinylated secondary antibodies from donkey (Dianova, Hamburg, Germany; 1/200), standard avidin-biotin-peroxidase techniques (Vectastain Elite ABC kit, Boehringer, Germany; 1/500), and finally with 3,3’-diaminobenzidine (Sigma, Deisenhofen, Germany) and ammonium nickel sulfate (Fluka, Buchs, Switzerland), resulting in a dark blue/black staining, or by indirect immunofluorescence with fluorochrome-conjugated secondary antibodies (Dianova; 1/200). For visualization of two different antigens in the same tissue section double immunofluorescence was done. Otherwise serial immunostainings were carried out on adjacent sections. The primary antibodies used are listed in Table 1. Recombinant COX1 and COX2 peptides (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used for preabsorption experiments of antibodies against COX1 and COX2 on monkey brain tissue sections. Stainings of IHC were analyzed and documented with Olympus AX70 microscope or with Olympus Fluoview confocal laser scanning microscope (Olympus Optical, Hamburg, Germany).
Table 1.
List of antibodies used in this study.
| Antigen | Epitope | Species | Dilution | Catalog no | Source |
|---|---|---|---|---|---|
| Human COX1 | Peptide, C-term 15–25 aa | Goat polyclonal | 1:8000/1:600 (f) | sc-1752 | SCB |
| Mouse COX1 | Peptide, C-term 15–25 aa | Goat polyclonal | 1:200–1:8000 | sc-1754 | SCB |
| Mouse COX1 | Peptide, aa 274–288 | Rabbit polyclona l | 1:200–1:8000 | 16109 | CC |
| Ovine COX1 | Protein, epitope n.d. | Mouse monoclonal | 1:200–1:8000 | 16110 | CC |
| Human COX2 | Peptide, C-term 15–25 aa | Goat polyclonal | 1:5000/1:400 (f) | sc-1745 | SCB |
| Mouse COX2 | Peptide, C-term 15–25 aa | Goat polyclonal | 1:8000 | sc-1747 | SCB |
| Rat COX2 | Peptide, N-term 15–25 aa | Goat polyclonal | 1:200–1:8000 | sc-1746 | SCB |
| Human COX2 | Peptide, aa 50–111 | Rabbit polyclonal | 1:200–1:8000 | sc-7951 | SCB |
| Mouse COX2 | Peptide, aa 570–598 | Rabbit polyclonal | 1:200–1:8000 | 160106 | CC |
| Mouse NeuN | Protein, epitope n.d. | Mouse monoclonal | 1:300 (f) | MAB377 | CI |
| Human Iba1 | Peptide, C-term | Rabbit polyclonal | 1:300 (f) | 019-19741 | WC |
| Human CD68 | Protein, epitope n.d. | Mouse monoclonal | 1:30 (f) | KP1 | D |
| Human CNPase | Protein epitope n.d. | Mouse monoclonal | 1:50 (f) | AB-1 | NM |
| Human GFAP | Protein, epitope n.d. | Guinea pig polyclonal | 1:600 (f) | GP-52 | P |
| Human vWF | Protein, epitope n.d. | Rabbit polyclonal | 1:200 (f) | A0082 | D |
| SIVmac251 gp120 | Peptide, aa 311–340 | Mouse monoclonal | 1:100 (f) | 2318 | NIHARP |
Abbreviations: aa, amino acids; AIDS, acquired immunodeficiency syndrome; BM, Boehringer, Mannheim, Germany; CC, Cayman Chemical, Ann Arbor, MI; CD68, cluster of differentiation 68; CI, Chemicon International, Temecula, CA; C/N-term, C/N-terminal; CNPase, 2’,3’-cyclic nucleotide 3’-phosphodiesterase; COX1/2, cyclooxygenase isotype-1/2; D, DAKO, Hamburg, Germany; f, dilution for immunofluorescence; GFAP, glial fibrillary acid protein; Iba1, ionized calcium binding adaptor molecule 1; n.d., not determined; NeuN, neuronal nuclear antigen; NIHARP, National Institutes of Health AIDS Research and Reference Program, Germantown, MD; NM, Neomarkers, Fremont, CA; P, Progen, Heidelberg, Germany; SCB, Santa Cruz Biotechnology, Santa Cruz, CA; SIV, simian immunodeficiency virus; vWF: van Willebrand factor; WC, WAKO Chemicals, Neuss, Germany.
Quantification of immunohistochemistry
Per monkey, 3 to 5 interval sections per anatomical area with IHC for COX1 and COX2 were analyzed in high-power fields (magnification representing 0.1 mm2) which allowed the discrimination of cellular features. Immunopositive cells were counted in more than 4 randomly chosen areas per section of frontal, parietal and occipital cortices, striatum and corpus callosum. Each multinucleated giant cell and cluster of cells, where the individual cell borders could not be distinguished, were counted as single cells. Quantitative image analysis was performed with the MCID M4 image analysis system (Imaging Research, St. Catherines, ON, Canada). Data were expressed as mean number (± s.e.m.) of cells per 0.1 mm2 area per experimental group. All immunohistochemical parameters and procedures relevant for COX1 or COX2 staining quantifications were kept constant (e.g. antibody concentrations, reaction and washing times, temperatures, illumination and image analysis settings).
Statistics
One way non-parametric analysis of variance (ANOVA) and the post-hoc Newman-Keuls Multiple Comparison Test were used to evaluate statistical differences between the experimental groups. Values of p less than 0.05 were considered statistically significant.
Results
Brain tissue sections of non-infected control monkeys (Ctrl), SIV-infected monkeys without AIDS (SIV,-AIDS), and SIV-infected monkeys exhibiting AIDS (SIV,+AIDS) were analyzed. An additional group consisted of monkeys with high viremia and increased viral load in cerebrospinal fluid at initiation of antiretroviral treatment and suffering from AIDS (SIV,+AIDS,+ddG).
Specificity of antibodies against COX1 and COX2 on brain tissue sections of rhesus macaque
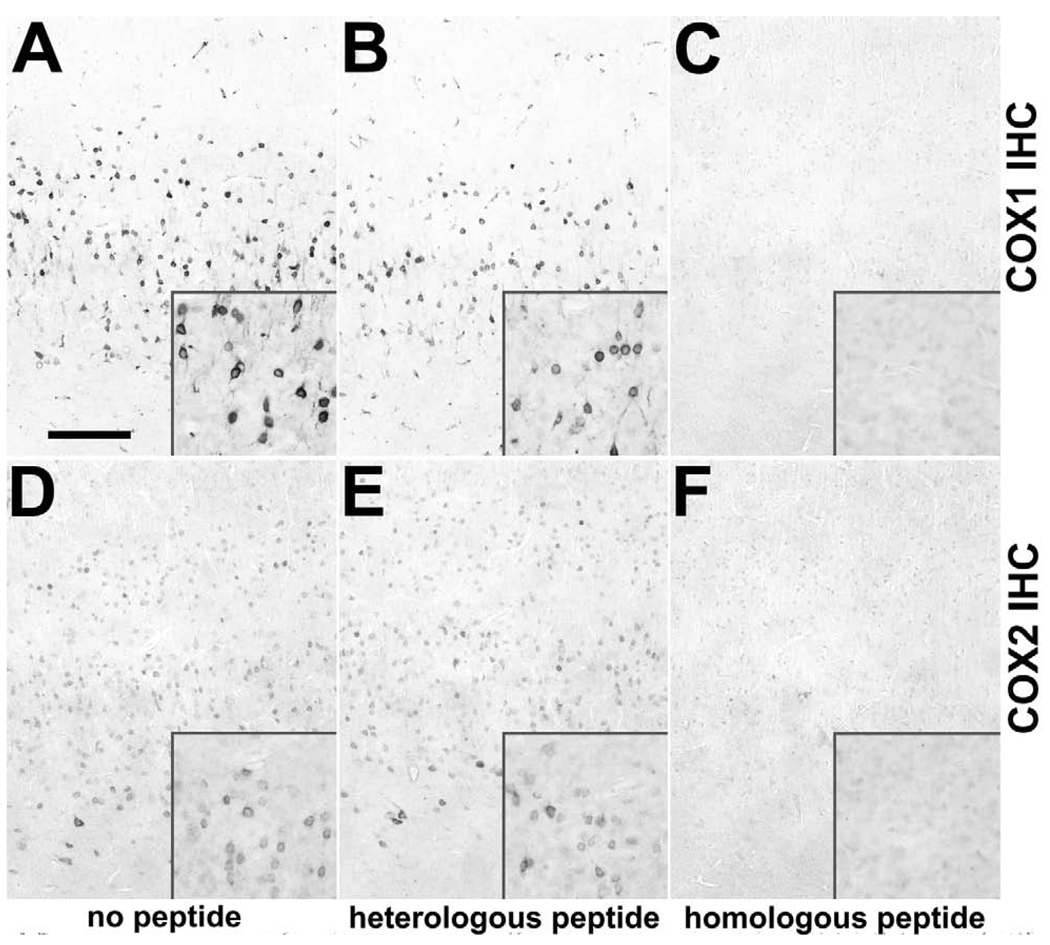
We initiated our studies by screening several antibodies against COX1 and COX2 for immunohistochemical specificity (Table 1). Only those antibodies giving staining which was preabsorbed exclusively with their cognate immunogen peptides were assumed to be specific and used for further studies. Normal monkey neocortical tissue sections were used as control in which neurons are known to express COX1 and COX2 (Tsubokura et al., 1991; Breder et al., 1992; 1995; Yamagata et al., 1993). As shown in Figure 1 (A, D), IHC for COX1 and COX2 demonstrated immunostaining of neurons in occipital cortex. These stainings were fully abolished when antibodies were preincubated with their recognizing homologous peptides (Figure 1 C, F), but not with heterologous peptides (Figure 1 B, E).
Fig. 1.
Expression of COX1 and COX2 in rhesus monkey neocortex and specificity of COX1 and COX2 antibodies. Rows demonstrate immunohistochemistry (IHC) against COX1 (A–C) and COX2 (D–F). Columns demonstrate preincubation of antibodies with no peptide (A, D), heterologous peptide (B, E) and homologous peptide (C, F). As shown in representative low-and medium-power images of IHC on adjacent sections COX1 (A with inset) and COX2 (D with inset) are detected in neurons in the occipital cortex of a control rhesus monkey. The antibodies recognize specifically their cognate peptides as their immunostaining are not preabsorbed with heterologous peptides (B, E), but with excess homologous peptides (C, F). Scale bars = (A–F) 200 µm; (insets in A–F) 100 µm.
Expression of COX1 and COX2 in multiple cellular compartments in rhesus macaque brain
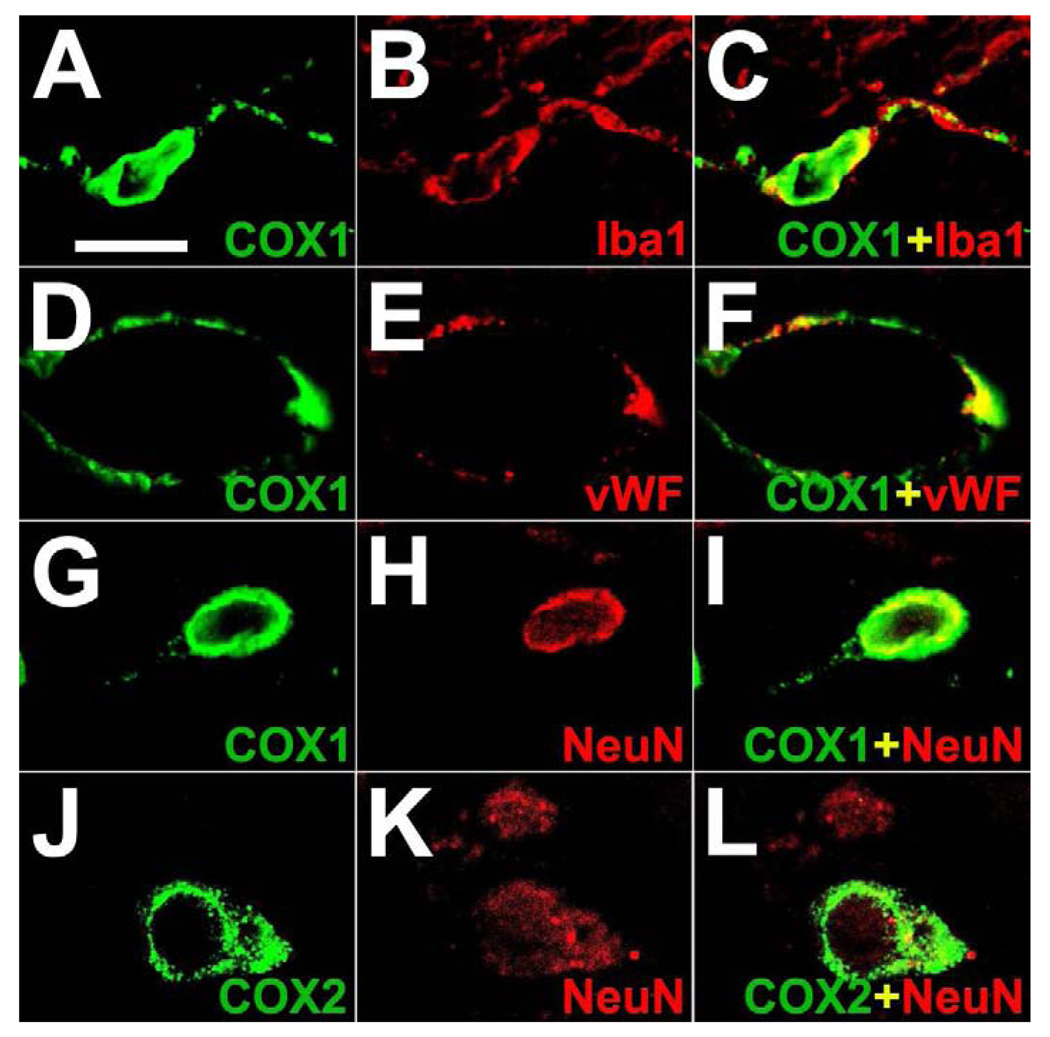
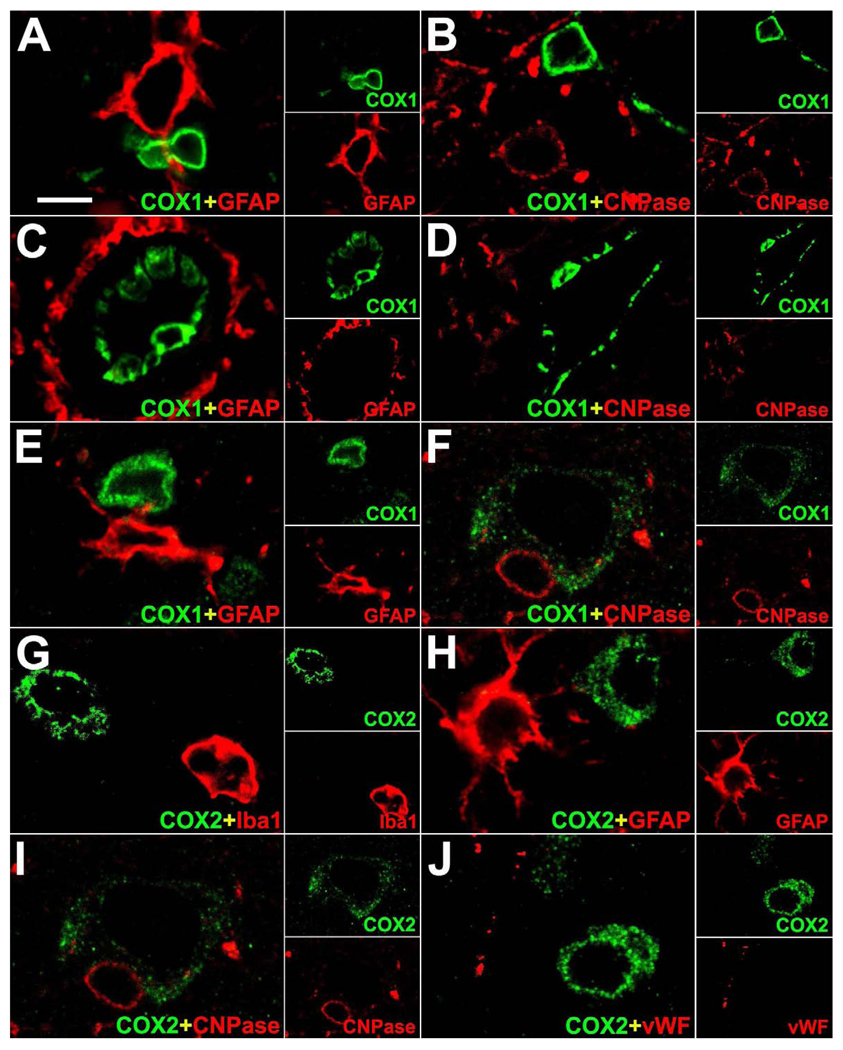
In control monkey brain, COX1 was immunostained in some neurons of the cortex (Figure 1 A) as well as in some subcortical neuronal cell groups. COX1 expression was also found in microglia and endothelial cells throughout the brain. High-power confocal double immunofluorescence analysis of COX1 with brain resident cellular markers confirmed that COX1 was co-localized with Iba1 in microglia, vWF in endothelial cells and with NeuN in cortical neurons (Figure 2 A–I). COX1 was absent from GFAP-positive astrocytes and CNPase-positive oligodendrocytes (Figure 3 A–F). In contrast, COX2 was expressed in some neurons of the cortex (Figure 1 D) and was found also in some subcortical neurons. Double immunofluorescence with brain resident cellular markers revealed that non-neuronal cells did not synthesize COX2 in the normal monkey brain (Figure 3 G–J). Ependymal and choroidal plexus epithelial cells were devoid of COX1 and COX2 immunoreactivity (not shown).
Fig. 2.
Cellular localization analysis of COX1 and COX2 in control rhesus monkey brain. Representative high-power confocal double immunofluorescence images demonstrate co-staining of COX1 (green) with ionized calcium binding adaptor molecule 1 (Iba1; red) in microglia (A–C), with von Willebrand factor (vWF; red) in endothelial cells (D–F) and with neuronal nuclear protein (NeuN; red) in cortical neurons (G–I). COX2 (green) is localized in NeuN (red)-positive neurons of the cortex (J–L). Single immunofluorescence images (green, left column; red, middle column) are merged (yellow, right column). Scale bars = (A–L) 15 µm.
Fig. 3.
Mutual exclusion analysis of COX1 and COX2 with brain resident cellular markers in control rhesus monkey brain. Representative high-power confocal double immunofluorescence images demonstrate expression of COX1 (green) in microglia (A, B), endothelial cells (C, D) and in neurons (E, F) in close proximity to glial fibrillary acidic protein (GFAP; red)-positive astrocytes (A, C, E) and 2’,3’-cyclic nucleotide 3’-phosphodiesterase (CNPase; red)-positive oligodendrocytes (B, D, F). COX2 (green) is expressed in neurons and is absent in neighboring ionized calcium binding adaptor molecule 1 (Iba1; red)-positive microglia (G), GFAP (red)-positive astrocytes (H), CNPase (red)-positive oligodendrocytes (I) and von Willebrand factor (vWF; red)-positive endothelial cells (J). Single immunofluorescence images (green and red) are shown in insets. Scale bars = (A–J) 15 µm; (insets in A–J) 30 µm.
Inflammatory changes of constitutive COX1 and inducible COX2 expression in rhesus macaque brain in early and late stage lentiviral infection, and effects of antiretroviral treatment with 6-Cl-ddG
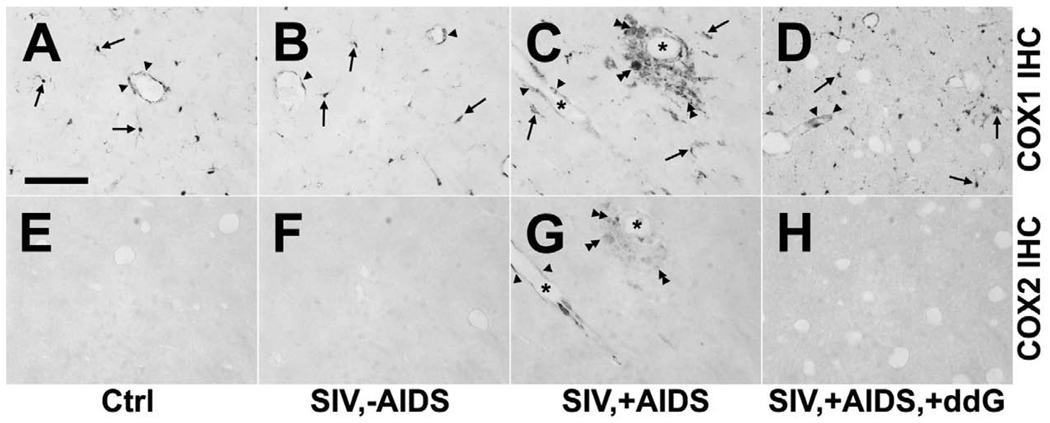
Constitutive expression of COX1 in microglia was not significantly altered in SIV,-AIDS as compared to Ctrl (Figure 4 A, B). In the brain of animals of the SIV,+AIDS group signs of SIV-induced encephalitis occurred as detected with COX1. Morphologically activated microglia, infiltrating monocytes, microglia/macrophage nodules and multinucleated giant cells were COX1-positive (Figure 4 C). In contrast, COX2 was not detected in non-neuronal cells in the brain in SIV,-AIDS, as it was in Ctrl (Fig. 4 E, F), but was induced in areas of productive inflammation and infiltrates in SIV,+AIDS (Figure 4 G).
Fig. 4.
Inflammatory changes of COX1 and COX2 expression in rhesus monkey brain in control, after simian immunodeficiency virus (SIV) infection and antiretroviral treatment with 6-Cl-ddG. Rows demonstrate immunohistochemistry (IHC) against COX1 (A–D) and COX2 (E–H). Columns show representative COX1 and COX2 stainings of subcortical white matter tissue sections of control monkey (Ctrl; A, E), SIV-infected monkey without acquired immunodeficiency syndrome (SIV,-AIDS; B, F), SIV-infected monkey with AIDS (SIV,+AIDS; C, G), and SIV-infected monkey with AIDS and antiretroviral treatment (SIV,+AIDS,+ddG; D, H). (A–C) Parenchymal and juxtavascular ramified COX1-positive microglia (arrows) as seen in Ctrl and SIV,-AIDS become morphologically activated (arrows) and demonstrate typical signs of SIV-induced encephalitis like increased brain monocytic infiltration and formation of microglia/macrophage nodules and multinucleated giant cells (double arrow heads) in SIV,+AIDS. COX1 expression is constitutively found in endothelial cells and is not significantly altered during SIV disease (arrow heads). (E–G) In contrast, COX2 is absent in microglia and endothelial cells in Ctrl and SIV,-AIDS but is induced in some perivascular, nodule and syncytium forming macrophages (double arrow heads) and endothelial cells (arrow heads) in areas of inflammatory infiltrates in SIV,+AIDS. (D, H) Signs of COX1-positive inflammatory reactions as well as induction of COX2 in endothelial cells and macrophages are reversed by antiretroviral treatment with 6-Cl-ddG. But the density of COX1-positive microglia (arrows) is still increased without changes of COX1 expression in endothelial cells (arrow heads) in SIV,+AIDS,+ddG. Note partially overlapped expression of COX1 and COX2 in endothelial cells (arrow heads) and multinucleated giant cells (double arrow heads) in SIV,+AIDS as demonstrated by stainings on adjacent sections (C, G). Asterisks mark the same blood vessel (C, G). Scale bars = (A–H) 100 µm.
To determine the influence of viral burden on COX1 and COX2 expression, immunostaining of COX1 and COX2 were performed on brain tissue sections of monkeys which were antiretrovirally treated with the lipophilic 6-Cl-ddG in late stage disease (Depboylu et al., 2004, 2005, 2006, 2007). The effectiveness of 6-Cl-ddG was proven as no signs of SIV-driven, COX1-positive productive inflammation (mononuclear infiltrates, nodules and giant cells) were observed in the brain of 6-Cl-ddG-treated monkeys (Figure 4 D). However, the density of diffuse COX1-positive microglia was still increased (Figure 4 D). Expression of COX1 in endothelial cells was not altered in the SIV,+AIDS,+ddG group (Figure 4 D). Induction of COX2 was prevented by 6-Cl-ddG treatment (Figure 4 H), except in 1 animal, which showed some COX2 expression albeit at very low levels (data not shown).
To quantify the observed non-neuronal changes of COX1 and COX2 expression during SIV infection and antiretroviral treatment, monocyte-derived cells immunopositive for COX1 or COX2 were counted in different brain regions. A dramatic increase in the number of microglia, macrophages and multinucleated giant cells expressing COX1 was observed in the frontal, parietal and occipital cortices, striatum and corpus callosum of SIV,+AIDS monkeys as compared to the animals of the Ctrl and SIV,-AIDS groups (Table 2). COX2-expressing monocyte-derived cells were almost exclusively found and counted in the SIV,+AIDS group as compared to Ctrl and SIV,-AIDS (Table 2). Treatment with 6-Cl-ddG significantly reduced the number of COX1-positive monocyte-derived cells to levels as observed between the SIV,-AIDS and SIV,+AIDS groups, and the number of COX2-positive monocyte-derived cells was decreased to levels of Ctrl and SIV,-AIDS (Table 2).
Table 2.
Numbers of monocyte-derived cells immunopositive for COX1 and COX2 in different brain regions during SIV infection and antiretroviral treatment.
| Brain regionsa | Ctrl | SIV,−AIDS | SIV,+AIDS | SIV,+AIDS,+ddG |
|---|---|---|---|---|
| COX1 | ||||
| Frontal cortex | 21.8±3.2 | 22.4±2.5 | 49.6±8.4b | 35.1±5.1c |
| Parietal cortex | 20.8±1.9 | 19.7±3.8 | 53.4±5.8b | 33.5±3.5c |
| Occipital cortex | 19.5±2.5 | 21.6±3.1 | 47.4±6.7b | 30.6±4.2 |
| Striatum | 23.1±1.1 | 24.4±2.6 | 55.4±9.1b | 36.1±6.2c |
| Corpus callosum | 21.6±2.4 | 20.5±3.1 | 50.2±10.2b | 32.4±4.8c |
| COX2 | ||||
| Frontal cortex | 0 | 0.1 | 9.7±2.4b | 0.6±0.1c |
| Parietal cortex | 0 | 0 | 8.2±4.7b | 0.3±0.2 |
| Occipital cortex | 0 | 0 | 7.1±3.8b | 0.1±0.1 |
| Striatum | 0 | 0.2±0.1 | 11.2±5.3b | 1.8±0.7c |
| Corpus callosum | 0 | 0.1±0.1 | 8.8±4.1b | 1.1±0.4c |
Abbreviations: AIDS, acquired immunodeficiency syndrome; COX1/2, cyclooxygenase isotype-1/2; Ctrl, control; ddG, 6-chloro-2',3'-dideoxyguanosine; SIV, simian immunodeficiency virus.
Data expressed as mean number (± s.e.m.) of COX1- and COX2-positive cells per 0.1 mm2 area.
Statistically significant different as compared to the other animal groups for the same brain area (p<0.05).
Statistically significant different only as compared to Ctrl and SIV,-AIDS groups for the same brain area (p<0.05). ANOVA and the post-hoc Newman-Keuls Multiple Comparison Test are used to evaluate statistical differences.
Partial co-expression of COX2 with COX1 and SIV in rhesus macaque brain in late stage lentiviral infection
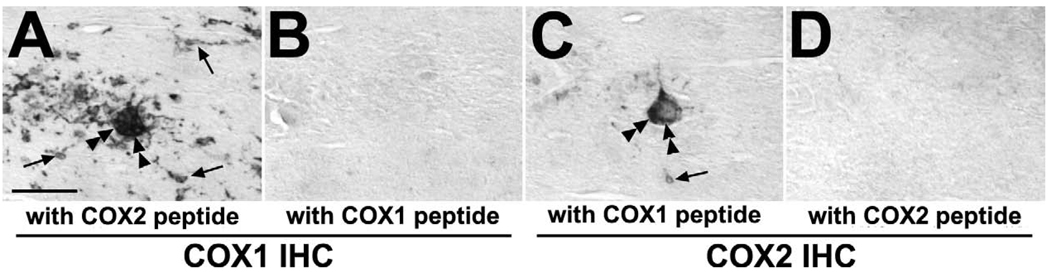
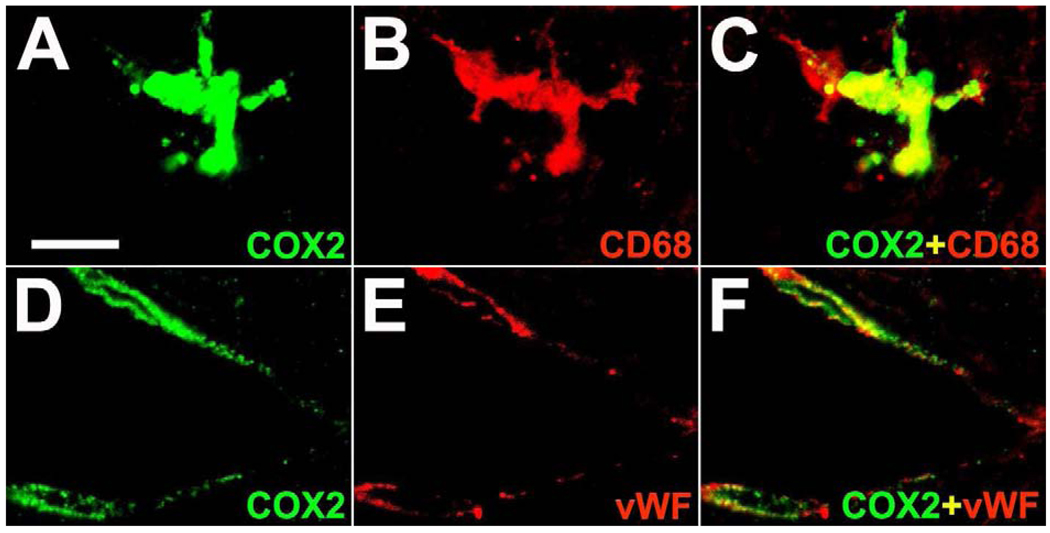
Double immunofluorescence analysis revealed that COX2 induction occurred in CD68-positive macrophages forming nodules and syncytia as well as in some vWF-positive endothelial cells in the brain of monkeys of the SIV,+AIDS group (Figure 5 A–F). Furthermore, as identified by alternate staining on adjacent sections COX1 and COX2 expressions were partially co-localized in macrophages, multinucleated giant cells and endothelial cells in the brain of monkeys of the SIV,+AIDS group (Figure 6 A, C; see also Figure 4 C, G). Immunohistochemical staining of COX1 and COX2 on brain tissue sections of these monkeys were specific as shown by preabsorption control experiments (Figure 6 A–D).
Fig. 5.
Representative high-power confocal double immunofluorescence images reveal expression of COX2 (green) in CD68-positive cells (red), a marker for activated monocyte-derived cells (A–C), and in von Willebrand factor (vWF)-positive endothelial cells (D–F) in the brain of a monkey with aquired immunodeficiency syndrome and encephalitis. Single immunofluorescence images (green and red) are merged (yellow; C, F). Scale bars = (A–F) 10 µm.
Fig. 6.
Specificity of COX1 and COX2 antibodies in simian immunodeficiency virus (SIV)-induced encephalitis. (A–D) As shown in images of serial sections with immunohistochemistry (IHC) against COX1 (A, B) and COX2 (C, D), stainings are not preabsorbed with excess heterologous peptides (A, C) but with their cognate homologous peptides (B, D), respectively, confirming that the antibodies specifically detect the partially overlapped expression of COX1 and COX2 in macrophages (arrows) and multinucleated giant cells (double arrow heads) in SIV-induced encephalitis as demonstrated on adjacent sections (A, C). Scale bars = (A–D) 50 µm.
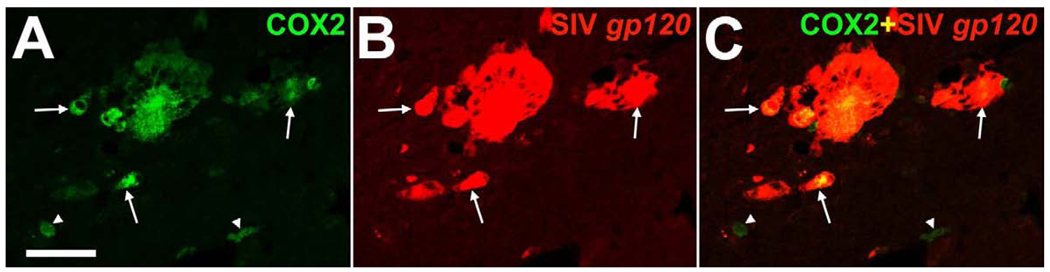
To assess the relationship of COX2 with SIV burden in the encephalitic brain, double immunofluorescence co-staining of COX2 with SIV glycoprotein gp120 was performed (Kent et al., 1992). Expression of COX2 co-incided majorly with SIV in macrophages and multinucleated giant cells or was in local contact with such SIV-infected cells in the brain of SIV,+AIDS monkeys (Figure 7 A–C).
Fig. 7.
Representative confocal double immunofluorescence images demonstrate major localization of COX2 (green) with simian immunodeficiency virus (SIV) protein gp120 (red) in macrophages and multinucleated giant cells (arrows) in the brain of a monkey with aquired immunodeficiency syndrome and encephalitis (A–C). Note that singular SIV gp120-negative macrophages express COX2 (arrow heads; A–C). Single immunofluorescence images (green and red) are merged (yellow; C). Scale bars = (A–C) 50 µm.
Discussion
The essential findings of this study are as follows: 1) both isoforms of COX are spatially and temporally differentially expressed and regulated in non-neuronal cellular compartments in rhesus macaque brain in health and during SIV infection; 2) COX1 can be considered to be a constitutive cellular marker of microglia and endothelial cells, and COX1 was globally upregulated during encephalitis due to increased proliferation, mobilization and infiltration of microglia/macrophages in the brain; 3) in contrast, COX2 was induced in a subset of microglia/macrophages and endothelial cells in inflammatory foci with SIV burden; and 4) the susceptibility to antiretroviral treatment with 6-Cl-ddG demonstrated that focal COX1 and COX2 reactions were directly related to brain SIV burden.
Differential COX1 and COX2 expression in microglia/macrophages and endothelial cells in health and SIV infection
Constitutive COX1 expression in microglia and endothelial cells suggest that microglia and endothelial cells continuously require or generate basal levels of lipid mediators possibly for physiological tissue homeostasis (Miller, 2006). In contrast, induction of COX2 in microglia and endothelial cells indicate that both cell types are able to increase the production of lipid mediators upon stimulation in pathological conditions (Miller, 2006). Focused induction of COX2 may be a consequence of local elevation of pro-inflammatory cytokines like IL-1 and TNF-alpha, or production of gp120 by SIV-infected cells (Wahl et al., 1989; Jones et al., 1993). Thus, SIV-induced COX2 biosynthesis may be both initiated and sustained by these pro-inflammatory cytokines known to be increased in the brain during SIV infection (Orandle et al., 2002). Direct cellular interactions of SIV-infected macrophages with brain endothelial cells during brain infiltration might enhance COX2 expression at the BBB (Pereira et al., 2000). Consistently, we observed that COX2 was majorly induced in SIV-positive macrophages and multinucleated giant cells or were in mutual contact with such cells.
Role of non-neuronal COX1 and COX2 expression in SIV infection
Localization of COX1 and COX2 to areas of SIV burden implies that COX-mediated prostaglandin generation is highly restricted rather than globally activated in SIV-induced encephalitis. Focused elevated levels of prostaglandins might mobilize neighboring microglia and/or direct monocyte-derived cells from the blood stream to enter the parenchyma (Legler et al., 2006; Tajima et al., 2008) through BBB leakages (Schmidley et al., 1992) to enhance brain inflammatory reactions. Accordingly, we found that monocytic infiltrates and macrophage nodules were overlapping with focal COX1 and COX2 expression in the encephalitic monkey brain.
Elevated levels of prostaglandins have been found in the cerebrospinal fluid of patients with HIV-associated dementia and have been linked with the severity of cognitive impairment. Because COX1 mRNA was found to be upregulated approximately 2-fold in HIV-demented compared with the non-demented patients, whereas COX2 mRNA expression was net unchanged, the increase in the prostaglandin levels was likely to be selectively mediated by COX1 (Griffin et al., 1994). The neuropathological hallmark of HIV-associated dementia is the presence of microgliosis as well as multinucleated giant cells (Budka, 1986; Glass et al., 1995), as found in SIV-induced encephalitis. Thus, our study and that of Griffin et al. suggest that COX1 might be a major neuroinflammatory player and source of prostaglandin synthesis in lentiviral encephalopathy.
As prostaglandins are also known to be toxic to neurons (Kawano et al., 2006; Takadera and Ohyashiki, 2006), how does neurodegeneration during SIV disease originate from these foci of increased prostaglandin production and spread diffusely throughout the brain? In part, this may be due to the highly diffusible, lipophilic character of prostaglandins. Activation of COX2 is functionally coupled to that of indoleamine-2,3 dioxygenase (IDO) and inducible nitric oxide synthase (iNOS) (Alberati-Giani et al., 1997; Ye et al., 2008; Jung et al., 2010). Like COX2, IDO and iNOS are upregulated by pro-inflammatory cytokines (Takikawa et al., 1988; Melillo et al., 1994) and found to be increased in areas of SIV replication (Li et al., 1999; Depboylu et al., 2004). Expression of IDO and iNOS results in the generation of highly diffusible quinolinic acid and NO, which may synergize with prostaglandins to exacerbate neuronal damage from sites of their synthesis. Furthermore, it was reported that genetic deletion of COX1 significantly reduced lipopolysaccharide-induced expression of both superoxide (O2-) and NO-forming enzymes and subsequent brain injury secondary to inflammation (Choi et al., 2008). Although, the precise mechanism(s) by which COX1 regulated free radical-generating enzymes in inflammatory cascade have not been clearly established, it is possible that because of its predominant localization in microglia, COX1 can modulate the induction of O2-, as well as NO, from NADPH oxidase and iNOS, which, in turn, can enhance the production of more potent free radicals such as peroxynitrite (ONOO-). In addition, O2- and NO act as potent cell signaling molecules and amplify production of TNF-alpha and prostaglandins by upregulation of COX2 (Qin et al., 2004). These initial effects combined with the activation of secondary signaling cascades might activate a robust immune response that consequently causes neuronal damage and death. Finally, COX2 induction may enhance glutamate-mediated neurotoxicity (Sang et al., 2007), further augmenting the wide-spread neurotoxicity of prostaglandins. The location and intensity of increased COX1 as well as COX2 expression shown here should be useful for interpreting prostaglandin neurotoxicity as arising from either COX1 or COX2 expression, depending on stage of disease and brain region affected.
Reversible and irreversible changes of COX1 and COX2 expression in SIV infection
Brain-permeant antiretroviral treatment with 6-Cl-ddG has previously allowed us to identify 2 distinct types of SIV-induced inflammatory changes (Depboylu et al., 2004, 2005, 2006, 2007), manifested in altered COX expression. The 1st is represented by the global non-neuronal increase in COX1 expression, which was generally resistant to antiretroviral treatment. The 2nd is the focal non-neuronal COX2 expression which is reversible by antiretroviral treatment. Recent evidence indicates that COX1 and COX2 have distinct roles in initiation and maintenance of immune responses like microglial activation, inflammatory cell infiltration, BBB dysruption, and expression of inflammatory mediators (Aid et al., 2009; Choi et al., 2009a, b). Inhibition of COX1 activity was suggested to be beneficial whereas COX2 inhibition detrimental during acute neuroinflammation (Choi et al., 2009b). Thus, increased COX1 expression in diffusely distributed microglia is most likely a sign of ongoing (neurodegeneration-associated) inflammation becoming irreversible and harmful at some point in chronic SIV disease at which antiretroviral treatment is insufficient.
Concluding remarks
Our present and previous results (Depboylu et al., 2004, 2005, 2006, 2007), and that of others (Witwer et al., 2009), suggest that early initiation of (brain-penetrating) antiretroviral therapy should be the primary treatment strategy in lentiviral encephalopathy to sustain control of lentivirus-associated inflammation and damage in the brain. To date, COX1-selective antagonistic treatment options are inadvisable as further studies are needed dealing with the role of COX1 in microglia and possibly in neurons in lentiviral brain disease. Finally, attention should be paid to prostaglandin receptors that are expressed on multiple cell types in the brain and mediating distinct and functionally antagonistic effects (Shie et al., 2005; Sugimoto and Narumiya, 2007).
Acknowledgements
The financial support of the Volkswagen-Stiftung is gratefully acknowledged. This project was supported in part by the NIMH-IRP. We thank Hiroaki Mitsuya of the Experimental Retrovirology Section, HIV and AIDS Malignancy Branch, Center for Clinical Research, NCI, NIH for his collaboration in the initial stages of this project and continued interest in it. We are especially grateful to Dianne Rausch, Division of Mental Disorders, Behavioral Research and AIDS, NIMH, for advice and encouragement in the conduct of the work, and the preparation of this manuscript. The National Institutes of Health AIDS Research and Reference Program kindly provided us with antibodies against SIV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aid S, Langenbach R, Bosetti F. Neuroinflammatory response to lipopolysaccharide is exacerbated in mice genetically deficient in cyclooxygenase-2. J. Neuroinflammation. 2008;5:17. doi: 10.1186/1742-2094-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aid S, Silva AC, Candelario-Jalil E, Choi SH, Rosenberg GA, Bosetti F. Cyclooxygenase-1 and −2 differentially modulate lipopolysaccharide-induced blood-brain barrier disruption through matrix metalloproteinase activity. J. Cereb. Blood Flow Metab. 2010;30:370–380. doi: 10.1038/jcbfm.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberati-Giani D, Malherbe P, Ricciardi-Castagnoli P, Köhler C, Denis-Donini S, Cesura AM. Differential regulation of indoleamine 2,3-dioxygenase expression by nitric oxide and inflammatory mediators in IFN-gamma-activated murine macrophages and microglial cells. J. Immunol. 1997;159:419–426. [PubMed] [Google Scholar]
- Bissel SJ, Wang G, Ghosh M, Reinhart TA, Capuano S, 3rd, Stefano Cole K, Murphey-Corb M, Piatak M, Jr, Lifson JD, Wiley CA. Macrophages relate presynaptic and postsynaptic damage in simian immunodeficiency virus encephalitis. Am. J. Pathol. 2002;160:927–941. doi: 10.1016/S0002-9440(10)64915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosetti F. Arachidonic acid metabolism in brain physiology and pathology: lessons from genetically altered mouse models. J. Neurochem. 2007;102:577–586. doi: 10.1111/j.1471-4159.2007.04558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breder CD, Smith WL, Raz A, Masferrer J, Seibert K, Needleman P, Saper CB. Distribution and characterization of cyclooxygenase immunoreactivity in the ovine brain. J. Comp. Neurol. 1992;322:409–438. doi: 10.1002/cne.903220309. [DOI] [PubMed] [Google Scholar]
- Breder CD, Dewitt D, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J. Comp. Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budka H. Multinucleated giant cells in brain: a hallmark of the acquired immune deficiency syndrome (AIDS) Acta Neuropathol. 1986;69:253–258. doi: 10.1007/BF00688301. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, de Oliveira AC, Gräf S, Bhatia HS, Hüll M, Muñoz E, Fiebich BL. Resveratrol potently reduces prostaglandin E2 production and free radical formation in lipopolysaccharide-activated primary rat microglia. J. Neuroinflammation. 2007;4:25. doi: 10.1186/1742-2094-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Langenbach R, Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 2008;22:1491–1501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and −2 in neuroinflammation: implications for translational research. Trends Pharmacol. Sci. 2009a;30:174–181. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Aid S, Choi U, Bosetti F. Cyclooxygenases-1 and −2 differentially modulate leukocyte recruitment into the inflamed brain. Pharmacogenomics J. 2009b Dec 29; doi: 10.1038/tpj.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depboylu C, Reinhart TA, Takikawa O, Imai Y, Maeda H, Mitsuya H, Rausch D, Eiden LE, Weihe E. Brain virus burden and indoleamine-2,3-dioxygenase expression during lentiviral infection of rhesus monkey are concomitantly lowered by 6-chloro-2',3'-dideoxyguanosine. Eur. J. Neurosci. 2004;19:2997–3005. doi: 10.1111/j.0953-816X.2004.03404.x. [DOI] [PubMed] [Google Scholar]
- Depboylu C, Schäfer MK, Schwaeble WJ, Reinhart TA, Maeda H, Mitsuya H, Damadzic R, Rausch DM, Eiden LE, Weihe E. Increase of C1q biosynthesis in brain microglia and macrophages during lentivirus infection in the rhesus macaque is sensitive to antiretroviral treatment with 6-chloro-2',3'-dideoxyguanosine. Neurobiol. Dis. 2005;20:12–26. doi: 10.1016/j.nbd.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Depboylu C, Eiden LE, Schäfer MK, Reinhart TA, Mitsuya H, Schall TJ, Weihe E. Fractalkine expression in the rhesus monkey brain during lentivirus infection and its control by 6-chloro-2',3'-dideoxyguanosine. J. Neuropathol. Exp. Neurol. 2006;65:1170–1180. doi: 10.1097/01.jnen.0000248550.22585.5e. [DOI] [PubMed] [Google Scholar]
- Depboylu C, Eiden LE, Weihe E. Increased APOBEC3G expression is associated with extensive G-to-A hypermutation in viral DNA in rhesus macaque brain during lentiviral infection. J. Neuropathol. Exp. Neurol. 2007;66:901–912. doi: 10.1097/nen.0b013e3181567a59. [DOI] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann. Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Griffin DE, Wesselingh SL, McArthur JC. Elevated central nervous system prostaglandins in human immunodeficiency virus-associated dementia. Ann. Neurol. 1994;35:592–597. doi: 10.1002/ana.410350513. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJ, Rozemuller JM, van Haastert ES, Veerhuis R, Eikelenboom P. Cyclooxygenase-1 and −2 in the different stages of Alzheimer's disease pathology. Curr. Pharm. Des. 2008;14:1419–1427. doi: 10.2174/138161208784480171. [DOI] [PubMed] [Google Scholar]
- Jones DA, Carlton DP, McIntyre TM, Zimmerman GA, Prescott SM. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J. Biol. Chem. 1993;268:9049–9054. [PubMed] [Google Scholar]
- Jung ID, Jeong YI, Lee CM, Noh KT, Jeong SK, Chun SH, Choi OH, Park WS, Han J, Shin YK, Kim HW, Yun CH, Park YM. COX-2 and PGE2 signaling is essential for the regulation of IDO expression by curcumin in murine bone marrow-derived dendritic cells. Int. Immunopharmacol. 2010 Apr 22; doi: 10.1016/j.intimp.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat. Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- Kent KA, Rud E, Corcoran T, Powell C, Thiriart C, Collignon C, Stott EJ. Identification of two neutralizing and 8 non-neutralizing epitopes on simian immunodeficiency virus envelope using monoclonal antibodies. AIDS Res. Hum. Retroviruses. 1992;8:1147–1151. doi: 10.1089/aid.1992.8.1147. [DOI] [PubMed] [Google Scholar]
- Lane JH, Sasseville VG, Smith MO, Vogel P, Pauley DR, Heyes MP, Lackner AA. Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation. J. Neurovirol. 1996;2:423–432. doi: 10.3109/13550289609146909. [DOI] [PubMed] [Google Scholar]
- Legler DF, Krause P, Scandella E, Singer E, Groettrup M. Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. J. Immunol. 2006;176:966–973. doi: 10.4049/jimmunol.176.2.966. [DOI] [PubMed] [Google Scholar]
- Li Q, Eiden LE, Cavert W, Reinhart TA, Rausch DM, Murray EA, Weihe E, Haase AT. Increased expression of nitric oxide synthase and dendritic injury in simian immunodeficiency virus encephalitis. J. Hum. Virol. 1999;2:139–145. [PubMed] [Google Scholar]
- Luabeya MK, Dallasta LM, Achim CL, Pauza CD, Hamilton RL. Blood-brain barrier disruption in simian immunodeficiency virus encephalitis. Neuropathol. Appl. Neurobiol. 2000;26:454–462. doi: 10.1046/j.1365-2990.2000.00275.x. [DOI] [PubMed] [Google Scholar]
- Luthert PJ, Montgomery MM, Dean AF, Cook RW, Baskerville A, Lantos PL. Hippocampal neuronal atrophy occurs in rhesus macaques following infection with simian immunodeficiency virus. Neuropathol. Appl. Neurobiol. 1995;21:529–534. doi: 10.1111/j.1365-2990.1995.tb01099.x. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Probst-Cousin S, Bergmann M, Neuhuber W, Neundörfer B, Heuss D. Expression and localization of cyclooxygenase-1 and −2 in human sporadic amyotrophic lateral sclerosis. Eur. J. Neurosci. 2003;18:1527–1534. doi: 10.1046/j.1460-9568.2003.02879.x. [DOI] [PubMed] [Google Scholar]
- Melillo G, Cox GW, Biragyn A, Sheffler LA, Varesio L. Regulation of nitric-oxide synthase mRNA expression by interferon-gamma and picolinic acid. J. Biol. Chem. 1994;269:8128–8133. [PubMed] [Google Scholar]
- Miller SB. Prostaglandins in health and disease: an overview. Semin. Arthritis Rheum. 2006;36:37–49. doi: 10.1016/j.semarthrit.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog. Lipid Res. 2004;43:3–35. doi: 10.1016/s0163-7827(03)00037-7. [DOI] [PubMed] [Google Scholar]
- Murray EA, Rausch DM, Lendvay J, Sharer LR, Eiden LE. Cognitive and motor impairments associated with SIV infection in rhesus monkeys. Science. 1992;255:1246–1249. doi: 10.1126/science.1546323. [DOI] [PubMed] [Google Scholar]
- Orandle MS, MacLean AG, Sasseville VG, Alvarez X, Lackner AA. Enhanced expression of proinflammatory cytokines in the central nervous system is associated with neuroinvasion by simian immunodeficiency virus and the development of encephalitis. J. Virol. 2002;76:5797–5802. doi: 10.1128/JVI.76.11.5797-5802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepicelli O, Fedele E, Berardi M, Raiteri M, Levi G, Greco A, Ajmone-Cat MA, Minghetti L. Cyclo-oxygenase-1 and −2 differently contribute to prostaglandin E2 synthesis and lipid peroxidation after in vivo activation of N-methyl-D-aspartate receptors in rat hippocampus. J Neurochem. 2005;93:1561–1567. doi: 10.1111/j.1471-4159.2005.03150.x. [DOI] [PubMed] [Google Scholar]
- Pereira CF, Boven LA, Middel J, Verhoef J, Nottet HS. Induction of cyclooxygenase-2 expression during HIV-1-infected monocyte-derived macrophage and human brain microvascular endothelial cell interactions. J. Leukoc. Biol. 2000;68:423–428. [PubMed] [Google Scholar]
- Phillis JW, Horrocks LA, Farooqui AA. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res. Rev. 2006;52:201–243. doi: 10.1016/j.brainresrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Price DL, Martin LJ, Clatterbuck RE, Koliatsos VE, Sisodia SS, Walker LC, Cork LC. Neuronal degeneration in human diseases and animal models. J. Neurobiol. 1992;23:1277–1294. doi: 10.1002/neu.480230916. [DOI] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Chen C. COX-2 oxidative metabolite of endocannabinoid 2-AG enhances excitatory glutamatergic synaptic transmission and induces neurotoxicity. J. Neurochem. 2007;102:1966–1977. doi: 10.1111/j.1471-4159.2007.04668.x. [DOI] [PubMed] [Google Scholar]
- Schmidley JW, Dadson J, Iyer RS, Salomon RG. Brain tissue injury and blood-brain barrier opening induced by injection of LGE2 or PGE2. Prostaglandins Leukot. Essent. Fatty Acids. 1992;47:105–110. doi: 10.1016/0952-3278(92)90145-9. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Beschorner R, Meyermann R, Gözalan F, Schluesener HJ. Persistent accumulation of cyclooxygenase-1-expressing microglial cells and macrophages and transient upregulation by endothelium in human brain injury. J Neurosurg. 2002;96:892–899. doi: 10.3171/jns.2002.96.5.0892. [DOI] [PubMed] [Google Scholar]
- Shie FS, Montine KS, Breyer RM, Montine TJ. Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia. 2005;52:70–77. doi: 10.1002/glia.20220. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S. Prostaglandin E receptors. J. Biol. Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- Tajima T, Murata T, Aritake K, Urade Y, Hirai H, Nakamura M, Ozaki H, Hori M. Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2) J. Pharmacol. Exp. Ther. 2008;326:493–501. doi: 10.1124/jpet.108.137992. [DOI] [PubMed] [Google Scholar]
- Takadera T, Ohyashiki T. Prostaglandin E2 deteriorates N-methyl-D-aspartate receptor-mediated cytotoxicity possibly by activating EP2 receptors in cultured cortical neurons. Life Sci. 2006;78:1878–1883. doi: 10.1016/j.lfs.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J. Biol. Chem. 1988;263:2041–2048. [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubokura S, Watanabe Y, Ehara H, Imamura K, Sugimoto O, Kagamiyama H, Yamamoto S, Hayaishi O. Localization of prostaglandin endoperoxide synthase in neurons and glia in monkey brain. Brain Res. 1991;543:15–24. doi: 10.1016/0006-8993(91)91043-z. [DOI] [PubMed] [Google Scholar]
- Wahl LM, Corcoran ML, Pyle SW, Arthur LO, Harel-Bellan A, Farrar WL. Human immunodeficiency virus glycoprotein (gp120) induction of monocyte arachidonic acid metabolites and interleukin 1. Proc. Natl. Acad. Sci. U. S. A. 1989;86:621–625. doi: 10.1073/pnas.86.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed MR, Gold LH, Polis I, Koob GF, Fox HS, Taffe MA. Impaired performance on a rhesus monkey neuropsychological testing battery following simian immunodeficiency virus infection. AIDS Res. Hum. Retroviruses. 2004;20:77–89. doi: 10.1089/088922204322749521. [DOI] [PubMed] [Google Scholar]
- Weihe E, Nohr D, Sharer L, Murray E, Rausch D, Eiden L. Cortical astrocytosis in juvenile rhesus monkeys infected with simian immunodeficiency virus. Neuroreport. 1993;4:263–266. doi: 10.1097/00001756-199303000-00009. [DOI] [PubMed] [Google Scholar]
- Williams R, Bokhari S, Silverstein P, Pinson D, Kumar A, Buch S. Nonhuman primate models of NeuroAIDS. J Neurovirol. 2008;14:292–300. doi: 10.1080/13550280802074539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer KW, Gama L, Li M, Bartizal CM, Queen SE, Varrone JJ, Brice AK, Graham DR, Tarwater PM, Mankowski JL, Zink MC, Clements JE. Coordinated regulation of SIV replication and immune responses in the CNS. PLoS One. 2009;4:e8129. doi: 10.1371/journal.pone.0008129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synapticactivity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Ye Y, Martinez JD, Perez-Polo RJ, Lin Y, Uretsky BF, Birnbaum Y. The role of eNOS, iNOS, and NF-kappaB in upregulation and activation of cyclooxygenase-2 and infarct size reduction by atorvastatin. Am. J. Physiol. Heart Circ. Physiol. 2008;295:343–351. doi: 10.1152/ajpheart.01350.2007. [DOI] [PubMed] [Google Scholar]