Dear Editor:
In the past few years, compounding pharmacies and pharmaceutical manufacturers have commercialized ready-to-use preservative-free triamcinolone acetonide (PFTA). PFTA marketing is fueled by the potential safety issues with the off-label intravitreous (IV) injection of Kenalog (TA; Bristol-Myers Squibb, Princeton, NJ), which is not specifically formulated for the eye and contains benzalkonium chloride (BAK), a toxic preservative associated with sterile endophthalmitis.1 Compounded PFTA (C-PFTA) was reported as a safe and effective replacement for TA,2 yet it can be prepared by many techniques, each with variable results on yield, concentration, and stability.3,4 Prior to the availability of pharmaceutical-manufactured PFTA products (P-PFTA, e.g. Triesence; Alcon, Fort Worth, TX), we purchased several C-PFTA vials from a regional compounding pharmacy. During the utilization of a particular lot of C-PFTA, we observed a dramatic increase in acute and severe elevations in intraocular pressure (IOP) after IV injection.
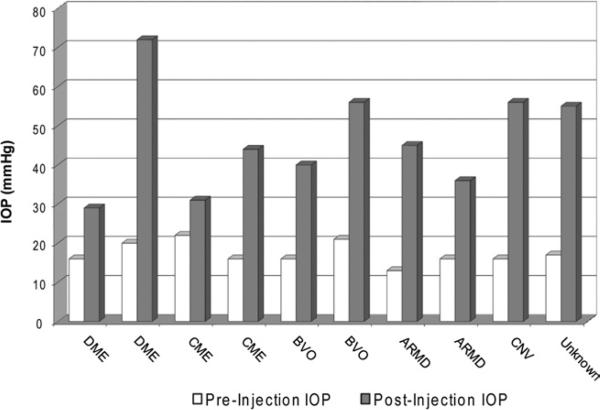
In 1 month, 28 eyes with severe retinal disease were treated with IV C-PFTA (vials derived from single lot). None of the patients had a history of glaucoma or uveitis. We were surprised that 10 patients (35.7%) had significantly elevated IOP (≥29 mmHg) at 7 days with an average increase of 29.1±3.1 mmHg from baseline (P<0.001, Mann-Whitney U) (Fig. 1, available at http://aaojournal.org). An acute rise (within 72 hours) was evident in 5 patients, and 2 required filtering surgery. No pseudo-hypopyon or angle deposition of particulate matter was observed.
Figure 1.
Ten of 28 eyes from patients with various retinal diseases (diabetic macular edema, cystoid macular edema, age-related macular degeneration, branch vein occlusion, and non-AMD related choroidal neovascularization, CNV) were treated with a single injection of IV C-PFTA and developed acute and severe elevations in IOP measuring 29.1±13.1 mmHg from baseline. ARMD = age-related macular degeneration; BVO = branch vein occlusion; CME = cystoid macular edema; CNV = choroidal neovascularization; C-PFTA = compounded PFTA; DME = diabetic macular edema; IOP = intraocular pressure; IV = intravitreous.
In response to this case series, we initiated an investigation of the affected C-PFTA lot. Firstly, its usage was immediately halted, and we contacted the regional compounding pharmacy to report the events. The manufacturer re-tested the lot both in-house and at an independent laboratory with confirmation that there were no abnormal levels of contaminants or endotoxin. Given that C-PFTA preparation methods have been hampered by wide variability, we conducted particle characterization analyses to determine C-PFTA crystal size distributions compared with TA controls.
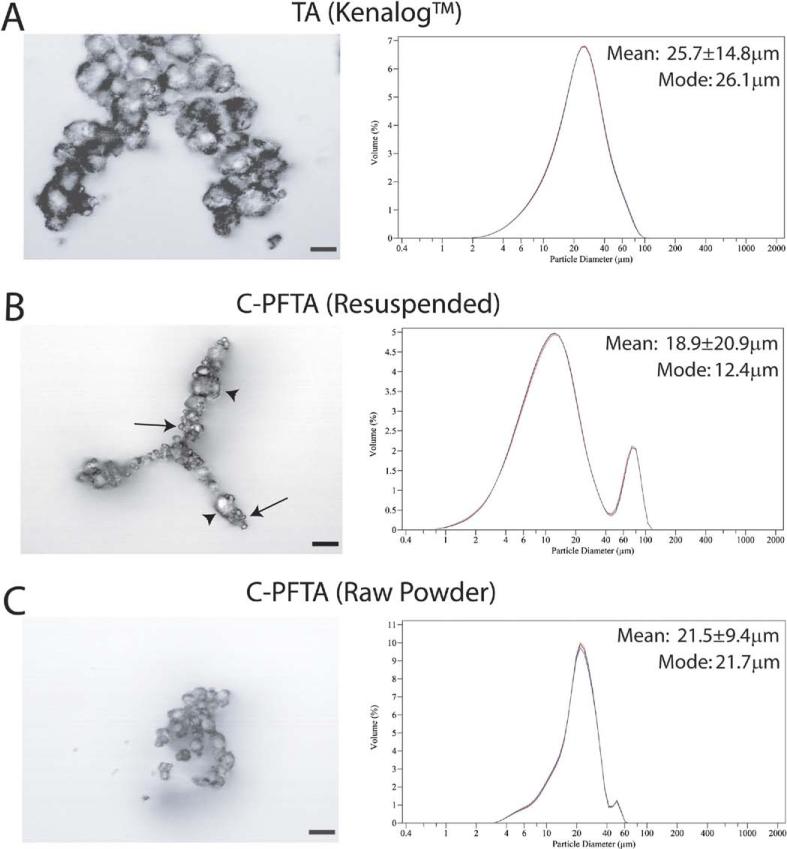
TA control samples examined with phase-contrast microscopy revealed an average particle diameter of 11.8±1.2 μm and the formation of large (100-200 μm) aggregates. Using a more advanced laser diffraction technique (Beckman Coulter [Brea, CA], LS100Q), we observed unimodal (26.1 μm) crystal size distributions in TA samples with 80% of particle diameters measuring between 10 μm and 45 μm without aggregates. In contrast, microscopic studies of the affected C-PFTA samples and stock powder showed large aggregates and significantly decreased average particle diameters of 4.6±0.7 μm and 5.3±0.7 μm, respectively (P<0.001). Laser diffraction analyses of C-PFTA demonstrated smaller crystal size distributions (mode 12.4 μm) with 80% of particles between 4.2 μm and 55.0 μm and <10% of total particle population forming aggregates between 50-120 μm (Fig. 2, available at http://aaojournal.org). Raw stock powder showed slightly decreased differences (mode 21.7 μm).
Figure 2.
Crystal size analyses of TA (Kenalog) and C-PFTA samples were conducted using techniques in phase-contrast microscopy (left) and high-accuracy laser diffraction (right). With microscopy, TA crystals appeared uniform in aggregates (A) in comparison to the mix of small (arrows) and large (arrowheads) crystal sizes in the C-PFTA samples of the affected lot (B) and the raw stock powder (C) (scale bar, 20 μm). With the more advanced laser diffraction technique, TA crystal diameter distributions were unimodal with a peak at 26.1 μm, whereas studies of C-PFTA samples of the affected lot and the raw stock powder demonstrate decreased particle size distributions and increased aggregate formation. C-PFTA = compounded PFTA; PFTA = preservative-free triamcinolone acetonide; TA = Kenalog.
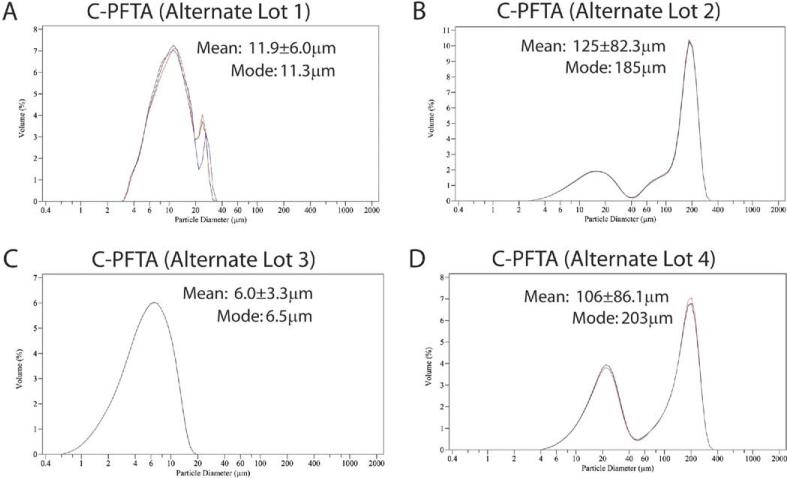
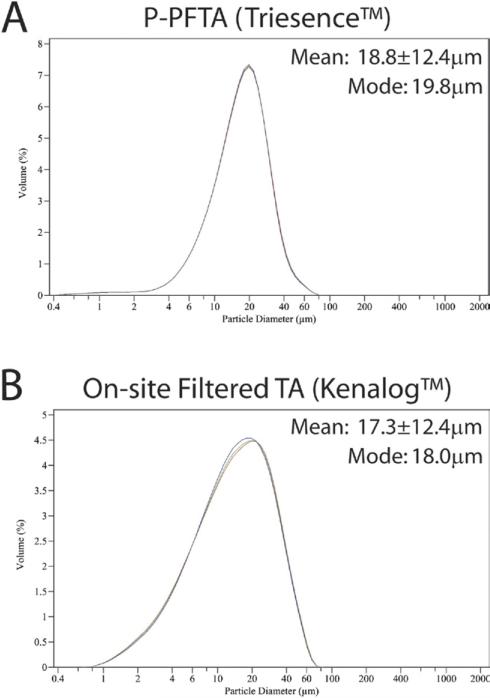
We then purchased 3 additional samples from 2 different major compounding pharmacies, as well as P-PFTA (Triesence) for analysis. For comparison, crystal size distributions for TA both in vehicle and washed in balanced salt solution (BSS) were evaluated. Strikingly, reduced crystal sizes and highly variable aggregation were observed in all C-PFTA samples tested (Fig. 3, available at http://aaojournal.org). Two of these samples exhibited submicron crystal populations ranging from 0.5 to 4 μm, but these had been previously administered by us without such adverse effects. Our finding of increased particle aggregates in PFTA samples supports a recently published article,5 but we observed these aggregates in all of the C-PFTA samples tested suggesting that they may not be the issue. Instead, we hypothesized that the modal distribution of particles (~12 μm) in the affected lot may physically obstruct the trabecular meshwork resulting in acute and severe IOP spikes. In our final studies, we found that P-PFTA and TA washed and resuspended in BSS also showed reduced particle diameters compared with TA in vehicle (P<0.01) but with improved retention of unimodal distributions and minimal aggregate formation (Fig. 4, available at http://aaojournal.org).
Figure 3.
Further laser diffraction studies conducted on other commercial sources of C-PFTA demonstrated wide variability with regards to crystal size distributions and aggregate formation; however, a trend of decreased individual crystal size was identified in all PFTA samples. C-PFTA = compounded PFTA; PFTA = preservative-free triamcinolone acetonide.
Figure 4.
Laser diffraction particle analyses of P-PFTA (Triesence) (A) and on-site pre-procedural filtered TA (B) revealed improved retention of unimodal distributions and crystal size parameters compared to the C-PFTA samples. C-PFTA = compounded PFTA; PFTA = preservative-free triamcinolone acetonide; P-PFTA = pharmaceutical manufactured PFTA; TA = Kenalog.
The question arose as to why these particle size variations in PFTA preparations had not been previously reported. In a follow-up study using a Hiac-Royco Particulate Analyzer (Pacific Scientific, Silver Spring, MD), a gold-standard in the pharmaceutical industry, no significant crystal size variations were detected between our C-PFTA samples. While there are fundamental differences in the detectors used by the 2 machines (light scatter versus obscuration), we suggest that in order to reliably assess deviations in PFTA crystal size populations and standardize uniformity, post-purification quality controls will need to be conducted with more highly sensitive particle sizing instruments.
References
- 1.Jonisch J, Lai JC, Deramo VA, et al. Increased incidence of sterile endophthalmitis following intravitreal preserved triamcinolone acetonide. Br J Ophthalmol. 2008;92:1051–4. doi: 10.1136/bjo.2007.136069. [DOI] [PubMed] [Google Scholar]
- 2.Kim H, Csaky KG, Gravlin L, et al. Safety and pharmacokinetics of a preservative-free triamcinolone acetonide formulation for intravitreal administration. Retina. 2006;26:523–30. doi: 10.1097/00006982-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Szurman P, Kaczmarek R, Jaissle GB, et al. Influence of different purification techniques on triamcinolone yield and particle size spectrum. Graefes Arch Clin Exp Ophthalmol. 2007;245:689–96. doi: 10.1007/s00417-006-0436-x. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Arumi J, Boixadera A, Giralt J, et al. Comparison of different techniques for purification of triamcinolone acetonide suspension for intravitreal use. Br J Ophthalmol. 2005;89:1112–4. doi: 10.1136/bjo.2005.067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moshfeghi AA, Nugent AK, Nomoto H, et al. Triamcinolone acetonide preparations: impact of crystal size on in vitro behavior. Retina. 2009;29:689–98. doi: 10.1097/IAE.0b013e31819e390a. [DOI] [PubMed] [Google Scholar]