Abstract
We performed a systematic mapping of interaction domains on COP I subunits to gain novel insights into the architecture of coatomer. Using the two-hybrid system, we characterize the domain structure of the α-, β′-, ε-COP and β-, γ-, δ-, ζ-COP coatomer subcomplexes and identify links between them that contribute to coatomer integrity. Our results demonstrate that the domain organization of the β-, γ-, δ-, ζ-COP subcomplex and AP adaptor complexes is related. Through in vivo analysis of α-COP truncation mutants, we characterize distinct functional domains on α-COP. Its N-terminal WD40 domain is dispensable for yeast cell viability and overall coatomer function, but is required for KKXX-dependent trafficking. The last ∼170 amino acids of α-COP are also non-essential for cell viability, but required for ε-COP incorporation into coatomer and maintainance of normal ε-COP levels. Further, we demonstrate novel direct interactions of coatomer subunits with regulatory proteins: β′- and γ-COP interact with the ARF-GTP-activating protein (GAP) Glo3p, but not Gcs1p, and β- and ε-COP interact with ARF-GTP. Glo3p also interacts with intact coatomer in vitro.
Keywords: ARF-GAP/clathrin/coatomer/COP I/Glo3/Golgi
Introduction
The COP I and COP II vesicle coats direct traffic between early compartments of the secretory pathway in eukaryotic cells (Rothman and Wieland, 1996; Schekman and Orci, 1996). COP II mediates selective protein export from the endoplasmic reticulum (ER), whereas COP I has a role in Golgi-to-ER retrieval (Letourneur et al., 1994). This model for COP I function is based on convergent genetic and biochemical evidence in yeast. First, coatomer from wild-type cells can interact with the KKXX trafficking motif (Cosson and Letourneur, 1994). Secondly, a genetic screen for mutants defective in KKXX retrieval (ret mutants) yielded mutants in α-, γ-, δ- and ζ-COP (Letourneur et al., 1994; Cosson et al., 1996). Thirdly, an α-COP mutant, ret1-1, displays a strong defect in the Golgi-to-ER retrieval of KKXX-tagged proteins even at permissive temperature. Coatomer from these ret1-1 cells is unable to interact with the KKXX motif (Letourneur et al., 1994). Additionally, COP I may function in intra-Golgi retrograde transport of Golgi enzymes (Lanoix et al., 1999) and transport of anterograde cargo within the Golgi complex (Orci et al., 1997).
The COP I coat consists of coatomer, a stable 700–800 kDa complex that is the cytosolic precursor of the coat, and the small ras-like GTPase, ARF, in its GTP-bound form. Coatomer comprises seven equimolar subunits, α-, β-, β′-, γ-, δ-, ε-RET1 (α), SEC26 (β), SEC27 (β′), SEC21 (γ), RET2 (δ), SEC28 (ε) and RET3 (ζ) (Gaynor et al., 1998). Each of these genes is essential for yeast cell viability except SEC28. ε-COP functions as a structural component of coatomer, stabilizing α-COP and thus coatomer at elevated temperatures (Duden et al., 1998).
Once assembled, coatomer is a stable complex with a half-life of ∼28 h in mammalian cells and no exchange of subunits (Lowe and Kreis, 1996). Using the two-hybrid system, four interacting pairs of coatomer subunits have been identified (Faulstich et al., 1996): β/δ-COPs, γ/ζ-COPs, α/ε-COPs and α/β′-COPs. In vitro, coatomer can be disintegrated in high salt buffers into subcomplexes that retain partial function (Cosson and Letourneur, 1994; Lowe and Kreis, 1995; Fiedler et al., 1996; Pavel et al., 1998). Under these conditions, a stable α-, β′-, ε-COP subcomplex is generated which interacts with KKXX motifs (Cosson and Letourneur, 1994; Lowe and Kreis, 1995). Further, a β-, γ-, δ-, ζ-COP subcomplex of coatomer may be generated (Fiedler et al., 1996), which tends to disassemble further into two stable heterodimers, consisting of β/δ COP and γ/ζ COP (Lowe and Kreis, 1995; Pavel et al., 1998). The β/δ COP heterodimer can bind to Golgi membranes in an ARF- and GTP-γ-S dependent manner (Pavel et al., 1998). The internal domain structure of both coatomer subcomplexes is unknown, as are the ‘links’ between them that maintain the structural integrity of coatomer.
The GTP/GDP cycle of ARF proteins is regulated, and is linked to the cycle of COP I coat recruitment, vesicle budding and uncoating. Upon binding of GTP, which is assisted by Golgi membrane-associated nucleotide exchange factors, ARF-GEFs (Jackson and Casanova, 2000), ARF undergoes a conformational change that exposes its myristoylated, hydrophobic N-terminus, allowing its membrane association. Binding of ARF-GTP to Golgi membranes leads to recruitment of coatomer, deformation of the membrane and budding of COP I vesicles (Rothman and Wieland, 1996).
In yeast, two ARF genes, ARF1 and ARF2 (96% identical), provide essential, overlapping functions in secretory protein traffic (Gaynor et al., 1998). GTP hydrolysis by ARF is a prerequisite for vesicle uncoating (Rothman and Wieland, 1996). Since ARFs possess a low intrinsic GTPase activity, GTPase-activating proteins (GAPs) are necessary for ARF function. Two yeast ARF-GAPs, Gcs1p and Glo3p, provide an overlapping essential function in retrograde Golgi-to-ER transport (Dogic et al., 1999; Poon et al., 1999). Interestingly, regulated GTP hydrolysis on ARF may also be required for cargo selection into COP I vesicles (Lanoix et al., 1999; Malsam et al., 1999).
We wish to understand the molecular architecture of coatomer and its interactions with regulatory molecules and cargo. In this study, we performed a systematic mapping of interaction domains on coatomer which are necessary for coatomer integrity. We further identify novel two-hybrid interactions of coatomer subunits with ARF-GTP and with the ARF-GAP, Glo3p.
Results
Molecular characterization of two unique α-COP mutations, ret1-3 and sec33-1
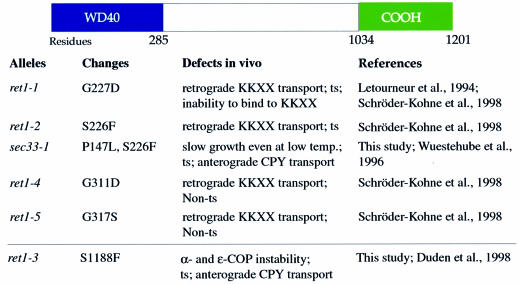
We wish to understand the function of individual domains on the four large coatomer subunits. α-COP and β′-COP across distant species both have a highly conserved N-terminal domain (∼285 residues) comprising six and five WD40 repeats, respectively. WD40 repeats are conserved sequence motifs predicted to fold into a structure composed of β-strands and turns, a so-called β-propeller (Neer and Smith, 2000). All α-COP point mutations characterized so far are closely spaced within 85 residues of α-COP, either within or immediately adjacent to the WD40 repeats (Figure 1). The ret1-1, ret1-2, ret1-4 and ret1-5 mutants were most illuminating as far as coatomer function is concerned, as they display a strong defect in KKXX retrieval in vivo, but no anterograde transport defect (Letourneur et al., 1994; Schröder-Kohne et al., 1998; see Figure 1). Based on these mutants, it has been suggested that the α-COP WD40 domain may be involved in interaction with COP I vesicle cargo, e.g. KKXX-tagged proteins (Schröder-Kohne et al., 1998).
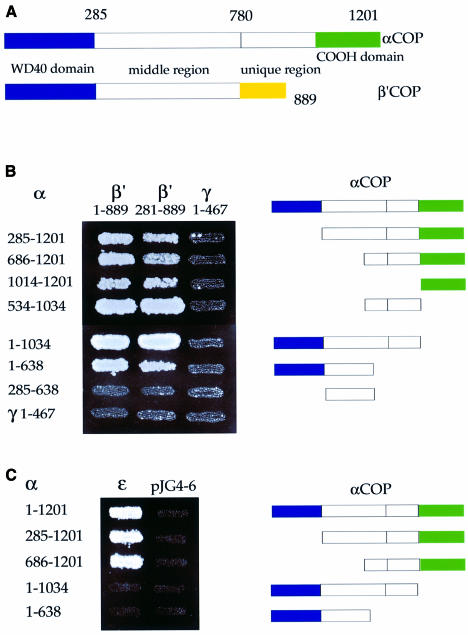
Fig. 1. A tentative domain structure of α-COP. The location of α-COP point mutations and the corresponding phenotypes of mutant α-COP strains are indicated. In this study, we show that the last ∼170 amino acids of α-COP are important for interactions with β′-COP and ε-COP, and thus refer to this region as the ‘C-terminal domain’.
Since the sequence of the remaining ∼900 residues on α-COP is uninformative, we considered whether as yet unsequenced α-COP point mutations might help us to identify α-COP domains. We previously described two yeast α-COP mutants with unusual phenotypes, sec33-1 and ret1-3 (Wuestehube et al., 1996; Duden et al., 1998). Both mutants display a forward transport defect for the secreted pheromone α-factor at restrictive temperature. sec33-1 cells grow very slowly and have a forward transport defect even at permissive temperature (Wuestehube et al., 1996). ret1-3 cells display structural alterations to coatomer, and α-COP degradation and forward transport defects at restrictive temperature; all these phenotypes can be rescued by overexpression of ε-COP (Duden et al., 1998).
We mapped and sequenced the sec33-1 and ret1-3 mutations using gap repair. sec33-1 harbours two mutations in the WD40 domain, changing proline at position 147 to leucine, and serine at position 226 to phenylalanine. The ret1-3 mutation, on the other hand, changes residue 1188 from serine to phenylalanine. Thus, ret1-3 is a unique α-COP mutation which is located at the C-terminus, and in this study we used this mutant to characterize a novel functional domain on α-COP. A tentative domain structure of α-COP is shown in Figure 1.
Since point mutations in the α-COP WD40 domain cause a broad range of phenotypes (Figure 1), it is possible that all existing mutants affecting this domain may only partially impair the function of the WD40 domain. We decided to investigate the role of the N- and C-terminal regions of α-COP in vivo using truncation mutants, thus analysing null phenotypes for these domains.
Novel α-COP mutants lacking the WD40 domain or a C-terminal region are viable
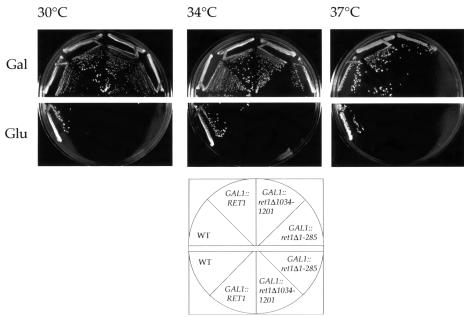
The RET1 gene is essential for yeast cell viability (Letourneur et al., 1994). To test whether the N- or C-terminal domain of α-COP is essential, we constructed strains in which the only copy of RET1 present was expressed under the control of the regulated GAL1 promoter from a single-copy plasmid. Two truncated versions of Ret1p, lacking either the entire WD40 domain (pGAL1::ret1Δ1–285) or the C-terminal ∼170 residues (pGAL1::ret1Δ1034–1201), supported yeast cell viability. Both α-COP mutants grow like wild-type cells up to 34°C (Figure 2). However, GAL1::ret1Δ1–285 cells are temperature-sensitive, ceasing growth at 37°C, whereas GAL1::ret1Δ1034–1201 cells grow like wild-type cells at this temperature (Figure 2).
Fig. 2. Novel α-COP mutants lacking the the WD40 domain or a C-terminal region are viable. GAL1::ret1Δ1–285 and GAL1::ret1Δ1034–1201 cells were streaked onto minimal plates containing either galactose (Gal) or glucose (Glu) and grown for 3 days at the temperatures indicated. Strains were, as indicated: wild-type control strain YAE7, GAL1::RET1 (YAE3), GAL1::ret1Δ1034–1201 (YAE4) and GAL1::ret1Δ1–285 (YAE5). Note that GAL1::ret1Δ1–285 cells are temperature-sensitive.
We tested whether high level expression of the α-COP truncations is necessary for yeast viability, by expressing the ret1Δ1–285 and ret1Δ1034–1201 mutant proteins at near endogenous levels from a CEN plasmid under the endogenous RET1 promoter. Indeed, pCEN::ret1Δ1–285 supported life of ret1Δ::HIS3 cells. ret1Δ1–285 mutant cells grow only slightly more slowly than wild-type cells at 30°C, but grow poorly at 37°C (not shown). On the other hand, low level expression of the C-terminally truncated α-COP (pCEN::ret1Δ1034–1201) did not retain viability in an α-COP-deleted strain. Since overexpression of mutant Ret1-3p partially rescues the thermosensitivity of ret1-3 cells, which is due to the instability of Ret1-3p at elevated temperatures (Duden et al., 1998; our unpublished data), the lethal phenotype of CEN::ret1Δ1034–1201 cells may be due to instability of truncated α-COP. In summary, neither the WD40 domain nor the C-terminal ∼170 amino acids of α-COP are essential for yeast cell viability.
ret1Δ1–285 α-COP cells display defects in KKXX trafficking in vivo and KKXX binding in vitro
To gain further insight into the function of the WD40 domain in vivo, we compared the ability of ret1Δ1–285 cells and ret1-1 cells to retrieve a KKXX-tagged membrane protein at permissive temperature. For these experiments, we used ret1Δ1–285 cells in which the truncated Ret1p is expressed at an endogenous level.
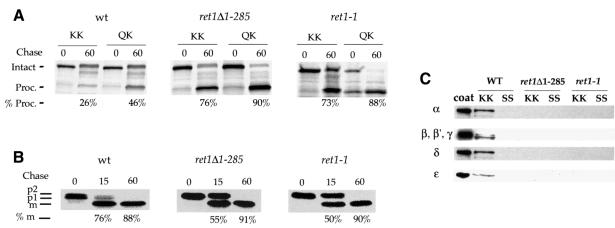
As a reporter, we used a chimeric protein consisting of invertase fused to the transmembrane and cytoplasmic domains of Wbp1p, a KKXX-tagged protein resident in the ER (Inv.-Wbp1p) (Gaynor et al., 1994). In wild-type cells, this chimera undergoes continuous Golgi-to-ER retrieval, whereas in coatomer mutants defective for KKXX retrieval, Inv.-Wbp1p escapes to the vacuole where it is cleaved. ret1Δ1–285 cells, congenic wild-type cells and ret1-1 cells were pulse-labelled for 10 min and chased for 0 and 60 min. Fusion proteins were recovered by immunoprecipitation, treated with endoglycosidase H and resolved by SDS–PAGE (Figure 3A). Values obtained for cleavage of the KKXX fusion protein were almost identical between ret1Δ1–285 cells and ret1-1 cells, 76 and 73%, respectively, compared with only 26% for wild-type cells. As a control, an Inv.-Wbp1p fusion protein harbouring a poor retrieval signal, QKXX, was tested. Efficient cleavage of this QKXX fusion protein after 60 min chase indicates that forward passage through the Golgi and to the vacuole is normal. We obtained values of 88% for ret1-1 cells, 90% for ret1Δ1–285 cells and 46% for wild-type cells (Figure 3A). The fact that this protein is transported more efficiently to the vacuole in ret1Δ1–285 cells (or ret1-1 cells) than in wild-type cells indicates that the QKXX motif still has a residual activity resulting in some Golgi-to-ER retrieval, as previously noticed (Gaynor et al., 1994; Duden et al., 1998). To adjust for this, we calculated a KKXX/QKXX fraction: values are 0.57 for wild-type, and 0.83 and 0.84 for the ret1-1 and ret1Δ1–285 cells. Thus, like ret1-1 cells, ret1Δ1–285 cells display a strong defect in the retrieval of a KKXX-tagged protein.
Fig. 3. (A) ret1Δ1–285 α-COP mutant cells display defects in KKXX trafficking in vivo. ret1Δ1–285 cells, congenic wild-type cells (YAE7) and ret1-1 cells were transformed with plasmids expressing Inv.-Wbp1 fusions carrying either the KKXX motif (KK) or a mutated motif, QKXX (QK). Cells were grown to log phase at 30°C, and pulse-labelled with [35S]Promix for 10 min at 30°C. Chase time points were taken at 0 and 60 min. Intact and PEP4-processed fusion proteins migrate at 70 and 56 kDa. The percentage of processing at 60 min was quantified using a PhosphorImager. The asterisk marks a band that may represent non-specific, cross-reactive material and was not included in the quantitation. (B) ret1Δ1–285 cells display a minor delay in CPY transport. Pulse–chase analysis of CPY transport in ret1Δ1–285 cells, congenic wild-type strain YAE7 (wt) and ret1-1 cells. Cells were radiolabelled with [35S]Promix label for 10 min at 30°C and chase time points taken at 0, 15 and 60 min. p1, p2 and mature forms of CPY are indicated, and processing to the mature form is quantified at 15 and 60 min. (C) Coatomer from ret1Δ1–285 cells is unable to bind to the KKXX motif. Whole-cell lysate of wild-type cells (WT), ret1Δ1–285 cells or ret1-1 cells was incubated for 2 h at 4°C with immobilized GST fusion proteins harbouring the KKXX motif (KK) or a mutated motif, SSXX (SS). Bound proteins were eluted from the beads, separated by SDS–PAGE and analysed by immunoblot using anti-coatomer antiserum. Purified coatomer (coat) is loaded to define positions of the COPs. Note that binding of coatomer subunits from wild-type cells, but not ret1-1 and ret1Δ1–285 cells, to the KKXX fusion protein.
We observe only a minor kinetic defect in forward transport of the vacuolar hydrolase carboxypeptidase Y in ret1Δ1–285 cells compared with isogenic wild-type cells, and very similar to ret1-1 cells (Figure 3B), indicating that anterograde transport in the mutants is virtually unaffected.
To assess the ability of coatomer from ret1Δ1–285 mutant cells to bind to the KKXX motif, in vitro binding experiments were performed, using glutathione S-transferase (GST) fusion proteins harbouring the cytoplasmic tail of Wbp1p (KKXX) or a non-functional motif (SSXX). Whole-cell extracts from mutant and wild-type cells grown at 30°C were prepared, and binding experiments were done as described by Cosson and Letourneur (1994). Coatomer from ret1Δ1–285 cells is unable to bind to the GST–Wbp1p fusion protein (Figure 3C), comparable with coatomer from ret1-1 cells (Letourneur et al., 1994). Thus, ret1Δ1–285 mutant cells and ret1-1 cells show similar phenotypes both in vivo and in vitro. In summary, the WD40 domain of α-COP is dispensable for yeast cell viability, but is required for KKXX-dependent trafficking and KKXX binding.
In ret1Δ1034–1201 mutant cells, α-COP is not bound to coatomer
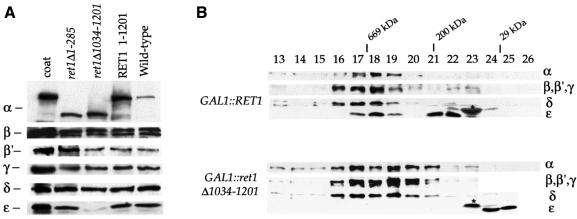
Since the GAL1::ret1Δ1034–1201 mutant lacks the region to which we have mapped the ret1-3 mutation, we next tested whether it displays a phenotype similar to ret1-3. In ret1-3 cells, α-COP levels fall rapidly after shift to 37°C, accompanied by a slower decrease in ε-COP levels (Duden et al., 1998). Whole-cell extracts of GAL1::ret1Δ1–285 cells, GAL1::ret1Δ1034–1201 cells, GAL::RET1 cells and wild-type cells were tested for levels of coatomer subunits by immunoblotting (Figure 4A). As expected, wild-type or truncated Ret1p expressed from the GAL1 promoter is present at higher levels compared with Ret1p in wild-type cells, whereas levels of β′-, β-, γ-, δ- and ζ-COP are comparable. Interestingly, ε-COP levels are severely reduced in GAL1::ret1Δ1034–1201 cells (Figure 4A). Coatomer complex integrity in wild-type cells, GAL1::ret1Δ1034–1201 cells and GAL1::RET1 cells grown at 30°C was analysed by gel filtration (Figure 4B). Coatomer in cytosol from wild-type cells eluted with a molecular mass of 700–800 kDa in fractions 17 and 18, as expected (not shown; see Duden et al., 1998). GAL1::RET1 cells showed a very similar, if slightly broader peak of coatomer in fractions 17 and 18, in which all subunits including ε-COP co-fractionated (Figure 4B). Additional ε-COP was present in a prominent peak (fractions 21 + 22) corresponding to a partial complex of ∼200 kDa. Probably, in GAL1::RET1 cells, excess monomeric α-COP is proteolytically degraded and such α-COP fragments bind to ε-COP. Significantly, when cytosol from GAL1::ret1Δ1034–1201 cells was fractionated, ε-COP was absent from the fractions corresponding to intact coatomer complex. Instead, ε-COP was found solely in monomeric form. Thus, the last ∼170 residues of α-COP are essential for ε-COP incorporation into coatomer and maintenance of normal levels of ε-COP.
Fig. 4. (A) ε-COP levels are severely reduced in ret1Δ1034–1201 mutant cells. ret1Δ strains expressing full-length α-COP (RET1) or the α-COP truncations (ret1Δ1–285 or ret1Δ1034–1201) from a CEN plasmid under the control of the GAL1 promoter, as well as a congenic wild-type control were grown to log phase at 30°C. Total cell extracts were analysed by immunoblotting. α-COP was detetected with anti-coatomer antiserum; the other COPs were detected with subunit-specific antisera. Note the higher levels of GAL1-overexpressed α-COP compared with wild-type cells, as expected, and the size differences between full-length and truncated α-COP. Further, note the dramatic depletion of ε-COP in ret1Δ1034–1201 cells, and comparable levels of β-, β′-, γ- and δ-COP in all four strains. (B) In ret1Δ1034–1201 mutant cells, ε-COP is not bound to coatomer. Superose 6 gel filtration of cytosolic proteins from GAL1::RET1 cells and GAL1::ret1Δ1034–1201 cells. SDS–PAGE-separated proteins of column fractions were probed with anti-coatomer antiserum to detect α-COP, β′-, β-, γ-COP (which co-migrate as a triplet) and δ-COP; an anti-ε-COP serum was used to detect ε-COP; the asterisk marks a non-specific, cross-reactive band. To visualize the reduced amounts of ε-COP in GAL1::ret1Δ1034–1201 cells, the ε-COP lane required longer exposure than for GAL1::RET1 cells. Note that coatomer from GAL1::RET1 cells elutes with a molecular mass of 700–800 kDa (fractions 17 + 18), similar to coatomer from wild-type cells (Duden et al., 1998), and that ε-COP co-fractionates with coatomer. Additional ε-COP is present in a prominent peak (∼200 kDa), most likely containing proteolytic fragments of the overexpressed, monomeric α-COP not detectable with anti-coatomer serum (fractions 21 + 22). Note that in GAL1::ret1Δ1034–1201 cells, ε-COP is absent from the broad coatomer peak around fractions 17–20. ε-COP is present exclusively in low molecular weight fractions in monomeric form. Fraction numbers and positions of marker proteins are indicated: thyroglobulin (669 000), β-amylase (200 000) and carboanhydrase (29 000).
Interactions between α-, β′- and ε-COP
In order to understand the phenotype of the two classes of α-COP mutants in more detail, we looked at interactions of full-length coatomer subunits by two-hybrid analysis (Figure 5). At first, we analysed interactions within the α-, β′-, ε-COP subcomplex, a stable heterotrimer obtained upon coatomer dissociation which still can bind KKXX peptides in vitro (Letourneur et al., 1994; Lowe and Kreis, 1995). Using the sensitive LexA two-hybrid system (Golemis et al., 1996), we detect the previously demonstrated interactions between α-COP and β′-COP, and α-COP and ε-COP (see Faulstich et al., 1996). We also find a novel direct interaction between β′-COP and ε-COP (Figure 5).
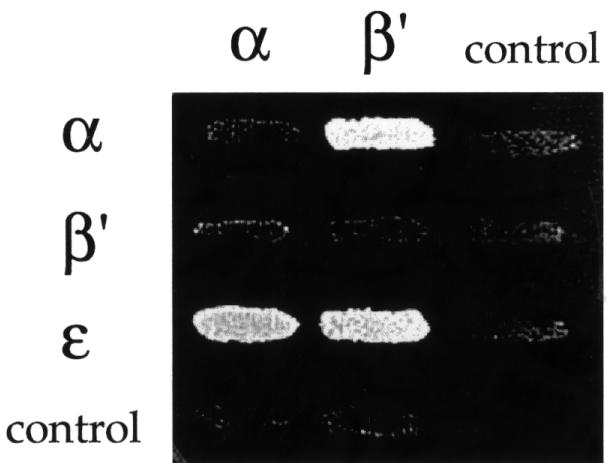
Fig. 5. Interactions within the α-, β′-, ε-COP subcomplex. On the horizontal axis, full-length α- and β′-COP are expressed in the bait vector; on the vertical axis, full-length α-, β′- and ε-COP are expressed in the prey vector. Empty vector is used as a control. ε-COP in the bait vector displayed high intrinsic transcriptional activation and could not be tested. Note the interactions between α-COP and β′-COP, and α-COP and ε-COP, and a novel direct interaction between β′-COP and ε-COP. Results shown are from the growth assay on minimal plates lacking leucine. As is typical of two-hybrid studies (Estojak et al., 1995), the same proteins expressed in the two different vectors (bait and prey) may show different signal intensities. Note the interaction between α-COP and β′-COP for an example.
Figure 6A depicts a tentative domain structure of α- and β′-COP based on sequence analysis. Both proteins harbour a conserved N-terminal WD40 domain (∼285 residues). When performing database homology searches (Ψ BLAST; Altschul et al., 1997) with α-COP lacking the WD40 repeats, we noticed a sequence relationship between α-COP and β′-COP that extends to the region comprising residues ∼285 to ∼780, resulting in a Ψ BLAST alignment score of 504 between yeast α-COP and β′-COP. This sequence relationship is readily apparent in multiple sequence alignments of α- and β′-COP from distant species (not shown); however, in pairwise alignments, sequence identities are low, e.g. 17% identity and 33% similarity between yeast α- and β′-COP. We here refer to this region with previously unrecognized sequence similarity between α- and β′-COP as the ‘middle region’. A ‘unique region’ (∼200 residues for β′-COP and ∼400 residues for α-COP) with no recognizable sequence relationship between the two proteins is present at their C-termini.
Fig. 6. Mapping of domain interactions within the α-, β′-, ε-COP subcomplex. (A) Tentative domain structure of α- and β′-COP, based on a multiple alignment of α- and β′-COP from distant species. Both proteins harbour an N-terminal WD40 domain (∼285 residues). A middle region (residues ∼285 to ∼780 on both proteins) shares sequence similarity in α-COP and β′-COP. A ‘unique region’ (length ∼200 residues for β′-COP and ∼400 residues for α-COP) with no recognizable sequence relationship between the two proteins is present at the C-terminus. The C-terminal domain on α-COP is indicated. (B) Mapping of α-/β′-COP interactions. α-COP truncations expressed in the prey plasmid were tested against β′-COP in the bait plasmid; amino acids of α- and β′-COP encoded by the two-hybrid fusions are indicated. The N-terminal half of γ-COP served as negative control. Results shown are from the growth assay on minimal plates lacking leucine. Note that three separable regions of α-COP, comprising residues 534–1034, 1014–1201 or the N-terminal half (residues 1–638), are able to interact with β′-COP. Note further that the WD40 domain of β′-COP is not required for these interactions. (C) Mapping of α-/ε-COP interactions. α-COP truncations were expressed in the bait vector and ε-COP in the prey vector; empty prey vector was used as a control. α-COP residues encoded by the two-hybrid fusions are indicated. Results shown are from the growth assay on minimal plates lacking leucine. ε-COP interacts with the C-terminal half of α-COP. Note that the last ∼170 residues of α-COP are essential for this interaction.
To identify ‘domains’ on α-COP involved in interactions with β′-COP or ε-COP, a set of overlapping α-COP truncations from both ends were made, covering the entire length of the protein (Figure 6B and C). The WD40 domain alone was untestable in the two-hybrid system as it displayed high intrinsic transcriptional activation in both the bait and the prey vectors. The α-COP truncations were tested against full-length β′-COP (1–889) or a β′-COP truncation lacking its WD40 domain (Figure 6B). Three separable regions of α-COP are able to interact with β′-COP in a manner independent of the WD40 domain of β′-COP. Thus, our data suggest that α-COP tightly interacts with β′-COP over three independent binding sites.
To confirm our prediction that the C-terminus of α-COP is required for interaction with ε-COP, we used the α-COP truncations described above. We find that the C-terminal half of α-COP interacts with ε-COP. Truncation of the last ∼170 residues of α-COP abolishes binding to ε-COP without affecting interaction with β′-COP (Figure 6C). Thus, consistent with our biochemical data, we find that the C-terminal ∼170 amino acids of α-COP are required for direct interaction with ε-COP in the two-hybrid system.
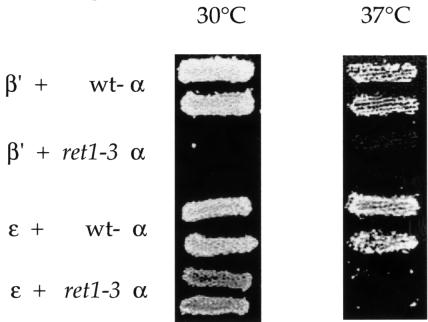
ret1-3 mutant α-COP does not interact with β′-COP and displays a temperature-sensitive interaction with ε-COP
The S1188F mutation present in the ret1-3 strain resides in a region of α-COP which we have shown to be involved in interactions with β′- and ε-COP. We next tested whether the ret1-3 mutation affects these interactions. We tested Ret1-3p, and wild-type Ret1p as a control, with β′-COP or ε-COP at different temperatures. No interaction of ret1-3 mutant α-COP with β′-COP is detectable at 30°C, whereas ε-COP is still able to interact with ret1-3 α-COP (Figure 7); identical results were obtained at 24°C (not shown). As expected, at 37°C, ret1-3 mutant α-COP displays no interaction with either β′-COP or ε-COP, since Ret1-3p is unstable at 37°C (Duden et al., 1998). Our data indicate that the ret1-3 mutation causes structural alterations which affect the two-hybrid interaction with β′-COP even at permissive temperature.
Fig. 7. ret1-3 α-COP cannot interact with β′-COP and is temperature-sensitive for interaction with ε-COP. Wild-type or ret1-3 mutant α-COP C-terminal halves (residues 685–1201) were expressed in the bait vector and tested for interaction with full-length β′-COP or ε-COP in the prey vector at 30 or 37°C. Results shown are from the growth assay on minimal plates lacking leucine. Note that Ret1-3p, in contrast to wild-type α-COP, does not interact with β′-COP at either temperature. Note further that Ret1-3p interacts with ε-COP at 30°C, but not at 37°C.
In vivo, the ret1-3 mutation leads to a dramatic disintegration of coatomer and degradation of α-COP at restrictive temperature (Duden et al., 1998; our unpublished data). Surprisingly, ret1-3 cells grow like wild-type cells at 24°C, display no trafficking defects, and coatomer from such cells fractionates like wild-type coatomer upon gel filtration (Duden et al., 1998). Thus, at 24°C, Ret1-3p mutant α-COP may rely on as yet unidentified interactions to remain integrated in coatomer. We endeavoured to search for links between the COP I subcomplexes that contribute to coatomer integrity.
The α-, β′-, ε-COP subcomplex interacts with β- and α-COP
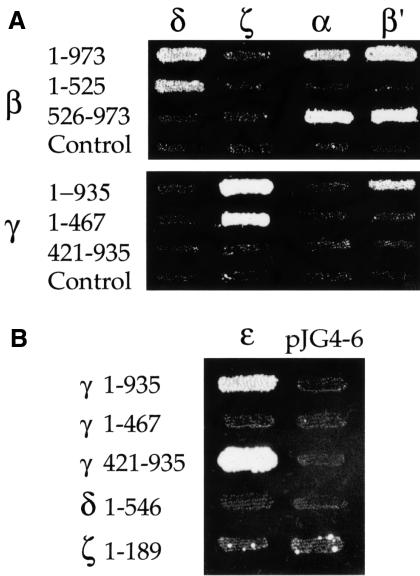
In contrast to other coat proteins (e.g. COP II, AP adaptor complexes and clathrin), the building blocks for the COP I coat are found in the cytoplasm in a large pre-assembled form, the heptameric coatomer complex. In order to find interactions contributing to the assembly of the α-, β′-, ε-COP subcomplex with the rest of the complex, we tested interactions of α-, β′- and ε-COP with the remaining coatomer subunits. The high sensitivity of the LexA-based two-hybrid system allowed us to reveal previously undetected interactions of β-COP with α-COP and β′-COP, and of γ-COP with β′-COP (Figure 8A). Further, we detected another novel interaction between γ-COP and ε-COP (Figure 8B). The interactions of α-COP and β′-COP with β-COP map to the C-terminal half of β-COP (Figure 8A). The interaction of γ-COP with ε-COP relies on the C-terminal half of γ-COP (Figure 8B).
Fig. 8. Interactions of α-, β′-, ε-COP with β- and γ-COP. (A) Regions of β-COP and γ-COP involved in interactions with other coatomer subunits were mapped. The N- and C-terminal halves of β-COP and γ-COP were cloned into the prey vector pJG4-5; amino acid residues of the COPs encoded by the two-hybrid fusions are indicated. These prey fusions were tested against other coatomer subunits in the bait vector; empty pJG4-5 served as a control. Results shown are from the growth assay on minimal plates lacking leucine. Interactions with δ-, α- and β′-COP are shown in reporter strain EGY48; interactions with ζ-COP are shown using the less sensitive reporter strain EGY191. Note that the interactions of β-COP with δ-COP, and γ-COP with ζ-COP, map to the N-terminal halves of β- and γ-COP, respectively. Note further three novel interactions: β-COP with α-COP and β′-COP, and γ-COP with β′-COP. The C-terminal region of β-COP (β 526–973) interacts with α- and β′-COP; the interaction of γ-COP with β′-COP could not be mapped. (B) The N-terminal half of γ-COP interacts with ε-COP. The N- and C-terminal halves of β-COP and γ-COP were cloned into the bait vector pEG203; amino acid residues of the COPs encoded by the two-hybrid fusions are indicated. Bait fusions were tested against all other coatomer subunits in the prey vector; δ- and ζ-COP in the bait vector served as controls. Results shown are from the growth assay on minimal plates lacking leucine.
Strong interactions between full-length β-COP and δ-COP, and full-length γ-COP and ζ-COP have been shown previously, both by the two-hybrid system (Faulstich et al., 1996) and biochemically (Lowe and Kreis, 1995; Faulstich et al., 1996; Pavel et al., 1998). We show here that these interactions between β- and δ-COP, as well as between γ- and ζ-COP, are mediated by the N-terminal halves of β-COP and γ-COP, respectively (Figure 8A). Our findings suggest that the β-, γ-, δ-, ζ-COP subcomplex has a domain structure very similar to that of adaptor complexes. We further conclude that the C-terminal domains of β- and γ-COP are required for coatomer integrity through binary interactions with subunits of the α-, β′-, ε-COP subcomplex.
ARF1 interacts directly with β-COP in the two-hybrid system
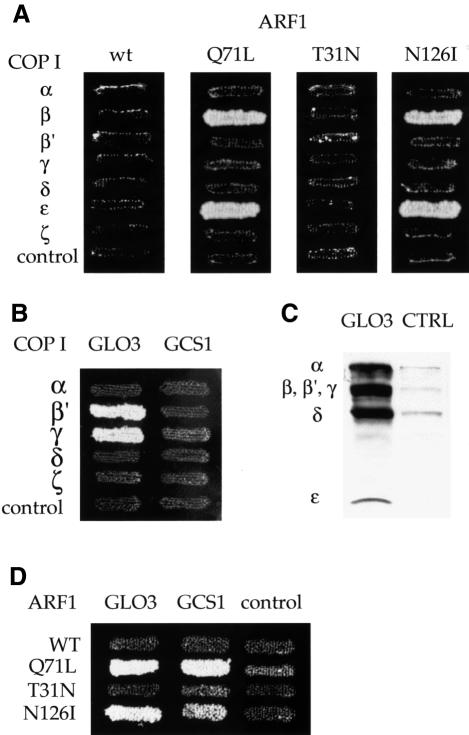
The small GTPase ARF is a component of the COP I coat and a key regulator of its assembly and disassembly. We wished to analyse direct interactions of yeast ARF1 with subunits of coatomer in the two-hybrid system. Arf1p in its active form can be photo-cross-linked to β-COP in vitro, demonstrating that these proteins are in close proximity on the Golgi membrane (Zhao et al., 1997). However, in a previous study (Faulstich et al., 1996), no two-hybrid signals had been detected with full-length ARF1 constructs, using either wild-type ARF1 or a mutant form of ARF, Q71L-ARF. Q71L-ARF is stabilized in the GTP-bound, active form of ARF (Dascher and Balch, 1994). Interestingly, unlike full-length ARF1, a truncated form of mammalian ARF1 (ΔN17) lacking the first 17 hydrophobic residues can bind to coatomer in vitro (Goldberg, 1999). The ΔN17 truncation creates a soluble ARF1 protein that retains full activity in exchange factor assays (Paris et al., 1997), and ΔN17-ARF1 can compete with wild-type myristoylated ARF1 to prevent coatomer binding to membranes.
Therefore, we tested whether ΔN17-ARF1 would interact with coatomer subunits in the two-hybrid system. We generated two-hybrid constructs expressing truncated yeast ΔN17-Arf1p in the bait vector: wild-type ARF1 and three mutants, Q71L-ARF1 (GTP-ARF), T31N-ARF1 (GDP-ARF) and the dominant-negative ARF mutant, N126I-ARF1. Expression of full-length myristoylated N126I-ARF in mammalian cells results in disassembly of the Golgi complex and redistribution of coatomer into punctate cytoplasmic structures. This mutant may thus bind to the soluble pool of coatomer, causing its aggregation (Dascher and Balch, 1994). We tested these constructs for interactions with the COPs in the prey vector. We detect an interaction of β-COP with ΔN17-Q71L-ARF1 and ΔN17-N126I-ARF1, but not ΔN17-wild-type Arf1p (Figure 9A). Interactions with the same ARF constructs are also seen for ε-COP (Figure 9A). The interaction with T31N-ARF1 needed to be tested in the less sensitive reporter strain EGY191 because of a background problem with this construct in the standard strain EGY48. Therefore, a direct comparison between Q71L-ARF1 and T31N-ARF1 could not be drawn. However, the T31N-ARF1 construct is functional, since, as expected, it can interact with a GEF, Gea1p, in the less sensitive reporter strain EGY191 (A.Eugster and R.Duden, unpublished data).
Fig. 9. (A) ARF1 interacts directly with β- and ε-COP in the two-hybrid system. N-terminally truncated (ΔN17) Arf1p in the bait vector was tested against the coatomer subunits in the prey vector. Wild-type ARF1 and the mutants Q71L (GTP-ARF), T31N (GDP-ARF) and N126I were tested. Empty prey vector was a negative control. Results shown are from the growth assay on minimal plates lacking leucine. Interactions with Q71L-ARF1, N126I-ARF1 and wild-type ARF1 are shown in reporter strain EGY48; interaction with T31N-ARF1 is shown using the less sensitive reporter strain EGY191. Note that β-COP and ε-COP interact with Q71L-ARF1 and N126I-ARF1, but not wild-type ARF1 or T31N-ARF1. (B) The ARF-GAP Glo3p interacts with β′- and γ-COP in the two-hybrid system. Glo3p or Gcs1p in the prey vector was tested against coatomer subunits in the bait vector, using empty bait vector as a control. Note that Glo3p interacts with β′- and γ-COP. (C) His6-tagged Glo3p (GLO3) can pull down whole coatomer from yeast cytosol in vitro, whereas empty beads (CTRL) or a control protein (His6-Rab-GDI; not shown), cannot. Bound proteins were analysed using anti-coatomer serum; the positions of the COPs are indicated. For details, see Materials and methods. (D) Glo3p and Gcs1p interact with Arf1p. Glo3p or Gcs1p in the prey vector was tested against wild-type or mutant ΔN17-ARF1 in the bait vector. Note that Glo3p and Gcs1p both interact with Q71L- and N126I-ARF1, but not wild-type ARF1 or T31N-ARF1. Reporter strains used were the same as in (A) above.
Thus, in contrast to earlier studies, we can detect direct interactions of Q71L-ARF with β-COP and ε-COP in the LexA two-hybrid system, using a truncated ARF1 (ΔN17) lacking its N-terminal α-helix.
The ARF-GAP Glo3p, but not Gcs1p, interacts with β′- and γ-COP
We wished to identify interactions of coatomer subunits with proteins that regulate the cycle of COP I coat assembly and disassembly. Two related ARF-specific GAPs (ARF-GAPs), Glo3p and Gcs1p, provide an overlapping essential function in retrograde Golgi-to-ER transport (Poon et al., 1999). Glo3p and Gcs1p are each sufficient for Golgi-to-ER transport, but a glo3Δ gcs1Δ double mutant is inviable (Poon et al., 1999). Recently, ret4-1, a temperature-sensitive mutant in GLO3, has been isolated based on its defect in retrieval of KKXX proteins from the Golgi complex (Dogic et al., 1999). As expected, the T66I mutation of ret4-1 which is located within the GTPase-activating domain of Glo3p reduces the GAP activity of mutant Glo3p towards ARF1 in vitro. Analysis of knockout strains of all six GLO3-related proteins in yeast revealed that glo3Δ alone results in a defect in KKXX retrieval from the Golgi (Dogic et al., 1999). Furthermore, the glo3Δ mutant shows strong synthetic lethality with sec21-1, a γ-COP mutant (Poon et al., 1999). Thus, it appears that GLO3 is the main ARF-GAP involved in COP I-dependent Golgi-to-ER transport.
We first investigated possible interactions of Glo3p, and Gcs1p, with all COPs in the two-hybrid system. As shown in Figure 9B, indeed β′-COP and γ-COP interact with Glo3p. We also tested Ret4-1p, i.e. T66I-Glo3p, against the COPs, which behaved identically to wild-type Glo3p (not shown). Somewhat surprisingly, the related ARF-GAP Gcs1p is unable to undergo interactions with COPs.
For the Glo3p interaction to be physiologically relevant, it should also be detectable with intact coatomer. To test this, we performed in vitro binding experiments using His6-tagged Glo3p in ‘pull-down’ experiments from yeast cytosol. Significantly, we observe a strong interaction of Glo3p with intact coatomer (Figure 9C).
As a control for the two-hybrid interactions, we tested whether Glo3p, or Gcs1p, can interact with Arf1p. We find an interaction of both Glo3p and Gcs1p with ΔN17-Q71L-ARF1, but not with wild-type ΔN17-ARF1 or ΔN17-T31N-ARF; the latter was again tested in strain EGY191 (Figure 9D). Interestingly, the T66I mutation in Glo3p had no effect on the affinity of Glo3p for Q71L-ARF1 in the two-hybrid system (not shown). Thus, we can detect signals for both ARF-GAPs with ΔN17-ARF1 in the two-hybrid system.
In summary, we report that although both ARF-GAPs implicated in Golgi-to-ER transport interact with Q71L-ARF1, only Glo3p interacts directly with subunits of the coatomer complex. Most interestingly, this novel interaction could be confirmed biochemically. Purified His6-tagged Glo3p can interact strongly with intact coatomer from yeast cytosol in vitro.
Discussion
We wish to understand domain interactions of coatomer subunits that contribute to the remarkable stability of the coatomer complex. For this purpose, we used the two-hybrid system and analysis of in vivo phenotypes of α-COP truncation mutants. Most interestingly, we further identify novel direct interactions of coatomer subunits with ARF and with an ARF-GAP, Glo3p.
Novel molecular interactions that contribute to coatomer integrity
To study binary interactions between COPs that contribute to coatomer integrity, we employed a modern version of the two-hybrid system, the LexA system or ‘interaction trap’ (Gyuris et al., 1993; Estojak et al., 1995). The power of the LexA system is the sensitivity and reliability of the growth assay which is a major improvement over earlier versions of the two-hybrid system. Other useful features include the availability of reporter strains and plasmids with a differing number of LexA operators, making it possible to increase or decrease sensitivity of the system, depending on the observed signal-to-noise ratio (Estojak et al., 1995; Golemis et al., 1996). We confirm the four pairs of binary interactions between coatomer subunits that had been identified previously (Faulstich et al., 1996), namely β/δ-COP, γ/ζ-COP, α/ε-COP and α/β′-COP. More interactions between coatomer subunits should exist, given the overall stability of the coatomer complex. We therefore asked which are the links between the separate subcomplexes of coatomer observed after disintegration in high salt buffers. Due to the high sensitivity of the LexA system, we found five novel direct interactions: β′ 2pt>/ε-COP, α/β-COP, β′ 2pt>/β-COP, β′ 2pt>/γ-COP and γ/ε-COP. Although these binary interactions are not very strong, they seem to be sufficient to maintain the stability of the coatomer complex.
The molecular organization of coatomer: similarity to AP adaptor complexes
The small GTPase ARF1 in its GTP-bound form is involved in the membrane recruitment of several types of vesicle coats: coatomer/COPI as well as the AP-1 and AP-3 adaptor coat complexes, thus suggesting a shared structural principle. Adaptors are heterotetrameric protein complexes involved in coat formation in distinct membrane transport steps: AP-1 in trafficking of soluble lysosomal hydrolases from the trans-Golgi; AP-2 in endocytosis; and AP-3 in Golgi-to-vacuole transport of membrane proteins, e.g. alkaline phosphatase (Hirst and Robinson, 1998). In fact, the β-, γ-, δ-, ζ-COP subunits of coatomer all display low but significant sequence similarity to adaptor subunits. β- and γ-COP share similarity with the large subunits AP-1, AP-2 and AP-3 in their N-terminal domains (∼450 residues) (Duden et al., 1991; Gaynor et al., 1998). δ-COP displays sequence similarity to µ AP subunits, and ζ-COP with σ AP subunits (Cosson et al., 1996; Faulstich et al., 1996; Schledzewski et al., 1999).
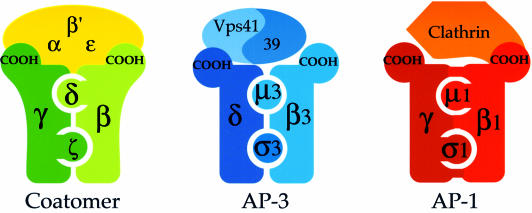
Here we set out to investigate whether COP I is really analogous to AP complexes, and that is what we have indeed found. Internal interactions within coatomer identified in this study are depicted schematically in Figure 10, together with known interactions within AP complexes and with their respective coat partners (i.e. clathrin for AP-1 and Vps41/39p for AP-3). In the adaptor complexes that have been shown to bind to clathrin, AP-1 and AP-2, the N-terminal part of the large subunits binds the small subunits (Page and Robinson, 1995) and the hinge domain of the β-subunit which is part of the C-terminal halves binds to clathrin (Shih et al., 1995). As for AP-3, the C-terminus of the δ-subunit binds to Vps41p, a protein required for vesicle formation in this pathway which is part of the coat of AP-3 vesicles (Rehling et al., 1999). Thus, our two-hybrid results suggest that the domain organization of adaptor complexes and of the β-, γ-, δ-, ζ-COP coatomer subcomplex is related, and that these coat complexes may share a common basic structural arrangement.
Fig. 10. Schematic depiction of overall topology and internal interactions within three different, but structurally related coat complexes: coatomer; AP-1 and clathrin, and AP-3 adaptor with the presumed coat proteins Vps41/Vps39p. Domain interactions of proteins within these complexes that have been identified by two-hybrid or biochemical analysis are indicated by coloured bars connecting these proteins. For details see Discussion.
However, there are also noticeable differences between adaptors and coatomer. For example, the large subunits of the AP1 complex interact with each other in the two-hybrid system (Page and Robinson, 1995), whereas neither we nor Faulstich et al. (1996) could detect interactions between β- and γ-COP. Consistent with this, upon treatment of coatomer with high salt buffers or the reagent DMMA (dimethyl maleic anhydride), the β-, γ-, δ-, ζ-COP subcomplex disintegrates into two heterodimers, β/δ-COP and γ/ζ-COP (Pavel et al., 1998), whereas the adaptor complexes are stable (Hirst and Robinson, 1998).
Another common feature between coatomer and adaptor/clathrin coats is a sequence motif (∼145 residues) defined by structural comparisons (Ybe et al., 1999) as well as earlier sequence comparisons based on profile searches (Conibear and Stevens, 1998). The motif, called the clathrin heavy chain repeat, is found in seven copies in clathrin heavy chains from different species (Ybe et al., 1999). One copy of this motif is also found in α- and β′-COP (residues 655–699 in α-COP; 664–724 in β′ COP), as well as in Vps39p and Vps41p (Conibear and Stevens, 1998); the latter proteins form a hetero-oligomer. Interestingly, this conserved motif is present in the α-COP truncation which gives rise to the strongest two-hybrid interactions with β′-COP. Perhaps this motif mediates or regulates protein–protein interactions between these coat proteins.
The α-COP WD40 domain is essential for KKXX trafficking and the C-terminal domain is required for α-COP stability
Using truncation mutants, we gained insights into the functions of domains on α-COP. The α-COP N-terminal domain consists of six WD40 repeats and is implicated in interactions with KKXX motifs (Letourneur et al., 1994). WD40 repeats are conserved sequence motifs that are predicted to fold into a structure composed of β-strands and turns, a so-called β-propeller structure, in which each WD40 repeat contributes to a blade of the propeller (Neer and Smith, 2000). Surprisingly, cells expressing ret1Δ1–285 lacking the WD40 domain as the only copy of the RET1 gene at an endogenous level grow comparably with wild-type cells at physiological temperatures. Thus, the WD40-domain of α-COP is dispensable for yeast cell viability and coatomer integrity/subunit stoichiometry. Consistent with earlier findings (Letourneur et al., 1994), our data establish that the WD40 domain as a whole is required for KKXX-dependent Golgi-to-ER recycling in vivo and KKXX binding in vitro. However, photo-cross-linking data have implicated γ-COP in KKXX interactions (Harter and Wieland, 1998). The α-COP WD40 domain may either constitute a sufficient binding region for direct interactions with the KKXX motif, or it may merely alter the conformation of γ-COP to allow its interactions with the KKXX motif. Future experiments will need to resolve this question.
Consistent with earlier findings, our data argue that the essential function of coatomer indeed resides in the trafficking of cargo other than KKXX-tagged proteins. Well established candidates for such essential cargo molecules are the ER-to-Golgi v-SNAREs Bet1p/Bos1p/Sec22p, the cis-Golgi t-SNARE Sed5p, Golgi-resident enzymes and the HDEL receptor, Erd2p (Lewis and Pelham, 1996).
We demonstrate that an ∼170 residue C-terminal region of α-COP (residues 1034–1201) is required for interactions with β′-COP and ε-COP. In contrast to ret1Δ1–285 cells, deletion of this α-COP region does affect the essential function of coatomer, since near endogenous levels of this C-terminally truncated α-COP are insufficient to keep cells alive. In GAL1::ret1Δ1034–1201 cells, a very low level of ε-COP is detected. Further, ε-COP is absent from the coatomer peak in ret1Δ1034–1201 cells upon gel filtration. Consistent with this, C-terminally truncated α-COP does not interact with ε-COP in the two-hybrid system.
A point mutation in this region (S1188F) present in ret1-3 cells causes structural alterations to coatomer at restrictive temperature, instability of the mutant α-COP and subsequent cell death (Duden et al., 1998). Using the two-hybrid system, we show that the ret1-3 mutation affects ε-COP binding at a restrictive temperature, 37°C, and β′-COP binding at all temperatures (even 24°C). In summary, the C-terminal region of α-COP is required for incorporation of ε-COP into coatomer, and thus contributes to the integrity of the α-, β′-, ε-COP subcomplex and therefore the coatomer complex as a whole.
Direct interactions of coatomer subunits with ARF and an ARF-GAP
We identify novel interactions of coatomer subunits with an ARF-GAP, Glo3p, and with ARF1. Direct interactions of ARF with coatomer have been emerging through several lines of evidence (Zhao et al., 1997; Goldberg, 1999). Here we find that truncation of the N-terminal α-helix of ARF1 is critical to obtain fusion proteins that are able to interact in the two-hybrid system. This truncation (ΔN17) creates a soluble protein that retains full activity in vitro (Paris et al., 1997; Goldberg, 1999). Consistent with photo-cross-linking data (Zhao et al., 1997), we find a direct interaction of β-COP with the GTP-bound mutant form of ARF, Q71L-ARF.
More interestingly, we find strong direct interactions of Glo3p, the main ARF-GAP involved in retrograde traffic (Dogic et al., 1999; Poon et al., 1999), with γ-COP and β′-COP in the two-hybrid system. Further, His6-tagged purified Glo3p can interact with intact coatomer from cytosol in vitro. Surprisingly, the functionally related (Poon et al., 1999) ARF-GAP Gcs1p is unable to undergo two-hybrid interactions with the COPs. We expect this novel and unexpected direct interaction of Glo3p with coatomer to be physiologically important. It could be involved in the targeting of Glo3p to Golgi membranes, or in the regulation of COP I budding, cargo selection or vesicle uncoating. Future experiments will address these questions.
As expected, Glo3p and Gcs1p can interact with ARF1 in the two-hybrid system, consistent with their well characterized GAP activity on ARF1 in vitro. We find that the GTP-stabilized mutant Q71L-ARF1 can interact with both ARF-GAPs, whereas wild-type ARF1, which presumably in vivo is a mixture of GTP- and GDP-ARF, cannot. The dominant-negative mutant N126I-ARF1 behaved like Q71L-ARF1 in all two-hybrid interactions observed.
Goldberg (1999) has implicated a ternary complex consisting of coatomer, ARF-GAP and ARF in the regulation of GTP hydrolysis on ARF. First, GTP-bound ARF can bind to coatomer in vitro. Secondly, addition of coatomer to in vitro reactions containing GTP-loaded ARF and ARF-GAP stimulated GTP hydrolysis a further 1000-fold (Goldberg, 1999). Thus, coatomer (the ARF ‘effector’) participates directly in the GTPase reaction. COP I coat recruitment, budding and uncoating are highly regulated events in vivo, and therefore such a ternary complex is likely to be at the core of the regulatory mechanism.
We have dissected the binding of ARF and ARF-GAP to coatomer down to direct binary interactions, identifying the coatomer subunits engaged in the complex. Future experiments will map the regions on Glo3p and β-, β′- and γ-COP involved in these interactions. The fact that ARF binds β-COP, Glo3p binds γ- and β′-COP, γ-COP binds β′-COP, and β-COP binds β′- and α-COP in a ‘mappable’ fashion starts to suggest possible three-dimensional structural arrangements of these molecules on and within coatomer. Our two-hybrid data provide an important step towards the characterization of a tripartite complex of ARF, ARF-GAP and coatomer.
Materials and methods
Yeast strains and media
Yeast strains used are listed in Table I. Yeast media were either rich medium (YP), consisting of 1% Bacto yeast extract (Difco), 2% Bacto peptone (Difco) and supplemented with either 2% glucose (YP-Glu) or 2% galactose/2% glycerol (YP-Gal/Gly), or minimal medium, contaning 0.7% yeast nitrogen base (Difco) supplemented with appropriate amino acids (Sigma).
Table I. Yeast strains.
| Strain | Genotype | Source or reference |
|---|---|---|
| EGY48 | MATα, his3, trp1, ura3-52, leu2::pLEU2-LEXAop3 | Estojak et al. (1995) |
| EGY191 | MATα, his3, trp1, ura3-52, leu2::pLEU2-LEXAop1 | Estojak et al. (1995) |
| RSY255 | MATα, ura3-52, leu2-3,-112 | D.Botstein |
| PC70 | MATα, ret1-1, ura3, leu2, trp1 | Letourneur et al. (1994) |
| RDY193 | MATa, ret1-3, ura3-52, leu2-3,-112 | Duden et al. (1998) |
| RDY260 | MATa, sec33-1, ura3-52, leu2-3,-112 | Wuestehube et al. (1996) |
| YPH501 | MATa/α, ura3-52, lys2, ade2-101, trp1, his3-Δ200, leu2-1 | Sikorski and Hieter (1989) |
| YAE1 | MATa/α, ura3-52, lys2, ade2-101, trp1, his3-Δ200, leu2-1, RET1/ret1Δ::HIS3 | this study |
| YAE2-1c | MATa, ura3-52, lys2, ade2-101, trp1, his3-Δ200, leu2-1, ret1Δ::HIS3 + pCEN::RET1 (CEN, URA3) | this study |
| YAE3 | MATa, ura3-52, lys2, ade2-101, trp1, his3-Δ200, leu2-1, ret1Δ::HIS3 + pGAL::RET1 (CEN, LEU2) | this study |
| YAE4 | MATa, ura3-52, lys2, ade2-101, trp1, his3-Δ200, leu2-1, ret1Δ::HIS3 + pGAL1::ret1Δ1034–1201 (CEN, LEU2) | this study |
| YAE5 | MATa, ura3-52, lys2, ade2-101, trp1, his3-Δ200, leu2-1, ret1Δ::HIS3 + pGAL1::ret1Δ1–285 (CEN, LEU2) | this study |
| YAE6 | MATa, ura3-52, lys2, ade2-101, trp1, his3-Δ200, leu2-1, ret1Δ::HIS3 + pCEN::ret1Δ1–285 (CEN, LEU2) | this study |
| YAE7 | MATa, ura3-52, lys2, ade2-101, trp1, his3-Δ200, leu2-1, ret1Δ::HIS3 + pCEN::RET1 (CEN, LEU2) | this study |
DNA cloning, sequencing, computer analysis and plasmids
Escherichia coli strain DH5α was used for plasmid isolation, and PCRs using Vent DNA polymerase, restriction enzyme digests and ligations were performed by standard methods. Database searches were performed using the BLAST and Ψ BLAST servers at NIH. Multiple alignments were done with the program MEGALIGN using the CLUSTAL method.
Plasmids pEG1-KK and pEG1-QK encoding Inv.-Wbp1p fusion proteins harbouring a KKXX or QKXX motif (Gaynor et al., 1994) were used in pulse–chase experiments (Duden et al., 1998). For in vitro binding studies, pGST-WBP1-KK and pGST-WBP1-SS (plasmids pFL67 and pFL68) encoding GST fusions with the cytoplasmic tail of Wbp1p harbouring either a KKXX or SSXX motif were used (Cosson and Letourneur, 1994).
Plasmid and strain construction
A RET1/ret1Δ::HIS3 heterozygous diploid strain, YAE1, harbouring one deleted copy of the RET1 gene was generated using a PCR strategy, and transformed with a plasmid expressing RET1 under its own promoter (pRS316::RET1; URA3 CEN) to support life of haploid ret1Δ::HIS3 cells. His+, Ura+ transformants were sporulated, and tetrads were dissected and analysed for correct marker segregation.
LEU2 plasmids expressing full-length RET1, or truncated versions of RET1 (Δ1–285 and Δ1034–1201) from the GAL1 promoter were constructed: pGAL1::RET1, pGAL1::Δ1–285 and pGAL1::Δ1034–1201 [derivatives of YCplac111galp (CEN, LEU2, GAL1 promoter)].
Plasmids expressing RET1 or RET1 truncations from the endogenous RET1 promoter were constructed, based on pRS415 (CEN, LEU2).
To obtain haploid strains expressing truncated or wild-type Ret1p from the GAL1 promoter, strain YAE1-1c (ret1Δ::HIS3; harbouring pCEN::RET1, URA3) was transformed with the LEU2 plasmids containing pGAL::RET1, pGAL::ret1Δ1–285 or pGAL::ret1Δ1034–1201, and Ura–, Leu+ colonies were selected on 5-fluoro-orotic acid (5-FOA) plates. To obtain haploid strains expressing truncated or wild-type Ret1p from the RET1 promoter, strain YAE1-1c was transformed with pCEN::ret1Δ1–285, pCEN::ret1Δ1034–1201 or pCEN::RET1 and again Ura–, Leu+ colonies were selected on 5-FOA plates. Transformation with pCEN::ret1Δ1034–1201 did not yield viable Ura–, Leu+ colonies.
Fusion constructs, point mutants and two-hybrid assays
The LexA two-hybrid system was used (Estojak et al., 1995; Golemis et al., 1996). Yeast reporter strains EGY48 or EGY191 (Table I), as well as the bait plasmids pEG202 or pEG203 and the prey plasmids pJG4-5 or pJG4-6 were used (Golemis et al., 1996); plasmids and strains were obtained from Erica Golemis (Philadelphia) and Michael Hall (Basel). Prey fusions are under the control of the GAL1 promoter.
Fusion constructs were created by ligating PCR products made using custom primers into the vectors above, generating in-frame fusions with LexA (bait vector) or ‘acid blob B42’ (prey vector). For full-length fusions of the COPs, the chimeras started at the first ATG codon of the open reading frames. For truncations, amino acids on the COPs expressed are depicted in Figures 6 and 8. The T66I-GLO3 mutation, and the N126I-ARF1 and T31N-ARF1 mutations were introduced using the QuikChange mutagenesis kit (Promega). The Q71L-ARF1 mutant was provided by Randy Schekman (Berkeley). To create fusion constructs that contain the ret1-3 mutation, regions of α-COP were PCR amplified using genomic DNA prepared from ret1-3 cells, and fragments subcloned. Constructs were verified by DNA sequencing.
Reporter strains EGY48 or EGY191, harbouring the LacZ reporter plasmid pSH18-34 (URA3; see Golemis et al., 1996), were co-transformed with a bait and a prey fusion construct and transformants selected on –HIS, –TRP, –URA plates. To assess growth on media lacking leucine, four independent transformants were streaked onto plates lacking histidine, tryptophan, uracil and leucine, containing 2% galactose/1% raffinose or 2% glucose, and incubated at 30°C for 3 days. β-galactosidase activity was tested on minimal media plates containing X-GAL (Sigma). Unless otherwise indicated, reporter strain EGY48 was used. Each experiment was repeated at least three times.
Immunoblotting, radiolabelling and immunoprecipitation
SDS–PAGE, immunoblot analysis using ECL, radiolabelling of cells and immunoprecipitation have been described (Duden et al., 1994, 1998). Immunoprecipitates were analysed by SDS–PAGE followed by PhosphorImager quantitation. Rabbit antisera used were: anti-coatomer; anti-Sec26p (β-COP) and anti-Sec27p (β′-COP) (Duden et al., 1994); anti-Sec21p (γ-COP) (Hosobuchi et al., 1992); anti-Sec28p (ε-COP) (this study); anti-CPY and anti-invertase (kindly provided by Randy Schekman and Scott Emr).
Cytosol preparation, gel filtration and in vitro binding to KKXX motifs and to Glo3p
Prepraration of cytosol and Superose 6 gel filtration analysis were as described (Duden et al., 1998). GST fusion proteins of Wbp1p carrying a KKXX or SSXX motif expressed in E.coli were purified on glutathione–Sepharose 4B (Amersham). For binding experiments, yeast spheroplasts were lysed in 100 OD600/ml HEPES–Triton buffer [50 mM HEPES, pH 7.3, 90 mM KCl, 0.5% Triton X-100, 1 mM aminoethylbenzenesulfonyl fluoride (AEBSF)]. Cell lysates were spun for 15 min at 20 000 g. Supernatants were incubated twice for 1 h at 4°C with beads containing GST only, followed by 2 h with beads harbouring the fusion proteins. Beads were washed once in HEPES–Triton buffer and five times in 50 mM HEPES, pH 7.3, and bound proteins were eluted in SDS sample buffer.
Wild-type GLO3 cloned in pET21b (Novagen) was expressed in λDE3 lysogens of strain BL21 harbouring pLysS and purified by Ni-NTA chromatography as described by Dogic et al. (1999). A 3 mg aliquot of wild-type cytosol prepared as above was pre-absorbed with 25 µl of Ni-NTA beads for 1 h at 4°C in 1 ml total volume, and then incubated with 40 µg of purified Glo3p for 1 h. Buffer conditions were 50 mM NaHPO4 pH 8.0, 150 mM NaCl, 20 mM imidazole. Glo3p was pulled down with 25 µl of Ni-NTA beads, washed three times with buffer, and bound proteins were eluted in SDS sample buffer. As controls, either empty beads or a His6-tagged purified control protein (Rab-GDI) were used. Bound proteins were analysed by SDS–PAGE and immunoblot with anti-coatomer serum.
Acknowledgments
Acknowledgements
We are grateful to Drs Pierre Cosson, Scott Emr, Erica Golemis, Francois Letourneur, Michael Hall and Randy Schekman for providing strains, plasmids and antibodies. We thank Dr Jussi Jantti for advice on in vitro binding assays, and Drs Jussi Jantti, Margaret ‘Scottie’ Robinson and Sebastian Springer for helpful comments on the manuscript. R.D. is a Senior Research Fellow of The Wellcome Trust (047578).
References
- Altschul S.F., Madden,T.L., Schäffer,A.A., Zhang,J., Zhang,Z., Miller,W. and Lipman,D.J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res., 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear E. and Stevens,T.H. (1998) Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim. Biophys. Acta, 1404, 211–230. [DOI] [PubMed] [Google Scholar]
- Cosson P. and Letourneur,F. (1994) Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science, 263, 1629–1631. [DOI] [PubMed] [Google Scholar]
- Cosson P., Demolliere,C., Hennecke,S., Duden,R. and Letourneur,F. (1996) δ- and ζ-COP, two coatomer subunits homologous to clathrin-associated proteins, are involved in ER retrieval. EMBO J., 15, 1792–1798. [PMC free article] [PubMed] [Google Scholar]
- Dascher C. and Balch,W.E. (1994) Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J. Biol. Chem., 269, 1437–1448. [PubMed] [Google Scholar]
- Dogic D., de Chassey,B., Pick,E., Cassel,D., Lefkir,Y., Hennecke,S., Cosson,P. and Letourneur,F. (1999) The ADP-ribosylation factor GTPase-activating protein Glo3p is involved in ER retrieval. Eur. J. Cell Biol., 78, 305–310. [DOI] [PubMed] [Google Scholar]
- Duden R., Griffiths,G., Frank,R., Argos,P. and Kreis,T.E. (1991) β-COP, a 110 kDa protein associated with non-clathrin coated vesicles and the Golgi complex, shows homology to β-adaptin. Cell, 64, 649–665. [DOI] [PubMed] [Google Scholar]
- Duden R., Hosobuchi,M., Hamamoto,S., Winey,M., Byers,B. and Schekman,R. (1994) Yeast β- and β′-coat proteins (COP): two coatomer subunits essential for ER-to-Golgi traffic. J. Biol. Chem., 269, 24486–24495. [PubMed] [Google Scholar]
- Duden R., Kajikawa,L., Wuestehube,L. and Schekman,R. (1998) ε-COP is a structural component of coatomer that functions to stabilize α-COP. EMBO J., 17, 985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estojak J., Brent,R. and Golemis,E.A. (1995) Correlation of two-hybrid affinity data with in vitro measurements. Mol. Cell. Biol., 15, 5820–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulstich D. et al. (1996) Architecture of coatomer: molecular characterization of δ-COP and protein interactions within the complex. J. Cell Biol., 135, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K., Veit,M., Stamnes,M.A. and Rothman,J.E. (1996) Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science, 273, 1396–1399. [DOI] [PubMed] [Google Scholar]
- Gaynor E.C., Te Heesen,S., Graham,T.R., Aebi,M. and Emr,S.D. (1994) Signal-mediated retrieval of a membrane protein from the Golgi to the ER in yeast. J. Cell Biol., 127, 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor E.C., Graham,T.R. and Emr,S.D. (1998) COPI in ER/Golgi and intra-Golgi transport: do yeast COPI mutants point the way? Biochim. Biophys. Acta, 1404, 33–51. [DOI] [PubMed] [Google Scholar]
- Goldberg J. (1999) Structural and functional analysis of the ARF1–ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell, 96, 893–902. [DOI] [PubMed] [Google Scholar]
- Golemis E.A., Gyuris,J. and Brent,R. (1996) Interaction trap/two hybrid system to identify interacting proteins. In Ausubel,F.M. et al. (eds), Current Protocols in Molecular Biology. John Wiley and Sons, Inc. pp. 20.1.1–20.1.28. [Google Scholar]
- Gyuris J., Golemis,E., Chertkov,H. and Brent,R. (1993) Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell, 75, 791–803. [DOI] [PubMed] [Google Scholar]
- Harter C. and Wieland,F.T. (1998) A single binding site for dilysine retrieval motifs and p23 within the γ-subunit of coatomer. Proc. Natl Acad. Sci. USA, 95, 11649–11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J. and Robinson,M.S. (1998) Clathrin and adaptors. Biochim. Biophys. Acta, 1404, 173–193. [DOI] [PubMed] [Google Scholar]
- Hosobuchi M., Kreis,T.E. and Schekman,R. (1992) SEC21 is a gene required for ER to Golgi protein transport that encodes a subunit of a yeast coatomer. Nature, 360, 603–605. [DOI] [PubMed] [Google Scholar]
- Jackson C.L. and Casanova,J.E. (2000) Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol., 10, 60–67. [DOI] [PubMed] [Google Scholar]
- Lanoix J., Ouwendijk,J., Lin,C.C., Stark,A., Love,H.D., Ostermann,J. and Nilsson,T. (1999) GTP hydrolysis by arf-1 mediates sorting and concentration of Golgi resident enzymes into functional COP I vesicles. EMBO J., 18, 4935–4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F., Gaynor,E.C., Hennecke,S., Demolliere,C., Duden,R., Emr,S.D., Riezman,H. and Cosson,P. (1994) Coatomer is essential for retrieval of dilysine-tagged proteins to the ER. Cell, 79, 1199–1207. [DOI] [PubMed] [Google Scholar]
- Lewis M.J. and Pelham,H.R.B. (1996) SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell, 85, 205–215. [DOI] [PubMed] [Google Scholar]
- Lowe M. and Kreis,T.E. (1995) In vitro assembly and disassembly of coatomer. J. Biol. Chem., 270, 31364–31371. [DOI] [PubMed] [Google Scholar]
- Lowe M. and Kreis,T.E. (1996) In vivo assembly of coatomer, the COP-I coat precursor. J. Biol. Chem., 271, 30725–30730. [DOI] [PubMed] [Google Scholar]
- Malsam J., Gommel,D., Wieland,F.T. and Nickel,W. (1999) A role for ADP ribosylation factor in the control of cargo uptake during COPI-coated vesicle biogenesis. FEBS Lett., 462, 267–272. [DOI] [PubMed] [Google Scholar]
- Neer E.J. and Smith,T.F. (2000) A groovy new structure. Proc. Natl Acad. Sci. USA, 97, 960–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Stamnes,M., Ravazzola,M., Amherdt,M., Perrelet,A., Sollner,T.H. and Rothman,J.E. (1997) Bidirectional transport by distinct populations of COPI-coated vesicles. Cell, 90, 335–349. [DOI] [PubMed] [Google Scholar]
- Page L. and Robinson,M.S. (1995) Targeting signals and subunit interactions in coated vesicle adaptor complexes. J. Cell Biol., 131, 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavel J., Harter,C. and Wieland,F.T. (1998) Reversible dissociation of coatomer: functional characterization of a β/δ-coat protein subcomplex. Proc. Natl Acad. Sci. USA, 95, 2140–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris S., Berand-Dufour,S., Robineau,S., Bigay,J., Antonny,B., Chabre,M. and Chardin,P. (1997) Role of protein–phospholipid interactions in the activation of ARF1 by the guanine nucleotide exchange factor ARNO. J. Biol. Chem., 272, 22221–22226. [DOI] [PubMed] [Google Scholar]
- Poon P.P., Cassel,D., Spang,A., Rotman,M., Pick,E., Singer,R.A. and Johnston,G.C. (1999) Retrograde transport from the yeast Golgi is mediated by two ARF GAP proteins with overlapping function. EMBO J., 18, 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehling P., Darsow,T., Katzmann,D.J. and Emr,S.D. (1999) Formation of AP-3 transport intermediates requires Vps41 function. Nature Cell Biol., 1, 346–353. [DOI] [PubMed] [Google Scholar]
- Rothman J.E. and Wieland,F.T. (1996) Protein sorting by transport vesicles. Science, 272, 227–234. [DOI] [PubMed] [Google Scholar]
- Schekman R. and Orci,L. (1996) Coat proteins and vesicle budding. Science, 271, 1526–1533. [DOI] [PubMed] [Google Scholar]
- Schledzewski K., Brinkmann,H. and Mendel,R.R. (1999) Phylogenetic analysis of components of the eukaryotic vesicle transport system reveals a common origin of adaptor complexes 1, 2, and 3 and the F subcomplex of coatomer. J. Mol. Evol., 48, 770–778. [DOI] [PubMed] [Google Scholar]
- Schröder-Kohne S., Letourneur,F. and Riezman,H. (1998) α-COP can discriminate between distinct, functional di-lysine signals in vitro and regulates access into retrograde transport. J. Cell Sci., 111, 3459–3470. [DOI] [PubMed] [Google Scholar]
- Shih W., Gallusser,A. and Kirchhausen,T. (1995) A clathrin-binding site in the hinge of the β2 chain of mammalian AP-2 complexes. J. Biol. Chem., 270, 31083–31090. [DOI] [PubMed] [Google Scholar]
- Sikorski R.S. and Hieter,P. (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics, 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuestehube L.J., Duden,R., Eun,A., Hamamoto,S., Korn,P., Ram,R. and Schekman,R. (1996) New mutants of Saccharomyces cerevisiae affected in the transport of proteins from the endoplasmic reticulum to the Golgi complex. Genetics, 142, 393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybe J.A, Brodsky,F.M., Hofmann,K., Lin,K., Liu,S.H., Chen,L., Earnest,T.N., Fletterick,R.J. and Hwang,P.K. (1999) Clathrin self-assembly is mediated by a tandemly repeated superhelix. Nature, 399, 371–375. [DOI] [PubMed] [Google Scholar]
- Zhao L., Helms,J.B., Brugger,B., Harter,C., Martoglio,B., Graf,R., Brunner,J. and Wieland,F.T. (1997) Direct and GTP-dependent interaction of ADP ribosylation factor 1 with coatomer subunit β. Proc. Natl Acad. Sci. USA, 94, 4418–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]