Abstract
Background
Numerous national antiretroviral (ARV) treatment initiatives offering protease-inhibitor (PI)-sparing combination antiretroviral therapy (cART) have recently commenced in southern Africa, the first of which began in Botswana in January 2002. Evaluation of the efficacy and tolerability of various PI-sparing cART regimens requires intensive study in the region, as does investigation of the development of drug resistance and the optimal means of sustaining adherence. The Tshepo Study is the first large-scale randomized clinical trial which addresses these important issues among HIV-1 subtype C infected, ARV-treatment naïve adults in southern Africa.
Methods
The Tshepo Study is a completed open-labeled randomized study that enrolled 650 ARV-naïve adults between December 2002 and December 2004. The study is a 3 × 2 × 2 factorial design comparing the efficacy and tolerability among factors: (i) three combinations of nucleoside reverse transcriptase inhibitors (NRTIs): zidovudine (ZDV) + lamivudine (3TC); ZDV + didanosine (ddI); and stavudine (d4T) + 3TC; (ii) two different Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs): nevirapine (NVP) and efavirenz (EFV); and (iii) two different adherence strategies: the current national “Standard of Care (SOC)” versus an “intensified adherence strategy”, incorporating “community-based Directly Observed Therapy (Com-DOT)”. Study patients were stratified into two balanced CD4+ T cell count groups: less than 201 cells/mm3 versus 201-350 cells/mm3 with viral load greater than 55,000 copies/mL. Following DSMB recommendations in April 2006, ZDV/ddI-containing arms were discontinued due to inferiority in primary endpoint, namely, virologic failure with resistance. We report both overall data and pooled data from patients receiving ZDV/ddI- versus ZDV/3TC- and d4T/3TC-containing cART through 1 April 2006.
Results
Four hundred fifty-one (69.4%) females and 199 males with a median age of 33.3 years were enrolled into the study. The median follow-up as of 1 April 2006 was 104 weeks, and loss to follow-up rate at two years was 4.1%. The median baseline CD4+ T cell count was 199 cells/mm3 [IQR 136-252] and the median plasma HIV-1 RNA level was 193,500 copies/mL [IQR 69-250, 472-500]. The proportion of participants with virologic failure and genotypic resistance mutations was 11% in those receiving ZDV/ddI-based cART versus 2% in those receiving either ZDV/3TC- or d4T/3TC-based cART (p=0.002). The median CD4+ T cell count increase at one year was 137cells/mm3 [IQR 74-223] and 199 cells/mm3 [IQR 112-322] at two years with significantly lower gain in the ZDV/ddI arm. At one and two years, respectively, 92.0% and 88.8% of patients had an undetectable plasma HIV-1 RNA level (≤400 copies/mL). Kaplan-Meier survival estimates at one and two years were 96.6% and 95.4%. One hundred twenty (18.2%) patients had treatment modifying toxicities, of which the most common were lipodystrophy, anemia, neutropenia, and Stevens-Johnson syndrome. There was a trend towards difference in time to treatment modifying toxicity by pooled dual NRTI combination, and no difference in death rates.
Conclusions
The preliminary study results show overall excellent efficacy and tolerability of NNRTI-based cART among HIV-1 subtype C infected adults. ZDV/ddI-containing cART, however, is inferior to the dual NRTIs d4T/3TC or ZDV/3TC when used with an NNRTI for first-line cART.
Keywords: HIV/AIDS, cART, Africa, Randomized clinical trial
INTRODUCTION
In recent years, combination antiretroviral therapy (cART) has been introduced on a large scale throughout sub-Saharan Africa. Initial reports from ARV pilot studies in Côte d’Ivoire [1], Senegal [2], Uganda [3], Khayelitsha, South Africa [4], and Botswana [5], as well as preliminary data from larger public cART initiatives in Malawi [6] [7] and Zambia [8] have been very encouraging and reported impressive clinical, immunologic, and virologic responses among the vast majority of cART-treated individuals. These African cART outcomes have surpassed previous expectations and offer hope for the vast numbers of people in dire need of ARV treatment [9-12].
These published outcomes also raise numerous issues which need to be addressed to further improve ARV treatment protocols. Important considerations include: the selection of optimal regimens, especially with regards to tolerability and efficacy; the determination of the optimal time for cART initiation; the optimal means to promote and sustain ARV medication adherence; and the issue of drug resistance among persons infected with non-B subtypes [13] [14] [15]. Data from randomized clinical trials conducted in Africa to evaluate these critical questions are very much needed [16] [17] [18].
The Tshepo Study was begun in December 2002 in urban Botswana to compare six different cART regimens with regard to their efficacy and tolerability and to describe the development and overall kinetics of drug resistance. The study was also designed to identify the optimal means of promoting adherence by evaluating an intensified adherence strategy (Com-DOT) versus the standard of care. We herein report outcomes among adults randomized to ZDV/ddI-based cART versus ZDV/3TC- (given as Lamzid™) or d4T/3TC-based cART following the discontinuation of the ZDV/ddI-containing study treatment arms due to documented inferiority in efficacy as part of the study's third interim Data Safety Monitoring Board review. The word Tshepo means “hope” in Setswana.
METHODS
Study Design
The Tshepo Study is an open-label, randomized, 3 × 2 × 2 factorial design study conducted at Princess Marina Hospital in Gaborone, Botswana. This study was designed to address many important questions by evaluating the efficacy, tolerability, and the development of drug resistance of six different first-line cART regimens. The evaluated regimens were: ZDV/3TC/NVP (Arm A); ZDV/3TC/EFV (Arm B); ZDV/ddI/NVP (Arm C); ZDV/ddI/EFV (Arm D); d4T/3TC/NVP (Arm E), and d4T/3TC/EFV (Arm F). The study also compared two different adherence strategies: standard-of-care (SOC) versus standard-of-care plus community-based supervision (Com-DOT) to determine the better method for promoting adherence among treatment-naïve adults initiating cART.
The primary endpoints of the study were: (i) the development of virologic failure with significant drug resistance and (ii) the development of treatment-related toxicity, as defined by first incidence of a grade 3 or higher adverse event. Secondary endpoints were the time-to-occurrence of AIDS defining events and/or death for any reason.
The study was approved by the institutional review boards of the Botswana Ministry of Health (Health Research Development Committee) and the Harvard School of Public Health (Human Subjects Committee) and written informed consent was obtained from all participants.
Study Population
Adult (≥18 years of age), HIV-infected, cART-naïve Botswana citizens who attended one of the five ARV treatment screening clinics in Gaborone were approached for possible enrollment. All potentially eligible adults had to qualify for cART, based on existing Botswana National ARV Treatment guidelines [19] of having an AIDS-defining illness and/or CD4+ T cell count of equal or less than 200 cells/mm3 or meet this study's upper CD4 stratum eligibility criteria which was a CD4+ T cell count between 201 and 350 cells/mm3 with plasma HIV-1 RNA level greater than 55,000 copies/mL. Potentially eligible adults also had to reside within the study catchment area for the duration of the study. Other inclusion criteria were as follows: hemoglobin value greater than 8.0 grams/dL; absolute neutrophil count greater than or equal to 1.0 x 103/mm3; aminotransferase levels less than five times the upper limit of the normal; serum alkaline phosphatase level of less than three times the upper limits of the normal; and for women of child-bearing potential, a willingness to maintain active contraception throughout the duration of the study and a negative urine pregnancy test within 14 days of study enrollment. Exclusion criteria were: poor Karnofsky performance score (40 or below); an AIDS-related malignancy other than mucocutaneous Kaposi's sarcoma; grade 2 or higher peripheral neuropathy; major psychiatric illness; and for women, actively breastfeeding and/or less than six months post-partum.
Additional treatment steps, defined as protease-inhibitor (PI)-containing regimens, were available for all participants with confirmed virologic failure, toxicities, or concomitant medical conditions that required the use of PI's.
Data Collection and Follow-up
Clinical and adherence assessment were done monthly at the study clinic. To monitor treatment efficacy, CD4+ T cell counts (FACS Calibur flow cytometer, Becton Dickinson, San Jose, CA, USA) and plasma HIV-1 RNA levels (Amplicor HIV-1 Monitor test, version 1.5 Roche Diagnostics Systems, Branchburg, NJ), were obtained at enrollment and then every two months for the duration of the study. Laboratory safety monitoring included comprehensive chemistry and complete blood count (hematology) specimens at study enrollment, then every months for the first six months of the study, every two months during months 6-12 of study participation, and every four months during the remainder of participation, for safety monitoring purposes. In addition, all patients had lipid chemistries (total, LDL, and HDL cholesterol, glucose, and serum triglycerides) performed at the time of study initiation and then every six months for the duration of the study. Laboratory values were graded according to the 1994 DAIDS laboratory grading scale, except lipid chemistry values which were graded using the DAIDS December 2004 grading scale. Additional routine clinical assessment included peripheral neuropathy assessments every two months, lipodystrophy and performance assessments every six months, and annual screening for the presence of other sexually transmitted infections (hepatitis B and syphilis), proteinuria/glycosuria, and chest x-ray abnormalities. Patients also received an ophthalmologic evaluation at baseline which was repeated at least every two years on study. All female patients had baseline Papanicolau smears performed which were repeated at least annually or more frequently, as clinically indicated. Colposcopic examinations were performed when clinically indicated. All women of reproductive potential had monthly urine pregnancy tests performed.
Comprehensive care for study participants was provided in accordance with existing national policy and free-of-charge [20],[21]. Opportunistic infections were diagnosed using available laboratory, imaging and histopathologic services as well as specialist consultation. Pulmonary and extra-pulmonary TB were diagnosed using acid fast bacilli (AFB) staining, CSF microscopy and radiographic imaging (chest radiography, computed tomography (CT) scanning and ultrasound). Pneumocystis jiroveci (formerly carinii) pneumonia (PCP) was diagnosed using clinical expertise and radiographic imaging (chest radiography). Cryptococcal meningitis was ascertained by India Ink staining of the cerebrospinal fluid (CSF). Cytomegalovirus (CMV) retinitis was diagnosed by fundoscopic examinations which were performed by specially trained ophthalmologists. Malignancies (invasive cervical carcinoma, Kaposi's sarcoma and non-Hodgkin's lymphoma) were diagnosed by histology and expert pathologist and/or oncologist review. Clinical expertise alone was used to diagnose Candida esophagitis and herpes zoster. Prophylaxis for opportunistic infections included 6 months of isoniazid (INH) plus pyridoxine (vitamin B6) preventative therapy if determined that participant was without clinically active tuberculosis disease and one oral double-strength cotrimoxasole tablet three times per week (or once daily) for the prevention of Pneumocystis jiroveci (PCP) pneumonia if the CD4+ T cell count was less than 200 cells/mm3 .
ARV medication adherence was defined as being ‘excellent’ (greater than 90 percent) based on a composite adherence measure which included: (i) patient self-reporting (four day and one month), (ii) patient ARV self-demonstration (including verbal reporting on timing of doses, number of tablets per dose, and food requirements), (iii) four-day recall, and (iv) ARV pill counts.
Initially, virologic failure was defined as a confirmed plasma HIV-1 RNA level of greater than 5,000 copies/mL at 16 or more weeks following cART initiation. If the repeat plasma HIV-1 RNA level following an intensified adherence intervention still exceeded 5,000 copies/mL, the patient underwent a step change and was initiated on two different NRTIs and a PI in accordance with the 2002 and 2005 Botswana national ARV treatment guidelines [19] [21]. Effective 1 April 2006, the study virologic failure definition was changed to any confirmed viremia (greater than the lower limit of detection, which is 400 copies/mL) in accordance with new literature [22] [23] [24] and existing national guidelines [21]. All participants with confirmed virologic failure underwent an intensified adherence intervention which included an initial adherence assessment and adherence education, followed by a two-to-four week period of Com-DOT. A repeat viral load measurement was done at the end of the intervention. Genotypic resistance testing was done using Roche ViroSeq v 2.0™, an integrated system for sequence-based analysis of drug resistance mutations in HIV-1, as per the manufacturer's instructions. In cases of first line cART regimen virologic failures, the study physician was blinded to genotypic resistance results.
On 6 April 2006, as part of the third interim analysis, our independent Data Safety Monitoring Board (DSMB) recommended discontinuing the ZDV/ddI-containing study treatment arms due to inferiority in efficacy, specifically higher virologic failure rates among cohort-treated participants receiving ZDV/ddI-containing cART compared to those receiving ZDV/3TC- and d4T/3TC-containing cART regimens. The Board recommended that all cART-treated patients who were receiving the dual NRTI combination of ZDV/ddI be switched to ZDV/3TC.
Statistical Analysis
All primary analyses and the majority of secondary analyses were performed on an “intent-to-treat” basis. Kaplan-Meier (K-M) survival curves including 95% confidence intervals at one and two years and Cox proportional hazards models were used to compare study participants receiving ZDV/ddI-containing cART to patients who received cART regimens that did not contain ZDV/ddI, with respect to event rates for virologic failure, death, toxicities, opportunistic infections, and non-adherence. For our analyses, observations were censored on 1 April 2006 or when the participant died or was lost to follow-up, if before that date. All statistical analyses were conducted using SAS software.
RESULTS
Study Recruitment
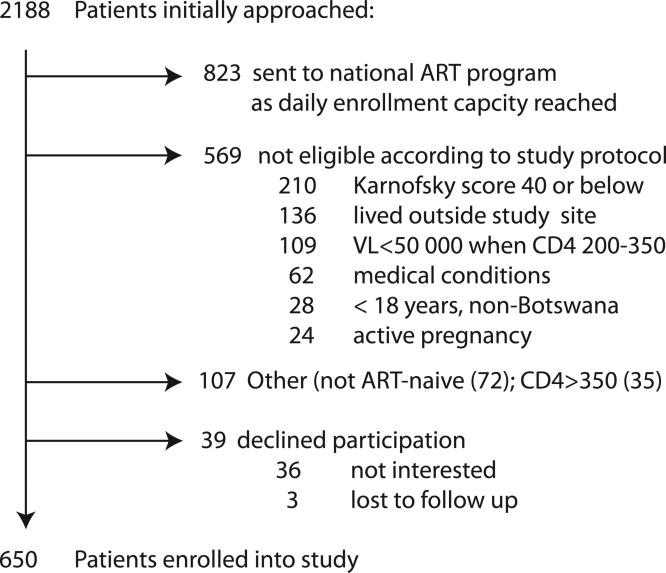
Between December 2002 and December 2004, 2188 patients were screened for possible study enrollment at the adult Infectious Disease Care Clinic (IDCC) of Princess Marina Hospital and four designated local Gaborone City Council “CD4+ screening” clinics. In total, 650 adults were eligible, consented, and enrolled in the study. Figure 1 summarizes the main reasons for non-enrollment into the study. In short, 823 patients were referred to the Botswana national ARV program at the adjacent adult PMH IDCC for continued longitudinal care due to our study team reaching its’ daily limit in total number of enrolled patients. Five-hundred and sixty-nine patients were not eligible according to the study protocol. Of these 569, 210 patients had a Karnofsky score equal or below 40, and 65 patients were deemed ineligible based on the presence of the following active medical conditions and/or laboratory abnormalities; namely neutropenia (19), anemia (18), active tuberculosis infection not yet on appropriate therapy (9), and other health related conditions (19) such as grade 2 or greater peripheral neuropathy or elevated liver enzymes and active/recent pregnancy. An additional 109 patients were not eligible due to virologic criteria, namely having a CD4+ count between 201 and 350 cells/mm3 but having a plasma viral load below 55,000 copies/mL, as our viral load cutoff was based on existing WHO and DHHS guidelines at the time the study was designed. 107 patients had CD4+ count of greater than 350 cells/mm3 (35), or were not ART-naive (72). 39 patients declined study participation (36) or were lost to follow-up (3) during the screening process.
Fig 1.
Recruitment Flow Chart
Baseline Characteristics
Of the 650 enrolled adults, 451 (69.4%) were female. Forty-three percent had advanced WHO clinical disease (Stage 3 or 4). Table 1 summarizes the key baseline characteristics.
Table 1.
Baseline Characteristics of Study Population
| ZDV/ddI N=216 | non-ZDV/ddI N=434 | Overall N=650 | ||
|---|---|---|---|---|
| Gender N (%) | female | 157 (24.1) | 294 (45.2) | 451 (69.4) |
| male | 59 (9.1) | 140 (21.5) | 199 (30.6) | |
| Age in yrs, median (IQR) | 32.9 (29, 38.1) | 33.6 (28.8, 38.8) | 33.3 (28.9, 38.7) | |
| Weight in kg, mean (SD) | 59.7 (12.5) | 59.3 (12.4) | 59.4 (12.5) | |
| BMI, median (IQR) | 21.1 (19.4, 24.9) | 21.4 (19.1, 24.1) | 21.2 (19.2, 24.3) | |
| F | 22 (19.7, 26.7) | 21.7 (19.3, 25) | 21.8 (19.4, 25.3) | |
| M | 20.5 (18.5, 21.5) | 20.8 (19, 23.3) | 20.7 (18.8, 22.7) | |
| WHO clinical staging, N (%) | I | 59 (9.3) | 139 (22) | 198 (31.3) |
| II | 50 (7.9) | 111 (17.5) | 161 (25.4) | |
| III | 85 (13.4) | 131 (20.7) | 216 (34.1) | |
| IV | 14 (2.2) | 44 (6.9) | 58 (9.2) | |
| CD4+ T cells, cells/μL, median (IQR) | Overall | 197 (140, 258) | 199 (134, 249) | 199 (136, 252) |
| CD4 strata ≤ 200 | 140 (86, 171) | 135 (79, 169.5) | 137 (81, 170) | |
| CD4 strata 201-350 | 259 (225,288) | 250 (222, 294) | 252 (222.5, 293) | |
| CD4, N (%) | <50 | 14 (2.15) | 30 (4.6) | 44 (6.8) |
| 50-200 | 96 (14.8) | 190 (29.2) | 286 (44) | |
| 201-350 | 106 (16.3) | 214 (32.9) | 320 (49.2) | |
| Viral Load, copies/mL, median (IQR) | 207 000 (79 000, 499 000) | 190 000 (64 900, 460 000) | 193 500 (69 250, 472 500) | |
| Opportunistic Infections, N (%) | ||||
| Wasting syndrome** N (%) | 36 (5.6) | 85 (13.2) | 121 (18.7) | |
| Tuberculosis (any site) | 33 (5.1) | 73 (11.2) | 106 (16.3) | |
| Anemia HB<10 g% | 34 (5.3) | 67 (10.5) | 101 (15.9) | |
| HB 10-12 g% | 93 (14.6) | 170 (26.7) | 263 (41.3) | |
| Herpes zoster (VZV) | 32 (4.9) | 55 (8.5) | 87 (13.4) | |
| Peripheral Neuropathy | 29 (4.5) | 44 (6.8) | 73 (11.2) | |
| Esophageal candidiasis | 5( 0.8) | 10 (1.5) | 15 (2.3) | |
| Chronic, recurrent diarrhea | 2 (0.3) | 10 (1.5) | 12 (1.9) | |
| Kaposi's sarcoma | 3 (0.5) | 8 (1.2) | 11 (1.7) | |
| Pneumocystis jiroveci pneumonia | 1 (0.2) | 5 (0.8) | 6 (0.9) | |
| Cryptococcal meningitis | 2 (0.3) | 1 (0.2) | 3 (0.5) |
wasting syndrome defined as BMI<18.5
Baseline characteristics of patients in the ZDV/ddI arms versus the other four arms (d4T/3TC- and ZDV/3TC-containing) were evenly balanced at entry. Three hundred twenty-five participants were randomized to the intensified adherence (Com-DOT) arm. Three hundred thirty (50.9%) patients were enrolled in the lower CD4+ T cell count stratum with a median CD4+ T cell count of 137 cells/mm3. Three hundred twenty (49.1%) patients were enrolled in the upper CD4+ T cell count stratum (CD4+ T cell count value between 201 and 350 cells/mm3 and plasma HIV-1 RNA above 55,000 copies/mL) with a median CD4+ T cell count of 252 cells/mm3.
Follow-Up
The amount of study follow-up was approximately 1,308 person-years, with a median follow-up time of 104 weeks [IQR 78-136]. Ninety-eight percent of all scheduled follow-up visits were attended. As of 1 April 2006, four years and three months into the study, 31 of the 650 enrolled patients were lost to follow up with regard to primary endpoint information. Eighteen (58%) of the 31 had moved out of the study catchment area, six (19%) declined further participation, and for seven (23%), no further information was available despite repeated attempts by the study team to contact them. The overall loss to follow-up rates were 2.4% [CI: 1.4%, 3.9%] and 4.1% [CI: 2.6%, 5.2%] at one and two years, respectively. The sociodemographic and clinical characteristics of participants who were lost to follow-up did not differ from those participants who completed the trial.
HAART Outcomes
Immunologic
The median increase in CD4+ T cell count was 137 cells/mm3 at one year [IQR 74-223] and 199 cells/mm3 at two years [IQR 112-322]. There was a significant difference by treatment arms with a median CD4+ T cell gain from baseline in the ZDV/ddI arm of 106 [IQR 34-176] and in the non- ZDV/ddI arm of 156 [IQR 94-242] cells/mm3 at one year and 163 [IQR 70-250] and 238 [IQR 138-333] cells/mm3 at two years respectively (p=0.0001). CD4+ T cell count increases were significantly higher in women (p=0.0094). No significant difference was found in relation to anemia (baseline hemoglobin), body mass index, or age greater than 40 years. Concordant virologic and immunologic responses were found in 75%, 76% and 81% of participants at 6, 12, and 24 months respectively. At 12 and 24 months, significantly more patients in the ZDV/ddI group showed discordant responses. Additional analyses are planned to look more in-depth at potential reasons for a small subset of patients who have discordant immunologic and virologic responses.
Virologic
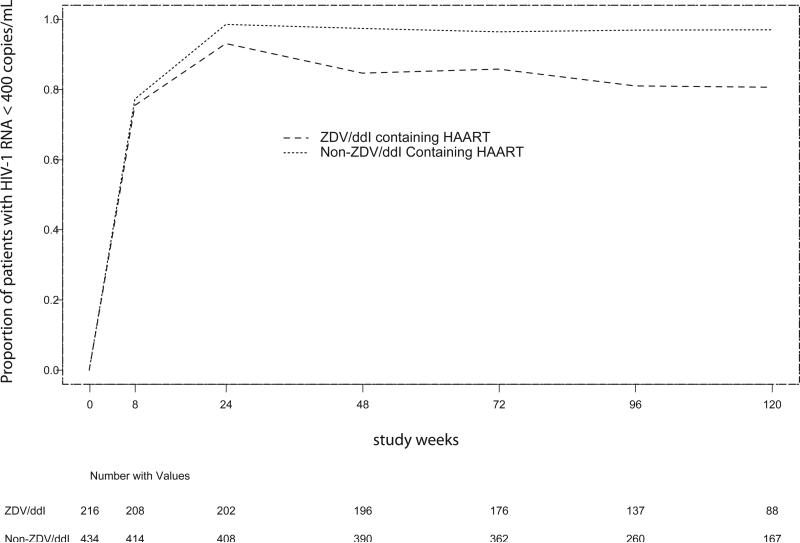
Figure 2 shows the proportion of all study participants who had undetectable plasma HIV-1 RNA levels at weeks 8, 24, 48, 72, and 96; stratified by whether they were receiving ZDV/ddI-containing cART or non-ZDV/ddI-containing (ZDV/3TC- or d4T/3TC-based) cART. Three hundred sixty-one (56%) of study participants had undetectable (<400 copies/mL) plasma HIV-1 RNA levels at four weeks following cART initiation. Of those whose HIV-1 RNA suppressed to undetectable levels at eight weeks following cART initiation, 70.8% (64.1%, 76.4%) receiving ZDV/ddI based cART and 85.6% (81.9%, 88.7%) receiving non-ZDV/ddI based cART remained consistently suppressed at one year. At two years, the percentages remaining consistently suppressed were 57.6% (50.4%, 64.2%) for ZDV/ddl arms and 78.5% (74.0%, 82.2%) for the non-ZDV/ddl arms. Following DSMB recommendations, the ZDV/ddI-containing cART arms were discontinued (after the cutoff date for this analysis); and all patients substituted 3TC for ddI due to inferiority in primary endpoint, namely virologic failure.
Figure 2.
Proportion of Patients with HIV-1 RNA Level of less than 400 copies/mL
As of 1 April 2006, 55 (8.5%) study participants had developed virologic failure, as defined by the study protocol of whom 52 cases were successfully genotyped. In two cases no genotyping data was available due to a missed confirmatory visit, in one case viral amplification was unsuccessful. Thirty-eight (70%) of available 52 genotypes were found to have virologic failure with primary genotypic resistance mutations. Figure 3 is a Kaplan-Meier plot of time to first virologic failure with resistance. Rates of virologic failure with resistance were significantly higher in the ZDV/ddI-containing treatment arms when compared to the non-ZDV/ddI-containing treatment arms (p-value <0.0001). At one year, 5.3% (3.0%, 9.4%) of patients receiving ZDV/ddI –containing cART had virologic failure with resistance, compared to 1.0% (0.1%, 1.7%) of those receiving non-ZDV/ddI-containing cART. At two years, 13.5% (9.2%, 19.4%) of those receiving ZDV/ddI -containing cART and 3.2% (1.8%, 5.8%) of patients receiving non-ZDV/ddI-containing cART had virologic failure with resistance.
Figure 3.
Kaplan Meier Curves for Virologic Failure with Resistance by Treatment Arms
Out of 38 cases of virologic failure with resistance mutations 25 cases occurred in NVP-containing regimens versus 13 cases of EFV-containing regimens. NRTI mutations were present in 27 cases. Of note, a unique pattern of TAM mutations, namely the 67N 70R 215Y pathway was found in patients failing ZDV/ddI-containing first-line cART at the time of virologic failure. Among all patients failing ZDV/ddI-containoing cART, the two most common resistance genotypes were the 67N 70R 215Y genotype, present in 7 of 19 (37%) of cases, and a single T215Y mutation, present in 5 of 19 (26%) of cases. No 210W or 219Q genotypes were present among analyzed failures. With the exception of three virologic failure cases, namely one patient having the 67N 70R 215Y genotype and two cases having an isolated M41L mutation, all other failing patients having primary NRTI mutations also had concomitant primary NNRTI mutations.
As of April 2006, 43 patients had been changed from first-line study treatment to PI-based cART. Reasons for the change to second- or third-line PI-based cART included: virologic failure (63%), pregnancy protection (23%), and severe ARV-related toxicity (14%), the majority of which were due to moderate-severe symptomatic hyperlactatemia or lactic acidosis syndrome [25].
Clinical
The mean body weight increase following two years of cART was 6.2 kilograms for patients with pre-existing wasting syndrome and 3.0 kilograms for patients without existing HIV-associated wasting syndrome at the time of study enrollment.
Survival
As of 1 April 2006, 32 (5%) of enrolled study participants had died. Eight (25%) of these 32 deaths occurred within the first three months following cART initiation. Among theses 8 cases, 4 were due to advanced AIDS, 2 were non-AIDS related and 2 were possibly related to ARV treatment. Overall, of the 32 deaths, seven (23%) were deemed “possibly related to ARV treatment, with four related to lactic acidosis/pancreatitis, two related to traditional medication use/abuse with possible underlying pancreatitis, and one from nevirapine-associated fulminant hepatic failure potentially exacerbated by isoniazid which the patient was taking for TB prophylaxis. 12 (37.5%) of the 32 deaths were due to an opportunistic infection.
The Kaplan-Meier one-year and two-year survival estimates were 96.6% [CI: 94.8%, 97.7%] and 95.4% [CI: 93.5%, 96.8%], respectively, for the entire cohort. There were no statistically significant differences across the two pooled treatment groups, ZDV/ddI-treated versus non-ZDV/ddI-treated, (p=0.93). In univariate analysis, death was significantly related to anemia (baseline hemoglobin value <10.0 gr/dL), poor performance status (Karnofsky score less than 90), and HIV-associated wasting syndrome (BMI <18.5).
Safety and Tolerability
Overall, 208 clinically relevant grade 3 and 4 serious adverse events occurred in 99 patients, excluding laboratory events which did not change clinical management such as amylase, gammaglutaryl transaminase (GGT), and alkaline phosphatase elevations. These serious adverse events were distributed equally among the pooled treatment groups (ZDV/ddI-treated versus ZDV/3TC- and d4T/3TC-treated, log-rank p=0.2575). At one and two years, 11.3% [9.1%, 14.0%] and 14.5% [11.9%, 17.5%] of patients had had a grade 3 or 4 serious adverse event, respectively.
Thirty-one (14.8%) patients in the ZDV-ddI group and 89 (19.8%) patients in the non-ZDV/ddI group had 140 treatment modifying toxicities (log-rank p=0.0647), with lipodystrophy (50), neutropenia (23), anemia (22), and cutaneous hypersensitivity reactions (16) being the most common (Table 2). At one year, 8.9% [5.8%, 13.6%] of patients receiving ZDV/ddI-containing cART had a treatment-modifying toxicity, compared to 12.2% [9.5%, 15.7%] of those who received non-ZDV/ddI-containing cART At two years, 12.6% [8.8%, 18.0%] of patients who received ZDV/ddI-containing cART and 16.7% (13.8%, 20.7%) of those receiving non-ZDV/ddI-containing cART had a treatment-modifying toxicity.
Table 2.
Adverse Events Resulting in Treatment Modification
| Cause | ZDV-ddI patients n (% of 216) | Non ZDV-ddI patients n (% of 434) | Total n (% of 650) |
|---|---|---|---|
| Lipodystrophy | 9 (4.2) | 41 (9.4) | 50 (7.7) |
| Neutropenia | 5 (2.3) | 18 (4.1) | 23 (3.5) |
| Anemia | 8 (3.7) | 14 (3.2) | 22 (3.4) |
| Rash/Hypersensitivity Reaction | 5 (2.3) | 11 (2.5) | 16 (2.5) |
| Hepatotoxicity | 2 (0.9) | 6 (1.4) | 8 (1.2) |
| Pancreatitis | 5 (2.3) | 2 (0.5) | 7 (1.1) |
| Lactic Acidosis | 0 | 4 (0.9) | 4 (0.6) |
| Neuropsychiatric Symptoms | 1 (0.5) | 4 (0.9) | 5 (0.8) |
| Peripheral Neuropathy | 0 | 2 (0.5) | 2 (0.3) |
| Diarrhea | 1 (0.5) | 0 | 1 (0.2) |
| Vomiting | 1 (0.5) | 0 | 1 (0.2) |
| Pancytopenia | 0 | 1 (0.2) | 1 (0.2) |
| Total number of patients* | 31 | 89 | 120 |
Note: Columns do not add correctly because a patient may have more than one treatment-modifying toxicity.
Incident Opportunistic Infections
One hundred and six incident opportunistic infections (OIs) were diagnosed in 93 study participants. The most common OIs included (i) varicella zoster virus (VZV) infection (Shingles) (40 patients), (ii) pulmonary TB (36 patients), and (iii) extra-pulmonary TB (13 patients, 11 with miliary/disseminated TB, and two with TB meningitis) (Table 3). At one year 12.8% [9.0%, 18.1%] of patients receiving ZDV/-containing cART had had an OI, compared to 7.5% [5.4%, 10.5%] of patients who received non-ZDV/ddI-containing cART. At two years, 16.6% [12.0%, 22.2%] of patients receiving ZDV/ddI-containing cART and 11.9% [9.1%, 15.6%] of patients who received non-ZDV/ddI-containing cART had had an OI. The log-rank test by treatment group was statistically significant (p=0.042), with ZDV/ddI-treated patients having a shorter time to first OI compared to non-ZDV/ddI-treated patients.
Table 3.
Incident Opportunistic Infections on HAART
| |
0-3 months on HAART n (% of 106) |
4-6 months on HAART n (% of 106) |
> 6 months on HAART n (% of 106) |
Total n (% of 106) |
|---|---|---|---|---|
| Herpes Zoster (VZV) |
9 (8.5) |
18 (17) |
13 (12.3) |
40 (37.7) |
| Pulmonary TB |
11 (10.4) |
19 (17.9) |
6 (5.7) |
36 (34) |
| Extra-pulmonary TB |
2 (1.9) |
2 (1.9) |
9 (8.5) |
13 12.3) |
| Malignancies | ||||
| Kaposi's sarcoma | 2 (1.9) | 0 | 0 | 2 (1.9) |
| Invasive cervical carcinoma | 1 (.9) | 1 (0.9) | 1 (0.9) | 3 (2.8) |
| Squamous cell penile carcinoma | 0 | 0 | 1 | 1 (0.0) |
| Non-Hodgkin's lymphoma |
0 |
1 (0.9) |
0 |
1 (0.9) |
| Cryptococcal Meningitis |
1 (0.9) |
3 (2.8) |
0 |
4 (3.8) |
|
Pneumocystis jiroveci (carinii) pneumonia |
1 (0.9) |
2 (1.9) |
0 |
3 (2.8) |
| Esophageal candidiasis |
0 |
2 (1.9) |
0 |
2(1.9) |
|
Cytomegalovirus (CMV) Retinitis |
0 |
0 |
1 (0.9) |
1 (0.9) |
| Total | 27 (25.5) | 48 (45.3) | 31 (29.2) | 106 (100) |
Adherence
Medication adherence was reported to be excellent (i.e. greater than 90% at all measured time-points, as per monthly clinic adherence assessments) in 89.8 % of study participants after one year of follow-up and 81.2 % after two years of study follow-up. There was a statistically significant difference by dual NRTI combination, with ZDV/ddI-treated patients having a shorter time to first report of non-adherence when compared to those receiving ZDV/3TC- and d4T/3TC-based cART regimens (p=0.03) and anecdotally, study participants frequently expressed difficulties in following the specific food-related instructions when taking the non-enteric coated ddI. Pooled treatment group analysis also showed statistically significant differences in adherence by gender, with males having a shorter time to non-adherence (p=0.006).
DISCUSSION
We report herein our two-year findings among adult patients enrolled in Botswana's ongoing Tshepo Study, one of the first large-scale randomized clinical trials focusing on cART outcomes to be conducted in sub-Saharan Africa. Our interim study results, backed by an impressive retention rate of study participants during the two years of follow-up, demonstrate an overall excellent immunologic and virologic response to NRTI/NNRTI regimens among HIV-1C infected adults. The low virologic failure rate with resistance mutations, 1.0% at year 1 and 3.2% at year 2, in the four non-ZDV/ddI-containing arms is superior to results obtained from cART-treated cohorts in industrialized countries [26].
Our data clearly demonstrate the inferiority of the dual NRTI combination of ZDV/ddl compared to the dual NRTI combinations of ZDV/3TC and d4T/3TC, when given together with either NVP or EFV in HIV-1C infected adults. These findings prompted the closure of the ZDV/ddl containing treatment arms as recommended by our DSMB. Interestingly, a novel reverse transcriptase 67N 70R 215Y genotype was the predominant TAM pathway among HIV-1C infected individuals treated with ZDV/ddI as part of their first-line regimen. This mixture of a TAM-1 (41L/210W/215Y) and TAM-2 67N/70R/215F/219Q) pathways might represent an HIV-1 subtype C specific resistance pathway to first line ZDV/ddI containing regimens [27].
While recommended as an alternative first-line regimen by World Health Organization (WHO) guidelines at the time our randomized trial began, information on the efficacy of the dual NRTI combination of ZDV/ddI in NNRTI-containing cART regimens has been scarce [28] [29] [30] [31] [32]. Our findings of a novel TAM resistance pathway selected by HIV-1C in the presence of ZDV/ddl make a compelling case against the use of this dual NRTI combination for first-line cART in the developing world and support the most recent WHO [33] and international guidelines [34] which now recommend that 3TC (or FTC) be given with either tenofovir or ZDV for first-line cART with NVP or EFV.
ARV Treatment programs are rapidly scaling-up in resource-limited settings in sub-Saharan Africa. Most programs have chosen either Triommune™ (d4T/3TC/NVP) or Combivir/Lamzid™ (ZDV/3TC) based cART as the first-line regimen [35] [36]. Didanosine still plays an important role for second line cART regimens [37], especially where newer NRTIs (abacavir) and nucleotide reverse transcriptase inhibitors (tenofovir) are not uniformly available, largely due to high cost. Additional studies are needed to characterize the activity of ZDV/ddl-based cART regimens given with a PI as the second-line regimen in settings where d4T and 3TC combinations are used as the NRTI backbones in first-line regimens [38].
The one-year (3.4%) and two-year (4.6%) survival outcomes among cART-treated adults in this large cohort are impressive when compared to data from other cohorts in resource-limited settings [39]. Our study did not include patients that were severely ill at baseline which may have certainly influenced our overall favorable clinical outcomes (i.e. low mortality rates), but as a team we did make every effort to include ill patients as evidenced by the numbers with advanced immunosuppression, advanced WHO clinical stage (3 and/or 4), and high plasma HIV-1 RNA levels at baseline. Nearly a quarter of all deaths were deemed “possibly related to study treatment” with the majority of ARV-related deaths related to severe mitochondrial toxicities, especially lactic acidosis and/or pancreatitis, emphasizing the importance of replacing d4T with a safer alternative. Cohort data is still needed to characterize long-term cART outcomes in the region especially as co-morbid medical illness, and particularly cardiovascular causes of death, comprise an increasing percentage of deaths, especially among predominantly NNRTI, non-PI-treated adults.
The sustainability of ART programs largely depends on the tolerability of ARV medication. While a number of studies in Africa have reported toxicity outcomes and regimen switch rates, these studies used different regimens, observation times, and severity grading systems. A comprehensive analysis of toxicities related to the individual ARV drugs used in this study is currently performed. The interim two-year outcomes data of this controlled clinical trial show that all six first-line cART regimens are well tolerated. The overall rate of treatment modifying toxicity was low when compared to results from observational studies followed in the region [40], [41], [42], [43]. This may, in part, reflect the fact that many Tshepo Study participants initiated cART at higher baseline CD4+ T cell counts than in other cohorts. Didanosine- related toxicities such as pancreatitis, peripheral neuropathy and diarrhea were rarely a reason for treatment modification in the ZDV/ddI containing treatment arm. Importantly, the occurrence of lipodystrophy and lactic acidosis was disproportionately more frequent among patients in the pooled, d4T- containing treatment arms. This finding is consistent with the result of other studies that have shown higher rates of mitochondrial toxicity among d4T-treated patients [41], [44].
Incident opportunistic infection rates are important markers for understanding the clinical course of treated HIV disease and for the development of treatment guidelines and plan health services. Unfortunately, these conditions have often not been reported in a standardized fashion. Limited diagnostic facilities, high TB rates, and difficulties defining IRIS make the task more difficult for African settings [4] [5] [40]. Patients on ZDV/ddI containing cART experienced significantly higher OI rates in the first two years of ARV treatment as compared to those on non-ZDV/ddI regimens. This finding is most probably a consequence of the higher virologic failure rate and the poorer immunologic recovery among patients in the ZDV/ddI arm of the study.
Of note, seven of our intensively monitored participants developed cancer-related diagnoses. As cART-treated adults survive longer in the region, improved diagnostic capacity at the referral hospital level and education and training to enable healthcare providers to more efficiently diagnose and manage these potentially life-threatening conditions will be important regional needs over the next 5-10 years.
We also report excellent one-year adherence rates, which support rates reported by other ART programs in Africa [8] [11] [45] [46] [47]. However, the decline in medication adherence in the second year, especially among male participants, is a concern. As the long-term sustainability of cART programs in Africa largely depends upon sustained cART adherence rates, it is of paramount importance for healthcare personnel to provide ongoing ARV adherence support. This support must be continually adapted to meet patients’ needs, while at the same time addressing the needs of those most at risk for poor adherence, with particular attention paid to men and patients with ongoing psychosocial, financial, or physical needs.
In summary, interim results from Tshepo Study show the importance and feasibility of conducting large clinical research initiatives in resource-limited settings. Our preliminary findings document remarkable immunologic and virologic responses to first-line PI-sparing cART regimens and excellent cohort retention rates. These outcomes surpass many reported in Western Europe and the United States. Continued long term cohort follow-up is needed, as are new randomized clinical trials designed to address important regional considerations such as optimal first-line cART regimens, the optimal timing of cART initiation, and the kinetics and development of drug resistance which appears to differ from what has been reported among HIV-1 subtype B infected, cART-treated individuals.
ACKNOWLEDGEMENTS
We would like to formally acknowledge the Botswana Ministry of Health, the Princess Marina Hospital administration, outpatient adult Infectious Disease Care Clinic (IDCC), and inpatient Medical Ward teams, the entire Tshepo Study team and our sponsor, the Bristol-Myers Squibb Foundation, Secure the Future, for their support of this research initiative.
We also want to formally acknowledge and thank all study participants.
The project described was also supported by grant number K23AI073141 (C. William Wester, M.D.) from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Lastly, we would like to acknowledge and personally thank Erika Färdig (Administration, Harvard School of Public Health, Boston, MA, USA) for her administrative oversight, review of this manuscript, and overall technical assistance and expertise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Djomand G, et al. Virologic and immunologic outcomes and programmatic challenges of an antiretroviral treatment pilot project in Abidjan, Cote d'Ivoire. AIDS. 2003;17(Suppl 3):S5–15. doi: 10.1097/00002030-200317003-00002. [DOI] [PubMed] [Google Scholar]
- 2.Laurent C, et al. The Senegalese government's highly active antiretroviral therapy initiative: an 18-month follow-up study. AIDS. 2002;16(10):1363–70. doi: 10.1097/00002030-200207050-00008. [DOI] [PubMed] [Google Scholar]
- 3.Weidle PJ, et al. Assessment of a pilot antiretroviral drug therapy programme in Uganda: patients’ response, survival, and drug resistance. Lancet. 2002;360(9326):34–40. doi: 10.1016/S0140-6736(02)09330-3. [DOI] [PubMed] [Google Scholar]
- 4.Coetzee D, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18(6):887–95. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 5.Wester CW, et al. Initial response to highly active antiretroviral therapy in HIV-1C-infected adults in a public sector treatment program in Botswana. J Acquir Immune Defic Syndr. 2005;40(3):336–43. doi: 10.1097/01.qai.0000159668.80207.5b. [DOI] [PubMed] [Google Scholar]
- 6.Ferradini L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367(9519):1335–42. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 7.Harries AD, et al. Monitoring the response to antiretroviral therapy in resource-poor settings: the Malawi model. Trans R Soc Trop Med Hyg. 2004;98(12):695–701. doi: 10.1016/j.trstmh.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Stringer JS, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296(7):782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 9.Binswanger HP. Public health. HIV/AIDS treatment for millions. Science. 2001;292(5515):221–3. doi: 10.1126/science.1057504. [DOI] [PubMed] [Google Scholar]
- 10.De Cock KM, Mbori-Ngacha D, Marum E. Shadow on the continent: public health and HIV/AIDS in Africa in the 21st century. Lancet. 2002;360(9326):67–72. doi: 10.1016/S0140-6736(02)09337-6. [DOI] [PubMed] [Google Scholar]
- 11.Akileswaran C, et al. Lessons learned from use of highly active antiretroviral therapy in Africa. Clin Infect Dis. 2005;41(3):376–85. doi: 10.1086/431482. [DOI] [PubMed] [Google Scholar]
- 12.Hanson S. AIDS control in sub-Saharan Africa--are more drugs and money the solution? Lancet Infect Dis. 2002;2(2):71–2. doi: 10.1016/s1473-3099(02)00180-9. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins C, Murphy R. Adherence to antiretroviral therapy in resource-limited settings: everything matters. AIDS. 2007;21(8):1041–2. doi: 10.1097/QAD.0b013e3281900eb9. [DOI] [PubMed] [Google Scholar]
- 14.Bangsberg DR, et al. High levels of adherence do not prevent accumulation of HIV drug resistance mutations. AIDS. 2003;17(13):1925–32. doi: 10.1097/00002030-200309050-00011. [DOI] [PubMed] [Google Scholar]
- 15.Colebunders R, et al. A new model to monitor the virological efficacy of antiretroviral treatment in resource-poor countries. Lancet Infect Dis. 2006;6(1):53–9. doi: 10.1016/S1473-3099(05)70327-3. [DOI] [PubMed] [Google Scholar]
- 16.Colebunders R, et al. First-line antiretroviral therapy in Africa--how evidence-base are our recommendations? AIDS Rev. 2005;7(3):148–54. [PubMed] [Google Scholar]
- 17.Rates of disease progression according to initial highly active antiretroviral therapy regimen: a collaborative analysis of 12 prospective cohort studies. J Infect Dis. 2006;194(5):612–22. doi: 10.1086/506362. [DOI] [PubMed] [Google Scholar]
- 18.Hughes MD. Initial treatment of HIV infection: randomized trials with clinical end points are still needed. J Infect Dis. 2006;194(5):542–4. doi: 10.1086/506369. [DOI] [PubMed] [Google Scholar]
- 19.Guidelines on Antiretroviral Treatment, 2002 version. Ministry of Health, Botswana; Gaborone: 2002. [Google Scholar]
- 20.Botswana Guidelines on Antiretroviral Treatment. Ministry of Health; Gaborone: 2002. [Google Scholar]
- 21.Botswana Guidelines on Antiretroviral Treatment. 2005 version. Ministry of Health, Botswana; Gaborone, Botswana: 2005. [Google Scholar]
- 22.Tenorio AR, et al. HIV-1-infected antiretroviral-treated patients with prolonged partial viral suppression: clinical, virologic, and immunologic course. J Acquir Immune Defic Syndr. 2003;34(5):491–6. doi: 10.1097/00126334-200312150-00007. [DOI] [PubMed] [Google Scholar]
- 23.Nettles RE, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293(7):817–29. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 24.Cohen Stuart JW, et al. Transient relapses (“blips”) of plasma HIV RNA levels during HAART are associated with drug resistance. J Acquir Immune Defic Syndr. 2001;28(2):105–13. doi: 10.1097/00042560-200110010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Wester CW, et al. Higher-Than-Expected Rates of Lactic Acidosis Among HAART-Treated Adults in Botswana: Preliminary Results from a Large Randomized Clinical Trial. J Acquir Immune Defic Syndr. 2007 doi: 10.1097/QAI.0b013e3181568e3f. [DOI] [PubMed] [Google Scholar]
- 26.May MT, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368(9534):451–8. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- 27.Novitsky V, et al. The reverse transcriptase 67N 70R 215Y genotype is the predominant TAM pathway associated with virologic failure among HIV type 1C-infected adults treated with ZDV/ddI-containing HAART in southern Africa. AIDS Res Hum Retroviruses. 2007;23(7):868–78. doi: 10.1089/aid.2006.0298. [DOI] [PubMed] [Google Scholar]
- 28.Montaner JS, et al. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS Trial. Italy, The Netherlands, Canada and Australia Study. JAMA. 1998;279(12):930–7. doi: 10.1001/jama.279.12.930. [DOI] [PubMed] [Google Scholar]
- 29.D'Aquila RT, et al. Nevirapine, zidovudine, and didanosine compared with zidovudine and didanosine in patients with HIV-1 infection. A randomized, double-blind, placebo-controlled trial. National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group Protocol 241 Investigators. Ann Intern Med. 1996;124(12):1019–30. doi: 10.7326/0003-4819-124-12-199606150-00001. [DOI] [PubMed] [Google Scholar]
- 30.Henry K, et al. A randomized, controlled, double-blind study comparing the survival benefit of four different reverse transcriptase inhibitor therapies (three-drug, two-drug, and alternating drug) for the treatment of advanced AIDS. AIDS Clinical Trial Group 193A Study Team. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19(4):339–49. doi: 10.1097/00042560-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 31.Young B. The role of nucleoside and nucleotide reverse transcriptase inhibitor backbones in antiretroviral therapy. J Acquir Immune Defic Syndr. 2004;37(Suppl 1):S13–20. doi: 10.1097/01.qai.0000137002.17634.4e. [DOI] [PubMed] [Google Scholar]
- 32.Zhou XJ, et al. Population pharmacokinetics of nevirapine, zidovudine, and didanosine in human immunodeficiency virus-infected patients. The National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group Protocol 241 Investigators. Antimicrob Agents Chemother. 1999;43(1):121–8. doi: 10.1128/aac.43.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO Antiretroviral therapy for HIV Infection in Adults and adolescents in resource limited settings: Towards universal access. 2006.
- 34.Hammer SM, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296(7):827–43. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 35.Beck EJ, et al. National adult antiretroviral therapy guidelines in resource-limited countries: concordance with 2003 WHO guidelines? AIDS. 2006;20(11):1497–502. doi: 10.1097/01.aids.0000237365.18747.13. [DOI] [PubMed] [Google Scholar]
- 36.Egger M. Outcomes of ART in Resource-limited and Industrialized Countries.. 14th Conference on Retroviruses and Opportunistic Infections.; Los Angeles, USA. 2007. [Google Scholar]
- 37.Gilkes C, Vitoria M. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a public health approach. World Health Organization; Geneva: 2006. [PubMed] [Google Scholar]
- 38.Ferradini L, et al. Efficacy of Kaletra-based Second-line ART in Cambodia.. 14th Conference on Retroviruses and Opportunistic Infections.; Los Angeles, USA. 2007. [Google Scholar]
- 39.Braitstein P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 40.Hawkins C, et al. Antiretroviral durability and tolerability in HIV-infected adults living in urban Kenya. J Acquir Immune Defic Syndr. 2007;45(3):304–10. doi: 10.1097/QAI.0b013e318050d66c. [DOI] [PubMed] [Google Scholar]
- 41.Boulle A, et al. Substitutions due to antiretroviral toxicity or contraindication in the first 3 years of antiretroviral therapy in a large South African cohort. Antivir Ther. 2007;12(5):753–60. doi: 10.1177/135965350701200508. [DOI] [PubMed] [Google Scholar]
- 42.Sow PS, et al. Implementation of an antiretroviral access program for HIV-1-infected individuals in resource-limited settings: clinical results from 4 African countries. J Acquir Immune Defic Syndr. 2007;44(3):262–7. doi: 10.1097/QAI.0b013e31802bf109. [DOI] [PubMed] [Google Scholar]
- 43.Forna F, et al. Clinical toxicity of highly active antiretroviral therapy in a home-based AIDS care program in rural Uganda. J Acquir Immune Defic Syndr. 2007;44(4):456–62. doi: 10.1097/QAI.0b013e318033ffa1. [DOI] [PubMed] [Google Scholar]
- 44.Gallant JE, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 45.Coetzee D, et al. Promoting adherence to antiretroviral therapy: the experience from a primary care setting in Khayelitsha, South Africa. AIDS. 2004;18(Suppl 3):S27–31. doi: 10.1097/00002030-200406003-00006. [DOI] [PubMed] [Google Scholar]
- 46.Orrell C, et al. Adherence is not a barrier to successful antiretroviral therapy in South Africa. AIDS. 2003;17(9):1369–75. doi: 10.1097/00002030-200306130-00011. [DOI] [PubMed] [Google Scholar]
- 47.Laniece I, et al. Adherence to HAART and its principal determinants in a cohort of Senegalese adults. AIDS. 2003;17(Suppl 3):S103–8. doi: 10.1097/00002030-200317003-00014. [DOI] [PubMed] [Google Scholar]