Abstract
Amyloid-β (Aβ) is cleared from the brain by both proteolytic digestion and transport across the blood-brain-barrier into the peripheral circulatory system. To investigate the role peripheral Aβ levels play in regulating Aβ brain clearance, we measured the clearance of [125I]-Aβ1–40 injected into the brains of liver-ligated rats that allowed peripheral Aβ levels to be maintained at elevated levels for approximately one hour with/without a single peripheral bolus of unlabeled Aβ1–40. We found that elevating peripheral Aβ levels significantly decreased [125I]-Aβ1–40 brain clearance, thus supporting the hypothesis that peripheral Aβ levels regulate Aβ clearance from the central nervous system.
Keywords: Alzheimer’s disease, blood-brain-barrier, hepatic clearance, liver, passive immunization, peripheral sink
INTRODUCTION
Molecules that bind amyloid-β (Aβ) in plasma are sometimes referred to as Aβ sinks [1,2] and have been shown to alter Aβ levels in the brain [3–5]. How these Aβ-sequestering strategies work is not well understood [1]. One hypothesis is that by reducing the amount of bio-available Aβ in plasma, the dynamic equilibrium between Aβ in the periphery versus the central nervous system (CNS) shifts to favor increased clearance from the brain [2]. Consistent with this idea are data showing that peripherally injected soluble lipoprotein receptors and Aβ antibodies reduce free Aβ levels in plasma and correspondingly reduce Aβ burden in the CNS [3,5]. However, there are also data that show increased plasma Aβ concentrations do not alter [125I]-Aβ1–40 efflux from the brain [6].
Addressing this issue directly is challenging from the standpoint of measuring clearance of known amounts of Aβ in the CNS while at the same time limiting peripheral Aβ disposal, a process that occurs within minutes. In this regard, several reports indicate the liver plays an important role in peripheral Aβ clearance [7–9]. We therefore reasoned that if liver-mediated clearance were transiently disrupted, it might be possible to hold or “clamp” peripheral Aβ levels long enough to determine the effects of elevating plasma Aβ on the clearance of [125I]-Aβ1–40 injected into the brain.
MATERIAL AND METHODS
Surgical procedures
Experiments were approved by the VAPSHCS institutional animal care and use committee (IACUC). Sprague Dawley male rats were randomly assigned a sham surgery or liver ligation. Following isoflurane anesthesia both jugular veins were cannulated and the portal vein and hepatic artery (ciliac axis) were ligated, while control animals received loose ligatures that did not restrict blood flow. A 2 µCi bolus of human [125I]-Aβ1–40 (Amersham, Piscataway, NJ) supplemented with 10 µg of unlabeled human Aβ1–40 (Bachem, Torrance, CA) in 0.1% BSA/10 µ-units/ml−1 heparin/0.9% saline was injected into the right jugular vein followed by 200 µl of heparin/saline solution. Blood (200 µl) was collected from the left jugular vein. Plasma proteins were precipitated with 20% Trichloroacetic acid to determine both intact and degraded [125I]-Aβ1–40 levels using a gamma counter (CobraII, Packard Bell). In brain clearance experiments (see below), animals undergoing liver ligations also received [125I]-Aβ1–40/[14C]-inulin brain injections where animals were divided in two groups, one receiving and one not receiving a bolus injection of 10 µg of unlabeled Aβ1–40 in 200 µl of saline via jugular catheter over 30 seconds followed by 200 µl of saline to ensure complete delivery of the bolus.
Pharmacokinetic analysis
Pharmacokinetic parameters were calculated using statistical moment analysis [10]. The area under the concentration versus time curve (AUC) was calculated for each plasma concentration (Cp) versus time (t) using the trapezoidal method. The terminal slope for each data set was estimated by log-linear regression after visually identifying the terminal portion of each curve. The mean residence time (MRT) was calculated as AUMC/AUC with AUMC as the area of the Cp*t versus t curve. Whole body clearance (Cl) was calculated as dose/AUC.
Determining Aβ Brain Clearance
Measuring [125I]-Aβ1–40 clearance from the brain followed an established brain efflux index (BEI) method [11]. Briefly, Sprague Dawley male rats (200–250 grams) were anesthetized with isoflurane and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA.) The brain injectate (1 micro liter) comprised 0.02 µCi [125I]-Aβ1–40 (Phoenix Pharmaceuticals, Burlingame, CA) and 0.01 µCi [14C]-inulin (PerkinElmer, Shelton, CT), used as a reference tracer molecule to account for non-specific diffusion or disruption of the blood brain barrier (BBB), dissolved in 122 mM NaCl, 25 mM NaHCO3, 3 mM KCl, 1.4 mM CaCl2, 1.2 mM MgSO4, 0.4 K2 mM HPO4, 10 mM D-glucose and 10 mM HEPES, pH 7.4. Each animal received a 1 µl injection into both the ipsilateral and contra-lateral hemispheres of parietal cortex (0.2 mm anterior and 5.5 mm lateral to the bregma at a depth of 4.5 mm) using a 5 µl microsyringe mounted in an ultramicro-syringe pump system (World Precision Instruments, Sarasota, FL). Prior to this, we verified that no radiolabeled compounds diffused into the non-injected hemisphere by injecting only one hemisphere and then counting the radiolabel in the injected and non-injected hemispheres. No radiolabel was detected in the non-injected hemisphere. By convention, the right (ipsilateral) cortex was injected 70 minutes before the contra-lateral cortex. Immediately following the contra-lateral injection, the animals were sacrificed and both hemispheres were excised. A multi-channel scintillation counter (Tri-Carb 2300TR; Packard Instruments) measured [125I] counts at 0–45 kilo electron volts (keV) and [14C] counts at 46–156 keV. The BEI(%) is defined as the relative percentage of Test drug (T, [125I]-Aβ1–40) in the brain compared to the Reference drug (R, [14C]-inulin) in the brain after 70 minutes and is described by the formula: BEI(%) = [1−((T70/R70)/(T0/R0))] × 100 with T0 and R0 representing the total amounts of test and reference compounds injected into the brain. Decreased BEI(%) indicated impaired Aβ clearance from the brain. Statistical comparisons were performed using analysis of variance (ANOVA).
RESULTS
We predicted that blocking liver-mediated Aβ disposal would permit a single peripheral injection of Aβ1–40 to elevate circulating plasma Aβ1–40 levels long enough to test the hypothesis that altering the Aβ concentration gradient between the CNS and the periphery changes Aβ clearance from the brain.
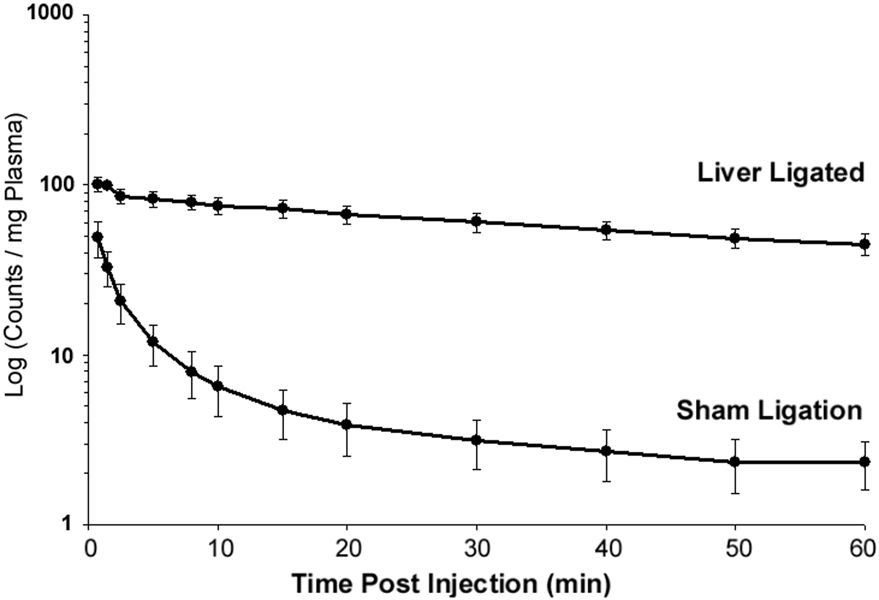
First, to test the utility of this approach, we measured peripheral clearance of [125I]-Aβ1–40 injected directly into the blood stream of anesthetized rats whose liver-mediated Aβ disposal was occluded by ligating the portal vein and celiac artery. Figure 1 shows that liver ligation, compared to sham surgery, caused an immediate and significant reduction in the Aβ1–40 clearance rate from plasma. The mean clearance rate was calculated as 22.8 ml/kg/min +/− 6.30 for liver ligated animals and 1.27 ml/kg/min ± 0.36 for liver intact animals (p < 0.001). Liver ligation also produced a significant increase in the mean residence time (mean MRT = 88.3 min ± 16.6 with liver ligated; mean MRT = 41.0 min ± 6.9 with liver intact; p < 0.01).
Fig. 1.
The mean retention time of [125I]-Aβ1–40 injected directly into the circulating blood stream of rats receiving surgical ligation of the portal vein and celiac artery (N = 5) was significantly reduced (p < 0.001) compared to non-ligated controls (N = 3). Error bars represent standard error of the mean (SEM).
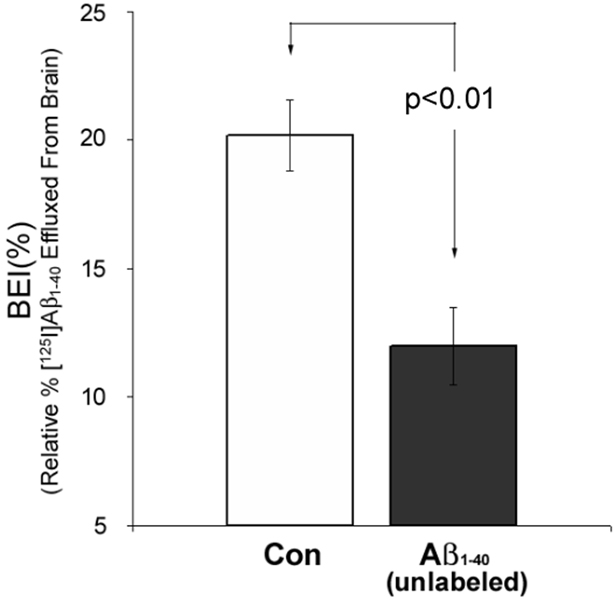
Having shown that liver ligation markedly retards peripheral Aβ1–40 clearance, we then examined the effect of elevating plasma Aβ levels on Aβ clearance from the CNS under liver-ligated conditions. To accomplish this we used the BEI method [11] to measure the specific clearance of [125I]-Aβ1–40 injected into the parietal cortex of rats. Figure 2 shows that with the liver ligated, a single bolus injection of unlabeled Aβ1–40 (10 µg) into the peripheral blood supply caused a statistically significant 40.7% decrease in the amount of [125I]-Aβ1–40 cleared from the brain compared to liver-ligated, untreated control animals (p < 0.01).
Fig. 2.
Brain efflux index BEI(%) indicates specific clearance of [125I]-Aβ1–40 injected into parietal cortex. BEI(%) was significantly reduced (p < 0.01) in liver-ligated animals receiving concomitant peripheral injections of unlabeled Aβ1–40 (closed histogram, N = 5) compared to liver-ligated untreated controls (Con, open histogram, N = 5). Error bars represent standard error of the mean (SEM).
DISCUSSION
Aβ is generated by many cell types throughout the body (including circulating platelets and leukocytes [12]), and traffics both in and out of the brain via receptor-mediated processes [2,13]. The biological significance of this dynamic relationship between CNS and peripheral Aβ pools has been underscored by data showing that peripheral Aβ-binding compounds reduce Aβ in the brain [3,4,14]. Current evidence supports a number of different, but not necessarily mutually exclusive, potential mechanisms that may help explain how these manipulations enhance Aβ clearance from the brain [1]. For example, peripherally injecting Aβ-binding antibodies reduces free plasma Aβ in a mouse model of AD [3], suggesting that increasing the Aβ concentration gradient between the brain and the periphery thermodynamically drives Aβ out of the brain. However, in these chronic treatment strategies other potential processes may also influence Aβ brain clearance, including increased CNS amyloid phagocytosis or altered oligomeric Aβ stability [1]. Further-more, investigators failed to detect a change in CNS [125I]-Aβ1–40 clearance following peripheral Aβ bolus injections [6]. This finding, which contrasts with the current report, may be explained by methodological differences. We measured clearance of [125I]-Aβ1–40 injected directly into the cortical parenchyma whereas in the previous study [6], [125I]-Aβ1–40 was delivered to the CNS indirectly by injecting radiolabeled Aβ1–40 into the periphery. The latter approach would make it challenging to distinguish between the clearance of [125I]-Aβ1–40 accumulated in luminal BBB domains versus that which had entered the CNS parenchyma.
In the current study, we examined CNS Aβ clearance under acute, short-term conditions where the effects of increased Aβ plasma levels would, in principle, be more easily addressed. To accomplish this it was also necessary to simultaneously retard peripheral Aβ disposal. With the liver occluded from the circulatory system, we found that elevating plasma Aβ levels significantly reduced that rate at which [125I]-Aβ1–40 was cleared from the CNS. In addition to addressing the role peripheral Aβ levels play in regulating CNS Aβ clearance, these data also support and extend previous findings [7–9] by illustrating the substantial contribution of the liver to peripheral Aβ clearance. To the extent the peripheral circulatory system can act as an Aβ sink, these data demonstrate that the liver is the primary drain.
In keeping with the majority of previously published studies that have examined in vivo Aβ clearance from the CNS, we focused on Aβ1–40 because it is the most abundant Aβ species in plasma, and since both Aβ40 and Aβ42 share the same transporter at the BBB [15], Aβ1–40 is likely to be representative of the family of circulating Aβ peptides. Importantly, Aβ1–40 is much less likely than Aβ1–42 to form oligomers/aggregates that would alter functional circulating Aβ concentrations in ways that would complicate interpreting these findings in terms of concentration gradients [1].
These results do not address the specific mechanism( s) by which peripheral Aβ concentrations influence Aβ clearance from the brain. One possibility is that elevated Aβ concentrations reduce cerebral blood flow [16] or promote degradation of receptors involved inAβ efflux from the brain [14]. Nonetheless, these data are consistent with the idea that central-peripheral Aβ concentration gradients regulate Aβ brain clearance. Moreover, these results highlight the potential significance of taking advantage of both the liver’s greater pharmacological accessibility (compared to the brain) and its dominating capacity to regulate the peripheral Aβ pool in order to devise new strategies to combat AD.
ACKNOWLEDGMENTS
This work was supported by a grant to DGC from the Veterans Affairs Office of Research and Development Medical Research Serve and an NIH fellowship to MAM (T32 AG000258).
References
- 1.Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative disorders. Ann Rev Neurosci. 2008;31:151–173. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zlokovic BV. Clearing amyloid through the blood-brain barrier. J Neurochem. 2004;89:807–811. doi: 10.1111/j.1471-4159.2004.02385.x. [DOI] [PubMed] [Google Scholar]
- 3.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuoka Y, Saito M, LaFrancois J, Gaynor K, Olm V, Wang L, Casey E, Lu Y, Shiratori C, Lemere C, Duff K. Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral administration of agents with an affinity to beta-amyloid. J Neurosci. 2003;23:29–33. doi: 10.1523/JNEUROSCI.23-01-00029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandimalla KK, Curran GL, Holasek SS, Gilles EJ, Wengenack TM, Poduslo JF. Pharmacokinetic analysis of the blood-brain barrier transport of 125I-amyloid beta protein 40 in wild-type and Alzheimer’s disease transgenic mice (APP,PS1) and its implications for amyloid plaque formation. J Pharmacol Exp Ther. 2005;313:1370–1378. doi: 10.1124/jpet.104.081901. [DOI] [PubMed] [Google Scholar]
- 7.Ghiso J, Shayo M, Calero M, Ng D, Tomidokoro Y, Gandy S, Rostagno A, Frangione B. Systemic catabolism of Alzheimer’s Abeta40 and Abeta42. J Biol Chem. 2004;279:45897–45908. doi: 10.1074/jbc.M407668200. [DOI] [PubMed] [Google Scholar]
- 8.Hone E, Martins IJ, Fonte J, Martins RN. Apolipoprotein E influences amyloid-beta clearance from the murine periphery. J Alzheimers Dis. 2003;5:1–8. doi: 10.3233/jad-2003-5101. [DOI] [PubMed] [Google Scholar]
- 9.Tamaki C, Ohtsuki S, Iwatsubo T, Hashimoto T, Yamada K, Yabuki C, Terasaki T. Major involvement of low-density lipoprotein receptor-related protein 1 in the clearance of plasma free amyloid beta-peptide by the liver. Pharm Res. 2006;23:1407–1416. doi: 10.1007/s11095-006-0208-7. [DOI] [PubMed] [Google Scholar]
- 10.Yamaoka K, Nakagawa T, Uno T. Statistical moments in pharmacokinetics. J Pharmacokinet Biopharm. 1978;6:547–548. doi: 10.1007/BF01062109. [DOI] [PubMed] [Google Scholar]
- 11.Kakee A, Terasaki T, Sugiyama Y. Brain efflux index as a novel method of analyzing efflux transport at the blood-brain barrier. J Pharmacol Exp Ther. 1996;277:1550–1559. [PubMed] [Google Scholar]
- 12.Li QX, Fuller SJ, Beyreuther K, Masters CL. The amyloid precursor protein of Alzheimer disease in human brain and blood. J Leukoc Biol. 1999;66:567–574. doi: 10.1002/jlb.66.4.567. [DOI] [PubMed] [Google Scholar]
- 13.Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer’s Abeta peptide: the many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Martel CL, Mackic JB, McComb JG, Ghiso J, Zlokovic BV. Blood-brain barrier uptake of the 40 and 42 amino acid sequences of circulating Alzheimer’s amyloid beta in guinea pigs. Neurosci Lett. 1996;206:157–160. doi: 10.1016/s0304-3940(96)12462-9. [DOI] [PubMed] [Google Scholar]
- 16.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]