Abstract
Owing to their potential for systematic analysis, complex networks have been widely used in proteomics. Representing a protein structure as a topology network provides novel insight into understanding protein folding mechanisms, stability and function. Here, we develop a new feature to reveal correlations between residues using a protein structure network. In an original attempt to quantify the effects of several key residues on catalytic residues, a power function was used to model interactions between residues. The results indicate that focusing on a few residues is a feasible approach to identifying catalytic residues. The spatial environment surrounding a catalytic residue was analyzed in a layered manner. We present evidence that correlation between residues is related to their distance apart most environmental parameters of the outer layer make a smaller contribution to prediction and ii catalytic residues tend to be located near key positions in enzyme folds. Feature analysis revealed satisfactory performance for our features, which were combined with several conventional features in a prediction model for catalytic residues using a comprehensive data set from the Catalytic Site Atlas. Values of 88.6 for sensitivity and 88.4 for specificity were obtained by 10fold crossvalidation. These results suggest that these features reveal the mutual dependence of residues and are promising for further study of structurefunction relationship.
Introduction
Enzymes participate in various cellular processes by temporarily binding to reactants, significantly decreasing the activation energy required and accelerating the reaction. Enzyme structure provides an insight into such catalytic mechanisms. Since the advent of structure genomics projects, many enzyme structures have been explored however, determining the correlation of functional information with structural data and extrapolation to a catalytic mechanism remains a challenging task. Commonly, only a few amino acids in the active site of an enzyme are involved directly in such bioreactions. The prediction of catalytic residues in newly solved protein structures is highly desirable in structural proteomics and should help to further our understanding of catalytic mechanisms, which will be useful in protein engineering and in functional annotation.
Many studies have been devoted to the identification of active enzyme residues. Various features have been mined for active site description and can be roughly divided into several categories. Sequence [1] or structure [2] conservation analysis performs well in correlating residues with function because functionally important residues under high selective pressure usually exhibit a higher degree of conservation than other residues. Other properties for singling out active site residues have been investigated extensively. As reported by Bartlett [3], catalytic residues have relatively low solvent accessibility, tend to be charged or polar, are less flexible, are located in an appropriate cavity [4] and occur in coil regions. Moreover, most catalytic residues are involved in hydrogen bonding via amino acid main chains or side chains [3]. BenShimon et al. found that catalytic residues are frequently located close to the enzyme center [5]. Thus, sequential and structural features characterizing catalytic residues, such as residue type, physicochemical properties, hydrogen bonding, secondary structure, solvent accessibility and Bfactors, have been investigated in depth. Combination of these properties with information on evolutionary conservation has led to the development of numerous prediction models [6]–[14].
The threedimensional structural patterns of catalytic residues are usually shared by functionally similar enzymes and prediction can be made by searching for spatial patterns or templates resembling known catalytic sites [15]–[18]. Phylogenetic motifs, which are regions around key functional sites that are conserved in the overall phylogeny of a family, are promising for functional site prediction [19]. A mechanical study revealed high force constants for catalytic residues [20] and theoretical titration has proved useful by indicating the location of active sites [21]–[23]. Therefore, it is desirable to develop effective methods for describing such mutual restraints between catalytic and other residues, as well as the spatial environment around a catalytic residue.
Protein structure, as a type of complex system, can be analyzed by complex network approaches whereby the structure is represented as a residue contact network in which vertices are the residues and edges are their interactions. This method provides a novel insight into protein folding mechanisms, stability and function. Studies by Bagler et al. have indicated the smallworld and even scalefree [24] properties of such a network, which is independent of the structural class [25]. Vendruscolo et al. determined that a limited set of vertices with large connectivity, which they termed hubs, play a key role in protein folding [26]–[28]. In another study, hubs were defined as residues with more than four links that bring together different secondary structural elements, suggesting that these hubs contribute to both protein folding and stability [29]. Together, these studies have demonstrated that complex networks provide a convenient approach for systematic analysis of protein structure. Particularly high residue closeness values are associated with sequence conservation and reflect the key role in protein structure [30]. By definition, closeness score of a vertex is relative to its distances from all other vertices in a network, which reflects the global role of a residue in the global structure. These concepts are widely accepted as important features and have been combined with other features for the prediction of active sites [79], . In this study, several other network topological parameters were calculated and used to predict catalytic residues.
We determined the extent to which catalytic and noncatalytic residues differ in terms of their interactions with other residues. For this purpose, we developed the novel descriptor description of network signal communication DNSC for catalytic residues to reveal the effects imposed on catalytic residues by other residues. Here, effects from only a few key residues are taken into account, because proteins have evolved to a relatively optimized design that is robust to mutations and changes of the environment and extremely sensitive to perturbations at crucial sites. Moreover, Amitai et al. [30] and del Sol et al. [31] revealed that several central residues are vital for signal communication in the protein structure networks assumed for integration and transmittance of signals from and to the other residues. Our analysis demonstrates that these few residues are informative for the identification of catalytic residues.
To investigate the environmental influence on catalytic residues, a multilayer strategy based on the shortest path concept was used to characterize the environment surrounding catalytic residues. Several studies have revealed that catalytic residues are usually found in an unfavorable environment. Mutations of functional residues usually decrease enzyme activity but often increase stability at the same time [32], [33]. Thus, the free energy difference between naturally occurring and mutated amino acids at each position is useful for imposing constraints on functionally and structurally important residues [34]. We found that catalytic residues are affected by the outer layer the second and third layers environment and the effects of environmental features are steadily decreased as the layer number is increased.
Finally, a prediction model was constructed by combining these new features with several features reported earlier. Our model yielded satisfactory performance and was robust when implemented for a comprehensive noncatalytic residue set.
Results
We used 10fold crossvalidation for the construction and testing of the model and the dataset was split at the protein level. To avoid an imbalance between catalytic and noncatalytic residues, the model was trained on a dataset with a ratio of 11 between catalytic and noncatalytic residues. Each residue was represented by a 130dimensional vector. Details of the features used for encoding a catalytic residue are given in Materials and Methods. The LIBSVM package was used for training the model http://www.csie.ntu.edu.tw/~cjlin/libsvm/ and we measured the results in terms of sensitivity recall, specificity, accuracy, precision and area under the curve AUC of the receiver operating characteristic ROC.
Analysis of residue interactions for catalytic and noncatalytic residues
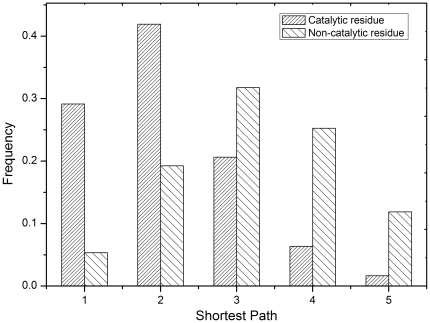
First, we analyzed the interactions between keyAAs central amino acids in a protein structure network see Materials and Methods and catalytic and noncatalytic residues. The five highestranked keyAAs were investigated for each enzyme. Interactions with a distance of 5 were considered, whereas those 5 were regarded as uninformative and were not used. The suitability of this approach was confirmed by analysis. Fig. 1 shows that catalytic residues exhibited a strong tendency to approach keyAAs, especially with direct contact or is the keyAA itself or at an interval of one residue. The rates for these two cases were significantly lower for noncatalytic residues, at only 15 and 12 of the rates for catalytic residues, respectively. However, the opposite was true when the interaction distance increased. It was found that the length of the shortest path between noncatalytic residues and keyAAs was usually 2. These results are in accord with our hypothesis that keyAAs are vital for catalytic activity and their effect on catalytic residues decreases as the interaction distance increases. Each keyAA was the subject of detailed investigation in Fig S1. Interestingly, the difference of distribution for each keyAA was quite small suggesting that several residues play key roles during protein folding and more than one position participates in formation of the exquisite scaffold for effective activity, some of which have a direct and others an indirect effect.
Figure 1. Observed frequency distribution of the shortest path between keyAAs and catalytic and non-catalytic residues.
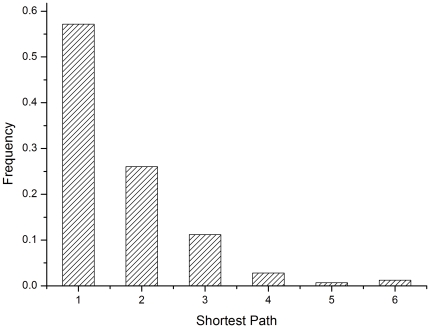
The shortest path between catalytic residues was analyzed Fig. 2. In most cases, intimate interactions were observed between catalytic residues. The fraction of interactions with direct contact and those with an interval of one residue are 57 and 26, respectively, which indicates collaboration between catalytic residues for effective function. In this method, some catalytic residues were also scored highly by closeness and were therefore treated as keyAAs. In this sense, correlations among catalytic residues are also, at least partially, implied by DNSC.
Figure 2. Observed frequency distribution of the shortest path between catalytic residues.
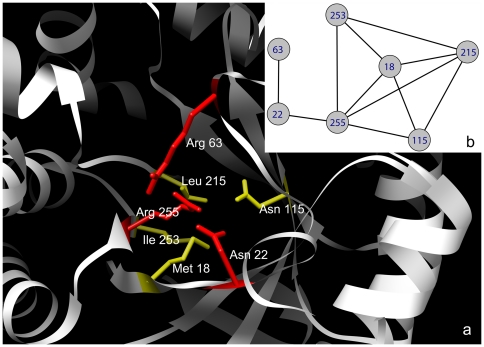
A detailed case study of dihydropteroate synthase PDB1aj0 is presented Fig. 3. Residues Met18, Asn115, Leu215, Ile253 and Arg255 are distant in the sequence but spatially close and were identified as the keyAAs in this structure. The catalytic site consists of the catalytic residues Asn22, Arg63 and Arg255, which was observed adjacent to keyAAs. The local interaction network for keyAAs and catalytic residues is shown in Fig. 3b. Arg255 was determined as a keyAA with direct interactions with other keyAAs. Asn22 has direct contact with Arg255, whereas the length of its shortest path to the other keyAAs is 2. Arg63 was far from the keyAAs however, close connections were found between this and the two other catalytic residues.
Figure 3. The spatial structure and local contact network for dihydropteroate synthase (1aj0).
(a) The local structure of the catalytic residues (yellow) and keyAAs (red). (b) The local contact network for the catalytic residues and keyAAs. Here, Asn22, Arg63, and Arg255 are catalytic residues, which were observed adjacent to keyAAs Met18, Asn115, Leu215, Ile253 and Arg255 and their interactions are shown.
Feature evaluation
To gauge the resolution limits of classification by our novel features in this prediction task, each feature alone was used to construct a prediction model for catalytic residues and compared to other features used in earlier studies Table 1. Models based on these individual features were trained using the scheme described above. DNSC achieved an average sensitivity of 69.6 and specificity of 79.0. Its specificity is 6 higher than the value for closeness. This means that, for identification of a catalytic residue, these limited keyAAs are as informative as all the rest of the residues in a protein together suggesting that not all residues within a protein are equally important for structure andor function. The conservation score performed best, with 76.4 sensitivity and 82.5 specificity. Catalytic residues are usually provided by charged and polar residues. So, the AAIdentity a 20dimensional vector used to denote a residue type performed well in identifying catalytic residues. However, it determined only 67.4 of noncatalytic residues.
Table 1. Performance for each feature by 10-fold cross-validation.
| Feature set | Sensitivity | Specificity | Accuracy | AUC |
| Conservation | 76.4 | 82.5 | 82.5 | 0.829 |
| Layer1 a | 82.8 | 80.2 | 80.2 | 0.894 |
| Layer2 b | 70.3 | 70.3 | 70.3 | 0.778 |
| Layer3 c | 68.4 | 69.0 | 69.0 | 0.749 |
| Neigs d | 84.0 | 83.9 | 83.9 | 0.907 |
| AA Identity | 75.8 | 67.4 | 67.5 | 0.753 |
| Network parameter | 77.1 | 76.7 | 76.7 | 0.835 |
| Closeness | 76.7 | 73.2 | 73.2 | 0.826 |
| DNSC | 69.6 | 79.0 | 79.0 | 0.781 |
Environmental features in the first layer.
Environmental features in the second layer.
Environmental features in the third layer.
Environmental features of all layers.
In Table 1, Layer1, Layer2 and Layer3 denote environmental features in layers 1, 2 and 3, respectively. Layer1 achieved the best performance with 82.8 sensitivity and 80.2 specificity. The performance of environmental features decreased steadily as the layer number increased. The environmental features of Layer2 correctly predicted 70.3 of catalytic residues and 70.3 of noncatalytic residues. This is in agreement with earlier reports and highlights the different selection pressure on the spatial environment of catalytic residues to maintain an efficient scaffold. The performance was enhanced when using features of all three layers, with 84.0 sensitivity and 83.9 specificity, which imply the dependence of catalytic residues on the neighboring environment. Network topological features were found to predict 76 of residues correctly. The AUC values for these features were calculated and are given in Table 1. These results suggest the feasibility of studying structurefunction relationship by revealing interactions between several residues.
CrossValidation and Feature Selection
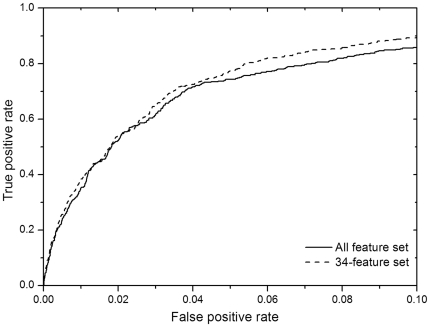
A prediction model was constructed for catalytic residues, by a combination of DNSC and network topological and environmental features with several conventional features for a detailed description see Materials and Methods. The average results over the 10fold crossvalidation are given in Table 2 and the sensitivity and specificity were 88.6 and 88.4, respectively. The ROC performance is shown in Fig. 4. Using our data set, our method achieved a recall value of 66.8 at a precision of 15.
Table 2. Performance for each feature set by 10-fold cross-validation.
| Feature set | Sensitivity | Specificity | Accuracy | AUC |
| All | 88.6 | 88.4 | 88.4 | 0.945 |
| 88 | 89.5 | 88.7 | 88.7 | 0.951 |
| 70 | 91.3 | 88.3 | 88.3 | 0.952 |
| 34 | 91.1 | 88.8 | 88.8 | 0.954 |
Here, the first 34, 70 and 88 features yielding the greatest contributions were selected to construct the prediction model for catalytic residues.
Figure 4. The ROC curves for the all-feature set and the 34-feature set.
To further analyze the impact of features on prediction performance and choose an optimized subset, feature evaluation was done by using the select attributes module in Weka 3.6.1 [35] according to the square of the weight assigned by the SVM [36]. In this step, elements in the vector of DNSC and AAIdentity features were treated individually. The merit of features is given in Table S1. It is evident that conservation score, polar and closeness make the greatest contributions to prediction. The environmental features, especially those in the first and second layers, appear to be very important for catalytic residues. Network topological features and physiochemical properties of amino acids in the first layer made great contributions to prediction. High scores were observed also for AAIdentity, PSSM and weighted frequencies. Interestingly, accessible surface area and relative accessible surface area made limited contributions to prediction, although they were used as the major predictors in earlier studies [30], [37].
The first 34, 70 and 88 features yielding the greatest contributions were selected to develop prediction models for catalytic residues. A 10fold crossvalidation was done and the average results are given in Table 2. Sensitivity and AUC were improved when the uninformative features were eliminated and both sensitivity and specificity were increased by using the 88feature set,. The sensitivity value of the 70feature set and the 34feature set was enhanced significantly by 3 however, specificity for the 70feature set was slightly decreased. Using the 34feature set, the best performance was obtained with a sensitivity value of 91.1 and a specificity value of 88.8. In this set, features in the second and third layer environment as well as the element of DNSC were included. The corresponding AUC values are 0.945, 0.951, 0.952 and 0.954. The ROC performance is shown in Fig. 4 where the curve for the allfeature set can be seen to be dominated by that for the 34feature set.
Model Evaluation
Six benchmark datasets that allow direct comparison with wellestablished methods were used to assess the performance of our method in Table 3. Models constructed by using the allfeature set model1 and the 34feature set model2 were used for comparison. We used 10fold crossvalidation on these datasets except for the data set from Chea et al. [37], on which 5fold crossvalidation was used instead. For the three datasets from Youn et al. [12], significant improvement of recall was observed for our methods at a precision corresponding to that reported by Youn et al. Our methods attained 10 greater recall at a precision of 14.9, Chea et al. obtained a recall value of 54.0, while model1 and model2 found 67.2 and 66.4 recall, respectively. Gutteridge et al. achieved a recall of 56.0 at a precision of 14.0 and the performance was enhanced remarkably by using spatial clustering with a recall of 68.0 and a precision of 16.0. We found our methods also performed well on their data set with 10 greater recall at 14.0 precision. The model2 achieved a recall even slightly higher than the refined result reported by Gutteridge et al. Petrova et al [7] reported a high recall value of 90 at a precision of 7. For this dataset, the recall was 64.1 for model1 and 67.3 for model2 at a precision of 18.0 on the basis of the crossvalidation. The satisfactory performance confirmed the robustness of our method. Thus, it is reasonable to believe that identifying catalytic residues by analyzing their interactions with other residues is both feasible and promising.
Table 3. Comparison with competing methods.
| MethodData set | EF familya | EF superfamilyb | EF foldc | HA superfamilyd | NNe | PCf | |
| gRecall18.5 | Recall16.9 | Recall17.1 | Recall14.9 | Recall14.0 | Recall16.0 | Recall7.0 | |
| Allfeature | 60.62 | 63.94 | 63.3 | 67.2 | 69.8 | 64.2 | 64.1 |
| 34feature set | 66.01 | 59.24 | 60.87 | 66.4 | 73.4 | 68.7 | 67.3 |
| Competing methods | 57.02 | 53.93 | 51.11 | 54.0 | 56.0 | 68.0 | 90.0 |
Results on the data set from Youn et al. at the SCOP family level.
Results on the data set from Youn et al. at the SCOP superfamily level.
Results on the data set from Youn et al. at the SCOP fold level.
Results on the data set from Chea et al. at the SCOP superfamily level.
Results on the data set from Gutteridge et al.
Results on the data set from Petrova et al. at the SCOP superfamily level.
Recall at the corresponding precision reported in earlier studies.
Discussion
Identification of catalytic residues can help to further our understanding of the catalytic mechanism of biological reactions. A great deal of effort has been devoted to the development of effective prediction models, for which good descriptors are a prerequisite. Complex networks enable systematic analysis of enzyme structure. On the basis of the results of the present study, we propose a novel feature, DNSC, which is based on an enzyme structure network. Unlike the reported closeness centrality, this feature focuses on the communication between keyAAs, instead of all the other residues and catalytic residues. Its satisfactory performance suggests its promise in describing the correlation between residues. Moreover, environmental parameters, especially those in Layer1 and Layer2, do help to discriminate between catalytic and noncatalytic residues. The limited contribution from Layer3 implies that more variation might occur in residues far from the catalytic site.
Our results confirm that systematic analysis has great potential for the analysis of protein structure. But the present study is only an initial step in this direction. Further studies will be complicated by virtual variations in protein structure. Residues interact mutually in various ways, including hydrogen bonding, interactions and hydrophobic interactions. The fact that two residues can be connected by more than one shortest path should be considered. Earlier research revealed that catalytic residues tend to be located in unfavorable environments which might be an important clue in distinguishing catalytic from neighboring residues. In conclusion, investigation of the correlations among residues and their links to protein structure and function remains an important challenge.
Materials and Methods
Dataset
The study data set was derived from PDB according to annotations in the Catalytic Site Atlas CSA database version 2.2.10 [38]. An enzyme entry was selected if i its PDB structure resolution is better than 2.5 and ii it was taken from the literature. The final data set consists of 140 enzyme structures that cover the six toplevel EC classifications and is filtered at the SCOP superfamily level. For comparison with previous methods, six benchmark data sets were prepared, including those from Petrova et al. [7] and Gutteridge et al. [6], the SCOP superfamily dataset from Chea et al. [37], and three datasets at different SCOP levels from Youn et al. [12].
Protein Structure Network
In this study, each chain was considered as a selfgoverned complex system, regardless of the possible interactions between chains. An enzyme structure was modeled as a network system in which residues are the vertices and connections between residues are the edges. Here, edges are defined such that two residues have a connection if the distance between any pair of atoms, one from each residue, is smaller than the sum of their van der Waals radii plus a threshold value of 2.
Feature extraction
Description of Network Signal Communication DNSC
The protein structure was treated as a selfgoverned complex system, and the active residue was treated as a terminus of the signal network i.e. the protein structure network that receives informative signals from other residues we call them signal sources in this context via direct andor indirect contacts. We attempted to quantify the intensity of these signals by postulating that the intensity decreases as distance between signal sources increases. It arises from the physiochemical intuition that a residue has stronger impacts on its closer neighbors. The signal transduction mode was generated for the protein structure network constructed according to the following assumptions i signal flows along the shortest path and ii the signal intensity is attenuated when passing through a vertex. Here, we postulate that the signal is regularly dampened and the intensity on reaching a vertex can be calculated as
| (1) |
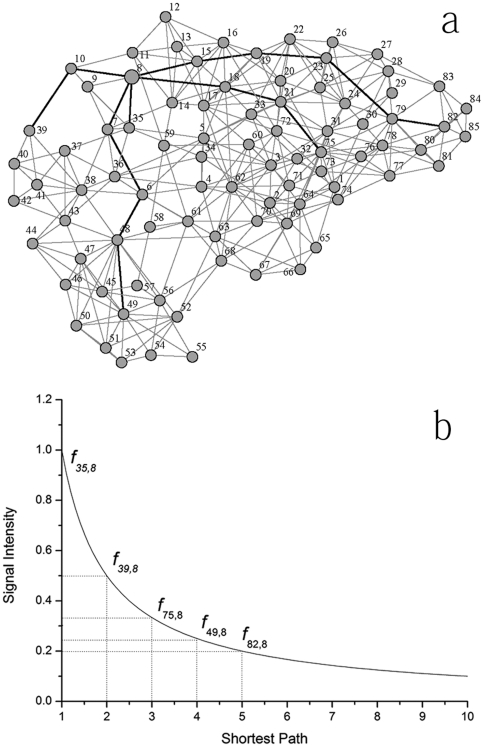
where fi ,j is the signal intensity at vertex j received from vertex i represented as a function of di ,j, the shortest path length between i and j. A power function was used to simulate signal attenuation with the exponential of a here a1 gs is the signal intensity at the signal source that was assumed to be 1 in this study. To illustrate this attenuation, the network representation of the whole structure of glutaredoxin 1 1qfn is shown in Fig. 5a. The bold line depicts the five shortest paths to Arg8 and the signal intensity along these paths is shown in Fig. 5b.
Figure 5. Description of residue interaction based on the protein structure network.
(a) Network representation of Glutaredoxin 1 (1QFN). (b) Depiction of signal attenuation model by power function. Here, vertex 8 was taken for advance and vertices 35, 39, 49, 75 and 82 were selected with the different shortest path length to vertex 8.
Although most residues are coupled by a shortest path of either long or short distance in this context, distance refers to the length of the shortest path, only information from residues playing a major role in protein structure key residues was considered. As reported, residues with high closeness values are considered to play a key role in protein folding. So, we used closeness of a residue as the measure of its structural importance. The question remains of how large a threshold is appropriate It is hard to establish a rigorous criterion because of the variety of protein structures. In the present study, residues in a protein were ranked by closeness and the topranked residues were taken into account in this context we call them keyAAs. Five keyAAs were used for protein encoding and this yielded satisfactory performance. A residue can therefore be described by a vector of signal intensities. For example, the catalytic residue Lys34 in DNA ligase 1a0i can be represented by the vector
where residues Leu149, Trp236, Glu32, Leu219 and Tyr35 are the top residues ranked by closeness.
Conventional Properties of Residues
Several conventional features were used to characterize the residues, including sequence conservation, amino acid type, polarity, hydrophobicity, volume, accessible surface area, relative accessible surface area, secondary structure, degree, cluster coefficient, hubscore, cocitation, coreness, constraint, betweenness and closeness. The last eight parameters were derived from the protein structure network. A detailed description of the features is given below.
Sequence conservation
Residues essential for protein function are conserved during evolution. Thus, conservation scores were calculated as one of the most important properties. Positionspecific iterated BLAST PSIBLAST [39] has been generally used in studies on proteomics. Here, it was implemented against the 90 nonredundant protein database with an Evalue cutoff of 1E3 and 3 iterations. The output positionspecific scoring matrix PSSM and weighted observed percentage were used to characterize a catalytic residue. Furthermore, the conservation score is defined as
| (2) |
where pi,j is the frequency of amino acid j at position i. A lower value suggests lower entropy more conserved at a position and vice versa.
Amino Acid Properties
As defined by Bartlett et al. [3], catalytic residues are directly involved in catalytic reactions as donors or acceptors or assist in reactions by exerting effects on the catalytic mechanism or the structural stability of the enzyme. Thus, residues occupying catalytic sites are usually polar or charged. A feature called AAType encodes charged DEKHR, polarity CNQSTY and hydrophobic residues AFGILMPVW as 0 0, 0 1 and 1 0, respectively. Physicochemical properties such as polarity and hydrophobicity and volume are used to further characterize catalytic residues quantitatively.
Accessible surface area and secondary structure
It is considered that catalytic residues are usually restricted in their correct position for enzyme function. Thus, in most cases catalytic residues exhibit a relatively low level of solvent accessibility. Accordingly, the accessible surface area and the relative accessible surface area were calculated for residues using DSSP [40]. As mentioned above, a single chain was regarded as an independent unit. Thus, values for chains were calculated separately, with ligands excluded for protein complexes. The secondary structure type for a residue was also derived by DSSP.
Network parameters
Translation of a protein structure to a network facilitates systematic analysis of the protein structure. In the present study, the igraph version 0.5.1 software package [41] was used to calculate network parameters. Eight network parameters, degree, cluster coefficient, hubscore, cocitation, coreness, constraint, betweenness and closeness, were used to describe residues. These parameters are described in detail by Watts and Newman et al. [42]–[45]
Layered Description of the Structural Environment
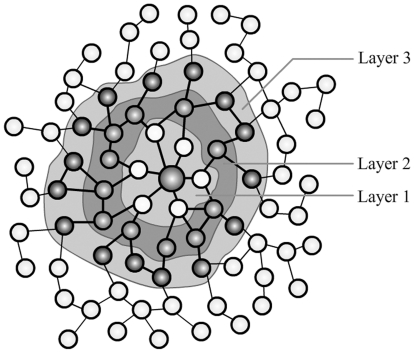
Functional residues tend to be located in unfavorable environments and therefore do not always satisfy structural requirements. Thus, it would be useful to introduce environmental parameters into schemes for the identification of catalytic residues. Moreover, as observed by Bartlett et al. [3], residue conservation is inversely proportional to the distance from catalytic residues. Thus, it is reasonable to believe that catalytic residues are more affected by residues that are closer. For this reason, we used a layered description of the structural environment. The structural network constructed in this study makes partition easy to implement. Based on the shortest path to catalytic residues, the surrounding residues naturally fall into three layers. The first layer consists of residues with a shortest path of 1 namely, in direct contact with the catalytic residues. The second and third layers comprise residues with shortest paths of 2 and 3, respectively. A sketch map of this layered description is shown in Fig. 6.
Figure 6. Layered description of the structural environment.
Layer 1 consists of residues with a shortest path of 1; i.e. in direct contact with the catalytic residues. Layer 2 and 3 consist of residues with shortest paths of 2 and 3, respectively.
The average values for all these features were used to reflect the physicochemical properties of surrounding residues and their importance in maintaining protein structure. Thus, a single layer of the environment can be represented simply by a 14dimensional vector. For each, a suffix of the layer number is added to each feature name as a distinctive mark. Thus, the layered environment was encoded by a 42dimensional vector.
Supporting Information
Observed frequency distribution of the shortest path between keyAA and catalytic and noncatalytic residues. Distribution of a shortest path to the first ranked keyAA b shortest path to the second ranked keyAA c shortest path to the third ranked keyAA d shortest path to the fourth ranked keyAA e shortest path to the fifth ranked keyAA.
TIF
The merit score for each feature.
DOC
Acknowledgments
We would like to thank Zheng Fang for preparing the figures Daichuan Ma for complex network methods.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National Nature Science Foundation of China No.20972103. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Capra JA, Singh M. Predicting functionally important residues from sequence conservation. Bioinformatics. 2007;23:1875–1882. doi: 10.1093/bioinformatics/btm270. [DOI] [PubMed] [Google Scholar]
- 2.Pazos F, Sternberg MJE. Automated prediction of protein function and detection of functional sites from structure. Proc Natl Acad Sci USA. 2004;101:14754–14759. doi: 10.1073/pnas.0404569101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett GJ, Porter CT, Borkakoti N, Thornton JM. Analysis of catalytic residues in enzyme active sites. J Mol Biol. 2002;324:105–121. doi: 10.1016/s0022-2836(02)01036-7. [DOI] [PubMed] [Google Scholar]
- 4.Ikura T, Kinoshita K, Ito N. A cavity with an appropriate size is the basis of the PPIase activity. Protein Eng Des Sel. 2008;21:83–89. doi: 10.1093/protein/gzm087. [DOI] [PubMed] [Google Scholar]
- 5.BenShimon A, Eisenstein M. Looking at enzymes from the inside out The proximity of catalytic residues to the molecular centroid can be used for detection of active sites and enzymeligand interfaces. J Mol Biol. 2005;351:309–326. doi: 10.1016/j.jmb.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 6.Gutteridge A, Bartlett GJ, Thornton JM. Using a neural network and spatial clustering to predict the location of active sites in enzymes. J Mol Biol. 2003;330:719–734. doi: 10.1016/s0022-2836(03)00515-1. [DOI] [PubMed] [Google Scholar]
- 7.Petrova NV, Wu CH. Prediction of catalytic residues using Support Vector Machine with selected protein sequence and structural properties. BMC Bioinformatics. 2006;7 doi: 10.1186/1471-2105-7-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang YR, Sheng ZY, Chen YZ, Zhang ZD. An improved prediction of catalytic residues in enzyme structures. Protein Eng Des Sel. 2008;21:295–302. doi: 10.1093/protein/gzn003. [DOI] [PubMed] [Google Scholar]
- 9.Pugalenthi G, Kumar KK, Suganthan PN, Gangal R. Identification of catalytic residues from protein structure using support vector machine with sequence and structural features. Biochem Biophys Res Commun. 2008;367:630–634. doi: 10.1016/j.bbrc.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Fischer JD, Mayer CE, Soding J. Prediction of protein functional residues from sequence by probability density estimation. Bioinformatics. 2008;24:613–620. doi: 10.1093/bioinformatics/btm626. [DOI] [PubMed] [Google Scholar]
- 11.Zhang T, Zhang H, Chen K, Shen S, Ruan J, et al. Accurate sequencebased prediction of catalytic residues. Bioinformatics. 2008;24:2329–2338. doi: 10.1093/bioinformatics/btn433. [DOI] [PubMed] [Google Scholar]
- 12.Youn E, Peters B, Radivojac P, Mooney SD. Evaluation of features for catalytic residue prediction in novel folds. Protein Sci. 2007;16:216–226. doi: 10.1110/ps.062523907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterner B, Singh R, Berger B. Predicting and annotating catalytic residues An information theoretic approach. J Comput Biol. 2007;14:1058–1073. doi: 10.1089/cmb.2007.0042. [DOI] [PubMed] [Google Scholar]
- 14.Sankararaman S, Sha F, Kirsch JF, Jordan MI, Sjolander K. Active site prediction using evolutionary and structural information. Bioinformatics. 2009;26:617–624. doi: 10.1093/bioinformatics/btq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stark A, Shkumatov A, Russell RB. Finding functional sites in structural genomics proteins. Structure. 2004;12:1405–1412. doi: 10.1016/j.str.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Wangikar PP, Tendulkar AV, Ramya S, Mail DN, Sarawagi S. Functional sites in protein families uncovered via an objective and automated graph theoretic approach. J Mol Biol. 2003;326:955–978. doi: 10.1016/s0022-2836(02)01384-0. [DOI] [PubMed] [Google Scholar]
- 17.Goyal K, Mohanty D, Mande SC. PAR3D a server to predict protein active site residues. Nucleic Acids Res. 2007;35:W503–W505. doi: 10.1093/nar/gkm252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torrance JW, Bartlett GJ, Porter CT, Thornton JM. Using a library of structural templates to recognise catalytic sites and explore their evolution in homologous families. J Mol Biol. 2005;347:565–581. doi: 10.1016/j.jmb.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 19.La D, Sutch B, Livesay DR. Predicting protein functional sites with phylogenetic motifs. Proteins. 2005;58:309–320. doi: 10.1002/prot.20321. [DOI] [PubMed] [Google Scholar]
- 20.SacquinMora S, Laforet E, Lavery R. Locating the active sites of enzymes using mechanical properties. Proteins. 2007;67:350–359. doi: 10.1002/prot.21353. [DOI] [PubMed] [Google Scholar]
- 21.Tong WX, Wei Y, Murga LF, Ondrechen MJ, Williams RJ. Partial Order Optimum Likelihood POOL Maximum Likelihood Prediction of Protein Active Site Residues Using 3D Structure and Sequence Properties. PLoS Comp Biol. 2009;5 doi: 10.1371/journal.pcbi.1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko J, Murga LF, Wei Y, Ondrechen MJ. Prediction of active sites for protein structures from computed chemical properties. Bioinformatics. 2005;21:I258–I265. doi: 10.1093/bioinformatics/bti1039. [DOI] [PubMed] [Google Scholar]
- 23.Ondrechen MJ, Clifton JG, Ringe D. THEMATICS A simple computational predictor of enzyme function from structure. Proc Natl Acad Sci USA. 2001;98:12473–12478. doi: 10.1073/pnas.211436698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greene LH, Higman VA. Uncovering network systems within protein structures. J Mol Biol. 2003;334:781–791. doi: 10.1016/j.jmb.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 25.Bagler G, Sinha S. Network properties of protein structures. Phys Stat Mech Appl. 2005;346:27–33. [Google Scholar]
- 26.Dokholyan NV, Li L, Ding F, Shakhnovich EI. Topological determinants of protein folding. Proc Natl Acad Sci USA. 2002;99:8637–8641. doi: 10.1073/pnas.122076099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vendruscolo M, Dokholyan NV, Paci E, Karplus M. Smallworld view of the amino acids that play a key role in protein folding. Phys Rev E. 2002;65 doi: 10.1103/PhysRevE.65.061910. [DOI] [PubMed] [Google Scholar]
- 28.Vendruscolo M, Paci E, Dobson CM, Karplus M. Three key residues form a critical contact network in a protein folding transition state. Nature. 2001;409:641–645. doi: 10.1038/35054591. [DOI] [PubMed] [Google Scholar]
- 29.Brinda KV, Vishveshwara S. A network representation of protein structures Implications for protein stability. Biophys J. 2005;89:4159–4170. doi: 10.1529/biophysj.105.064485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amitai G, Shemesh A, Sitbon E, Shklar M, Netanely D, et al. Network analysis of protein structures identifies functional residues. J Mol Biol. 2004;344:1135–1146. doi: 10.1016/j.jmb.2004.10.055. [DOI] [PubMed] [Google Scholar]
- 31.del Sol A, Fujihashi H, Amoros D, Nussinov R. Residue centrality, functionally important residues, and active site shape Analysis of enzyme and nonenzyme families. Protein Sci. 2006;15:2120–2128. doi: 10.1110/ps.062249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokuriki N, Stricher F, Serrano L, Tawfik DS. How Protein Stability and New Functions Trade Off. PLoS Comp Biol. 2008;4 doi: 10.1371/journal.pcbi.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoichet BK, Baase WA, Kuroki R, Matthews BW. A relationship between protein stability and protein function. Proc Natl Acad Sci USA. 1995;92:452–456. doi: 10.1073/pnas.92.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng G, Qian B, Samudrala R, Baker D. Improvement in protein functional site prediction by distinguishing structural and functional constraints on protein family evolution using computational design. Nucleic Acids Res. 2005;33:5861–5867. doi: 10.1093/nar/gki894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall MFE, Holmes G, Pfahringer B, Reutemann P, Witten IH. The WEKA Data Mining Software An Update. SIGKDD Explorations. 2009;11:10–18. [Google Scholar]
- 36.Guyon I, Weston J, Barnhill S, Vapnik V. Gene selection for cancer classification using support vector machines. Mach Learn. 2002;46:389–422. [Google Scholar]
- 37.Chea E, Livesay DR. How accurate and statistically robust are catalytic site predictions based on closeness centrality. BMC Bioinformatics. 2007;8 doi: 10.1186/1471-2105-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porter CT, Bartlett GJ, Thornton JM. The Catalytic Site Atlas a resource of catalytic sites and residues identified in enzymes using structural data. Nucleic Acids Res. 2004;32:D129–D133. doi: 10.1093/nar/gkh028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, et al. Gapped BLAST and PSIBLAST a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kabsch W, Sander C. Dictionary of protein secondary structure patternrecognition of hydrogenbonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 41.Gabor C, Tamas N. The igraph software package for complex network research. InterJournal Complex Systems. 2006:1695. [Google Scholar]
- 42.Newman MEJ. The structure and function of complex networks. SIAM Rev. 2003;45:167–256. [Google Scholar]
- 43.Watts DJ, Strogatz SH. Collective dynamics of smallworld networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- 44.Kleinberg JM. Authoritative sources in a hyperlinked environment. J ACM. 1999;46:604–632. [Google Scholar]
- 45.Burt RS. Structural holes and good ideas. Am J Sociology. 2004;110:349–399. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Observed frequency distribution of the shortest path between keyAA and catalytic and noncatalytic residues. Distribution of a shortest path to the first ranked keyAA b shortest path to the second ranked keyAA c shortest path to the third ranked keyAA d shortest path to the fourth ranked keyAA e shortest path to the fifth ranked keyAA.
TIF
The merit score for each feature.
DOC