Abstract
A radio frequency-free (RFF), analyzer-independent cell has been devised for electron-capture dissociation (ECD) of ions. The device is based on interleaving a series of electrostatic lenses with the periodic structure of magnetostatic lenses commonly found in a traveling wave tube. The RFF electrostatic/magnetostatic ECD cell was installed in a Finnigan TSQ700 ESI triple quadrupole (QqQ) spectrometer, and its performance was evaluated by recording product-ion spectra of doubly protonated substance P, doubly protonated gramicidin S, doubly protonated neurotensin, and triply protonated neurotensin. These spectra were readily obtained without recourse to a buffering gas or synchronizing electron injection with a specific phase of an RF field. The mass spectra produced with the modified instrument appear in all respects (other than resolution and mass accuracy, which were limited by the mass spectrometer used) to be at least as good for purposes of peptide identification as those recorded with Fourier transform ion cyclotron resonance (FT ICR) instruments; however, the effort and time to produce the mass spectra were much less than required to produce their FT ICR counterparts. The cell’s design and compact construction should allow it to be incorporated at relatively little cost into virtually any type of tandem mass spectrometer, for example, triple quadrupole, hybrid quadrupole ion trap, hybrid quadrupole time-of-flight, or even FT-ICR.
Mass spectrometry is performed on a molecular sample in multiple, tandem stages to probe incisively into the complexities of molecular structure and to markedly increase specificity and sensitivity in analyses of complex mixtures of molecules.1,2 In proteomic applications of tandem mass spectrometry (MS/MS), proteins and peptides can be induced to fragment to varying degrees by a number of physicochemical processes; of all of them, however, collision-induced dissociation (CID) has so far proven to be the most operationally practical and analytically robust. In CID, the internal energy of a cationic peptide-precursor is raised through gas-phase collisions between the latter and inert atoms or molecules; this thermal activation will in many instances induce a charge-directed cleavage of a peptide bond to produce a b-type or y-type fragment ion.3,4 Unfortunately, this type of fragmentation does not occur between all the residues in peptides of a given amino acid sequence. As a result, gaps are left in the sequence-data, and the heights of the fragments’ signals in the mass spectra vary from almost background level to that of the most intense signal in the spectrum. Since thermal activation favors fragmentation pathways that require the least energy, labile post-translational modifications and, along with them, information crucial for understanding physiological processes are readily lost in CID analyses.5,6 In the end, interpreting CID mass spectra can be much more tedious than acquiring them.
Electron-capture dissociation (ECD) has been shown to be an effective alternative to CID.6-13 In ECD, the exothermic capture of a free, low-energy (~1 eV) electron by a multiply protonated (cationic) peptidic precursor induces one of the peptide’s N–Cα backbone-bonds to break before the electronic excitation energy is distributed among all of the molecule’s degrees of freedom.6 This process generates almost exclusively c-type and z•-type (or c•-type and z-type) fragment ions,14 and these cleavages show almost no selectivity for particular amino acids–two exceptions being proline and disulphide bonds.15 Labile post-translational modifications, for example, phosphorylation,16,17 o-glycosylation,18,19 and ubiquination,20 remain intact during ECD MS/MS experiments thereby allowing the site and the nature of the modifications to be more reliably determined.21,22 Finally, ECD occurs at more backbone sites and, thus, provides more extensive coverage of a peptide’s sequence than does CID. Overall, ECD spectra are easier to interpret than CID spectra.
ECD requires that the precursor ions be forced to mingle with a dense population of low-energy electrons. Until quite recently, it has only been possible to meet this condition to any practical degree in Fourier transform ion cyclotron resonance (FT ICR) mass spectrometers. Expensive to purchase and operate, these instruments are not used widely despite their capability for very high mass resolution and mass accuracy. Although ECD of peptides and proteins was discovered on an FT ICR instrument, fundamental constraints on an FT ICR cell’s geometry and operation make it impossible to achieve more than crude control over electron energy and ionization efficiency in an ECD experiment. Consequently, it is impossible to exploit the phenomenon’s full potential in many applications.
In the past three years, four research groups have independently shown that ECD can be achieved in three-dimensional (3D) ion-traps and radio frequency (RF) multipoles. ECD product-ion spectra were produced in an ion-trap by using helium as a moderating gas and superimposing a moderate magnetic field (<0.1 T) along the instrument’s principal axis.23 In an independent study, product-ion spectra were obtained in an ion-trap by superimposing a digitally generated, rectangular-trapping, electric-field waveform onto the instrument’s RF-field.24 By creating a moderate magnetic field along the instrument’s longitudinal axis and using helium as a buffer gas, ECD was generated in a linear quadrupole.25 Similar results were obtained in a linear octapole without the aid of a moderating gas by using a solenoid to create the magnetic field along the instrument’s longitudinal axis.26
The authors of this report have devised a radio frequency-free (RFF), analyzer-independent device for ECD of ions that is based on interleaving a series of electrostatic lenses27 with the periodic structure of magnetostatic lenses28-30 commonly found in a traveling wave tube (TWT). Magnetostatic lenses, which have exceedingly high transmission efficiencies and are routinely employed, for example, in electron microscopes, linear accelerators, and traveling wave tubes,28-30 are not currently used in commercial mass spectrometers. This is most likely due to the historical fact that in the past century, permanent magnets that could be exploited in the design and fabrication of practical ion-optical components for mass spectrometers, other than sectors for purposes of mass-dispersion, were unavailable. The development of magnetic materials over the past few decades, however, has progressed to the point where it is now possible to fabricate very low-cost permanent magnets in sizes, shapes, strengths (0.1–1.5 T), and polarizations commensurate with components used to transport, trap, and fragment ions in mass spectrometers. We have taken advantage of these new magnets to create a new ECD cell; its design, construction, and operation are described in this article.
EXPERIMENTAL SECTION
Design and Fabrication of ECD Cell
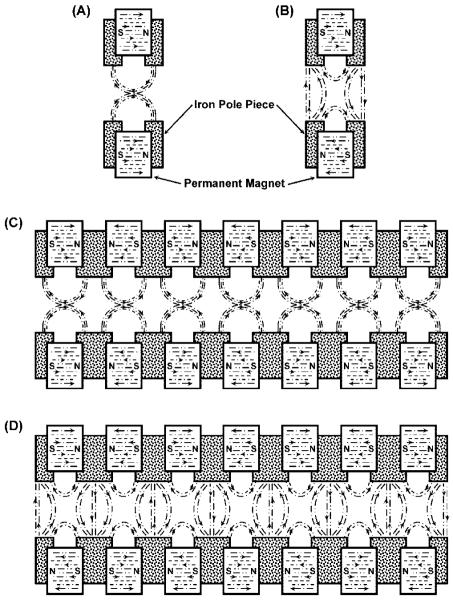
The ECD cell comprises a segmented, electrostatically focusing structure superimposed on a magnetostatic TWT design. Placing soft iron pole pieces on either side of a hole bored through a permanent magnet creates a magnetostatic-focusing lens for charged particles; the focusing action of this element can be made axial (Figure 1A) or radial (Figure 1B) depending on how the magnet is polarized.28-30 A TWT is formed when two or more of these magnetostatic lenses are arranged in a linear or curvilinear array so that the polarity of the lenses alternates periodically. Charged particles transiting a TWT are forced toward the cavity’s axis if the lens-elements focus axially (Figure 1C) or into sinusoidal paths if the lens-elements focus radially (Figure 1D).
Figure 1.
Cross sectional view through the axis of a cylindrical (A) axial focusing magnetic lens element; (B) radial focusing magnetic lens element; (C) periodic axial focusing traveling wave tube; and (D) periodic radial focusing traveling wave tube.
Electrically insulating the iron pole pieces that separate the magnets creates a hybrid device that provides strong periodic-magnetostatic-focusing/segmented-electrostatic-focusing through which electrons and ions with kinetic energies commonly found in mass spectrometers are simultaneously transported.
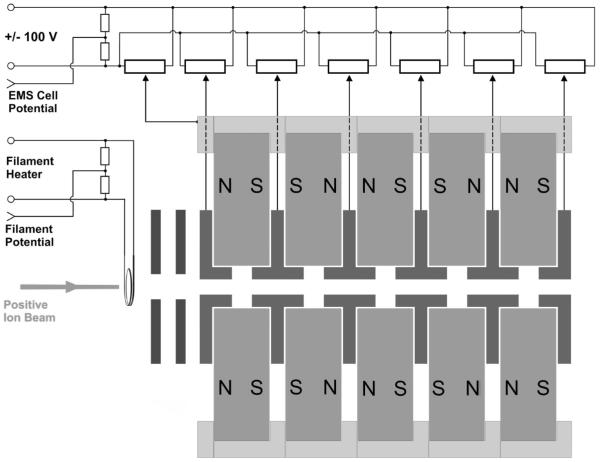
A five-lens electrostatic/magnetostatic (EMS) ECD cell was built for this study (Figure 2). Specifically, five axially magnetized N42SH-grade Nd–Fe–B ring-magnets (3.0″ in diameter, 0.5″ thick, and 0.375″ bore) were arranged in the alternating-polarity-structure of an axial TWT (Figure 1C). Each magnet was sheathed in an aluminum casing that provided the mechanical strength necessary for both forcing and holding (by means of screws from one aluminum piece to the next) the magnets together. Four soft-iron rings separated the five magnets and a soft-iron ring terminated the entire assembly at either end. Thin (0.010″ thick) Teflon rings insulated each iron ring from its adjacent magnets. Each of the six iron rings and the magnet’s aluminum housing is connected to an independently adjustable ±100 V channel of a 7-channel power supply (which can be floated up to 8 kV); the arrangement makes it possible for the iron rings to function as electrostatic lenses, as well as the pole pieces for the magnetostatic lenses. Electrons for ECD were emitted from a ring-shaped filament of tungsten–rhenium wire of 0.07″ (1.78 mm) diameter located concentrically on the axis of the five-magnet TWT at the end where the ions enter. Two titanium lenses were used to guide electrons into the ECD cell (Figure 2).
Figure 2.
Cross sectional view through the axis of a cylindrical five-lens RFF electrostatic/magnetostatic ECD cell.
Tandem Mass Spectrometry of Peptides
Sample solutions were prepared by dissolving standards of substance P, neurotensin (both American Peptide Co, Sunnyvale, CA, U.S.A.), and gramicidin S (Sigma Chem. Co., St.Louis, MO, U.S.A.) in H2O/MeOH (50:50, v/v) to a final concentration of 10−5 M. All mass spectrometry was performed on a commercial ESI triple quadrupole (QqQ) spectrometer (Finnigan TSQ700: Thermo Fisher Scientific, Inc., Waltham, MA, U.S.A.) that was modified by replacing the original RF octapole CID cell with the RFF electrostatic/magnetostatic ECD cell. The peptide solutions were separately electrosprayed at a flow rate of 0.2 μL/min, and doubly protonated substance P, doubly protonated gramicidin S, doubly protonated neurotensin, and triply protonated neurotensin were respectively selected as precursors. For this study, electron emission from the tungsten–rhenium filament was set at 5 μA, the filament and EMS cell potentials at −120 V, the potential on the first electrostatic lens at −115 V, the potential on the second lens at −20 V, and the potentials on all of the other lenses at −210 V.
RESULTS AND DISCUSSION
Analytical quality ECD product-ion spectra of doubly protonated substance P,31 doubly protonated gramicidin S (Figure 3A), triply protonated neurotensin (Figures 4A-left), and doubly protonated neurotensin (Figure 4B) were readily produced in the RFF electrostatic/magnetostatic cell. These spectra were obtained without recourse to an buffering gas, as was necessary in previous efforts to perform ECD MS/MS in non-FT ICR instruments23-25 (the authors’ own effort26 being the sole exception), or synchronizing electron injection with a specific phase of an RF field,23,24 as was necessary in previous attempts to attain ECD in ion-traps. The cell used in this study is installed in a 20-year-old, low-resolution mass spectrometer that is well suited to testing prototypes but cannot produce mass spectra that yield all of the inherent information available; nevertheless, the mass spectra produced with this instrument appear in all respects (other than the obvious exceptions of resolution and mass accuracy) to be at least as good for purposes of peptide identification as those produced by FT ICR instruments (Figures 3B, 3C, and 4A-right). The effort and time to produce these mass spectra, however, were much less than required to produce their FT ICR counterparts.
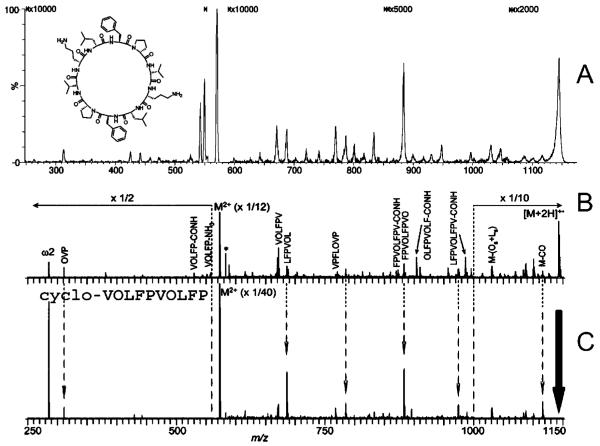
Figure 3.
Product-ion spectra of doubly protonated gramicidin S dissociated by (A) ECD in a flow-through five-lens RFF electrostatic/magnetostatic cell and by (B) ECD and (C) double-resonance ECD in an FT ICR cell. Mass spectra B and C reproduced from ref 33. Copyright 2006, reprinted with permission from Elsevier.
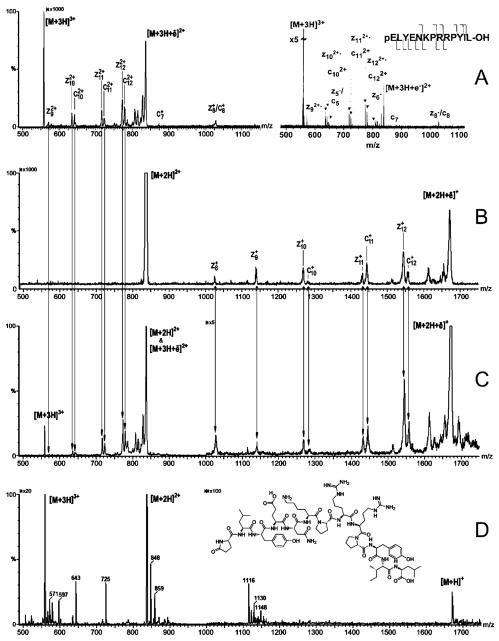
Figure 4.
Electrosprayed mass spectra of neurotensin produced by (A) selecting the triply protonated peptide ion (m/z 558) as sole precursor and performing ECD in a flow-through five-lens RFF electrostatic/magnetostatic cell (left) and by ECD in an FT ICR cell (right), (B) selecting the doubly protonated peptide ion (m/z 837) as sole precursor and then performing ECD, (C) selecting no precursor ion and performing ECD, and (D) selecting no precursor ion and performing no ECD. Mass spectrum A-right reproduced with permission from ref 37. Copyright 2001, American Chemical Society.
ECD of doubly protonated substance P carried out in the new cell was reported elsewhere.31 The spectrum produced from this trial will not be discussed herein other than to note that it qualitatively displays the same six c-type ions of substance P (i.e., c4–c10) observed in ECD product-ion spectra recorded with an FT ICR instrument.14,25
Product-ion mass spectra of doubly protonated cyclic peptides are considerably more complex than those of linear peptides. The initial ring-opening, which statistically can occur anywhere in the backbone of the peptide, creates a mixture of linear peptides any one of which can dissociate further to produce a secondary family of fragments.32 The ECD product-ion spectra of cyclic peptides are no exception to this tendency. An ECD product-ion spectrum of the repetitive cyclic peptide gramicidin S recorded during this project (Figure 3A) is shown for purposes of comparison with mass spectra produced on an FT ICR instrument33 via ECD (Figure 3B) and double-resonance ECD (Figure 3C). Examination of these three mass spectra and other published mass spectra of gramicidin S34,35 indicates that ECD in the RFF electrostatic/magnetostatic cell produces, with comparable signal-to-background, fragment-ions corresponding to the same losses of small molecules, amino acid residues, and side chains that are generally observed in ECD product-ion spectra of gramicidin S.
ECD of triply protonated neurotensin in the RFF electrostatic/magnetostatic cell produces a product-ion spectrum of both singly and doubly charged fragment ions (Figure 4A - left) that is qualitatively identical to that produced in an FT ICR cell (Figure 4A - right). Specifically, the RFF cell’s spectrum exhibits the same six c-type and seven z•-type ions, as well as the charge-reduced species [M+3H+e-]2+•observed in the FT ICR spec-trum36 sonly the bonds on the N-terminal side of the two prolines remained, as expected, intact.
ECD in an FT ICR cell is generally not commensurate with the time scale of liquid chromatography.37 By contrast, ECD in the RFF electrostatic/magnetostatic cell takes place in-flight through the device on a microsecond time scale (the time range for a singly protonated peptide of mass 1000 Da to travel through the 70 mm ECD cell31 ). It should eventually be possible, therefore,to carry out ECD in the RFF cell in time with the elution of peptides off an HPLC column.
To perform ECD efficiently, the precursor ions must be forced to mingle with a dense population of low-energy electrons. Since the reagent electrons and the multiply protonated precursor ions have opposite polarities and masses that differ by more than 6 orders of magnitude, the conditions for simultaneously confining them in the same volume of space cannot be satisfied in a purely electrostatic cell and can only be minimally satisfied in a cell in which an RF field is present. As the number of charged particles of a given polarity increases in an RF device, space-charge forces (i.e., repulsions between particles of the same polarity) result in lost particles (2D RF ion-traps) or degradation in analyzer-performance (3D ion-traps and FT ICR cells). In principle, a segmented-electrostatic-focusing/strong-periodic-magnetostatic-focusing device has a substantially greater charged-particle capacity than any RF-based device. Magnetic fluxes on the order of 1 T are more than strong enough to confine high volume-densities of ions and electrons with kinetic energies typically involved in electron capture reactions. An example of this was demonstrated using neurotensin as the sample. A regular mass spectrum of the electrosprayed neurotensin sample was recorded (Figure 4D) by operating the project’s modified mass spectrometer strictly in the Q3-mode (i.e., setting the first analyzer Q1 in a transmission only mode and the second analyzer Q3 in a scanning mode). In addition to the peaks corresponding respectively to singly, doubly, and triply protonated neurotensin nominally at m/z 1673, 837, and 558, peaks corresponding to a number of other species appear in the spectrum. The latter are presumably due to impurities in the sample. When electrons are introduced into the dissociation cell, all of the impurity peaks disappear, and peaks distinctly corresponding to the ECD product ions of doubly and triply protonated neurotensin appear in their place (Figure 4C). This becomes unequivocally evident when the composite ECD spectrum (Figure 4C) is compared with the individually produced ECD product-ion spectra of triply (Figure 4A) and doubly (Figure 4B) protonated neurotensin. Clearly, recombination with electrons was sufficiently high in the RFF electrostatic/magnetostatic cell to neutralize all of the impurity ions, which presumably but not necessarily were singly charged, recorded in the electrosprayed spectrum (Figure 4D) while efficiently producing fragment ions from the doubly and triply charged neurotensin ions.
In a RFF electrostatic/magnetostatic cell the reagent electrons cannot acquire kinetic energy from the magnetic field; however, their average energy can be controlled by the potentials applied to the electrostatic lenses. By abandoning RF-fields altogether in favor of segmented-electrostatic focusing in conjunction with strong-magnetostatic focusing, it should be possible to conduct ECD experiments on less costly instruments in which the average kinetic energies of the ions and electrons can be controlled with minimal loss of ions or electrons in the absence of an energy-moderating bath gas. This, in turn, could make it possible to increase the product-ion yields and, thus, the information to be gained from ECD reactions to levels that are much higher than possible in any RF-based cell.
The segmentation of the RFF electrostatic/magnetostatic structure makes it possible to study the energetics and kinetics of ECD reactions, as well as to exploit them in MS/MS analyses. For instance, decompositions of the radical precursor ions can be observed as a function of time by limiting electron capture events to the first entry-side segment of the cell and adjusting the potentials on the subsequent lenses to regulate the flight times of the product ions. This was easily demonstrated by producing an ECD product-ion spectrum of doubly protonated substance P ion at the front end of the cell and setting the potentials of the rest cell’s electrostatic elements for ion transport.31 This experimental capability could be used, for example, to investigate mechanisms like the recently proposed34 sequential formation of diagnostic c/z-type ions.
CONCLUSIONS
ECD has been achieved in a linear, RF-free, hybrid electrostatic/magnetostatic cell without the aid of a cooling gas. The cell is simple to fabricate, operate, and maintain. Even without the benefit of optimization, the device used in this study produces results that are at least comparable and potentially superior to those obtained with FT ICR instruments. The RFF cell could eventually make it possible to conduct ECD experiments on a subsecond time scale, thereby, enabling increased throughput for proteomic analyses. The cell’s design and compact construction should allow it to be incorporated at relatively low cost into virtually any type of tandem mass spectrometer, for example, triple quadrupole, hybrid quadrupole ion trap, hybrid quadrupole time-of-flight, or even FT-ICR.
ACKNOWLEDGMENT
The authors are indebted to Elsworth T. Hinke for his assistance in fabricating the RFF electrostatic/magnetostatic ECD cell used in this study. This work was supported directly by a grant from the W. M. Keck Foundation and indirectly by an Environmental Health Sciences Center Grant (ES00210) from the National Institute of Environmental Health Sciences.
References
- (1).Downard KM. Mass Spectrometry A Foundation Course. The Royal Society of Chemistry; Cambridge: 2004. [Google Scholar]
- (2).Gross JH. Mass Spectrometry A Textbook. Springer; New York: 2004. [Google Scholar]
- (3).Dongre AR, Jones JL, Somogyi A, Wysocki VH. J. Am. Chem. Soc. 1996;118:8365–8374. [Google Scholar]
- (4).Tsaprailis G, Nair H, Somogyi A, Wysocki VH, Zhong WQ, Futrell JH, Summerfield SG, Gaskell SJ. J. Am. Chem. Soc. 1999;121:5142–5154. [Google Scholar]
- (5).Annan RS, Carr SA. Anal. Chem. 1996;68:3413–3421. doi: 10.1021/ac960221g. [DOI] [PubMed] [Google Scholar]
- (6).Zubarev RA. Mass Spectrom. Rev. 2003;22:57–77. doi: 10.1002/mas.10042. [DOI] [PubMed] [Google Scholar]
- (7).Zubarev RA, Kelleher NL, McLafferty FW. J. Am. Chem. Soc. 1998;120:3265–3266. [Google Scholar]
- (8).Axelsson J, Palmblad M, Hakansson K, Hakansson P. Rapid Commun. Mass Spectrom. 1999;13:474–477. doi: 10.1002/(SICI)1097-0231(19990330)13:6<474::AID-RCM505>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- (9).McLafferty FW, Fridriksson EK, Horn DM, Lewis MA, Zubarev RA. Science. 1999;284:1289–1290. doi: 10.1126/science.284.5418.1289. [DOI] [PubMed] [Google Scholar]
- (10).Zubarev RA, Horn DM, Fridriksson EK, Kelleher NL, Kruger NA, Lewis MA, Carpenter BK, McLafferty FW. Anal. Chem. 2000;72:563–573. doi: 10.1021/ac990811p. [DOI] [PubMed] [Google Scholar]
- (11).McLafferty FW, Horn DM, Breuker K, Ge Y, Lewis MA, Cerda B, Zubarev RA, Carpenter BK. J. Am. Soc. Mass Spectrom. 2001;12:245–249. doi: 10.1016/S1044-0305(00)00223-3. [DOI] [PubMed] [Google Scholar]
- (12).Zubarev RA, Haselmann KF, Budnik BA, Kjeldsen F, Jensen F. Eur. J. Mass Spectrom. 2002;8:337–349. [Google Scholar]
- (13).Zubarev RA. Curr. Opin. Biotechnol. 2004;15:12–16. doi: 10.1016/j.copbio.2003.12.002. [DOI] [PubMed] [Google Scholar]
- (14).Cooper HJ, Hakansson K, Marshall AG. Mass Spectrom. Rev. 2005;24:201–222. doi: 10.1002/mas.20014. [DOI] [PubMed] [Google Scholar]
- (15).Zubarev RA, Kruger NA, Fridriksson EK, Lewis MA, Horn DM, Carpenter BK, McLafferty FW. J. Am. Chem. Soc. 1999;121:2857–2862. [Google Scholar]
- (16).Chalmers MJ, Quinn JP, Blakney GT, Emmett MR, Mischak H, Gaskell SJ, Marshall AG. J. Proteome. Res. 2003;2:373–382. doi: 10.1021/pr030004d. [DOI] [PubMed] [Google Scholar]
- (17).Chalmers MJ, Hakansson K, Johnson R, Smith R, Shen J, Emmett MR, Marshall AG. Proteomics. 2004;4:970–981. doi: 10.1002/pmic.200300650. [DOI] [PubMed] [Google Scholar]
- (18).Mirgorodskaya E, Roepstorff P, Zubarev RA. Anal. Chem. 1999;71:4431–4436. doi: 10.1021/ac990578v. [DOI] [PubMed] [Google Scholar]
- (19).Hakansson K, Cooper HJ, Emmett MR, Costello CE, Marshall AG, Nilsson CL. Anal. Chem. 2001;73:4530–4536. doi: 10.1021/ac0103470. [DOI] [PubMed] [Google Scholar]
- (20).Cooper HJ, Heath JK, Jaffray E, Hay RT, Lam TT, Marshall AG. Anal. Chem. 2004;76:6982–6988. doi: 10.1021/ac0401063. [DOI] [PubMed] [Google Scholar]
- (21).Kelleher NL, Zubarev RA, Bush K, Furie B, Furie BC, McLafferty FW, Walsh CT. Anal. Chem. 1999;71:4250–4253. doi: 10.1021/ac990684x. [DOI] [PubMed] [Google Scholar]
- (22).Mann M, Jensen ON. Nat. Biotechnol. 2003;21:255–261. doi: 10.1038/nbt0303-255. [DOI] [PubMed] [Google Scholar]
- (23).Silivra OA, Kjeldsen F, Ivonin IA, Zubarev RA. J. Am. Soc. Mass Spectrom. 2005;16:22–27. doi: 10.1016/j.jasms.2004.09.015. [DOI] [PubMed] [Google Scholar]
- (24).Ding L, Brancia FL. Anal. Chem. 2006;78:1995–2000. doi: 10.1021/ac0519007. [DOI] [PubMed] [Google Scholar]
- (25).(a) Baba T, Hashimoto Y, Hasegawa H, Hirabayashi A, Waki I. Anal. Chem. 2004;76:4263–4266. doi: 10.1021/ac049309h. [DOI] [PubMed] [Google Scholar]; (b) Satake H, Hasegawa H, Hirabayashi A, Hashimoto Y, Baba T. Anal. Chem. 2007;79:8755–8761. doi: 10.1021/ac071462z. [DOI] [PubMed] [Google Scholar]; (c) Deguchi K, Ito H, Baba T, Hirabayashi A, Nakagawa H, Fumoto M, Hinou H, Nishimura S. Rapid Commun. Mass Spectrom. 2007;21:691–698. doi: 10.1002/rcm.2885. [DOI] [PubMed] [Google Scholar]
- (26).Ji H, Voinov V, Deinzer ML, Barofsky DF. ECD in a Linear RF-Field without Collision Gas, 1381; Proceedings of the 55th ASMS Conference on Mass Spectrometry and Allied Topics; Indianapolis, Indiana. June 3-7, 2007. [Google Scholar]
- (27).Dahl P. Introduction to Electron and Ion Optics. Academic Press; New York: 1973. [Google Scholar]
- (28).Moskowitz LR. Permanent Magnet Design and Application Handbook. Cahners Books International; Boston: 1976. [Google Scholar]
- (29).McCaig M. Permanent Magnets in Theory and Practice. John Wiley & Sons; New York: 1977. [Google Scholar]
- (30).Campbell P. Permanent Magnet Materials and Their Application. Cambridge University Press; Cambridge: 1994. [Google Scholar]
- (31).Voinov VG, Deinzer ML, Barofsky DF. Rapid Commun. Mass Spectrom. 2008;22:3087–3088. doi: 10.1002/rcm.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Eckart KJ. Mass Spectrom. 1994;13:23–55. [Google Scholar]
- (33).Lin C, Cournoyer JJ, O’Connor PB. J. Am. Soc. Mass Spectrom. 2006;17:1605–1615. doi: 10.1016/j.jasms.2006.07.007. [DOI] [PubMed] [Google Scholar]
- (34).Leymarie N, Costello CE, O’Connor PB. J. Am. Chem. Soc. 2003;125:8949–8958. doi: 10.1021/ja028831n. [DOI] [PubMed] [Google Scholar]
- (35).Mihalca R, Kleinnijenhuis AJ, McDonnell LA, Heck AJR, Heeren RMA. J. Am. Soc. Mass Spectrom. 2004;15:1869–1873. doi: 10.1016/j.jasms.2004.09.007. [DOI] [PubMed] [Google Scholar]
- (36).Håkansson K, Emmett MR, Hendrickson CL, Marshall AG. Anal. Chem. 2001;73:3605–3610. doi: 10.1021/ac010141z. [DOI] [PubMed] [Google Scholar]
- (37).Creese AJ, Cooper HJ. J. Am. Soc. Mass Spectrom. 2008;19:1263–1274. doi: 10.1016/j.jasms.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]