Abstract
The influence of a single octarepeat expansion on the CuII and ZnII coordination environment within the octarepeat domain of the human prion protein is examined. Using X-ray absorption spectroscopy and diethyl pyrocarbonate labeling studies we find that at low copper concentrations the “normal” octarepeat domain (4 PHGGGWGQ repeats) coordinates ZnII in an (N/O)6 coordination environment with two histidine residues and CuII in a redox inactive (N/O)4 coordination environment using 1 imidazole residue. Expansion of the octarepeat region by one repeat (5 PHGGGWGQ repeats) yields a 3 histidine (N/O)6 coordination environment for ZnII and a 2 histidine (N/O)4 coordination environment for CuII at low copper concentrations. This CuII-((N/O)2-histidine2) coordination motif is redox active and capable of generating H2O2 under reducing aerobic conditions.
Transmissible spongiform encephalopathies (TSE) are a class of neurodegenerative disorders including kuru, bovine spongiform encephalopathy (Mad Cow disease) and Creutzfeldt-Jakob disease.1 These have been linked to the misfolding/misfunction of the ubiquitous neuronal membrane prion protein (PrP).1,2 Despite several decades of research, the specifics of the prion disease mechanism(s) or the role that the PrP plays in normal cellular physiology remain unknown. Because of its high affinity for Cu2+ it has been speculated that the PrP may be involved in copper trafficking or homeostasis, and that a disruption of this function may lead to TSEs.3–5
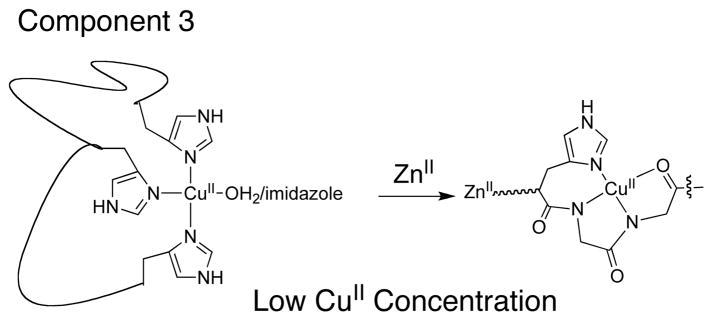
The PrP contains a disordered N-terminal region that comprises roughly half of the protein sequence. It has been shown that this region can normally bind up to six-equivalents of Cu2+.4–6 The most widely studied copper binding region of the PrP is the so-called octarepeat domain (one octarepeat = -PHGGGWGQ-), which coordinates Cu2+ in a redox-inactive square planar coordination geometry ({CuIIORP} Chart 1). In the normal human PrP there are 4 sequential octarepeats within the octarepeat domain. Recently Millhauser and colleagues demonstrated two interesting aspects concerning the PrP octarepeat domain. One is that the Cu2+ coordination environment is dependent upon Cu2+ concentration; different intraprotein histidine-based coordination modes can be observed as a function of copper concentration.7 Some of these copper-sites may lead to a redox active copper ion (e.g. the purported “component 3” motif (Scheme 1)).7 Another aspect concerning the octarepeat domain is that Zn2+ ion can “tie-up” several histidine residues yielding a {CuIIORP}-like center at low copper concentrations (Scheme 1).8 Increasing the Cu2+ concentration displaces the Zn2+ ion yielding a fully Cu2+-loaded octarepeat domain. These findings suggest that Zn2+ may offer neuroprotection by forcing Cu2+ to adopt a redox inactive Cu2+ coordination geometry under low copper loads. Furthermore, Millhauser has reported on the strong correlation between octarepeat expansions and the onset of Prion diseases.9
Chart 1.

Scheme 1.
Herein, we explore the structural basis of these findings. It will be demonstrated that the Zn2+ ion does induce Cu2+ to adopt a redox inactive coordination geometry for the normal octarepeat domain (i.e. 4 PHGGGWGQ repeats). Additionally we will show that one octarepeat addition to the normal octarepeat domain yields a different and redox-active Cu2+ coordination geometry upon Zn2+ addition.
The ORP, and 2– through 5–repeat fragments (Chart 1) were prepared by standard solid phase peptide synthesis as previously described.10,11 Metallopeptides were prepared by adding freshly prepared solutions of CuCl2 and/or ZnCl2 to peptide solutions at room temperature. All studies were performed using a solution pH of 7.4 (maintained by 50 mM N–ethylmorpholine (NEM) buffer). For all X-ray absorption experiments we used 1:1 solutions of the metallopeptide solutions and glycerol to: 1) avoid sample icing, and 2) to minimize photodegradation of the samples. The addition of glycerol to the solutions did not have any noticeable influence on the Zn2+ and Cu2+ coordination environments. Due to the sample requirements for X-ray absorption experiments, we utilized concentrations on the order of 0.5 mM (in peptide). We note that gel permeation chromatography indicated that there was no inter-protein metal coordination observed under these conditions.
The ability of these metal-coordinated fragments to produce H2O2 was probed using a quantitative peroxide assay (supporting information). When one equivalent of Cu2+ is complexed to either the 4- or 5-repeat fragments and ascorbate is added to solution under aerobic conditions we observe the rapid formation of H2O2. This is in stark contrast to {CuIIORP}, which does not promote the formation of H2O2 under identical conditions. When one equivalent of both Zn2+ and Cu2+ are coordinated to the 4- repeat there is a reduction in the production of H2O2 by 84% relative to a control complex ([Cu(im)4](ClO4)2). In contrast, we were unable to detect a significant decrease in the formation of H2O2 when one equivalent of Zn2+ and Cu2+ was added to the 5-repeat. Fully loading all of the octarepeats with Cu2+ in both the 4- and 5-repeat fragments reduced H2O2 production by 93–98%. It thus seems reasonable to suggest that: 1) within the 4-repeat fragment Zn2+ coordination is indeed modulating the Cu2+ redox chemistry, and thus its coordination environment, and 2) the expansion of the octarepeat domain by one octarepeat segment (i.e. the 5-repeat fragment) alters the redox chemistry and coordination environment of at least the Cu2+ center at low copper-concentration.
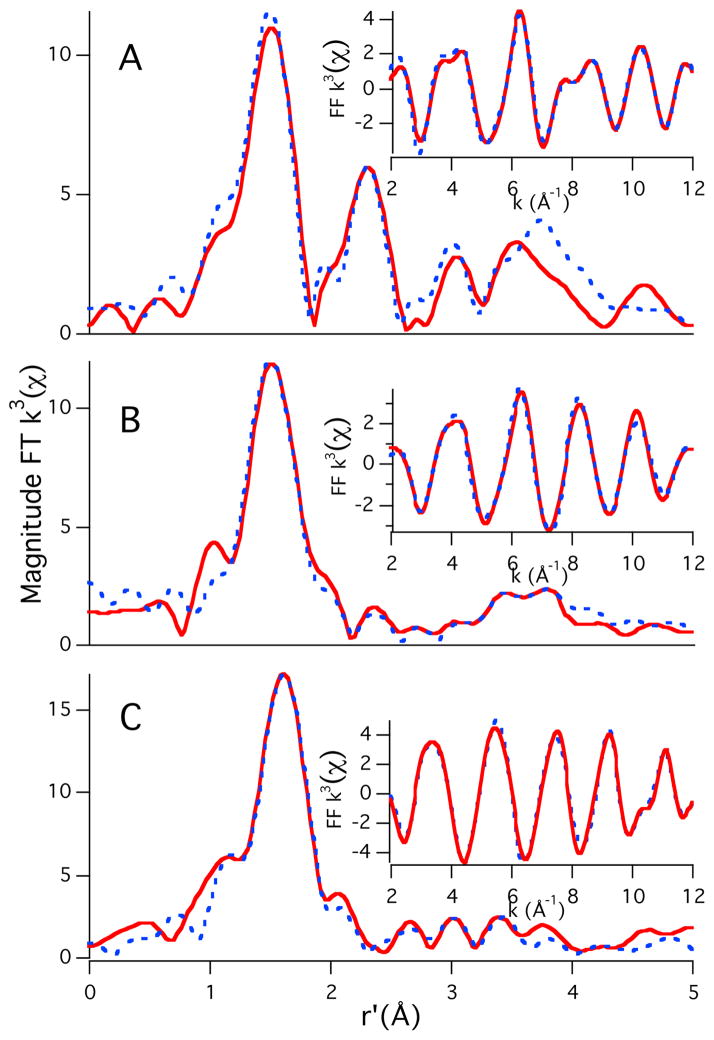
To gain insight into the structural changes induced by the octarepeat expansion in these PrP fragments we utilized both copper and zinc K-edge X-ray absorption spectroscopies (XAS). From our EXAFS analysis, we find that the addition of one equivalent of CuCl2 to the 4-repeat fragment yielded a square-planar (N/O)4 Cu-coordination geometry with 3 to 4 histidine imidazole groups ligated to the copper center at a distance of ~2 Å (Figure 1a; supporting information). Such a copper-center should be capable of undergoing redox cycling and produce reactive oxygen species, which is what is observed. The addition of one equivalent of Zn2+ to this solution leads to a dramatic change in the Cu2+ coordination environment; the copper center remains a 4-coordinate Cu(N/O)4 complex, but is now only ligated by 1 imidazole (rim = 2.01 Å; Figure 1b). As such the coordination environment is nearly identical to what is observed in the case of {CuIIORP}, as is supported by CD studies (supporting information). The EXAFS region of the Zn K-edge spectrum is best modeled with Zn2+ contained in a six coordinate ligand environment with two imidazole ligands (rim = 2.04 and 2.00 Å) and four additional N/O ligands (r = 2.09 Å; Figure 1c). We note that the observed coordination environment/affinity of Zn2+ for the PrP fragment may be influenced by the low temperature (20 K) under which the XAS data were obtained. The addition of 3 equivalents of Cu2+ (4 total) to the Cu2+/Zn2+ ligated 4-repeat fragment yields an average Cu2+ coordination environment that is identical to {CuIIORP}, suggesting that Zn2+ is displaced from the peptide, which was confirmed by Zn K-edge XAS.
Figure 1.
Magnitude FT k3(χ) and FF k3(χ) (insets) for (A) 4-repeat with 1 equivalent of Cu2+ (Cu-edge), (B) 4-repeat with 1 equivalent of Cu2+ and 1 equivalent of Zn2+ (Cu-edge) and (C) 4-repeat with 1 equivalent of Cu2+ and 1 equivalent of Zn2+ (Zn-edge). The refinement details can be found in the supporting information.
The above determined Cu2+ and Zn2+ coordination environments were indirectly supported by employing diethyl pyrocarbonate (DEPC) labeling studies, which identifies uncoordinated histidine residues.8,12 Histidine imidazoles are readily alkylated by DEPC except when coordinated to a metal-center. Thus, identification of alkylated peptides by HPLC and LC-MS can be used to identify metal-coordinated histidines. The addition of four eq of Cu2+ to the 4-repeat fragment eliminates the labeling of histidine residues indicating all four are coordinated to the metal-center. When only one eq. of Cu2+ is added to the 4-repeat fragment a large degree of non-specific mono- and bis-imidazole labeling is observed indicting a highly fluxional coordination motif. The addition of excess Zn2+ to this solution dramatically suppress all bis- and many of the mono-labeling events, but a large degree of mono-histidine labeling is still observed. This suggests one of the histidines remains uncoordinated per 4-repeat fragment when ZnII and CuII are coordinated to the PrP-fragment.
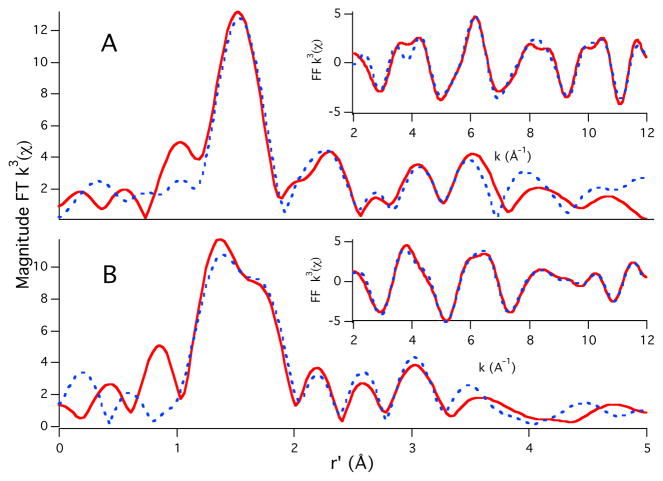
The addition of one eq. of Cu2+ to the 5-repeat fragment produces a mixture of copper coordination environments, with the square planar 3/4-histidine environment being the major contributor (rimidazole = 2.09 and 1.97 Å). Unlike the addition of one equivalent of Zn2+ to the 4-repeat fragment, the addition of one equivalent of Zn2+ to the 5-repeat fragment does not result in a copper coordination environment reminiscent of {CuIIORP}. Instead, it appears to be a mixture of different (N/O)4 Cu2+ coordination structures with a two-histidine coordination complex being the largest contributor (rimidazole = 1.97 and 2.03 Å; Figure 2a). Similar to the 4-repeat we see complete loading of the octarepeat domain when 5 equivalents of Cu2+ are added to the 5-repeat (i.e. the {CuIIORP} coordination motif is observed). DECP labeling supports these results (supporting information).
Figure 2.
Magnitude FT k3(χ) and FF k3(χ) (insets) for 5-repeat with 1 equivalent of CuII and ZnII at the (A) Cu-edge and (B) Zn-edge. Refinement details can be found in the supporting information.
The Zn2+ ion also appears to be in a different coordination environment in the 5- vs. 4-repeat fragments. When the Zn2+ ion is ligated to the 5-repeat peptide in the presence of Cu2+ zinc is contained within a six-coordinate (N/O)6 ligand environment utilizing three histidine residues (one at r = 1.94 Å and two at r = 2.00 Å; Figure 2b) and three other N/O ligands (rN/O = 1.98 Å). Such a situation is confirmed by DEPC labeling studies. The addition of Zn2+ to the 5-repeat fragment containing one equivalent of Cu2+ will suppressed labeling of all five imidazole groups. As with the 4-repeat fragment, when 5 eq of Cu2+ are added to the Zn2+ containing 5-repeat peptide fragment Zn2+ is released as the aqua-cation and five {CuII(ORP)}-like Cu2+ centers are formed.
The use of 2– and 3–repeat fragments suggests that the two-histidines used for Cu2+ coordination in the 5-repeat are not adjacent to one another. We find good agreement between the Cu-EXAFS data when one eq of Cu2+ is added to the 3–repeat fragment and those of the Zn2+/Cu2+ ligated 5-repeat fragment. In contrast, one (and two) equivalent(s) of Cu2+ added to the 2–repeat fragment produces a coordination environment reminiscent of {CuII(ORP)}.
These findings support and expand upon Millhauser’s previous assertions concerning octarepeat expansions and PrPs.6–9 Zn2+ aids in modulating the Cu2+ coordination environment within the “normal” 4-repeat PrP octarepeat domain. Furthermore, Zn2+ coordination shuts off ROS production under low-copper loads by forcing Cu2+ to adopt a redox inactive {CuII(ORP)} coordination environment, suggesting Zn2+ is neuroprotective. It also appears that octarepeat expansions are potentially neurotoxic. The addition of just one octarepeat expansion completely changes the copper and zinc coordination geometries. Zn2+ addition will no longer force the {CuII(ORP)} coordination geometry, but instead provide Cu2+ with a redox-active bis-histidine coordination environment. This opens a means for neurodegeneration through the production of ROSs in these PrP octarepeat expansion mutants. Studies probing this supposition are under-way.
Supplementary Material
Acknowledgments
The NIH (P20 RR-016464), NSF (CH-0844234) and PRF (PRF 49184-ND3) is acknowledged for financial support of this work. We are also indebted to Prof. Glenn Millhauser (UCSD) for valuable discussions. Use of the NSLS was supported by the DOE under contract no. DE-AC02-98CH10886.
Footnotes
SUPPORTING INFORMATION: Contains experimental details, additional XAS spectra, CD spectra, ROS production results and DEPC labeling assay results. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Prusiner SB. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissman C. Nat Rev Microbiol. 2004;2:861–871. doi: 10.1038/nrmicro1025. [DOI] [PubMed] [Google Scholar]
- 3.Brown DR, Qin K, Herms JW, Madling A, Manson J, Strome R, Fraser PE, Kruck T, von Bohlen A, Schulz-Schaeffer W, Giese A, Westaway D, Kretzschmar H. Nature. 1997;390:684–687. doi: 10.1038/37783. [DOI] [PubMed] [Google Scholar]
- 4.Millhauser GL. Acc Chem Res. 2004;37:79–85. doi: 10.1021/ar0301678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaggelli E, Kozlowski H, Valensin D, Valensin G. Chem Rev. 2006;106:1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- 6.Walter ED, Stevens DJ, Spevacek AR, Visconte MP, Dei Rossi A, Millhauser GL. Curr Protein Pep Sci. 2009;10:529–535. doi: 10.2174/138920309789352056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walter ED, Chattopadhyay M, Millhauser GL. Biochemistry. 2006;45:13083–13092. doi: 10.1021/bi060948r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter ED, Stevens DJ, Visconte MP, Millhauser GL. J Am Chem Soc. 2007;129:15440–15441. doi: 10.1021/ja077146j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens DJ, Walter ED, Rodriguez A, Draper D, Davies P, Brown DR, Millhauser GL. PLoS Pathogens. 2009;5:e1000390. doi: 10.1371/journal.ppat.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronoff-Soencer E, Burns CS, Avdievich NI, Gerfen GJ, Peisach J, Antholine WE, Ball HL, Cohen FE, Prusiner SB, Millhauser GL. Biochemistry. 2000;39:13760–13771. doi: 10.1021/bi001472t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shearer J, Soh P. Inorg Chem. 2007;46:710–719. doi: 10.1021/ic061236s. [DOI] [PubMed] [Google Scholar]
- 12.Qin K, Yang Y, Mastrangelo P, Westaway D. J Biol Chem. 2002;277:1981–90. doi: 10.1074/jbc.M108744200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.