Abstract
Purpose
Recent animal studies have shown that selective activation of pudendal nerve branches can evoke bladder responses through two distinct reflex pathways. In this study, we examined intraurethral electrical stimulation (IES) as a minimally invasive means to activate selectively these pathways in the cat.
Materials and Methods
The bladder responses evoked by IES were measured in α-chloralose anesthetized male cats at different stimulation frequencies, stimulation intensities, and intraurethral locations.
Results
IES evoked both inhibitory and excitatory bladder reflexes dependent on stimulation frequency and location. IES in the penile urethra (0–3 cm from the urethral meatus) at 33 Hz stimulation evoked bladder contraction and at 10 Hz stimulation evoked bladder relaxation, and these responses were abolished following bilateral transection of the dorsal penile nerves. Stimulation in the membranous urethra (5–7 cm from the urethral meatus) at 2, 10, and 33 Hz stimulation evoked bladder contractions, and these responses were abolished following bilateral transection of the cranial sensory nerves. Following acute spinal cord transection, bladder contractions were still evoked by 33 Hz stimulation in the penile urethra but not by stimulation at any frequency in the membranous urethra.
Conclusions
IES selectively evoked bladder responses by activation of two distinct pudendal afferent pathways, and the responses were dependent on the stimulation frequency and location. IES is a valid means to determine the pathways involved in bladder responses evoked by pudendal nerve stimulation.
Keywords: urinary bladder, neurogenic, electrical stimulation therapy, urethra, spinal cord injuries
Introduction
Spinal cord injury (SCI) or neurological disorders disrupt the neural control of continence and micturition and may cause urinary retention and/or urinary incontinence. Complications secondary to urinary dysfunction (e.g., frequent urinary tract infections) can lead to significant morbidity and substantially reduced quality of life. Electrical stimulation is a promising approach to restore control of urinary function to individuals with SCI or neurological disorders [1].
Electrical activation of somatovesical reflexes by stimulation of pudendal nerve afferents allows both inhibition and activation of the bladder. Two distinct sensory pathways, originating from the cranial sensory branch (CSN) and the dorsal genital branch (DNP) of the pudendal nerve, can produce bladder activation and inhibition in adult cats [2, 3]. The CSN and DNP innervate the membranous and penile urethra [4], and stimulation of the CSN and DNP evoke reflex bladder contractions through a supraspinal and spinal pathway, respectively [2]. In persons with SCI, robust bladder inhibition is evoked by stimulation of the DNP [5, 6]. Conversely, urethral fluid flow [7], urethral distension [8], and intraurethral electrical stimulation (IES) [9] can evoke bladder contractions, but the neural pathways mediating the responses to intraurethral activation are unclear. The objective of this study in the male cat was to determine the neural pathways mediating the effects of IES and to validate IES as a tool to investigate analogous pathways in human. The parameters of stimulation (frequency, location) enabled selective activation of the CSN and DNP pathways, and in humans IES may provide a means to determine the parameters of pudendal nerve stimulation that enable control of urinary function.
Materials and Methods
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at Duke University. Sixteen sexually intact male cats (2.8–4.6 kg) were anesthetized with ketamine KCl (35 mg/kg i.m.) and α-chloralose (65 mg/kg i.v. supplemented at 15 mg/kg as needed). The animals were intubated and respired artificially to maintain end tidal CO2 between 3.5 and 4.5 %. Body temperature was maintained at 38°C with a thermostatic heating pad, a catheter was inserted in the carotid artery to monitor the blood pressure, and IV fluids (lactated Ringer’s solution or saline/5% dextrose/sodium bicarbonate solution) were administered at 15 cc/kg/h. A 3.5 Fr or 5 Fr catheter was inserted into the bladder dome and secured with a purse-string suture. The catheter was connected to a solid-state pressure transducer (Deltran, Utah Medical) and a pump for infusion of room temperature saline into the bladder.
A 3.5 Fr catheter customized by adding a concentric platinum ring electrode 2–4 cm from the catheter tip was inserted into the urethra via the urethral meatus. Monophasic, cathodic stimulation pulses (10, 20, or 30 second trains of 100 μs pulses) were delivered at varying locations along the urethra (1–7 cm from the urethral meatus), frequencies (2, 10, or 33 Hz), and intensities (0.5–10 mA). The pudendal nerve was isolated in the ischiorectal fossa, and the sensory branch of the pudendal nerve was identified and traced until it branched into the CSN and DNP. Individual branches of the pudendal nerve were directly stimulated with monopolar cuff electrodes (platinum contact embedded in a silicon elastomeric cuff), and a needle in the hindlimb served as the return electrode for all stimulation.
In 3 animals the spinal cord was transected at the T10 vertebral level following collection of pre-spinalization data. A laminectomy was performed, the dura was incised, the spinal cord was elevated and transected with a scalpel, and a barrier of Avitene and Surgicel was placed between the transected ends of the spinal cord.
Changes in bladder pressure in response to 20 second trains of electrical stimulation were analyzed to quantify the bladder response to IES. The ability to evoke bladder contractions was investigated at volumes between 85 and 100% of the minimum volume at which distension evoked contractions occurred, and bladder inhibition was investigated at volumes at which distension evoked bladder contractions occurred. The mean bladder pressure for 3 seconds prior to stimulation was defined as the baseline pressure. A bladder contraction was determined to be evoked if the bladder pressure increased 10 cmH2O above the baseline pressure within 8 seconds of stimulation onset and remained above this threshold until stimulation ended. Bladder relaxation was determined to have occurred if stimulation during a distension evoked contraction caused the bladder pressure to decrease by 50% of the peak distension evoked pressure within 10 seconds of the onset of stimulation and remained below this threshold until stimulation ended. The mean contraction pressures were calculated from when the pressure reached the threshold until stimulation ended. Statistical analysis of bladder pressures at different stimulation locations and frequencies was done by one-way ANOVA with post-hoc comparisons made using Tukey-Kramer. All data are mean ± SE.
Results
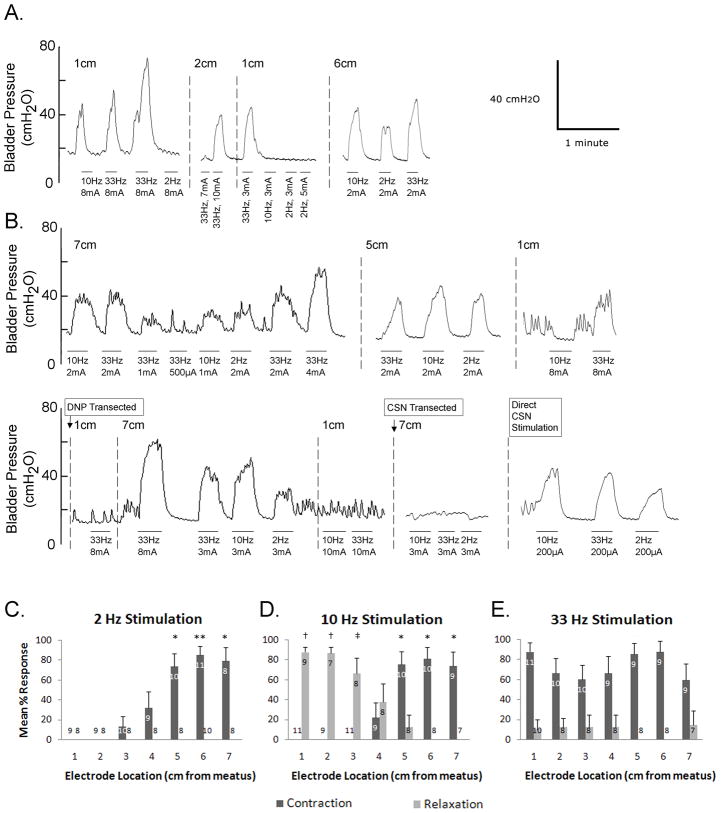
Intraurethral electrical stimulation (IES) evoked bladder responses in 13 of 16 cats, and the bladder responses to stimulation at 2, 10, and 33 Hz at locations 1–7 cm from the urethral meatus were investigated systematically in 11 cats. The ability to evoke a bladder response and the characteristics of the response depended on stimulation frequency, intensity, and location (fig. 1). Excitatory bladder responses were evoked only when the bladder volume was greater than ~70% of the threshold volume for distension evoked bladder contractions [2, 3, 10], while inhibitory bladder responses were only apparent when the bladder was contracting (i.e., at bladder volumes greater than the threshold volume for distension evoked bladder contractions). Average stimulation evoked bladder contraction magnitudes (27±1 cmH2O, range = 11 to 60 cmH2O) and baseline pressures (13±1 cmH2O, range = 8 to 20 cmH2O) varied across experiments
FIG. 1.
Bladder responses evoked by intraurethral electrical stimulation. (A, B) Bladder pressure versus time traces for two cats. Stimulation periods are indicated by horizontal bars under the pressure traces, stimulation frequency and intensity are indicated below each bar, and intraurethral electrode locations are indicated above the pressure traces. (C–E) Mean percentage of trials (+ standard error) when intraurethral stimulation at 1–7 cm from the urethral meatus evoked bladder contraction or relaxation. Numbers in the columns represent the number of cats in which at least 3 stimulation trials were performed at that stimulation location. (C) Stimulation at 2 Hz evoked bladder contractions dependent on stimulation location (p<0.001, one-way ANOVA). (*) Stimulation evoked contractions in a significantly greater percentage of trials than stimulation at 1–3 cm (p<0.05, Tukey-Kramer post-hoc comparison). (**) Stimulation evoked contractions in a significantly greater percentage of trials than stimulation at 1–4 cm (p<0.05). (D) Stimulation at 10 Hz evoked bladder contraction or relaxation dependent on stimulation location (p<0.001, one-way ANOVAs for contraction and relaxation). (*) Stimulation evoked contractions in a significantly greater percentage of trials than stimulation at 1–4 cm (p<0.05, Tukey-Kramer post-hoc comparison). (†) Stimulation evoked relaxation in a significantly greater percentage of trials than stimulation at 4–7 cm (p<0.05). (‡) Stimulation evoked relaxation in a significantly greater percentage of trials than stimulation at 5–7 cm (p<0.05). (E) Stimulation at 33 Hz evoked bladder contractions independent of stimulation location (p=.41, one-way ANOVA). Relaxation was evoked in a small number of trials, and the dependence on location was not statistically significant (p=.88, one-way ANOVA).
Bladder contractions were consistently evoked by 2 Hz stimulation in the membranous urethra, but 2 Hz stimulation did not evoke bladder relaxation at any location or intensity (fig. 1, C). IES at 10 Hz evoked either bladder contraction or bladder relaxation depending on location (fig. 1, D). 10 Hz stimulation in the membranous urethra evoked bladder contraction while 10 Hz stimulation in the penile urethra evoked relaxation. IES at 33 Hz consistently evoked bladder contractions at all locations but did not consistently evoke bladder relaxation at any location or intensity (fig. 1, E). Stimulation with excitatory parameters during distension evoked contractions often augmented distension evoked contraction magnitude (fig. 1, A).
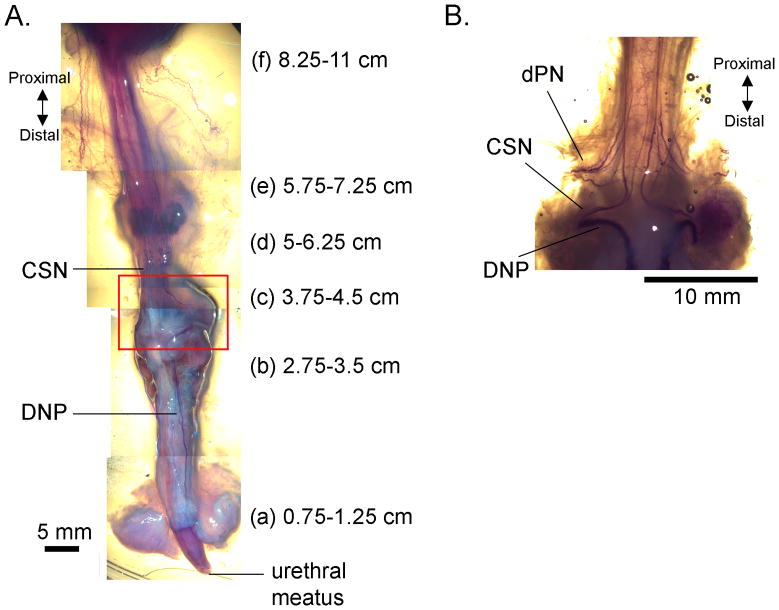
The distance from the urethral meatus to several anatomical landmarks was measured post-mortem in 6 cats (fig. 2). When positioned 1–3 cm from the urethral meatus the electrode was within the penile urethra, and at 5–7 cm from the urethral meatus the electrode was within the membranous urethra, extending into or just proximal to the prostate.
FIG. 2.
Anatomical relationship between intraurethral catheter locations along the male cat urethra and innervation by the cranial sensory (CSN) and dorsal penile (DNP) branches of the pudendal nerve. (A) A ventrolateral view of the lower urinary tract of a male cat with nerves rendered dark purple using Sihler’s method [15]. Distance from urethral meatus to several anatomical landmarks, including (a) the base of the glans penis, (b) the base of the penile body, (c) the proximal border of the bulb of the penis, (d) the distal border of the prostate, (e) the proximal border of the prostate, and (f) the bladder neck. All distances are accurate within ±0.5 cm. (B) Image of the ventral side of the membranous urethra and the proximal end of the bulb of the penis (box in (A)), with nerves rendered dark purple by Sihler’s method [15] illustrating the CSN, DNP, and deep perineal (dPN) branches of the pudendal nerve.
Selective transection of individual branches of the pudendal nerve was used to determine the pathways mediating the IES evoked responses. Bilateral transection of the DNP abolished the responses evoked by stimulation in the penile urethra, while bilateral transection of the CSN abolished responses evoked by stimulation in the membranous urethra (n=2 cats, fig. 1, B). Direct stimulation of the proximal stump of the transected DNP at 33 Hz or the proximal stump of the transected CSN at 2, 10, and 33 Hz still evoked bladder contractions (n=2 cats, fig. 1, B), demonstrating the continued functionality of the reflexes. Administration of α-bungarotoxin (2 cats, 100 μg/kg i.v.) abolished the reflex EMG evoked in the external anal sphincter by IES, but the bladder responses remained intact.
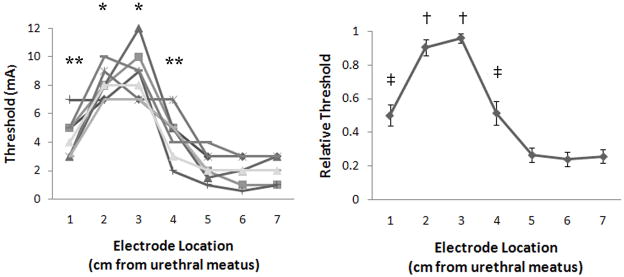
The stimulation intensity threshold to evoke a bladder contraction varied depending on stimulation location (fig. 3). Stimulation thresholds were highest 2–3 cm from the urethral meatus, and in some instances no bladder responses were evoked by stimulation at 2 cm (1 cat) or 3 cm (2 cats) from the urethral meatus, possibly because the thresholds were greater than 10 mA. In one cat stimulation thresholds were investigated above 10 mA, and the threshold to evoke contractions with 33 Hz stimulation at 3 cm from the urethral meatus was 12 mA. Thresholds were substantially lower in the membranous urethra (fig. 3), and stimulation at 2, 10, and 33 Hz evoked bladder responses at the same thresholds.
FIG. 3.
Stimulation intensity thresholds for evoking bladder contraction at different intraurethral electrode locations. (A) The stimulation intensity thresholds for 8 cats at 1–7 cm from the urethral meatus were dependent on the electrode location (p<0.001, one-way ANOVA). (*) Stimulation thresholds were significantly greater than at 1, 4, 5, 6, and 7 cm (p<0.05, Tukey-Kramer post-hoc comparison). (**) Stimulation thresholds were significantly greater than at 6 and 7cm (p<0.05). (B) Relative stimulation thresholds, normalized by the maximum threshold in each cat, versus electrode location. The relative intensities were dependent on electrode location (p<0.001, one-way ANOVA). (†) Relative thresholds were greater than thresholds at 1 cm and 4–7 cm (p<0.05, Tukey-Kramer post-hoc comparison). (‡) Relative thresholds were greater than those at 5–7 cm (p<0.05).
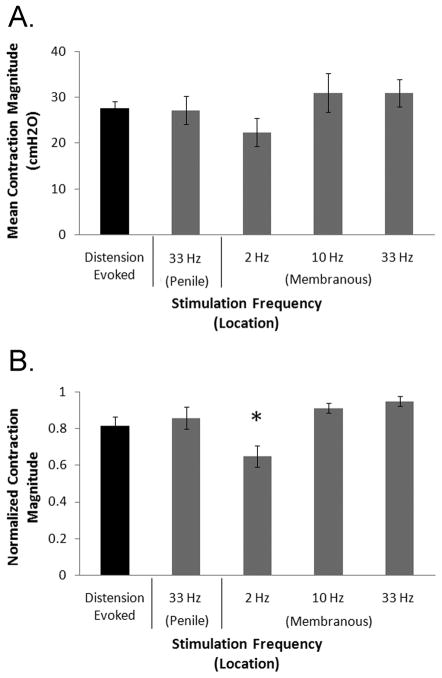
Contraction magnitudes were dependent on stimulation frequency and intensity. Stimulation in the penile urethra at 33 Hz and stimulation at 10 Hz and 33 Hz in the membranous urethra evoked larger bladder contractions than stimulation in the membranous urethra at 2 Hz (fig. 4). Stimulation at threshold intensities for evoking a bladder response evoked smaller bladder contractions (19±2 cmH2O) than stimulation at 1.5–2x threshold (28±2 cmH2O) and 2.25–3x threshold 29±3 cmH2O).
FIG. 4.
Magnitude of bladder contractions evoked by intraurethral electrical stimulation (IES) at different stimulation frequencies and locations. (A) The mean contraction magnitude of distension evoked reflex bladder contractions and contractions evoked by stimulation at 33 Hz in the penile urethra or by 2, 10, or 33 Hz in the membranous urethra. Distension evoked contractions were only observed and measure at bladder volumes greater than VTHR, the minimum volume at which distension evoked contractions were observed, while stimulation evoked contractions were measured at volumes between 85 and 100% of VTHR. (B) Average normalized magnitudes of distension evoked reflex contractions and IES evoked contractions at different frequencies and locations. The average contraction magnitudes were computed for each cat and normalized by dividing by the largest value. Normalized values were averaged across cats. Contractions evoked by stimulation at 2 Hz were smaller than contractions evoked by 33 Hz stimulation in the penile urethra and 10 and 33 Hz stimulation in the membranous urethra (*p<0.001, one-way ANOVA, p<0.05, Tukey-Kramer post-hoc comparison), but distension evoked contractions were not significantly different from stimulation evoked contractions at any stimulation parameters. VTHR is the minimum volume at which distension evoked contractions were observed, and stimulation evoked contractions were investigated at volumes between 85 and 100% of VTHR.
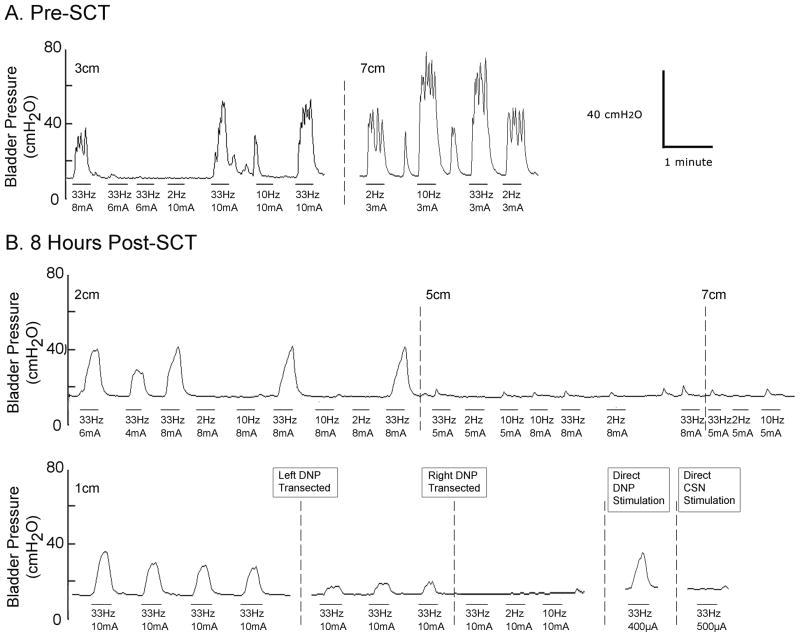
At 8–13 hours following SCT, IES at 33 Hz in the penile urethra evoked bladder contractions (fig. 5), while stimulation at 2 or 10 Hz did not evoke bladder contractions at any location. Stimulation in the membranous urethra occasionally evoked small magnitude (<5 cmH2O), transient rises in bladder pressure but never evoked a robust bladder response. In 2 SCT animals, the magnitude of bladder responses evoked by IES following unilateral DNP transection decreased by 23% (from 18±3 cmH2O to 14±1 cmH2O) and 62% (from 16±1 cmH2O to 6±0 cmH2O), and following bilateral transection of the DNP, IES no longer evoked bladder contractions. In SCT animals direct 33 Hz stimulation of the proximal stump of the transected DNP continued to evoke bladder contractions (2 cats), while direct stimulation of the proximal stump of the transected CSN did not produce contractions (1 cat).
FIG. 5.
Bladder responses evoked by intraurethral electrical stimulation before and after acute spinal cord transection. (A) Bladder pressure versus time traces prior to spinal cord transection. (B) Bladder pressure versus time traces 8 hours post spinal cord transection in the same cat from (A). The horizontal bars below the pressure trace indicate stimulation, the stimulation frequency and intensity are indicated below each bar, and stimulation locations are indicated above the pressure traces.
Discussion
IES evoked bladder responses through two distinct reflex pathways. IES at 2, 10, and 33 Hz in the membranous urethra evoked bladder contractions via afferents in the CSN branch of the pudendal nerve. These responses were no longer evoked following transection of the CSN or acute SCT. IES in the penile urethra at 10 Hz evoked bladder relaxation and at 33 Hz evoked bladder contraction via afferents in the DNP branch of the pudendal nerve. These responses were no longer evoked following DNP transection; however, following acute SCT, stimulation in the penile urethra at 33 Hz continued to evoke bladder contraction. Direct stimulation of the CSN and DNP [2, 3] evoked bladder contraction and relaxation at similar frequencies as IES in the membranous and penile urethra, respectively, and direct stimulation of the proximal stumps of the transected nerves in this study confirmed that the IES evoked responses were via sensory activation of reflex pathways. Direct CSN stimulation at 33 Hz was previously found not to evoke bladder contractions consistently across animals [2], but 33 Hz IES in the membranous urethra did consistently evoke bladder contractions across animals in this study. This difference is likely due to the different stimulation methods. Intraurethral stimulation may activate a different complement of sensory fibers in the CSN than direct CSN stimulation. Also, IES can activate the CSN bilaterally, and IES would be more effective at evoking bladder contractions if there is functional asymmetry in the CSN-bladder pathway [11] or if bilateral activation is more effective at evoking bladder contractions than unilateral activation.
Administration of α-chloralose anesthesia can effect bladder function leading to increased baseline pressures and reduced contraction magnitudes [12]. The contractions observed here are consistent with previous results in α-chloralose anesthetized cats, and a similar frequency dependence of somatovesical reflexes was observed in awake chronic SCI cats [13].
IES activated the pudendal, pelvic, and hypogastric nerves in the cat, evoking stronger hypogastric and pelvic nerve responses than pudendal nerve responses when stimulation was proximal to the prostate [14]. In the present study, the pudendal nerve was targeted by limiting IES to 1–7 cm from the urethral meatus, a length consistent with the urethral areas most heavily innervated by pudendal afferents (fig. 2) [15], and transection of the DNP and CSN verified that activation of pudendal afferents was required to evoke bladder contractions, and any spillover of stimulation to pelvic or hypogastric afferents was not sufficient to generate the observed responses.
Stimulation of efferent fibers in the deep perineal branch of the pudendal nerve activates the external urethral sphincter, and this motor response can generate sufficient sensory activation of the CSN to generate a bladder response [2]. Deep perineal nerve activation by IES in the area of the external urethral sphincter may have therefore contributed to the bladder responses observed in this study. However, evoked bladder responses were preserved following administration of α-bungarotoxin, demonstrating that this pathway did not contribute to the responses evoked by IES.
In a previous study of the bladder response to IES, stimulation at 2 Hz in the membranous urethra evoked bladder contractions in a SCT cat [16], but a similar response was not observed in this study. Direct CSN stimulation evoked small magnitude, transient bladder responses in acute SCT cats [2, 17], and in chronic SCI cats, 2 Hz stimulation did not evoke bladder contractions [18], consistent with the observed lack of robust bladder responses evoked by IES of the membranous urethra. In the previous cat study of IES, the nature of the contractions evoked by 2 Hz stimulation in the proximal urethra in the SCT cat was not described in detail, so it is likely that the contractions were small magnitude, transient responses [2, 17].
The ability to evoke bladder responses depended on bladder volume and stimulation intensity. Similar bladder volume dependence was observed in the previous studies of bladder responses evoked by pudendal afferent activation [2, 3, 10], as well as previous human IES experiments [9], and the volume dependence is mediate by neural rather than biomechanical factors (i.e. length-tension properties) [10]. Stimulation intensity thresholds were smaller in the membranous urethra than in the penile urethra, possibly because the post-prostatic and prostatic urethra are more heavily innervated by pudendal afferents than the penile urethra [15, 19] or because the CSN trunk is in closer proximity to the membranous urethral lumen than the DNP trunk is to the penile urethral lumen [15].
These results suggest that the DNP pathway should be the target for restoring bladder function in SCI humans. Clinical studies investigating the effect of DNP stimulation on bladder inhibition and urinary continence are promising [5, 6], but the ability to evoke bladder contractions and improve bladder voiding (i.e. treat urinary retention) by pudendal afferent stimulation has not been adequately investigated. High frequency direct stimulation of the DNP in the cat evoked bladder contraction and improved voiding (compared to distension evoked voiding) [3], but it is unknown how pudendal afferent evoked bladder contractions will effect bladder voiding and detrusor-sphincter dyssynergia in humans with SCI.
The CSN-to-bladder reflex may return at longer times post-SCT. While CSN stimulation does not appear to evoke bladder contractions in SCT cats, the organization of feline and human pudendal-to-bladder reflexes may differ. Bladder contractions evoked by IES in persons with SCI may have been evoked by activation of the human analog of the CSN. It is unclear if a human analog of the CSN exists, but the urethrovesical reflex in humans suggests somatic innervation of the proximal urethra [8, 20]. This study expanded the stimulation parameters used in previous cat experiments and identified a DNP mediated bladder pathway accessible by stimulation in the penile urethra, suggesting that expanded stimulation parameters in clinical studies will allow for full characterization of the pudendal-to-bladder reflexes that are present in humans. Additionally, IES enables reliable selective activation of pudendal afferent pathways compared to pudendal nerve trunk stimulation.
Conclusions
The bladder responses evoked by IES were dependent on stimulation location, frequency, and intensity. Two distinct reflex pathways were activated by IES to evoke bladder responses, but only the DNP-mediated pathway was present following SCI.
Acknowledgments
Grants
This work was supported by NIH Grant R01-NS050514 and the National Science Foundation Graduate Research Program.
Abbreviations
- CSN
cranial sensory nerve
- DNP
dorsal penile nerve
- IES
intraurethral electrical stimulation
- SCI
spinal cord injury
- SCT
spinal cord transection
References
- 1.Jezernik S, Craggs M, Grill WM, Creasey G, Rijkhoff NJ. Electrical stimulation for the treatment of bladder dysfunction: current status and future possibilities. Neurol Res. 2002;24:413. doi: 10.1179/016164102101200294. [DOI] [PubMed] [Google Scholar]
- 2.Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Exp Neurol. 2008;212:218. doi: 10.1016/j.expneurol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woock JP, Yoo PB, Grill WM. Activation and inhibition of the micturition reflex by penile afferents in the cat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1880. doi: 10.1152/ajpregu.00029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin WD, Fletcher TF, Bradley WE. Innervation of feline perineal musculature. Anat Rec. 1974;180:15. doi: 10.1002/ar.1091800104. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler JS, Jr, Walter JS, Zaszczurynski PJ. Bladder inhibition by penile nerve stimulation in spinal cord injury patients. J Urol. 1992;147:100. doi: 10.1016/s0022-5347(17)37145-8. [DOI] [PubMed] [Google Scholar]
- 6.Kirkham AP, Shah NC, Knight SL, Shah PJ, Craggs MD. The acute effects of continuous and conditional neuromodulation on the bladder in spinal cord injury. Spinal Cord. 2001;39:420. doi: 10.1038/sj.sc.3101177. [DOI] [PubMed] [Google Scholar]
- 7.Bump RC. The urethrodetrusor facilitative reflex in women: results of urethral perfusion studies. Am J Obstet Gynecol. 2000;182:794. doi: 10.1016/s0002-9378(00)70328-0. [DOI] [PubMed] [Google Scholar]
- 8.Shafik A, el-Sibai O, Ahmed I. Effect of urethral dilation on vesical motor activity: identification of the urethrovesical reflex and its role in voiding. J Urol. 2003;169:1017. doi: 10.1097/01.ju.0000046384.71563.51. [DOI] [PubMed] [Google Scholar]
- 9.Gustafson KJ, Creasey GH, Grill WM. A urethral afferent mediated excitatory bladder reflex exists in humans. Neurosci Lett. 2004;360:9. doi: 10.1016/j.neulet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol. 2005;93:2688. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- 11.Hamdy S, Enck P, Aziz Q, Uengoergil S, Hobson A, Thompson DG. Laterality effects of human pudendal nerve stimulation on corticoanal pathways: evidence for functional asymmetry. Gut. 1999;45:58. doi: 10.1136/gut.45.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudy D, Downie J, McAndrew J. alpha-Chloralose alters autonomic reflex function of the lower urinary tract. Am J Physiol. 1991;261:R1560. doi: 10.1152/ajpregu.1991.261.6.R1560. [DOI] [PubMed] [Google Scholar]
- 13.Tai C, Shen B, Wang J, Chancellor MB, Roppolo JR, de Groat WC. Inhibitory and Excitatory Perigenital-to-Bladder Spinal Reflexes in the Cat. Am J Physiol Renal Physiol. 2008;294:F591. doi: 10.1152/ajprenal.00443.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradley W, Griffin D, Teague C, Timm G. Sensory innervation of the mammalian urethra. Invest Urol. 1973;10:287. [PubMed] [Google Scholar]
- 15.Yoo PB, Woock JP, Grill WM. Somatic innervation of the feline lower urinary tract. Brain Research. 2008 doi: 10.1016/j.brainres.2008.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustafson KJ, Creasey GH, Grill WM. A catheter based method to activate urethral sensory nerve fibers. J Urol. 2003;170:126. doi: 10.1097/01.ju.0000070821.87785.14. [DOI] [PubMed] [Google Scholar]
- 17.Shefchyk SJ, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neurosci Lett. 1998;244:137. doi: 10.1016/s0304-3940(98)00155-4. [DOI] [PubMed] [Google Scholar]
- 18.Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp Neurol. 2006;197:225. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Todd JK. Afferent Impulses in the Pudendal Nerves of the Cat. Q J Exp Physiol Cogn Med Sci. 1964;49:258. doi: 10.1113/expphysiol.1964.sp001730. [DOI] [PubMed] [Google Scholar]
- 20.Shafik A, Shafik AA, El-Sibai O, Ahmed I. Role of positive urethrovesical feedback in vesical evacuation. The concept of a second micturition reflex: the urethrovesical reflex. World J Urol. 2003;21:167. doi: 10.1007/s00345-003-0340-5. [DOI] [PubMed] [Google Scholar]