Abstract
Background
It is not known if there is a relationship between gender and tissue outcome in human ischemic stroke. We sought to identify whether the proportion of initially ischemic to eventually infarcted tissue was different between men and women with ischemic stroke.
Methods
We studied 141 consecutive patients with acute ischemic stroke who had a baseline MRI obtained within 12 hours of symptom onset, a follow-up imaging on day 4 or later, and DWI/MTT mismatch on initial MRI. Lesion growth was calculated as percentage of mismatch tissue that underwent infarction on follow-up (Percentage Mismatch Lost or PML). Multivariable analyses explored the effect of gender and other predictors of tissue outcome on PML.
Results
There was no difference in median PML between men (19%) and women (11%) (p=0.720). There was, however, an interaction between gender and age; median PML was 7% (0–12%) in women and 18% (1–35%) in men younger than the population median (71 years, p=0.061). The PML was not different between men and women ≥71 years old (25% in both groups). The linear regression model revealed gender (p=0.027) and the interaction between age and gender (p=0.023) as independent predictors of PML.
Conclusion
There is an age-by-gender interaction in tissue outcome after ischemic stroke; brain infarcts in women younger than 70 years grow approximately 50% less than infarcts in their male counterparts. These findings extend the well-known concept that there is a differential age-by-gender effect on stroke incidence, mortality, and functional outcome to the tissue level.
Keywords: cerebral infarct, diffusion-weighted imaging, perfusion-weighted imaging, magnetic resonance imaging, gender, women & minorities, outcome
Brain imaging currently plays an essential role in the diagnosis of stroke, both in distinguishing between hemorrhage and ischemia, and in determining the extent and localization of the lesion. Recent advances in imaging have provided the ability to assist not only in the diagnosis but also to estimate the likelihood of irreversible injury within the ischemic territory1. Diffusion-weighted MRI (DWI) depicts tissue with evidence of ischemic injury. Perfusion-weighted MRI (PWI) reveals regions with impaired tissue perfusion. The mismatch tissue that occurs between abnormal tissue visualized on acute DWI and acute PWI has been postulated to represent a tissue at risk1. The outcome of the mismatch tissue is markedly variable in humans, ranging from no infarction, even without intervention (thrombolysis), to loss of all tissue at risk2. This is consistent with the notion that not all brains can handle an ischemic insult of similar degree and duration in the same manner3,4. Identification of determinants of tissue outcome in ischemic stroke is a useful step towards the understanding of evolution of stroke in humans and hence developing new potential therapeutic targets. Variables such as age5, the severity and volume of leukoaraiosis6, genetic susceptibility7, time of imaging relative to symptom onset8, and factors that alter the susceptibility to ischemic injury (arterial blood pressure, blood glucose, fever, etc.) might explain some of the variance in tissue outcome.
Published data in animals suggests that gender is also important in determining the amount of ischemic tissue turning into infarction. Young female animals are more resistant to cerebral ischemia and have smaller lesion volumes following experimental ischemic brain injury9,10. The favorable response to ischemia in female animals appears to diminish with age11,12, suggesting a possible role for sex hormones in the protection against ischemic injury13. The purpose of this study was to find out whether gender is a predictor of tissue outcome in human stroke. More specifically, we sought to understand whether the proportion of initially ischemic but eventually infarcted tissue was different between men and women with ischemic stroke.
Methods
Study population
We analyzed data that was prospectively collected as part of an NIH-funded study evaluating the utility of DWI and perfusion weighted imaging (PWI) in predicting tissue outcome in acute ischemic stroke (MRI Diffusion/Perfusion Mismatch in Human Acute Stroke). This study included consecutive patients with DWI and PWI performed within 12 hours of symptom onset who underwent a second imaging study (MRI or CT) on day 4 or later in order to assess the final infarct volume during an 8 year period between 2000 and 2008. Both CT and MRI were used for follow-up assessment because they both conspicuously define infarct limits after 24 hours of stroke onset14. Because of suboptimal reliability of PML determination at small mismatch volumes, we excluded patients with small DWI/PWI mismatch defined as “mean transit time (MTT) volume/DWI volume < 1.2” and “MTT volume – DWI volume < 10 mL”15,16. We also excluded those in whom assessment of final infarct was not possible due to low-quality follow-up images, hemicraniectomy, extensive hemorrhagic conversion, or massive brain edema, and who received experimental or intra-arterial thrombolytic treatment. The study protocol was approved by the local institutional review board.
Data collection
Demographic (age, gender) and clinical data (history of hypertension, diabetes mellitus, coronary artery disease, congestive heart failure, atrial fibrillation, admission blood glucose, admission mean arterial blood pressure, intravenous tissue plasminogen activator treatment, time from symptom onset to MRI, admission stroke severity, etiologic stroke subtype) were collected. Time of stroke onset was defined as the last time patient was seen normal. Admission stroke severity was assessed by the National Institute of Health Stroke Scale (NIHSS) score. Etiologic stroke subtype was determined using the Causative Classification of Stroke (CCS) system.17
Image acquisition and analysis
All acute and follow up MR images were performed by 1.5T (GE Medical Systems, Milwaukee, WI) or Siemens Sonata (Siemens Medical Solutions, Erlangen, Germany) scanners except for 10 patients who had their follow-up images obtained at 3T GE Signa or Siemens Trio scanners. Image acquisition and processing protocols were described in detail previously5. Briefly, DWI was obtained using echo planar imaging (EPI). Average DWI maps as well as ADC maps were computed from images corrected for motion and eddy-current distortions. Perfusion-weighted images were acquired using dynamic susceptibility contrast EPI. Mean transit time (MTT) and cerebral blood flow (CBF) maps were calculated as described previously5. The CT studies were performed by using a helical scanner (High-Speed Advantage, GE Medical Systems).
Ischemic lesions on admission DWI and MTT maps, and on FLAIR or CT images were manually outlined and lesion volumes were calculated using MRIcro software (University of Nottingham, UK). All lesion volumes were corrected for differences in overall brain size using mid-sagittal cross-sectional intracranial area (ICA) as a surrogate measure of the intracranial volume18. All MRI measurements were performed by a neurologist experienced in image analysis and processing (EMA) blinded to the clinical data. Methods used for calculation of infarct volume as well as intracranial area have been previously reported to have high inter-rater reliability15,18. The proportion of ischemic tissue undergoing infarction within the mismatch region was determined by percentage mismatch lost (PML) and was calculated according to the following formula5:
Statistical analysis
Statistical analyses explored the relationship between gender and tissue outcome (PML). Bivariate relationships between clinical and imaging variables and tissue outcome were explored using Mann-Whitney U and Kruskall-Wallis tests for categorical variables and Spearman’s correlation for continuous variables. A linear regression model was fitted using tissue outcome as the dependent and age, gender, and predictors of tissue outcome as independent variables using p<0.05 as retention criterion. Since tissue outcome metrics did not conform to normal distribution, they were each log-transformed before being introduced to the model. All categorical variables were entered as binary variables. Standard regression diagnostics were used to assess linear regression assumptions. There was no evidence of collinearity between the covariates or non-linearity between the dependent variable and independent variable. All numerical variables were expressed as mean±standard deviation (SD) or median (inter-quartile range, IQR). A two-tailed p value of <0.05 was considered significant. Statistical analyses were performed using SPSS 11.5.
Results
A total of 756 consecutive with DWI and PWI within the first 12 hours of symptom onset were screened during the study period. Patients were excluded if they did not provide informed consent for follow-up imaging or failed to return back for a follow-up study (435 patients), were enrolled into a therapeutic trial or underwent intra-arterial thrombolysis (90 patients), and had extensive hemorrhagic conversion, massive brain edema, or hemicraniectomy (28 patients). Of the remaining 203 patients, 62 (31%) did not have DWI/MTT mismatch according to the predefined criteria. Further analyses were restricted to the remaining 141 patients
The study population consisted of 53 women and 88 men, with a median (IQR) age of 71 (56–80) years. Initial and follow-up images were acquired after a median (IQR) of 5.3 (3.5–7.2) hours and 10 (6–33) days from stroke onset, respectively. Thirty-eight patients had follow-up studies performed after 30 days. The median (IQR) lesion volume was 14.7mL (4.6mL–48.7mL) on acute DWI and 34.5mL (10.3mL–94.6mL) on follow-up images. The median (IQR) PML was 16.4% (2.6%–47.8%). PML increased by increasing age, admission plasma glucose level, admission NIHSS score, DWI lesion volume, follow-up lesion volume, MTT lesion volume and by decreasing time from symptom onset to follow-up imaging (Table 1). The correlation between age and PML appeared to be driven by a steep increase in PML in patients older than 70 years as previously suggested5.
Table 1.
Univariate associations between baseline clinical and imaging variables and percentage mismatch lost
| PML median (IQR) or correlation coefficient | p | |
|---|---|---|
| Gender | ||
| Female (n=53) | 12 (3–55) % | 0.720 |
| Male (n=88) | 19 (2–46) % | |
| Iv-rtPA therapy | ||
| Yes (n=51) | 23 (6–55) % | 0.063 |
| No (n=90) | 11 (2–36) % | |
| CCS Subtype | ||
| Large artery atherosclerosis (n=36) | 19 (4–43) % | |
| Cardioaortic embolism (n=64) | 18 (5–46) % | 0.567 |
| Other (n=17) | 11 (0–70) % | |
| Undetermined (n=24) | 9 (0–58) % | |
| Type of follow-up imaging | ||
| Computed tomography (n=50) | 22 (4–59) % | 0.331 |
| Magnetic resonance imaging (n=91) | 12 (3–35) % | |
| Age | r=0.26 | 0.002 |
| Admission mean blood pressure | r=0.06 | 0.515 |
| Admission plasma glucose level | r=0.20 | 0.017 |
| Admission NIHSS score | r=0.46 | <0.001 |
| Time from onset to initial imaging | r=−0.14 | 0.095 |
| Time from onset to follow-up imaging | r=−0.23 | 0.006 |
| Admission DWI lesion volume | r=0.40 | <0.001 |
| Admission MTT volume | r=0.26 | 0.002 |
There was no significant association between PML and gender. Nevertheless, women ≤70 years old were more likely to have smaller PML (p=0.061) as compared to their male counterparts whereas there was no difference in PML between men and women >70 years (Table 2; Figure 1). The linear regression analysis showed that gender was not a predictor of PML (Table 3). Gender, however, was a significant predictor of PML (p=0.027) when age-by-gender interaction term was included into the linear regression model. The adjusted PML from the regression model was 0.44 fold (95% CI, 0.17–0.95; p=0.033) smaller in women younger than the group median (≤70 years old) as compared to men in the similar age strata. In contrast, in patients older than 70 years old, PML was 1.60 fold (95% CI, 0.90–2.81; p=0.091) higher in women as compared to men. Both gender and age-by-gender interaction remained significant when the regression model was repeated by using a forward stepwise selection approach including all variables presented in Table 1 as covariates.
Table 2.
Tissue MRI metrics according to age and gender
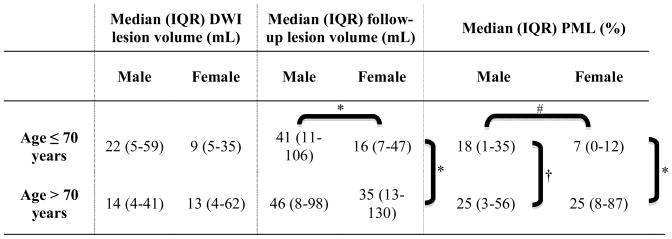 |
p<0.05;
p=0.061;
p=0.295
Figure 1.
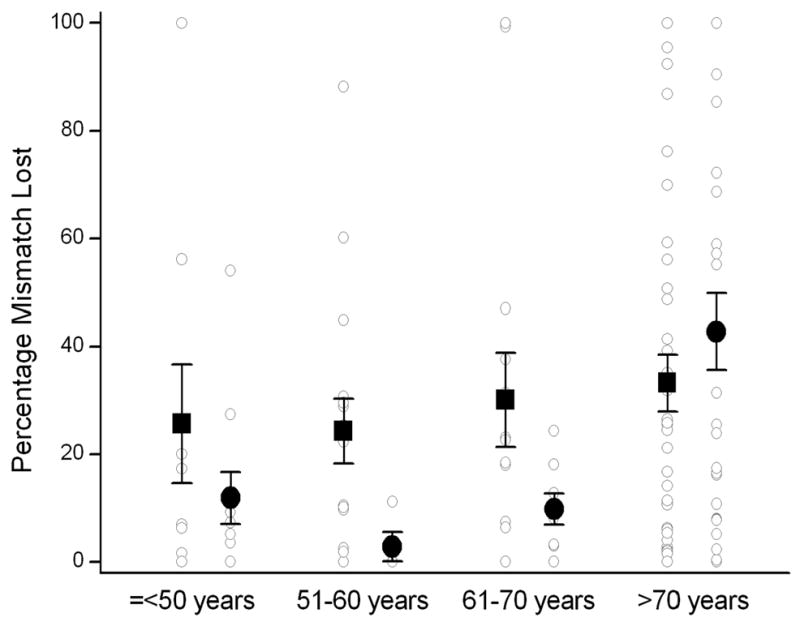
Percentage mismatch lost as a function of age and gender. The graph shows the individual data points (○), mean percentage mismatch lost for each age group (■: men; ●: women) and the corresponding standard error bars. Note that percentage mismatch lost is less in women compared to men among patients ≤70 years old, while there is no difference in percentage mismatch lost between men and women >70 years old.
Table 3.
Multivariable predictors of percentage mismatch lost with or without including age-by-gender interaction
| Without age-by-gender interaction | With age-by-gender interaction | |||
|---|---|---|---|---|
| β | p | β | p | |
| Age >70 years | 0.56 | 0.120 | −0.09 | 0.846 |
| Female Gender | −0.28 | 0.430 | −1.18 | 0.027 |
| Age × Gender interaction | - | - | 1.63 | 0.023 |
| Admission NIHSS score | 0.11 | <0.001 | 0.11 | <0.001 |
| Admission MTT volume | 0.002 | 0.477 | 0.002 | 0.449 |
| Admission DWI lesion volume | 0.01 | 0.285 | 0.003 | 0.498 |
| Admission plasma glucose level | 0.002 | 0.634 | 0.002 | 0.555 |
| Time to follow-up imaging | −0.03 | <0.001 | −0.03 | <0.001 |
The results did not change when the regression model with PML was repeated after including the 28 patients that were initially excluded from analysis due to massive edema, extensive hemorrhagic conversion or hemicraniectomy on follow-up imaging; assuming that the whole territory at risk underwent infarction in such patients (i.e. PML was 100%), both gender (p=0.039) and the interaction between age and gender (p=0.030) were significant predictors of PML. Similarly, there was no change in the results when the 10 patients who had follow-up MRI performed at 3T scanners were excluded (p for age-gender interaction 0.001). There were four women who were on hormone replacement therapy at the time of stroke onset. They had significantly lower PML when compared to the remaining 49 women (p=0.046). There was no change in the results when these 4 patients were excluded from the multivariable analyses (p for age-gender interaction 0.031).
Fifty-one patients received intravenous thrombolytic treatment. The median PML was higher in treated patients as compared to those who were not treated (23% versus 11%, p=0.063, Table 1). Patients who received intravenous thrombolytic treatment had more severe strokes at baseline (median NIHSS score: 14 in treated, 7 in untreated, p<0.001). The median PML was 14% (IQR, 8–92%) in women and 25% (IQR, 6–47%) in men among the 51 treated patients (p=0.750) and 9% (IQR, 1–26%) in women and 14% (IQR, 2–49%) in men in the 90 untreated patients (p=0.480).
Discussion
In this report of 141 patients, the relative percentage of initially ischemic tissue that eventually turned into infarction (percentage mismatch lost or PML) was similar between men and women with or without adjustment for important predictors of tissue outcome. However, there was an age-by-gender interaction; when age was considered in two strata (≤70 and >70 years), younger women had smaller PML (0.44 fold) compared to younger men whereas older women had marginally larger PML (1.60 fold) compared to older men.
Our finding that there is a differential age-dependent worsening in tissue outcome in men and women coincides with evidence from observations on the relationship between gender and clinical outcome. It is known that premenopausal women are at lower risk of developing cardiovascular diseases including stroke and vascular death compared to age-matched men. The risk of stroke increases in postmenopausal women and becomes equal to that of men by the time women are around 65 to 75 years old19. Mortality and functional outcome after stroke also show age-dependent change between men and women; the US age-specific stroke mortality statistics demonstrate that women aged 45–74 years have a lower risk of stroke mortality compared to men, whereas no such survival advantage is observed for women after the age of 75–8019. The mortality rates in older women even surpass the rates observed in their male counterparts20. The turnabout in stroke incidence, mortality, and functional outcome in women in around 65 to 75 years suggests that women continue to have protective mechanisms well beyond (>20 years) after menopause. It has been suggested that this extended protection is mediated by continued presence of lower levels of estrogen until a certain age after menopause. With aging, however, the effectiveness of estrogen on cerebral blood vessels starts to diminish, estrogen loses its protective effects on neural tissue and even exacerbates neural injury21,22. This may explain the disproportionate worsening in tissue outcome in women after 70 years of age. The change in estrogen level and activity with age suggests that estrogen may play role in the age-by-gender effect disclosed in the current study.
The estrogen hypothesis is further supported by data from animal models of cerebral ischemia. Estrogen has been shown to exert potent effects on the vascular wall; it induces vasodilation via nitric oxide dependent mechanisms or by directly acting on the vascular smooth muscle cells23. These effects lead to decreased vascular tone and increased cerebral blood flow under experimental conditions24. In addition, estrogen has anti-inflammatory, anti-oxidant, anti-apoptotic and neuroprotectant effects25,26. It has been shown that the effect of gender on infarct size disappears after ovariectomy10,11. It has also been reported that the size of infarction increases with aging in female mice after middle cerebral artery occlusion; infarct volume is smaller in younger and larger in older female animals as compared to their male counterparts12 – a pattern that completely overlaps with the findings of current study in humans.
This study is subject to certain limitations. The study population was selected based on the availability of baseline imaging within a relatively narrow time window (12 hours of stroke onset). This may have caused exclusion of patients with severe strokes who were not clinically stable enough for an early MRI, patients with mild stroke with delayed hospital admission, patients with grave short-term prognosis, and patients with contraindications for MRI. This might have led to a bias towards selection of a subset with a specific growth pattern. Nevertheless, the impact of this would not be clinically significant because this study represented a population in which infarct growth was the most clinically relevant. Second, follow-up images were obtained at different time points. This is inevitable in longitudinal research studies that are required to adjust their research priorities according to the medical condition and availability of patients. Infarct growth typically occurs within the first 48 hours of stroke onset but ischemic lesions continue to expand beyond this point because of vasogenic edema. It has been suggested that 30-day lesion volume is a reasonable approximation for final infarct volume27. The median time to follow-up was 10 days in the current study. This might have caused overestimation of infarct growth because of contribution of vasogenic edema. Nevertheless, this would not be expected to change our results significantly because “time from onset to follow-up imaging” was taken into account in multivariable models as a covariate.
The clinical outcome after stroke is less favorable in older women compared to younger women and men. It has been suggested that limited access to primary stroke prevention28 and high prevalence of risk factors such as atrial fibrillation and metabolic syndrome29 in older women could explain the gender effect. Others have highlighted the importance of more indirect post-stroke factors in older women such as social isolation, lower social status, issues with accessibility to healthcare, and frequent presence of co-morbidities such as depression, all of which can impede access and response to rehabilitation following stroke30. The current findings extend our knowledge on differential age-by-gender effect in clinical outcome to the tissue level and add to the accumulated body of evidence that intrinsic mechanisms that govern the brain’s susceptibility to ischemia may also differ by age and gender. Further studies are needed to elucidate the biological mechanisms that modulate the interaction between age, gender, and tissue outcome in response to ischemia.
Acknowledgments
Funding Information:
FG, EMA, TB, MV, PG, OW: None, ABS: NIH grants R01-NS051412, P50-NS051343, KLF: NIH grant P50-NS051343, AGS: NIH grant R01-NS038477, R01-NS063925, http://www.biomarkers.org/NewFiles/disclosures.html lists full disclosures, HA: NIH grant R01-NS059710
Footnotes
Author Contributions:
FG and EMA contributed equally to the manuscript; therefore they share the first authorship. FG, EMA and HA conceived and designed the study. FG, EMA, TB, PG and HA acquired and analyzed the data. FG, EMA, and MV performed statistical analysis. ABS, KLF and AGS contributed to the interpretation of results. FG, EMA and HA drafted the manuscript.
Contributor Information
Figen Gokcay, AA Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Ethem Murat Arsava, AA Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Tuna Baykaner, AA Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Mark Vangel, AA Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Priya Garg, AA Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Ona Wu, AA Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Aneesh B. Singhal, Stroke Service, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Karen L. Furie, Stroke Service, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
A. Gregory Sorensen, AA Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Hakan Ay, Stroke Service, Department of Neurology and AA Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
References
- 1.Sorensen AG, Buonanno FS, Gonzalez RG, Schwamm LH, Lev MH, Huang-Hellinger FR, Reese TG, Weisskoff RM, Davis TL, Suwanwela N, Can U, Moreira JA, Copen WA, Look RB, Finklestein SP, Rosen BR, Koroshetz WJ. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology. 1996;199:391–401. doi: 10.1148/radiology.199.2.8668784. [DOI] [PubMed] [Google Scholar]
- 2.Wu O, Christensen S, Hjort N, Dijkhuizen RM, Kucinski T, Fiehler J, Thomalla G, Röther J, Østergaard L. Characterizing physiological heterogeneity of infarction risk in acute human ischaemic stroke using MRI. Brain. 2006;129:2384–2393. doi: 10.1093/brain/awl183. [DOI] [PubMed] [Google Scholar]
- 3.Welch KM, Windham J, Knight RA, Nagesh V, Hugg JW, Jacobs M, Peck D, Booker P, Dereski MO, Levine SR. A model to predict the histopathology of human stroke using diffusion and T2-weighted magnetic resonance imaging. Stroke. 1995;26:1983–1989. doi: 10.1161/01.str.26.11.1983. [DOI] [PubMed] [Google Scholar]
- 4.Helpern JA, Dereski MO, Knight RA, Ordidge RJ, Chopp M, Qing ZX. Histopathological correlations of nuclear magnetic resonance imaging parameters in experimental cerebral ischemia. Magn Reson Imaging. 1993;11:241–246. doi: 10.1016/0730-725x(93)90028-c. [DOI] [PubMed] [Google Scholar]
- 5.Ay H, Koroshetz WJ, Vangel M, Benner T, Melinosky C, Zhu M, Menezes N, Lopez CJ, Sorensen AG. Conversion of ischemic brain tissue into infarction increases with age. Stroke. 2005;36:2632–2636. doi: 10.1161/01.STR.0000189991.23918.01. [DOI] [PubMed] [Google Scholar]
- 6.Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, Wu O, Gonzalez RG, Koroshetz WJ, Sorensen AG. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–1413. doi: 10.1161/STROKEAHA.107.501932. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Nuutinen J, Laakso MP, Karonen JO, Soimakallio S, Aronen HJ, Vanninen RL. ApoE polymorphism and acute stroke: a study with diffusion- and perfusion-weighted MRI and MR angiography. Acta Neurol Scand. 2006;114:323–328. doi: 10.1111/j.1600-0404.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 8.Darby DG, Barber PA, Gerraty RP, Desmond PM, Yang Q, Parsons M, Li T, Tress BM, Davis SM. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke. 1999;30:2043–2052. doi: 10.1161/01.str.30.10.2043. [DOI] [PubMed] [Google Scholar]
- 9.Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11:292–298. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- 10.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- 11.Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke. 2001;32:796–802. doi: 10.1161/01.str.32.3.796. [DOI] [PubMed] [Google Scholar]
- 14.Mohr JP, Biller J, Hilal SK, Yuh WT, Tatemichi TK, Hedges S, Tali E, Nguyen H, Mun I, Adams HP, Jr, Grimsman K, Marler JR. Magnetic resonance versus computed tomographic imaging in acute stroke. Stroke. 1995;26:807–812. doi: 10.1161/01.str.26.5.807. [DOI] [PubMed] [Google Scholar]
- 15.Ay H, Arsava EM, Vangel M, Oner B, Zhu M, Wu O, Singhal A, Koroshetz WJ, Sorensen AG. Interexaminer difference in infarct volume measurements on MRI: a source of variance in stroke research. Stroke. 2008;39:1171–1176. doi: 10.1161/STROKEAHA.107.502104. [DOI] [PubMed] [Google Scholar]
- 16.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G, Chalk JB, Fink JN, Kimber TE, Schultz D, Hand PJ, Frayne J, Hankey G, Muir K, Gerraty R, Tress BM, Desmond PM EPITHET investigators. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 17.Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, Jensen MB, Ayata C, Towfighi A, Smith EE, Chong JY, Koroshetz WJ, Sorensen AG. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke. 2007;38:2979–2984. doi: 10.1161/STROKEAHA.107.490896. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson KJ, Wardlaw JM, Edmond CL, Deary IJ, Maclullich AM. Intracranial area: a validated method for estimating intracranial volume. J Neuroimaging. 2005;15:76–78. doi: 10.1177/1051228404270243. [DOI] [PubMed] [Google Scholar]
- 19.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayala C, Croft JB, Greenlund KJ, Keenan NL, Donehoo RS, Malarcher AM, Mensah GA. Sex differences in US mortality rates for stroke and stroke subtypes by race/ethnicity and age, 1995–1998. Stroke. 2002;33:1197–1201. doi: 10.1161/01.str.0000015028.52771.d1. [DOI] [PubMed] [Google Scholar]
- 21.Sunday L, Osuna C, Krause DN, Duckles SP. Age alters cerebrovascular inflammation and effects of estrogen. Am J Physiol Heart Circ Physiol. 2007;292:H2333–H2340. doi: 10.1152/ajpheart.01057.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordell VL, Scarborough MM, Buchanan AK, Sohrabji F. Differential effects of estrogen in the injured forebrain of young adult and reproductive senescent animals. Neurobiol Aging. 2003;24:733–743. doi: 10.1016/s0197-4580(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 23.White RE. Estrogen and vascular function. Vascul Pharmacol. 2002;38:73–80. doi: 10.1016/s0306-3623(02)00129-5. [DOI] [PubMed] [Google Scholar]
- 24.Duckles SP, Krause DN. Cerebrovascular effects of oestrogen: multiplicity of action. Clin Exp Pharmacol Physiol. 2007;34:801–808. doi: 10.1111/j.1440-1681.2007.04683.x. [DOI] [PubMed] [Google Scholar]
- 25.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 26.Behl C. Oestrogen as a neuroprotective hormone. Nat Rev Neurosci. 2002;3:433–442. doi: 10.1038/nrn846. [DOI] [PubMed] [Google Scholar]
- 27.Gaudinski MR, Henning EC, Miracle A, Luby M, Warach S, Latour LL. Establishing final infarct volume: stroke lesion evolution past 30 days is insignificant. Stroke. 2008;39:2765–2768. doi: 10.1161/STROKEAHA.107.512269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dearborn JL, McCullough LD. Perception of risk and knowledge of risk factors in women at high risk for stroke. Stroke. 2009;40:1181–1186. doi: 10.1161/STROKEAHA.108.543272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holroyd-Leduc JM, Kapral MK, Austin PC, Tu JV. Sex differences and similarities in the management and outcome of stroke patients. Stroke. 2000;31:1833–1837. doi: 10.1161/01.str.31.8.1833. [DOI] [PubMed] [Google Scholar]
- 30.Kapral MK, Fang J, Hill MD, Silver F, Richards J, Jaigobin C, Cheung AM Investigators of the Registry of the Canadian Stroke Network. Sex differences in stroke care and outcomes: results from the Registry of the Canadian Stroke Network. Stroke. 2005;36:809–814. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]


