Abstract
Purpose
Localized prostate cancer (LPC) patients are faced with numerous treatment options, including observation or watchful waiting. The choice of treatment largely depends on their baseline health-adjusted life expectancy (HALE). By consensus, physicians recommend treatment if the patient’s HALE is ten or more years. However, the estimation of HALE is difficult. Although subjective by nature, self-rated health (SRH) is a robust predictor of mortality. We studied the usefulness of SRH in estimating HALE in patients who are considering treatment for LPC.
Methods
A total of 144 LPC patients from a large urology private practice in Norfolk, Virginia, were surveyed before they had chosen a treatment option.
Results
HALE determined by SRH correlated well with objective health measures, and was higher than age-based life expectancy by an average of 2 years. The observed difference in life expectancy due to SRH adjustment was higher among patients with a better socioeconomic and health profile.
Conclusions
SRH is an easy-to-use indicator of HALE in LPC patients. A table for HALE estimation by age and SRH is provided for men aged 70-80 years. Additional research with larger samples and prospective study designs are needed before the SRH method can be used in primary care and urology settings.
Keywords: Localized Prostate Cancer, Life Expectancy, Self-rated Health, Urology
Introduction
Prostate cancer is the most common cause of cancer death in non-smoking American men; however, whether patients should choose treatment for the cancer is controversial. In the United States, over 94% of patients choose treatment [1]. Due to widespread prostate-specific antigen (PSA) screening, over 90% of patients are diagnosed with localized prostate cancer (LPC). In about three-fourths of patients with screen-detected LPC, treatment may extend survival by only about six weeks [2]. The potentially deleterious effects of treatment on a patient’s health-related quality of life (HRQOL) are also well established. Choosing treatment or observation is difficult because no randomized trials have assessed the survival benefit of treatment in screen-detected patients. Central to decision-making is the patient’s estimated baseline health-adjusted life expectancy (HALE), because by consensus physicians recommend treatment if the patient’s HALE is ten or more years. Algorithms by organizations such as the National Comprehensive Cancer Network (NCCN) are also based on an estimated HALE. Yet, physician estimates of HALE are often imprecise [3] and an error of even a few years in the estimation of HALE could change a decision from treatment to observation or vice versa, because outcomes of treatment and observation are marginal. Several urologic nomograms have been published that simultaneously include co-morbidity scores with cancer and treatment characteristics to predict survival. However, patients and their physicians critically need an independent assessment of baseline HALE with which they can understand how the cancer or its treatment can affect their survival.
LPC is found at autopsy in 15-30% of American men 50 years and older and in 80% of men over 80 years of age [15], whereas one in six men will face a diagnosis of LPC in his lifetime [16]. By contrast, only 3% of men [16] and only one in seven diagnosed Americans will die due to untreated LPC [17]. The choice of treatment or observation depends on many factors including a patient’s baseline life expectancy according to age and co-morbidities [18]. With observation, prostate cancer-specific survival was 99.2% after 8 years of follow-up in 299 patients in a Canadian study [17] and 100% after 10 years of follow-up in 616 patients in a multi-center European study [19]. Nevertheless, over 90% of American patients choose treatments that can endanger their HRQOL, at a cost of about $10 000 to $25 000 (in 2000 dollars) for each potentially unnecessary prostatectomy or radiotherapy [20]. For 2000-2001, an over-treatment rate of 55% has been reported [21].
Patients diagnosed with LPC choose from a myriad of treatment techniques with marginally different HRQOL outcomes, and without clear numerical probabilities of the frequency, severity, and duration of side-effects. Finding the HRQOL outcome that can best match the patient’s preference can eclipse the bigger question of whether any treatment will enhance survival. Dahm et al. [22] have suggested that such complex decision-making should not be left to expert opinion; instead, national guidelines developed by panels of individuals who have devoted the necessary time to balance available evidence, can be used by physicians in order to prevent over- or under-treatment [22]. However, these national guidelines do not appear to be commonly used in selecting a treatment for LPC. Using combinations of search terms and medical subject headings, namely “prostate cancer”, “practice guidelines”, “NCCN”, “medical oncology/standards”, “evidence-based practice”, “urology/standards”, and “neoplasms/therapy”, we found several PUBMED articles, none of which implied the use of guidelines for LPC treatment selection. Guidelines typically require a patient’s baseline HALE, PSA level, cancer stage and grade, with HALE being the only undefined factor.
According to McCloskey and Kuttel, HALE cannot be predicted by currently available tools, and its uncertainty may make it difficult to use existing guidelines [23]. Estimates of HALE by urologists and radiation oncologists [3] as well as by primary care providers [24] also had a high margin of error. In one study, 64% of patients felt that their physicians were “unskilled, ill-equipped, and uncomfortable with the task” of estimating HALE [24]. Life expectancy according to age-based life tables cannot be used because co-morbidities are the strongest predictors of longevity in LPC patients [18] and because health is widely heterogeneous in older patients in general [25, 26]. In practice, even age-based life tables are not commonly used, and 70-90% of Americans select their treatment option in a single visit to the urologist after a positive biopsy [27].
For over two decades, it has been well known that Self-Rated Health (SRH) is a robust predictor of mortality risk. The correlation of SRH with longevity has been previously published [4]. By virtue of being a single question, SRH is easily self-administered and could serve as a valuable tool for busy physicians to quickly estimate a patient’s HALE, without having to rely on scales that measure co-morbidity or functional capacity. We hypothesized that newly diagnosed LPC patients will have SRH scores that will closely correlate to their co-morbidity and functional capacity scores, and that their estimated survival based on age alone will be significantly different from their estimated HALE based on SRH. If our hypothesis is correct, SRH may be useful for adjusting life expectancy based only on age. Longitudinal studies are needed to further address the predictive validity of SRH among LPC patients.
Methods
Study design and population
A detailed description of the study methodology has been provided elsewhere [5]. Briefly, a survey was conducted in patients who were newly diagnosed with LPC, after they had met with their urologists and before any treatment was performed. Biopsy-diagnosed LPC patients were recruited between March 2005 and October 2007 from a large urology private practice in Norfolk, Virginia. Patients were included if their prostate cancer was localized as implied by a cancer stage of T1a to T2c, irrespective of Gleason grade or severity of co-morbid conditions. They were excluded if they already had curative treatment with either surgery (including cryotherapy) or radiation (including brachytherapy). Patients who were unable to complete the survey because of neurological disorders such as dementia and those who could not read, write, or understand English were also excluded. Urology staff routinely contacted all patients regarding their interest to participate in a mailed survey. Patients who met all eligibility criteria and were willing to part-take in the survey were asked to provide their written informed consent. A $10 stipend was given to each survey participant. The self-administered questionnaire enquired about patient perceptions of their own health (SRH), co-morbidities, functional capacity, anxiety and depression, social support and other generic and symptom-specific measures of HRQOL. Demographic characteristics were obtained from patient records. The survey methods were approved by the institution’s review board. Surveys were mailed to 430 patients newly diagnosed with LPC, but in 69 the treatment had already been started by the time the patients received the surveys, three never received the surveys and two were found to be ineligible to participate as the cancer was not localized. Of the 356 remaining patients, 104 did not return the survey because they were ‘not interested’ in participating and 68 who did not return the surveys did not give a reason for not participating or could not be contacted. Furthermore, 184 of 356 patients (survey response rate of 52%) completed and returned the first pretreatment survey. 144 out of 184 patients who completed and returned the self-administered questionnaires also had valid SRH data. The mean (± standard deviation) age of these patients was 61.8 (± 7.1) years and 11.6% were African Americans. Whereas only 2.9% of these patients had not completed high school, 70% had a family income of more than $50 000.
Measures
Estimation of HALE by self-rated health (SRH)
SRH was evaluated by asking ‘In the last one month, how would you rate your health?’ Possible responses were ‘excellent’, ‘very good’, ‘good’, ‘fair’ or ‘poor’. HALE was calculated using these ratings to weight U.S. annual mortality rates (obtained from life tables for U.S. men [6]) by 0.52, 0.89, 1.0, 1.26, and 1.88 respectively. These weightings were obtained from a five-year follow-up study of 1 437 men older than 65 years who participated in the East Boston Senior Health Project [4] and were asked ‘Compared with others your age, would you rate your overall health as excellent, good, fair or poor?’ Men who indicated that their health was excellent had a lower mortality rate than ‘average’ (health status weight, 0.52), while those who indicated that their health was good, fair or poor had health status weights of 0.89, 1.26 and 1.88, respectively. In our study, LPC patients who indicated that their health was ‘good’ (1.0) were selected as the ‘average’ category, to which ‘excellent’ (0.52), ‘very good’ (0.89), ‘fair’ (1.26) and ‘poor’ (1.88) categories were compared. The main concern during LPC decision-making is whether or not the patient has a HALE of at least 10 years [7]. Applying the aforementioned weighting methodology, LPC patients older than 80 years would have a HALE of less than 10 years, irrespective of their SRH. Similarly, the HALE of LPC patients who are younger than 70 years would exceed 10 years even if they rated their health as “poor”. Thus, using SRH-based weightings [4] and age-based life expectancy in U.S. men [6], we have prepared a reference table of age-based life expectancy and HALE that can be applied in the context of LPC patients, 70-80 years of age (Table 1).
Table 1.
Estimation of life expectancy based on age and after adjustment for self-rated health
| Self-Related Health: “In the last one month, how would you rate your health?” |
||||||
|---|---|---|---|---|---|---|
| Age (Years) | Age-based life expectancy |
Excellent | Very Good | Good | Fair | Poor |
| 70 | 13.4 | 18.9 | 16.1 | 13.4 | 11.7 | 9.5 |
| 71 | 12.8 | 18.2 | 15.4 | 12.8 | 11.7 | 9 |
| 72 | 12.2 | 17.5 | 14.8 | 12.2 | 10.5 | 8.5 |
| 73 | 11.6 | 16.8 | 14.1 | 11.6 | 10.5 | 8 |
| 74 | 10.0 | 16.1 | 13.5 | 11 | 10 | 8 |
| 75 | 10.5 | 14.8 | 12.9 | 10.5 | 9 | 8 |
| 76 | 9.9 | 14.1 | 12.2 | 9.9 | 8.5 | 8 |
| 77 | 9.4 | 13.5 | 11.7 | 9.4 | 8 | 8 |
| 78 | 8.9 | 12.9 | 11.1 | 8.9 | 8 | 8 |
| 79 | 8.4 | 12.2 | 10.5 | 8.4 | 8 | 8 |
| 80 | 7.9 | 11.7 | 10 | 8 | 8 | 8 |
Note: SRH=self-rated health; Patients older than 80 years have a life expectancy of less than 10 years irrespective of SRH. Patients younger than age 70 have a life expectancy of at least 10 years irrespective of SRH. Cells with SRH-adjusted HALE of less than 10 years are shaded. Guidelines recommend watchful waiting for patients with less than a 10-year life expectancy.
Patient demographic characteristics
Age-based life expectancy and SRH-based HALE and the difference between the two measures were analyzed by the following demographic characteristics: 1) Age (< 60 years, 60-70 years, >70 years); 2) Race (Caucasian, African American); 3) Education (Less than high school, High school, College) and 4) Family income (< $50,000, ≥ $50,000).
Patient health characteristics
Age-based life expectancy and SRH-based HALE and the difference between the two measures were analyzed by the following health characteristics: Gleason grade, PSA level, Charlson comorbidity index (CCI), Duke Activity Status Index (DASI), the Hospital Anxiety and Depression (HAD) scale and the Medical Outcomes Study Social Support Scale (MOS-SSS). Gleason grade was defined as ‘6’, ‘7’ or ‘8+’, while PSA level was dichotomized as ‘≤10’ or ‘>10’. The CCI is widely used to measure the burden of co-morbid illnesses. We used a patient self-reported CCI which asks about the presence and severity of twelve chronic diseases; this version has been used by the multi-center Prostate Cancer Outcomes Study [8]. Of the twelve diseases, nine are counted only if they need a prescription or if they impact the patient’s life. Self-reported CCI had similar prediction of mortality to that predicted by co-morbidity assessed by a review of medical records of hospitalized patients [9]. The DASI scale is used to measure functional capacity and consists of binary questions that ask if a patient can perform twelve different activities with varying weights according to the level of exertion they require [10]. Summary scores obtained from the DASI have been previously correlated with treadmill and six-minute walk test results in cardiac rehabilitation patients [11] and with survival in a large younger-age population [12]. DASI scores can be expressed in units of oxygen consumption per minute or in Metabolic Equivalents (METS) [10]. We analyzed DASI score as a continuous variable and further categorized it as ‘Mild activities (< 3 METS)’, ‘Moderate activities (3-6 METS)’ and ‘Vigorous activities (6+ METS)’ [10]. The HAD scale is used to evaluate the presence and severity of anxiety and depression and has been extensively validated in geriatric and non-geriatric populations. This 14-item scale includes 7-item sub-scale for anxiety and a seven-item sub-scale for depression [13]. For each sub-scale, the total score can range between 0 and 21. Anxiety and depression sub-scales were analyzed as categorical variables [‘0-7 (None)’; ‘8-10 (Mild)’; ‘11-14 (Moderate)’; ‘15-21 (Clinical)’]. The MOS-SSS is a validated 19-item scale covering four domains, namely, ‘Emotional or Informational Support’ (8 items); ‘Tangible support’ (4 items); ‘Affectionate support’ (3 items); ‘Positive social interaction’ (3 items) and one additional item. MOS-SSS scores were transformed to a 0-100 scale and categorized as ‘<50’, ’50-<75’ and ’75-100’[14].
Statistical Analyses
All statistical analyses were performed using SAS software, version 9.1 (SAS Institute Inc., Cary, NC). Frequencies and percentages were used to describe categorical variables. Continuous variables were described using the means and standard deviations. Wilcoxon’s signed rank test was used to examine whether the difference between age-based life expectancy and SRH-based HALE was significantly different from zero, before and after stratifying by patient characteristics. The Wilcoxon signed-rank test is used to compare the distributions of two variables (considering both the sign and the magnitude of the differences between pairs) and find whether the difference scores are significantly different from zero (in the population). The Wilcoxon test makes less than maximum use of the data, in that it substitutes ranks for raw score differences. Finally, Spearman rank correlation was used to describe the relationship of SRH with other HRQOL measures. Two-sided statistical tests were conducted and statistical significance was declared at a conservative alpha level of 0.01, to adjust for multiple comparisons.
Results
As shown in Table 2, nearly 95% of our study sample rated their overall health as ‘good’, ‘very good’ or ‘excellent’. The mean (± standard deviation) life expectancy by age alone was 19.7±5.8 years and the mean (± standard deviation) HALE based on SRH was 21.6±6.6 years. SRH-based estimate of HALE was significantly higher than the age-based estimate of life expectancy, with a mean difference of 2 years (Wilcoxon signed rank test; P < 0.05). As shown in Tables 3 and 4, the mean difference between SRH- and age-based life expectancy varied according to patient demographic and health characteristics. SRH-based life expectancy was significantly higher than age-based life expectancy, irrespective of patient age, race, household income or PSA level. By contrast, patient education, Gleason grade, functional capacity, anxiety, depression and social support may be factors that need to be taken into consideration, as they can influence the ‘gap’ between SRH-based and age-based life expectancy. However, it is important to recognize the limited sample size which often resulted in a standard deviation that was higher than the mean for (SRH-LE – AGE-LE). A close examination suggests that the ‘gap’ in life expectancy was higher among patients with a better socioeconomic and health profile. The latter finding is supported by the weak association between age and SRH and the stronger correlation of SRH with various health indicators such as PSA level, CCI, DASI and depression (Table 5).
Table 2.
Patient distribution by self-rated health status in the study sample (n=144)
| Self-Rated Health | Categories | N (%) |
|---|---|---|
| N= 144 | Excellent | 39 (27.1) |
| Very Good | 68 (47.2) | |
| Good | 30 (20.8) | |
| Fair | 6 (4.2) | |
| Poor | 1 (0.7) |
Table 3.
Differences between age-based life expectancy and health-adjusted life expectancy according to self-rated health by demographic characteristics of study sample (n=144)
| Age (AGE-LE) |
Self-rated health(SRH-LE) | SRH-LE – AGE-LE | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| All, n=144 | 19.7 ± 5.8 | 21.7 ± 6.6 | 1.9 ± 3.0* |
|
| |||
| Age, n=143 | |||
| <60 yrs (n=57) | 25.6 ± 3.8 | 28.0 ± 4.5 | 2.3 ± 3.5* |
| 60-70 yrs (n=71) | 16.8 ± 1.9 | 18.5 ± 3.4 | 1.7 ± 2.8* |
| >70 yrs (n=15) | 11.2 ± 1.6 | 12.7 ± 2.2 | 1.5 ± 1.7* |
| Race, n=143 | |||
| AA (n=18) | 22.5 ± 6.8 | 23.6 ± 7.9 | 1.1 ± 2.9NS |
| CA (n=125) | 19.3 ± 5.6 | 21.4 ± 6.4 | 2.0 ± 3.0* |
| Education, n=140 | |||
| < H.S. (n=4) | 19.2 ± 4.4 | 19.9 ± 4.9 | 0.8 ± 0.7NS |
| H.S. (n=45) | 20.8 ± 5.9 | 22.0 ± 6.9 | 1.1 ± 2.7* |
| >H.S. (n=91) | 19.3 ± 5.7 | 21.9 ± 6.5 | 2.4 ± 3.2* |
| Income, n=141 | |||
| < $50 000 (n=41) | 18.7 ± 4.3 | 20.1 ± 5.9 | 1.3 ± 3.3* |
| > $50 000 (n=100) | 20.3 ± 6.3 | 22.5 ± 6.7 | 2.3 ± 2.9* |
Wilcoxon’s signed rank test, P < 0.01; AA=African American, CA=Caucasian, H.S.=High school; LE = Life expectancy, NS=Not significant, SRH=Self-rated health.
Table 4.
Differences between age-based life expectancy and health-adjusted life expectancy according to self-rated health by health characteristics of study sample (n=144)
| Age (AGE-LE) |
Self-rated health (SRH-LE) |
SRH-LE – AGE-LE |
|
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| All, n=144 | 19.7 ± 5.8 | 21.7 ± 6.6 | 1.9 ± 3.0 * |
| Gleason grade, n=143 | |||
| 6 (n=78) | 20.2 ± 5.7 | 22.4 ± 6.2 | 2.0 ± 3.2* |
| 7 (n=49) | 19.9 ± 5.6 | 22.3 ± 6.9 | 2.3 ± 2.9* |
| 8+ (n=16) | 16.7 ± 6.2 | 16.8 ± 5.9 | 0.1 ± 1.6NS |
| PSA, n=144 | |||
| ≤ 10 (n=127) | 19.8 ± 5.7 | 21.9 ± 6.5 | 2.1 ± 3.1* |
| > 10 (n=17) | 18.8 ± 6.8 | 19.7 ± 7.2 | 0.8 ± 1.9NS |
| CCI, n=143 | |||
| 0 (n=61) | 20.1 ± 6.7 | 23.0 ± 7.4 | 2.9 ± 3.2* |
| 1-2 (n=76) | 19.6 ± 5.2 | 21.0 ± 5.9 | 1.4 ± 2.6* |
| 3+ (n=6) | 17.7 ± 2.9 | 16.4 ± 2.4 | −1.9 ± 2.8NS |
| DASI, n=143 | |||
| 3-6 METS (n=11) | 17.4 ± 5.7 | 17.6 ± 6.0 | −0.03 ± 2.8NS |
| 6+ METS (n=132) | 19.9 ± 5.8 | 22.1 ± 6.6 | 2.1 ± 2.9* |
| HAD–Anxiety score, n=143 | |||
| None (0-7)(n=111) | 19.3 ± 5.9 | 21.2 ± 6.4 | 1.8 ± 2.8* |
| Mild (8-10)(n=21) | 20.0 ± 4.8 | 23.0 ± 6.7 | 2.9 ± 3.7* |
| Moderate (11-14) (n=9) | 24.7 ± 5.5 | 26.3 ± 7.0 | 1.3 ± 3.5NS |
| Clinical 15-21 (n=2) | 15.6 ± 2.4 | 15.9 ± 1.5 | 0.3 ± 0.9NS |
| HAD–Depression score, n=140 | |||
| None (0-7) (n=137) | 19.8 ± 5.8 | 21.8 ± 6.6 | 1.9 ± 3.0* |
| Mild (8-10) (n=3) | 21.4 ± 10.0 | 22.0 ± 10.5 | 0.7 ± 0.9NS |
| Moderate/Clinical (11-21) (n=0) | -- | -- | -- |
| MOS-SSS, n=143 | |||
| < 50th (n=6) | 18.0 ± 5.8 | 20.4 ± 7.6 | 2.4 ± 2.8NS |
| 50th -75th (n=23) | 21.6 ± 5.8 | 22.8 ± 6.7 | 1.0 ± 3.4NS |
| 75th -100th (n=114) | 19.4 ± 5.8 | 21.6 ± 6.6 | 2.0 ± 2.9* |
Wilcoxon’s signed rank test, P < 0.01; CCI=Charlson comorbidity index, DASI = Duke Activity Status Index, HAD = Hospital Anxiety and Depression, PSA = Prostate-specific antigen; LE=Life expectancy, MOS-SSS = Medical Outcomes Study – Social Support Scale, NS=Not significant, SRH=Self-rated health.
Table 5.
Correlation matrix for age, self-rated health and health characteristics of the study sample (n=140)
| Age | SRH | Gleason | PSA | CCI | DASI | Anxiety | Depression | MOS-SSS | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 0.01 | 0.14 | 0.09 | 0.05 | −0.42* | −0.33* | −0.08 | 0.09 | |
| SRH | 0.07 | 0.26* | 0.28* | −0.21* | 0.14 | 0.28* | −0.07 | ||
| Gleason | 0.23* | −0.03 | −0.06 | 0.06 | 0.14 | 0.12 | |||
| PSA | −0.08 | −0.23* | −0.09 | 0.03 | −0.04 | ||||
| CCI | −0.08 | 0.06 | −0.04 | −0.003 | |||||
| DASI | 0.22* | −0.05 | −0.07 | ||||||
| Anxiety | 0.48* | −0.14 | |||||||
| Depression | −0.09 | ||||||||
| MOS-SSS |
Spearman rank correlation, P < 0.01; CCI=Charlson comorbidity index, DASI = Duke Activity Status Index, PSA = Prostate-specific antigen, MOS-SSS = Medical Outcomes Study – Social Support Scale, SRH=Self-rated health.
Discussion
The SRH is one of several components of the Centers for Disease Control (CDC) HRQOL-4 definition adopted by the National Health and Nutrition Examination Surveys (NHANES) and the Behavioral Risk Factor Surveillance Systems (BRFSS). Whereas the NHANES is an ongoing series of cross-sectional surveys designed to be representative of the non-institutionalized, U.S. civilian population by using a complex, multistage probability design, the BRFSS is an ongoing, random-digit-dialed telephone survey of the non-institutionalized, U.S. civilian population aged ≥18 years conducted in all 50 states and the District of Columbia. In both surveys, the following HRQOL-4 measures were included: 1) SRH: ‘Would you say that in general your health is excellent, very good, good, fair, or poor?’; 2) Physically unhealthy days: ‘Now thinking about your physical health, which includes physical illness and injury, for how many days during the past 30 days was your physical health not good?; 3) Mentally unhealthy days: ’Now thinking about your mental health, which includes stress, depression, and problems with emotions, for how many days during the past 30 days was your mental health not good?’; 4) Activity limitation days: ‘During the past 30 days, for about how many days did poor physical or mental health keep you from doing your usual activities, such as self-care, work, school, or recreation?’ These HRQOL-4 measures, including SRH, were shown to predict morbidity, healthcare utilization and mortality, and were associated with chronic diseases, disability, risky health behaviors, and socio-demographic factors [28].
In this survey of newly diagnosed LPC patients, nearly 5% indicated that their SRH was fair or poor. Furthermore, SRH influenced HALE differentially across groups defined by their demographic, socioeconomic and health characteristics. We analyzed SRH data for older adults (≥ 50 years) from the 2005-2008 NHANES, which corresponds to the time period during which our survey was conducted. In general, over 15% of NHANES participants indicated that their SRH was fair or poor. As shown in Figure 1, non-Hispanic White men representing the vast majority of our study population were least likely to report a fair or poor SRH, compared to other racial and ethnic groups. It is important to note that while the 2005-2008 NHANES enquires about health ‘in general’, our survey enquires about health in the ‘last one month’. Yet, the staggering difference in the SRH distribution among these survey samples cannot be entirely attributed to distinct ways of phrasing the SRH question. It is plausible that our patient population was indeed healthier than the general population of the United States, given that the majority of recently diagnosed LPC patients were non-Hispanic White. A cautionary note is the issue of ‘response shift’ whereby patients may recalibrate or adjust the internal scale on which they are estimating their health. This has been hypothesized as a possible explanation for the observation that very ill patients often rate their health and/or their HRQOL as good, very good or excellent.
Figure 1.
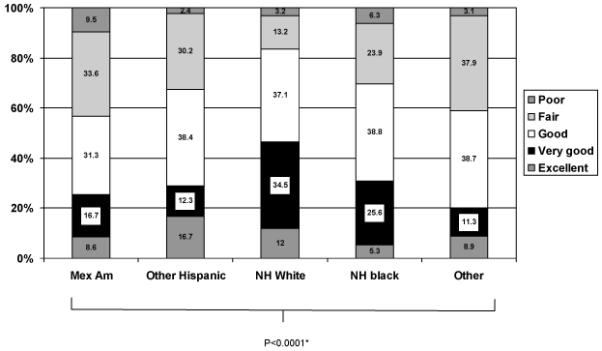
Distribution of self-rated health among men aged 50 years or more by race/ethnicity, NHANES 2005-08
*P-value derived from design-based F-value.
Although subjective in nature, SRH is known to predict mortality and thereby life expectancy as well as and beyond what is predicted by a patient’s health record [29]. Our results show that among LPC patients, SRH is independent of age and is a useful tool that can be used to compute HALE. Our results also suggest a significant and correlation with modest shared variance between SRH and two objective measures of health, namely CCI and DASI. Therefore, SRH may be as useful as and less cumbersome than measures of co-morbidity or physical functioning for estimating HALE in the context of LPC treatment selection. In our cross-sectional study, we did not evaluate whether SRH predicts long-term survival. However, by showing that SRH and CCI/DASI are correlated in LPC patients, our study implies that SRH may be useful for performing health adjustment of age-based life expectancy prior to LPC treatment selection, especially in settings where HALE is not routinely calculated.
Our survey was administered at a critical time, when the patients had just been diagnosed with LPC and had not yet been treated for the cancer. They had met with their urologist, and were actively considering treatment options in this narrow window of time. They would be expected to have done a careful evaluation of their health, and may have made an empiric calculation of their life expectancy with and without treatment. At such a time, when patients are acutely concerned about their health, SRH appears to be an excellent indicator of health status. SRH-based HALE and age-based life expectancy were found to differ significantly in LPC patients. Thus, weighting by SRH could alter age-based life expectancy by several years. As indicated in the reference table, at age 70, the life expectancy of patients who rate their SRH as “excellent” is 18.9 years as compared to only 9.5 years for patients who those rate their SRH as “poor”. Most of our patients had a high socioeconomic standing and adequate health profile as exemplified by having more than a high school level education, a family income of at least $50 000, a PSA ≤ 10 and a DASI ≥ 6 METS [5]. On the other hand, the presence of anxiety or depression could limit the validity of using SRH as an indicator of health status. Just after being diagnosed with cancer, patients are at risk of developing symptoms of anxiety and depression. Mood could potentially reduce the accuracy of SRH in predicting HALE, but SRH has been previously found to be a powerful predictor of life expectancy despite covariates such as depression [30].
Our study had several limitations. First, although the close relationship between SRH and other health indicators suggests that SRH can be used to weight age-based life expectancy, no long-term follow-up has been done in LPC patients to study if SRH does correlate with survival. Second, the survey response rate was 52%. We studied only those patients who had agreed to participate, and they were mostly Caucasian White, below 70 years of age, highly educated with a high family income. Self-selection bias is a plausible explanation for our study findings since these patients could have been healthier, more confident in filling out health surveys, and better able to rate their health. Nevertheless, the profile of our patients may also be typical of those who visit urologists in private practice, and about 80% of urologists in the United States are in private practice [31]. Third, this study is limited in terms of sample size and SRH profile. Out of 144 LPC patients, only 7 (4.9%) reported fair or poor SRH, and the use of SRH HALE generated an increased life expectancy which would increase the number of individuals who receive treatment if this approach was implemented. On the other hand, more favorable SRH scores for the men in this study translated into substantial increases in SRH-adjusted life expectancy, particularly among those in the better functioning clinical subcategories. Given the small samples of men who were categorized into the lowest functioning subcategories, the corresponding SRH-adjusted life expectancy estimates may be questionable. Finally, data used to represent age-adjusted life expectancy were obtained from 2003 vital statistics, weightings used to calculate SRH-adjusted HALE were obtained from a study conducted in 1996 (using vital statistics for the year 1991), while our own study was conducted several years later (2005-2007). Clearly, a larger study with a more advanced design is needed to confirm these preliminary findings.
In this study, SRH-adjusted life expectancy was generally higher than age-based life expectancy by around 2 years, among patients newly diagnosed with LPC. Yet, the ‘gap’ between these two measures of life expectancy was wider in LPC patients who reported better socioeconomic and health characteristics. Although SRH consists of only one item and is easy and quick to self-administer it should not be used as the only basis for clinical decision-making. Table 1 estimates life expectancy based on age alone and after SRH-based adjustment for individuals between 70 and 80 years of age. This table could be used by newly diagnosed LPC patients in this age group and their physicians to obtain an estimate of baseline HALE.
Acknowledgments
Support for this project was provided in part through grants from the Norfolk Foundation, and the US Health Resources Service Administration Department of Health and Human Services. In addition, the project was supported in part by the intramural research program of the NIH, National Institute on Aging. We would like to thank Mr. Brian Main for his help with the patient database.
Abbreviations
- BRFSS
Behavioral Risk Factor Surveillance Systems
- CCI
Charlson comorbidity index
- CDC
Centers for Disease Control and Prevention
- DASI
Duke Activity Status Index
- HAD
Hospital Anxiety and Depression
- HALE
health-adjusted life expectancy
- HRQOL
health-related quality of life
- LPC
localized prostate cancer
- METS
Metabolic Equivalents
- MOS-SSS
Medical Outcomes Study Social Support Scale
- NCCN
National Comprehensive Cancer Network
- PSA
prostate-specific antigen
- SRH
self-rated health
- NHANES
National Health and Nutrition Examination Surveys
Contributor Information
Ravinder Mohan, Department of Family and Community Medicine, Eastern Virginia Medical School, Norfolk, VA, USA, MOHANR@evms.edu.
Hind Beydoun, Graduate Program in Public Health, Eastern Virginia Medical School, Norfolk, VA, USA, BAYDOUHA@evms.edu.
May Beydoun, National Institute on Aging, Baltimore, MD, USA, BAYDOUNM@mail.nih.gov.
Myra Barnes-Eley, Center for Pediatric Research, Children’s Hospital of King’s Daughters, Norfolk, VA, USA, PEDIATRICS@chkd.org.
John Davis, Department of Urologic Oncology, M.D. Anderson Cancer Center, Houston, TX, USA, JOHNDAVIS@mdanderson.org.
Raymond Lance, Department of Urology, Eastern Virginia Medical School, Norfolk, VA, USA, LANCRS@evms.edu.
Paul Schellhammer, Department of Urology, Eastern Virginia Medical School, Norfolk, VA, USA, SCHELLPF@evms.edu.
References
- 1.Harlan SR, Cooperberg MR, Elkin EP, Lubeck DP, Meng MV, Mehta SS, Carroll PR. Time trends and characteristics of men choosing watchful waiting for initial treatment of localized prostate cancer: results from CaPSURE. The Journal of urology. 2003;170(5):1804–1807. doi: 10.1097/01.ju.0000091641.34674.11. [DOI] [PubMed] [Google Scholar]
- 2.Parker C, Muston D, Melia J, Moss S, Dearnaley D. A model of the natural history of screen-detected prostate cancer, and the effect of radical treatment on overall survival. British journal of cancer. 2006;94(10):1361–1368. doi: 10.1038/sj.bjc.6603105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson JR, Clarke MG, Ewings P, Graham JD, MacDonagh R. The assessment of patient life-expectancy: how accurate are urologists and oncologists? BJU international. 2005;95(6):794–798. doi: 10.1111/j.1464-410X.2005.05403.x. [DOI] [PubMed] [Google Scholar]
- 4.Welch HG, Albertsen PC, Nease RF, Bubolz TA, Wasson JH. Estimating treatment benefits for the elderly: the effect of competing risks. Annals of internal medicine. 1996;124(6):577–584. doi: 10.7326/0003-4819-124-6-199603150-00007. [DOI] [PubMed] [Google Scholar]
- 5.Mohan R, Beydoun H, Barnes-Ely ML, Lee L, Davis JW, Lance R, Schellhammer P. Patients’ survival expectations before localized prostate cancer treatment by treatment status. J Am Board Fam Med. 2009;22(3):247–256. doi: 10.3122/jabfm.2009.03.080200. [DOI] [PubMed] [Google Scholar]
- 6.NCHS . National Vital Statistics Report 54 (14) -- March 28, 2007. 2007. [Google Scholar]
- 7.Fowler FJ, Jr., McNaughton Collins M, Albertsen PC, Zietman A, Elliott DB, Barry MJ. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. Jama. 2000;283(24):3217–3222. doi: 10.1001/jama.283.24.3217. [DOI] [PubMed] [Google Scholar]
- 8.Potosky AL, Harlan LC, Stanford JL, Gilliland FD, Hamilton AS, Albertsen PC, Eley JW, Liff JM, Deapen D, Stephenson RA, et al. Prostate cancer practice patterns and quality of life: the Prostate Cancer Outcomes Study. Journal of the National Cancer Institute. 1999;91(20):1719–1724. doi: 10.1093/jnci/91.20.1719. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhry S, Jin L, Meltzer D. Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Medical care. 2005;43(6):607–615. doi: 10.1097/01.mlr.0000163658.65008.ec. [DOI] [PubMed] [Google Scholar]
- 10.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) The American journal of cardiology. 1989;64(10):651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton DM, Haennel RG. Validity and reliability of the 6-minute walk test in a cardiac rehabilitation population. Journal of cardiopulmonary rehabilitation. 2000;20(3):156–164. doi: 10.1097/00008483-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Lee IM, Hsieh CC, Paffenbarger RS., Jr. Exercise intensity and longevity in men. The Harvard Alumni Health Study. Jama. 1995;273(15):1179–1184. [PubMed] [Google Scholar]
- 13.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 14.Baigi A, Hildingh C, Virdhall H, Fridlund B. Sense of coherence as well as social support and network as perceived by patients with a suspected or manifest myocardial infarction: a short-term follow-up study. Clin Rehabil. 2008;22(7):646–652. doi: 10.1177/0269215507086237. [DOI] [PubMed] [Google Scholar]
- 15.Taichman RS, Loberg RD, Mehra R, Pienta KJ. The evolving biology and treatment of prostate cancer. J Clin Invest. 2007;117(9):2351–2361. doi: 10.1172/JCI31791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 17.Klotz L. Active surveillance for prostate cancer: for whom? J Clin Oncol. 2005;23(32):8165–8169. doi: 10.1200/JCO.2005.03.3134. [DOI] [PubMed] [Google Scholar]
- 18.Post PN, Hansen BE, Kil PJ, Janssen-Heijnen ML, Coebergh JW. The independent prognostic value of comorbidity among men aged < 75 years with localized prostate cancer: a population-based study. BJU Int. 2001;87(9):821–826. doi: 10.1046/j.1464-410x.2001.02189.x. [DOI] [PubMed] [Google Scholar]
- 19.van den Bergh RC, Roemeling S, Roobol MJ, Aus G, Hugosson J, Rannikko AS, Tammela TL, Bangma CH, Schroder FH. Outcomes of Men with Screen-Detected Prostate Cancer Eligible for Active Surveillance Who Were Managed Expectantly. Eur Urol. 2008 doi: 10.1016/j.eururo.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Ruchlin HS, Pellissier JM. An economic overview of prostate carcinoma. Cancer. 2001;92(11):2796–2810. doi: 10.1002/1097-0142(20011201)92:11<2796::aid-cncr10124>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 21.Miller DC, Gruber SB, Hollenbeck BK, Montie JE, Wei JT. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. Journal of the National Cancer Institute. 2006;98(16):1134–1141. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 22.Dahm P, Kunz R, Schunemann H. Evidence-based clinical practice guidelines for prostate cancer: the need for a unified approach. Curr Opin Urol. 2007;17(3):200–207. doi: 10.1097/MOU.0b013e3280eb1121. [DOI] [PubMed] [Google Scholar]
- 23.McCloskey SA, Kuettel MR. Counterpoint: prostate cancer life expectancy can not be accurately predicted from currently available tools. J Natl Compr Canc Netw. 2007;5(7):709–713. doi: 10.6004/jnccn.2007.0063. [DOI] [PubMed] [Google Scholar]
- 24.Kistler CE, Lewis CL, Amick HR, Bynum DL, Walter LC, Watson LC. Older adults’ beliefs about physician-estimated life expectancy: a cross-sectional survey. BMC family practice. 2006;7:9. doi: 10.1186/1471-2296-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walz J, Gallina A, Hutterer G, Perrotte P, Shariat SF, Graefen M, McCormack M, Benard F, Valiquette L, Saad F, et al. Accuracy of life tables in predicting overall survival in candidates for radiotherapy for prostate cancer. International journal of radiation oncology, biology, physics. 2007;69(1):88–94. doi: 10.1016/j.ijrobp.2007.02.022. 2007. [DOI] [PubMed] [Google Scholar]
- 26.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. Jama. 2001;285(21):2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 27.Cohen H, Britten N. Who decides about prostate cancer treatment? A qualitative study. Family practice. 2003;20(6):724–729. doi: 10.1093/fampra/cmg617. [DOI] [PubMed] [Google Scholar]
- 28.Zahran HS, Kobau R, Moriarty DG, Zack MM, Holt J, Donehoo R. Health-related quality of life surveillance--United States, 1993-2002. MMWR Surveill Summ. 2005;54(4):1–35. [PubMed] [Google Scholar]
- 29.Mossey JM, Shapiro E. Self-rated health: a predictor of mortality among the elderly. American journal of public health. 1982;72(8):800–808. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P. Mortality prediction with a single general self-rated health question. A meta-analysis. J Gen Intern Med. 2006;21(3):267–275. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carson C. Private practice or Academia-making the choice. Urology Times. 2007 Feb; 2007. [Google Scholar]


