Abstract
Introduction
Several studies have confirmed that gastroesophageal reflux disease (GERD) in lung transplant patients is a risk factor for the development and progression of bronchiolitis obliterans syndrome (BOS), a form of rejection after lung transplantation. Moreover, numerous reports indicate that surgical correction of GERD may control the decline in lung function characteristic of BOS. Although laparoscopic fundoplication is an accepted treatment option for these patients with GERD, the surgical technique, which often includes a laparoscopic pyloroplasty, has not been standardized.
Methods
The purpose of this article is to describe a step-by-step approach to the laparoscopic treatment of GERD in lung transplant patients. We also address specific technical concerns encountered in the surgical management of this high-risk patient population; we provide data on the safety of this operation; and we illustrate the evidence-based rationale for each technical step of the procedure.
Keywords: Gastroesophageal reflux disease (GERD), Laparoscopic antireflux surgery (LARS), Gastroparesis, Pyloroplasty, Lung transplantation
Introduction
Despite improvements in immunosuppressive strategies, the median survival of patients after lung transplantation is only 5 years, still inferior to the transplantation of any other solid organ.1–4 This low survival rate is largely due to the development of bronchiolitis obliterans syndrome (BOS).5–7 Unfortunately, the pathophysiology of BOS is poorly understood, though evidence suggests that BOS might represent a non-immunologic aberrant response to a chronic stimulus injury.7–9 Several reports have shown that GERD might be responsible for this chronic injury.10 Indeed, some studies have confirmed that GERD in lung transplant recipients is a risk factor for the development or progression of BOS and that surgical correction of GERD may control the decline in lung function.11–14 The purpose of this article is threefold: (1) to describe a step-by-step approach to the laparoscopic treatment of GERD in lung transplantation, illustrating the evidence-based rationale for each technical step; (2) to address specific technical concerns we have encountered in the management of this high-risk patient population; and (3) to provide the results of our approach in terms of perioperative safety and outcomes.
Preoperative Evaluation
All lung transplant patients who are potential candidates for laparoscopic surgical correction of GERD undergo a preoperative assessment that includes a symptomatic evaluation, a barium swallow, an upper endoscopy, a gastric emptying nuclear scan, and esophageal manometry with ambulatory pH monitoring. Each patient also undergoes rigorous pre-anesthesia testing, where critical anesthetic concerns are addressed (e.g., prevention of infection, airway trauma, fluid overload, respiratory depression, and pharmacological interaction between antirejection medications and anesthetic agents). Most important is the assessment of post-transplant pulmonary function. This predicts both the patient’s ability to tolerate general anesthesia and the success of ventilator weaning and extubation after the procedure. If the patient has developed BOS since the lung transplant, fibrosis and obliteration of the small airways may produce severe airway obstruction and the inability to have sufficient pulmonary function to allow extubation after the anesthetic.
Operative Planning and Anesthesiologic Considerations
Before induction, the patient is positioned with a beanbag on the operative table. Pneumatic compression stockings are always used as prophylaxis against deep vein thrombosis. However, subcutaneous heparin is usually not administered preoperatively. Preoperative antibiotics are administered prior to skin incisions. No stress dose of hydrocortisone is routinely administered. In most patients, invasive monitoring with a central line or an arterial line is not employed to minimize the risk of infectious complications. A Foley catheter is always inserted to monitor the fluid status, as crystalloid infusions are minimized to prevent fluid overload. Then, the patient is intubated carefully to avoid trauma to the site of the tracheal anastomosis. A rapid and careful intubation also protects against regurgitation and aspiration of gastric contents. During transplantation, resection of one or both lungs results in disruption of tracheal innervation, and stimulation of the bronchial mucosa during intubation may not elicit a cough reflex. This places the patient at increased risk of aspiration. Moreover, the prevalence of gastroparesis in these patients is high (about one third in our series) and further poses them at risk for an aspiration event. Even if the patient has electively fasted for greater than 8 h prior to the procedure, a totally empty state can never be guaranteed, especially in those with gastric atony. Therefore, the anesthesiologist performs a rapid sequence intubation technique to rapidly secure the airway. Further measures are also employed to diminish gastric volume and increase the pH of gastric fluid. If a laparoscopic pyloroplasty is planned, the patient is asked to maintain a liquid diet for the preceding 2 to 3 days. It is also our practice to administer H2 receptor blockers prior to surgery with 15–30 mL of a 0.3 M solution of sodium citrate 15–30 min before induction of anesthesia. After intubation, the beanbag is inflated and the lower extremities are placed in stirrups such that the surgeon stands between them. The abdomen is then prepped and draped and the patient is positioned in steep reverse Trendelenburg.
Operative Technique
Initial Access and Placement of Trocars
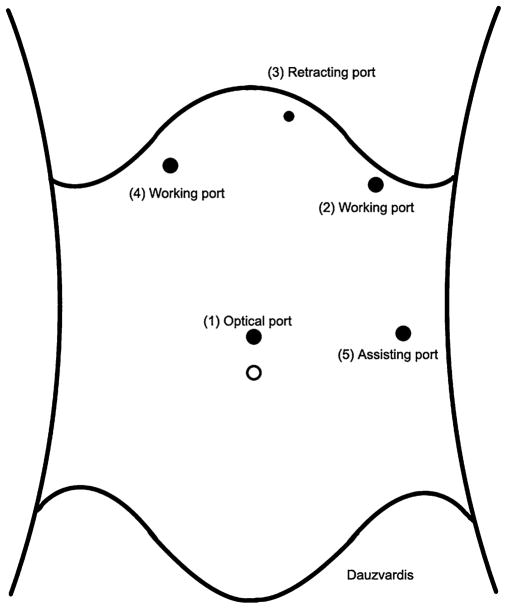
After complete neuromuscular paralysis is achieved, a 1-cm transverse midline incision is made in the skin 14 cm below the xiphoid process; the fascia is grasped with a Kocher clamp, pulled, and nicked with a #15 scalpel blade; the Veress needle is inserted, a water drop test is performed, and the abdomen is insufflated to 14 mmHg; then the Veress needle is removed and an 11-mm Kii Optical Fixation Trocars ™ (Applied Medical, Rancho Santa Margarita, CA) is inserted into the abdominal cavity under direct visualization with a 0° laparoscope. Maintaining the same alignment of the entry path, the introducer of the trocar is removed and the laparoscope is reinserted into the abdominal cavity to inspect the entry area, ensuring that no intrabdominal injuries were made upon entering the abdominal cavity. The 0° laparoscope is then exchanged for a 30° laparoscope, and the other trocars are placed under direct visualization in the order illustrated by Fig. 1. Port 2 is placed below the left costal margin in the mid-clavicular line and accommodates an 11-mm trocar. This is a working port through which the graspers, the laparoscopic Ligasure™ Vessel Sealing System (Valleylab, Boulder, CO), and the suturing instruments are introduced. This port is placed second in order, as it allows the introduction of an atraumatic grasper that facilitates the placement of the Nathanson retractor below the left lobe of the liver. Port 3 is inserted next and is placed in the epigastrium just to the left of the xiphoid process. A 5-mm incision is used to insert bluntly through the abdominal wall the tip of a Nathanson retractor. This retractor is placed to retract the left lobe of the liver away from the diaphragmatic hiatus and expose the gastroesophageal junction. The Nathanson retractor is then held in place by a self-retaining system attached to the operating table. Port 4 is placed below the right costal margin in the mid-clavicular line and holds an 11-mm trocar. This is a working port and is placed after retraction of the left lobe of the liver through the falciform ligament to achieve optimal exposure of the gastroesophageal junction. Port 5 is placed last, accommodates an 11-mm trocar, and is situated on the left anterior axillary line at the level of the optical port. It is used for (a) manipulation of a laparoscopic atraumatic Allis clamp; (b) a grasper, which will hold the Penrose drain once it has been placed around the esophagus; (c) the Ligasure™ to take down the short gastric vessels; and (d) to introduce a clip applier.
Fig. 1.
Position of operative ports in order of placement: (1) optical port, 14 cm below the xiphoid process; (2) left working port, below the left costal margin in the mid-clavicular line; (3) epigastric port for the Nathanson retractor; (4) right working port, below the right costal margin in the mid-clavicular line; (5) assisting port, on the left anterior axillary line at the level of the optical port.
Identification and Dissection of the Esophagus
Once all ports are placed, the assistant inserts the laparoscopic atraumatic Allis clamp through port 5, places it onto the stomach just distal to the gastroesophageal junction, and applies gentle lateral traction to facilitate the surgeon’s dissection. The surgeon uses ports 2 and 4 to start the dissection by dividing the gastrohepatic ligament with the Ligasure™ until the apex of the right diaphragmatic crus is identified. Subsequently, the phrenoesophageal ligament is divided anteriorly from the apex of the right crus to the apex of the left crus, and the anterior vagus nerve is identified. The esophagus is then bluntly dissected away from the right crus, and the posterior vagus nerve is identified. Finally, the right crus is dissected inferiorly toward the junction with the left crus.
Creation of the Retroesophageal Window
While the assistant maintains lateral and cephalad traction of the stomach just distal to the gastroesophageal junction with the laparoscopic Allis clamp, the surgeon creates a retroesophageal window by blunt dissection lateral to the left pillar of the crus, staying in the abdominal cavity, and not in the posterior mediastinum, and away from the mediastinal pleura. Through this window, a 1/4-in. Penrose drain, 6 in. long, is passed around the esophagus and the posterior vagus, and its tails are anchored with metal clips applied by a clip applier introduced through port 5. This drain is then used for the atraumatic traction of the gastroesophageal junction instead of the Allis clamp. This atraumatic traction onto the gastroesophageal junction helps in completing the dissection of the retroesophageal window which will later accommodate the fundoplication. This part of the dissection is completed only when the gastroesophageal junction is completely mobilized and freed from the attachments of the esophagus to the posterior mediastinum, and both borders of the diaphragmatic crura are cleared (when this step is completed, a classic “V”, which is represented by the two diaphragmatic pillars, is always demonstrated). The goal is to obtain at least 1 in. of intra-abdominal esophagus around which the wrap is fashioned.
Division of Short Gastric Vessels
While the assistant applies medial traction on the greater curvature of the stomach with the Allis clamp through port 4, the surgeon applies countertraction with a grasper introduced through port 2 and divides the short gastric vessels with the Ligasure™ starting 10–15 cm distally to the angle of His. Then, the dissection continues upward until all short gastric vessels and the posterior gastric artery, which originates from the splenic artery and which gives blood supply to the upper portion of the posterior wall of the stomach, are divided. This last step assures that the posterior wall of the stomach, which will constitute the fundoplication, is completely mobilized and available for a floppy wrap.
Closure of the Diaphragmatic Hiatus
The diaphragmatic crura are always closed with two or three intracorporeally tied, interrupted, #0 silk sutures with an Endostitch™ (Covidien, Norwalk, CT). The first stitch is placed just above the junction of the crura. One or two additional stitches are placed above the first one, 1 cm apart, with the uppermost being placed 1 cm posterior to the esophagus to avoid excessive tightening of the diaphragmatic hiatus.
Fundoplication
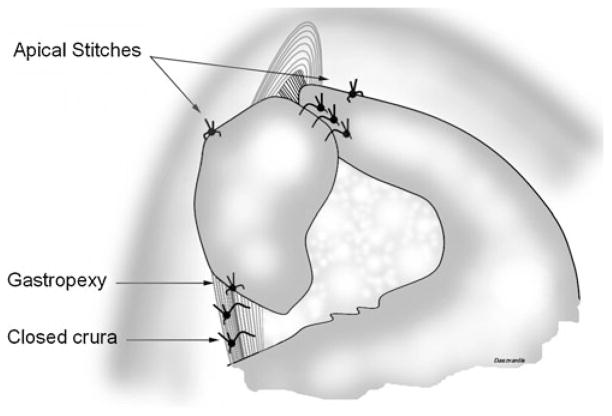
A total 360° Nissen fundoplication is usually performed. A partial 240° posterior fundoplication is usually reserved for those patients with advanced-stage scleroderma with absent esophageal motility on preoperative esophageal manometry. The surgeon gently grabs the gastric fundus and pulls it under the esophagus through the retroesophageal window with atraumatic graspers. A “shoeshine” maneuver is performed to ensure the adequate mobilization of the fundus of the stomach, especially its posterior wall. The left and right sides of the fundus are grabbed at the level of the stumps of the short gastric vessels and held together in place with the Allis clamp introduced through port 5. Then, three 2–0 silk sutures, spaced 1 cm apart, are placed to anchor the two ends of the fundoplication to each other and tied intracorporeally. None of these stitches include the esophagus. No bougie is passed. The Penrose drain is removed. Two stitches are then placed, one on each side of the fundoplication, to pexy the fundoplication and the esophagus to the diaphragm. These “apical” stitches incorporate the top of the fundoplication, the esophagus, and the uppermost portion of the crus (Fig. 2). Finally, one additional interrupted 2–0 silk suture is placed with the Endostitch™ between the posterior side of the fundoplication at the 6 o’clock position and the crura closed to fashion a posterior gastropexy (Fig. 2).
Fig. 2.
Position of operative ports for the pyloroplasty.
Laparoscopic Pyloroplasty
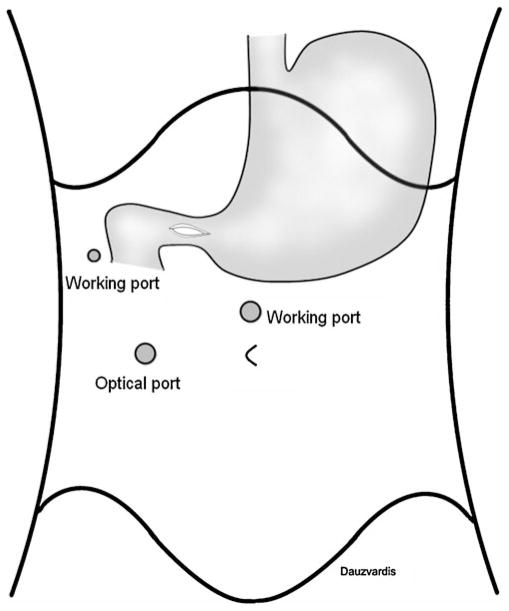
A laparoscopic Heineke–Mikulicz pyloroplasty is performed when severe gastric atony is preoperatively identified by dynamic scintigraphic nuclear medicine imaging in a symptomatic patient. Fig. 3 illustrates the port placement for the execution of the laparoscopic pyloroplasty. Port 6 is placed at the right mid-clavicular line at the level of the transverse umbilical line. This port holds an 11-mm optical trocar. Port 1 is then converted to a working port. Finally, a 5-mm working port (port 7) is placed at the right anterior axillary line, triangulating with Port 1 for combined manipulation of the suturing instruments. Special attention must be given to proper port placement, because if placed too high, the angle of suturing becomes too wide and suturing becomes difficult. Once the pylorus is identified, electrocautery is employed to score the anterior surface of the pylorus and first portion of the duodenum. The pylorus is then entered, and a 5-cm longitudinal enterotomy is carried distally in the duodenum and proximally in the antrum with the Ligasure™. Anchoring sutures are placed at the top and bottom of the enterotomy with interrupted 2–0 silk stitches with a V-20 needle intracorporeally. To prevent incorporation of the posterior wall of the pylorus during closure, a rolled piece of Gelfoam (created by placing 2–0 silk ties at both ends) is introduced into the lumen of the pylorus and left in place to later dissolve. The longitudinal enterotomy is then closed transversely in a single layer over the Gelfoam roll with interrupted 2–0 silk sutures. These are placed approximately 0.3–0.5 cm apart starting from the ends, progressing towards the middle, and tied intra-corporeally. A Maryland dissector is then used to assess for gaps between sutures, and simple 2–0 silk stitches are placed where appropriate. Finally, two metallic clips are placed on the top and the bottom of the pyloroplasty to facilitate the location of the pyloroplasty on subsequent barium swallow.
Fig. 3.
Completed Nissen fundoplication with collar stitches, posterior gastropexy and pyloroplasty.
Closure of Port Sites and Termination of Anesthesia
After a final inspection, the Nathanson retractor and all trocars are removed under direct visualization. The pneumoperitoneum is completely evacuated and the midline fascial incision of port 1 is closed with a figure-of-eight 2–0 absorbable suture. Fig. 3 shows a completed fundoplication. During the entire procedure, the anesthesiologist rigorously maintains the peak airway pressure less than 40 cm H20 and finally removes the endotracheal tube only when the patient is fully awake to minimize the risk of aspiration, as the cough reflex is impaired.
Postoperative Care
Postoperatively, all patients are closely monitored by both the surgical and the lung transplant teams in the Surgical Intensive Care (ICU) overnight. No chest X-ray is performed. They are started on a soft mechanical diet, the morning of postoperative day 1 and are asked to keep this dietary regimen for the first 2 weeks postoperatively and then to advance to more solid foods as tolerated. A barium swallow is never required before starting oral intake, unless a pyloroplasty is performed. In this case, a barium swallow is performed on postoperative day 1 to rule out a gastric leak. Patients are then discharged from the ICU after breakfast on postoperative day 1 and are able to resume regular activities in the next few days.
Specific Technical Concerns in the Lung Transplant Population
In our series of lung transplant patients with GERD who underwent laparoscopic antireflux surgery (LARS), we have noticed that the tissues of the esophagus and stomach are generally more friable and edematous, probably due to the use of steroids. Therefore, we advocate a very gentle handling of the organs. A perforation would have disastrous consequences and can be definitely avoided by handling the tissues with atraumatic graspers and by applying atraumatic traction of the gastroesophageal junction with a Penrose drain. This step of the operation should be accomplished early in the course of the procedure, as it will facilitate the remainder of the hiatal dissection. Moreover, we have found that the dissection of the esophagus from the posterior mediastinum can be challenging, as the pleura can be plastered to the esophagus, as a result of the previous lung transplantation. Therefore, if the mediastinal dissection is not carried out safely and meticulously, one may risk causing a unilateral or a bilateral pneumothoraces or even injuring the vagus nerves. This is especially true when the patient had a bilateral transplant or a re-transplant. In all cases, cautious dissection with the Ligasure™ has prevented symptomatic pneumothoraces and allowed for full esophageal mobilization, though we rarely encountered a hiatal hernia large enough to increase the difficulty of the dissection and the repositioning of the gastroesophageal junction to its proper anatomic location within the abdomen. We speculate that the rarity of a large hiatal hernia (we encountered only a small hiatal hernia in 24% of our series of 25 lung transplant) may be due to the adhesion of the pleura to the distal esophagus, which may prevent a hernia to develop after lung transplantation. Lastly, we noticed that when a replaced left hepatic artery is encountered (two patients, or 8%, in our series), a fundoplication is still feasible, and the aberrant vessel can always be preserved, although this may add time to the operation.
Results
Between November 2008 and February 2010, 25 consecutive lung transplant patients with GERD underwent laparoscopic Nissen fundoplication according to our standardized approach. A laparoscopic pyloroplasty was added in seven patients. The perioperative outcome of these lung transplant recipients was prospectively compared over the same time period to a control group of 23 patients without lung disease or transplantation (observational data submitted for publication). There was no in-hospital or 30-day mortality. The estimated blood loss, the duration of surgery, and length of hospital stay were similar between lung transplant patients and controls. There was no difference in complication or readmission rates after LARS between the lung transplant population and the control group despite the fact that these patients faced a significantly higher surgical risk (median ASA class, 3 vs. 2 for controls, p<0.0005). Overall, these results suggest that our approach to LARS is as safe for lung transplant patients as it is for the general population with GERD.
Discussion
Although laparoscopic fundoplication is an accepted treatment option for lung transplant patients with GERD, the surgical technique, which often includes a laparoscopic pyloroplasty, has not been standardized. The way we perform the operation takes into account several technical steps whose execution has proven successful in non-transplant patients. Such technical elements include a full mobilization of the esophagus with meticulous closure of the diaphragmatic crura, division of the short gastric vessels, a non-tailored approach when performing the fundoplication, and the addition of a laparoscopic pyloroplasty in patients with symptomatic and severe gastric atony. Below, we illustrate the evidence-based rationale for the technical details for each step of the operation.
Esophageal Mobilization, Closure of the Diaphragmatic Crura, and Pexy of the Fundoplication
A meticulous esophageal mobilization, closure of the diaphragmatic crura, and pexy of the fundoplication are essential to obtain good results. Soper et al. demonstrated an advantage to complete esophageal mobilization followed by meticulous closure of the diaphragmatic crura.15 They analyzed the outcomes of 290 patients who had undergone laparoscopic Nissen fundoplication over a 6-year period and found a significant difference in anatomic failure rate between those patients in whom the diaphragmatic crura were not routinely closed and those in whom the crura were routinely closed (19% vs. 4%; p<0.05). The most common cause of failure was intrathoracic wrap herniation. In addition, when the authors conducted a multivariate analysis, they identified large hiatal hernia size, postoperative emesis, diaphragmatic stressors, and early operative experience (at which time, the crura were not routinely closed) as factors predictive of failure. Horgan et al. also demonstrated the need of respecting important technical elements of the procedure in order to prevent failures.16 They identified three types of failure. Type I failure was identified when the gastroesophageal junction was herniated through the hiatus, either with the fundoplication (type IA) or without it (type IB), resulting in a telescoping of the stomach through the fundoplication; Type II failure involved a redundant stomach; and Type III failure was attributed to defective position or construction of the fundoplication. The authors demonstrated that the following technical details may play a role in the success of the operation: mobilization of the esophagus and placement of the gastroesophageal junction into the abdomen, meticulous closure of the hiatus, suturing the fundoplication to the esophagus to avoid telescoping, and suturing the fundoplication to the closed crura (posterior gastropexy) to prevent its herniation into the chest.
Division of the Short Gastric Vessels
Although controversy still exists between advocates and opponents of dividing the short gastric vessels, we prefer to routinely divide them to allow for a tension-free and floppy fundoplication. Our approach is supported by the data of Bell et al. and Wu et al. Specifically, in the study by Bell et al., the non-division of the short gastric vessels accounted for almost two-thirds of operative failures (p=0.045).17 In addition, Wu et al. noted a complete absence of wrap slippage into the chest in those patients in whom the division of the short gastric vessels was employed together with a posterior crural closure and pexy of the wrap to the crus.18
Type of Fundoplication
We prefer to perform a total 360° Nissen fundoplication, and we reserve a partial 240° posterior fundoplication for those patients with absent esophageal motility on preoperative esophageal manometry. Several trials have shown that the tailored approach provides less-than-optimal results. In a controlled trial from 2001, which 200 patients were stratified according to the presence or absence of esophageal dysmotility and randomized to either 360° (Nissen) or 270° (Toupet) fundoplication, Fibbe et al. showed that clinical outcome and reflux recurrence were similar (21% vs. 14%) in patients with and without dysmotility.19 The authors concluded that esophageal dysmotility (1) does not affect postoperative clinical outcome, (2) that it is not corrected by fundoplication, regardless of the surgical procedure performed, and (3) that it does not require a tailored approach. Then, in 2004, Patti et al. conducted a retrospective study of 235 patients in whom a tailored approach was used between October 1992 and December 1999 (141 patients, partial fundoplication and 94 patients, total fundoplication).20 They showed that heartburn from reflux on pH monitoring recurred in 19% of patients after partial fundoplication and in 4% after total fundoplication. They also showed that in 122 patients in whom a non-selective approach was used after December 1999 (total fundoplication regardless of quality of peristalsis), heartburn recurred in only 4% of patients after total fundoplication. In addition, this group found that the incidence of postoperative dysphagia was similar regardless of the procedure performed. The authors concluded that laparoscopic partial fundoplication was less effective than total fundoplication, and that, compared with a partial (240°) fundoplication, a total (360°) fundoplication was not followed by more dysphagia, even when esophageal peristalsis was weak (esophageal peristalsis was considered weak if the amplitude in the distal esophagus was equal or less than 40 mmHg). These results were confirmed in a multicenter retrospective review by Novitsky et al. in 2007 in which they showed that patients with severely disordered esophageal peristalsis (defined as an esophageal amplitude of 30 mmHg or less and/or 70% or more non-peristaltic esophageal body contractions) can safely undergo a laparoscopic total fundoplication with expected low rates of long term postoperative dysphagia (4%).21
Specific to lung transplantation, prior studies allow for minimal assessment of the operative approach to antireflux surgery. Most reports limit their discussion to the percentage of those undergoing total versus partial fundoplication as opposed to a focus on their respective technique and outcomes.11–13,22 The study of Burton et al. in 2009 is one of the few, comparing partial to total fundoplication in lung transplant patients, demonstrating no difference in the chosen technique on gas bloat, satisfaction, or dysphagia score.14 Though Burton et al. indicate a preference for partial fundoplication, other centers typically reserve a partial fundoplication for poor esophageal acid clearance or absent esophageal motility.11,22
Pyloroplasty
Gastroparesis is prevalent after lung transplantation. Studies from other lung transplant centers report a prevalence ranging from 23% to 92%.22–28 Among lung transplant patients with GERD studied at our institution by nuclear medicine imaging, 36% had severe delayed gastric emptying. Because gastroparesis has been shown to be implicated in the pathogenesis of GERD and associated with aspiration and allograft compromise, we prefer to perform a pyloroplasty at the time of LARS in the lung transplant patient with objectively identified GERD and symptomatic and severe gastric atony (defined as when <30% of the radiolabeled gastric contents were emptied into the small bowel by 90 min).27,28
Conclusion
Laparoscopic surgical correction of reflux, with or without pyloroplasty, is accepted and safe in lung transplant patients and may preserve pulmonary function by preventing aspiration of gastroduodenal contents. The respect for specific anesthesiologic details and technical aspects of the operation in this high-risk patient population is essential. The important technical elements of the operation, including meticulous closure of the hiatus, division of the short gastric vessels, and a 360° fundoplication in all but those with absent esophageal motility, should be respected. This effort, in combination with the appropriate patient selection and a standardized management, may provide the lung transplant recipient an effective treatment for GERD and reduce their risk of aspiration.
Acknowledgments
The authors would like to thank Michael Dauzvardis, PhD, for his assistance with the medical illustrations.
Contributor Information
Christopher S. Davis, Departments of Surgery and Anesthesia, Loyola University Medical Center, Maywood, IL, USA
W. Scott Jellish, Departments of Surgery and Anesthesia, Loyola University Medical Center, Maywood, IL, USA.
P. Marco Fisichella, Email: marco6370@yahoo.com, Departments of Surgery and Anesthesia, Loyola University Medical Center, Maywood, IL, USA, Swallowing Center, Department of Surgery, Stritch School of Medicine, Loyola University Medical Center, 2160 South First Avenue—Room 3226, Maywood, IL 60153, USA.
References
- 1.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel A, Taylor DO, Kucheryavaya AY, Hertz MI. Registry for the International Society for Heart and Lung Transplantation: Twenty-fifth Official Adult Lung and Heart/Lung Transplantation Report. J Heart Lung Transplant. 2008;27(9):957–969. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Brown RS, Rush SH, Rosen HR, Langnas AN, Klintmalm GB, Hanto DW, Punch JD. Liver and intestine transplantation. Am J Transplant. 2004;4(Suppl 9):81–92. doi: 10.1111/j.1600-6135.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 3.Wynn JJ, Distant DA, Pirsch JD, Norman D, Gaber AO, Ashby VB, Leichtman AB. Kidney and pancreas transplantation. Am J Transplant. 2004;4(Suppl 9):72–80. doi: 10.1111/j.1600-6135.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 4.Pierson RN, 3rd, Barr ML, McCullough KP, Egan T, Garrity E, Jessup M, Murray S. Thoracic organ transplantation. Am J Transplant. 2004;4(Suppl 9):93–105. doi: 10.1111/j.1600-6135.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 5.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, Marchevsky A, Ohori NP, Ritter J, Stewart S, Tazelaar HD. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: lung rejection study group. J Heart Lung Transplant. 1996;15(1 Pt 1):1–15. [PubMed] [Google Scholar]
- 6.Grossman EJ, Shilling RA. Bronchiolitis obliterans in lung transplantation: the good, the bad, and the future. Transl Res. 2009;153(4):153–165. doi: 10.1016/j.trsl.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001, an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 8.Valentine VG, Robbins RC, Berry GJ, Patel HR, Reichenspurner H, Reitz BA, Theodore J. Actuarial survival of heart–lung and bilateral sequential lung transplant recipients with obliterative bronchiolitis. J Heart Lung Transplant. 1996;15(4):371–383. [PubMed] [Google Scholar]
- 9.Nicod LP. Mechanisms of airway obliteration after lung transplantation. Proc Am Thorac Soc. 2006;3(5):444–449. doi: 10.1513/pats.200601-007AW. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Hartwig MG, Appel JZ, Bush EL, Balsara KR, Holzknecht ZE, Collins BH, Howell DN, Parker W, Lin SS, Davis RD. Chronic aspiration of gastric fluid induces the development of obliterative bronchiolitis in rat lung transplants. Am J Transplant. 2008;8(8):1614–1621. doi: 10.1111/j.1600-6143.2008.02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantu E, 3rd, Appel JZ, 3rd, Hartwig MG, Woreta H, Green C, Messier R, Palmer SM, Davis RD, Jr J. Maxwell Chamberlain Memorial Paper. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg. 2004;78(4):1142–1151. doi: 10.1016/j.athoracsur.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Lau CL, Palmer SM, Howell DN, McMahon R, Hadjiliadis D, Gaca J, Pappas TN, Davis RD, Eubanks S. Laparoscopic antireflux surgery in the lung transplant population. Surg Endosc. 2002;16(12):1674–1678. doi: 10.1007/s00464-001-8251-2. [DOI] [PubMed] [Google Scholar]
- 13.Davis RD, Jr, Lau CL, Eubanks S, Messier RH, Hadjiliadis D, Steele MP, Palmer SM. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003;125(3):533–542. doi: 10.1067/mtc.2003.166. [DOI] [PubMed] [Google Scholar]
- 14.Burton PR, Button B, Brown W, Lee M, Roberts S, Hassen S, Bailey M, Smith A, Snell G. Medium-term outcome of fundoplication after lung transplantation. Dis Esophagus. 2009;22(8):642–648. doi: 10.1111/j.1442-2050.2009.00980.x. [DOI] [PubMed] [Google Scholar]
- 15.Soper NJ, Dunnegan D. Anatomic fundoplication failure after laparoscopic antireflux surgery. Ann Surg. 1999;229:669–676. doi: 10.1097/00000658-199905000-00009. discussion 76–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horgan S, Pohl D, Bogetti D, Eubanks T, Pellegrini C. Failed antireflux surgery: what have we learned from reoperations? Arch Surg. 1999;134(8):809–815. doi: 10.1001/archsurg.134.8.809. [DOI] [PubMed] [Google Scholar]
- 17.Bell RC, Hanna P, Mills MR, Bowrey D. Patterns of success and failure with laparoscopic Toupet fundoplication. Surg Endosc. 1999;13(12):1189–1194. doi: 10.1007/pl00009618. [DOI] [PubMed] [Google Scholar]
- 18.Wu JS, Dunnegan DL, Luttmann DR, Soper NJ. The influence of surgical technique on clinical outcome of laparoscopic Nissen fundoplication. Surg Endosc. 1996;10(12):1164–1169. doi: 10.1007/s004649900271. discussion 1169–1170. [DOI] [PubMed] [Google Scholar]
- 19.Fibbe C, Layer P, Keller J, Strate U, Emmermann A, Zornig C. Esophageal motility in reflux disease before and after fundoplication: a prospective, randomized, clinical, and manometric study. Gastroenterology. 2001;121(1):5–14. doi: 10.1053/gast.2001.25486. [DOI] [PubMed] [Google Scholar]
- 20.Patti MG, Robinson T, Galvani C, Gorodner MV, Fisichella PM, Way LW. Total fundoplication is superior to partial fundoplication even when esophageal peristalsis is weak. J Am Coll Surg. 2004;198(6):863–869. doi: 10.1016/j.jamcollsurg.2004.01.029. discussion 869–870. [DOI] [PubMed] [Google Scholar]
- 21.Novitsky YW, Wong J, Kercher KW, Litwin DE, Swanstrom LL, Heniford BT. Severely disordered esophageal peristalsis is not a contraindication to laparoscopic Nissen fundoplication. Surg Endosc. 2007;21(6):950–954. doi: 10.1007/s00464-006-9126-3. [DOI] [PubMed] [Google Scholar]
- 22.Gasper WJ, Sweet MP, Hoopes C, Leard LE, Kleinhenz ME, Hays SR, Golden JA, Patti MG. Antireflux surgery for patients with end-stage lung disease before and after lung transplantation. Surg Endosc. 2008;22(2):495–500. doi: 10.1007/s00464-007-9494-3. [DOI] [PubMed] [Google Scholar]
- 23.Hadjiliadis D, Davis RD, Steele MP, Messier RH, Lau CL, Eubanks SS, Palmer SM. Gastroesophageal reflux disease in lung transplant recipients. Clin Transplant. 2003;17(4):363–368. doi: 10.1034/j.1399-0012.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 24.Young LR, Hadjiliadis D, Davis RD, Palmer SM. Lung transplantation exacerbates gastroesophageal reflux disease. Chest. 2003;124(5):1689–1693. doi: 10.1378/chest.124.5.1689. [DOI] [PubMed] [Google Scholar]
- 25.D’Ovidio F, Mura M, Ridsdale R, Takahashi H, Waddell TK, Hutcheon M, Hadjiliadis D, Singer LG, Pierre A, Chaparro C, Gutierrez C, Miller L, Darling G, Liu M, Post M, Keshavjee S. The effect of reflux and bile acid aspiration on lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant. 2006;6(8):1930–1938. doi: 10.1111/j.1600-6143.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 26.Au J, Hawkins T, Venables C, Morritt G, Scott CD, Gascoigne AD, Corris PA, Hilton CJ, Dark JH. Upper gastrointestinal dysmotility in heart–lung transplant recipients. Ann Thorac Surg. 1993;55(1):94–97. doi: 10.1016/0003-4975(93)90480-6. [DOI] [PubMed] [Google Scholar]
- 27.Berkowitz N, Schulman LL, McGregor C, Markowitz D. Gastroparesis after lung transplantation. Potential role in postoperative respiratory complications. Chest. 1995;108(6):1602–1607. doi: 10.1378/chest.108.6.1602. [DOI] [PubMed] [Google Scholar]
- 28.Sodhi SS, Guo JP, Maurer AH, O’Brien G, Srinivasan R, Parkman HP. Gastroparesis after combined heart and lung transplantation. J Clin Gastroenterol. 2002;34(1):34–39. doi: 10.1097/00004836-200201000-00007. [DOI] [PubMed] [Google Scholar]