Abstract
Tourette Syndrome (TS) is a childhood-onset neuropsychiatric disorder that is familial and highly heritable. Although genetic influences are thought to play a significant role in the development of TS, no definite TS susceptibility genes have been identified to date. TS is believed to be genetically related to both obsessive-compulsive disorder (OCD) and grooming disorders (GD) such as trichotillomania (TTM). SAP90/PSD95-associated protein 3 (SAPAP3/DLGAP3) is a post-synaptic scaffolding protein that is highly expressed in glutamatergic synapses in the striatum and has recently been investigated as a candidate gene in both OCD and GD studies. Given the shared familial relationship between TS, OCD and TTM, DLGAP3 was evaluated as a candidate TS susceptibility gene. In a family-based sample of 289 TS trios, 22 common single nucleotide polymorphisms (SNPs) in the DLGAP3 region were analyzed. Nominally significant associations were identified between TS and rs11264126 and two haplotypes containing rs11264126 and rs12141243. Secondary analyses demonstrated that these results cannot be explained by the presence of comorbid OCD or TTM in the sample. Although none of these results remained significant after correction for multiple hypothesis testing, DLGAP3 remains a promising candidate gene for TS.
Keywords: tic disorders, SAPAP3, gene, glutamate, trichotillomania
INTRODUCTION
Tourette Syndrome (TS) is a childhood-onset neuropsychiatric disorder characterized by multiple motor tics and one or more vocal tic(s) that are present for at least one year [American Psychiatric Association, 2000]. The prevalence of TS in children and adolescents is estimated to be between 0.1% and 1% of the general population [Scharf and Pauls, 2007]. TS is highly familial with many large, multi-generational TS pedigrees reported in the literature and is also one of the most heritable non-Mendelian neuropsychiatric disorders [Pauls, 2003]. Family studies indicate that the risk of developing TS in first degree relatives of patients with the disorder is 5 to 15 times greater than the risk of developing TS in the general population [NIMH Genetics Workgroup, 1998; Pauls et al., 1991]. Unfortunately, no definitive TS susceptibility gene has been identified to date. Identification of the etiological factors of TS, including its genetic basis, is important to advance the understanding of TS pathogenesis and to discover new avenues of treatment.
Obsessive-compulsive disorder (OCD) and trichotillomania (TTM) (chronic hair-pulling) are two conditions believed to be genetically related to TS. OCD is a common comorbidity in TS patients, and the two disorders appear to share common genetic susceptibilities [Pauls et al., 1991; Scharf and Pauls, 2007]. Both TS and OCD are thought to arise from dysregulated cortico-striato-thalamo-cortical (CSTC) loops [Graybiel and Rauch, 2000; Mink, 2006]. TTM has clinical characteristics that overlap with both TS and OCD, including the presence of a premonitory urge and temporary relief after completion [Novak et al., 2009]. In relatives of probands with TTM there is an increased prevalence of both OCD and tics [King et al., 1995; Lenane et al., 1992]. The genetics of TTM are not well characterized, but family studies suggest that TS, OCD and TTM share common genetic factors [Bienvenu et al., 2009; King et al., 1995; Lenane et al., 1992; Pauls et al., 1995; Pauls et al., 1986].
SAP90/PSD95-associated protein 3 (SAPAP3/DLGAP3), located at 1p34.3, has been recently examined as a candidate gene in OCD-spectrum disorder studies [Bienvenu et al., 2009; Zuchner et al., 2009]. Although it has not been implicated in TS or OCD linkage studies and, therefore, is not a positional candidate, SAPAP3/DLGAP3 is a promising functional TS candidate gene. SAPAP3/DLGAP3 is a post-synaptic scaffolding protein that is highly expressed in glutamatergic synapses in the striatum and is thought to play a key role in regulating synaptic function and plasticity [Scannevin and Huganir, 2000; Welch et al., 2004]. Welch and colleagues [2007] demonstrated that mice with a targeted deletion of Sapap3 exhibited behaviors consistent with increased anxiety and compulsive over-grooming reminiscent of OCD and TTM as they present in humans. Sapap3-deficient mice (Sapap3−/−) were also found to have cortico-striatal synaptic deficits. Interestingly, treatment with selective serotonin reuptake inhibitors, which are used as a first-line treatment for OCD, and selective re-expression of Sapap3 in the striatum in Sapap3−/− mice eliminated the over-grooming behaviors and rescued the synaptic deficits [Welch et al., 2007]. Zuchner et al. [2009] recently reported an increased frequency of non-synonymous coding variants in human SAPAP3/DLGAP3 in 165 patients with either TTM or OCD compared to controls (4.2% vs. 1.1%). In addition, Bienvenu et al. [2008] reported nominal associations between multiple common single nucleotide polymorphisms (SNPs) in SAPAP3/DLGAP3 and grooming disorders (GDs), including TTM, in a family-based study of 383 families with GDs and/or OCD. Given the shared characteristics of TS, OCD, and TTM, and the evidence that these disorders are genetically related, DLGAP3 was investigated in the current study as a functional candidate TS susceptibility gene in a family-based sample.
MATERIALS AND METHODS
Subjects, including 1288 individuals from 423 independently ascertained nuclear families (423 parent-proband trios and 19 affected siblings), were recruited from tic disorder specialty clinics in the United States, Canada, Great Britain and the Netherlands for a family-based genetic study of TS. Assessments consisted of an in-person, semi-structured interview, using instruments documented previously to be valid and reliable for the diagnosis of TS (κ = 0.98) and OCD (κ = 0.97) [Pauls et al., 1995]. Diagnoses of TS and OCD were established using DSM-IV-TR criteria and were best-estimated by consensus between two independent TS clinical investigators. A diagnosis of probable TTM was made based on a screening question for the lifetime presence or absence of recurrent hair-pulling behavior in the context of the OCD and OCD-spectrum disorders semi-structured interview: “I pull my hair out. For example, you may pull your hair from your scalp, eyebrows, eyelashes, or pubic area. You may use your fingers or tweezers to pull your hair. You may produce bald spots on your scalp that require a wig, or pluck your eyelashes or eyebrows smooth”. All participants 18 years of age and older signed informed consent forms. Individuals under 18 years of age signed an assent form after a parent signed a consent form on their behalf.
Genomic DNA was extracted from either peripheral blood or buccal cells and purified using standard protocols (Gentra, Minneapolis, Minnesota, USA). Validated common (SNPs) from the genomic region containing DLGAP3 and 10kb of upstream and downstream flanking sequences (approximately 60 kb overall) were downloaded from the HapMap Phase II database [Frazer et al., 2007] (Suppl. Fig. 1). Twenty-two tag SNPs were selected by the program Tagger within Haploview using pairwise tagging of SNPs with minor allele frequencies >0.05 and an r2>0.8 [Barrett et al., 2005; de Bakker et al., 2005]. Although rs6682829 was excluded as a tag SNP due to an inability to design a valid assay from its flanking sequences, it was tagged by proxy SNP rs4652869 with an r2 of 0.743. The remaining 21 tag SNPs captured all 30 of the other common alleles (MAF>0.05) in the DLGAP3 region at r2>0.8 and a mean max r2 of 0.988. Three additional SNPs (rs1001616, rs11587343 and rs35688758) with validated minor allele frequencies ≥0.05 or within coding regions of DLGAP3 were added from the SNPper [Riva and Kohane, 2002] and dbSNP [dbSNP] databases for a total of 24 SNPs that were genotyped (Suppl. Fig. 1).
SNP genotyping was performed in a 384-well plate format on the Sequenom MassARRAY platform (Sequenom, San Diego, California, USA). Primers for polymerase chain reaction (PCR) amplification and single base extension (SBE) assays were designed using Assay Design 3.1 software (Sequenom, San Diego, California, USA) based on FASTA sequences surrounding the SNPs taken from SNPper [Riva and Kohane, 2002]. SNP genotyping was performed using multiplex PCR followed by a pooled SBE reaction using iPLEX® Gold SBE chemistry [Sequenom, 2009]. Samples were analyzed in automated mode by a MassARRAY RT mass spectrometer. The resulting spectra were analyzed by SpectroAnalyzer software after baseline correction and peak identification.
Prior to analysis, data cleaning was performed to exclude SNPs and individuals with call rates <90% or SNPs with Hardy-Weinberg Equilibrium p-values <10−6. Families and SNPs with Mendel error rates >5% were also excluded. Pairwise linkage disequilibrium between markers was calculated using the D’ and r2 statistics in Haploview. Haplotype blocks were defined according to the confidence interval method of [Gabriel et al., 2002]. Family-based association testing of single SNPs and haplotype blocks with frequencies ≥5% were performed using the Transmission Disequlibrium Test (TDT) in PLINK [Purcell, 2009; Purcell et al., 2007]. Correction for multiple hypothesis testing was implemented in PLINK using gene-dropping and max(T) permutation methods with 10,000 permutations.
RESULTS
During the quality control process, 2 SNPs (rs11583978 and rs35688758) and 141 families were excluded (Suppl. Fig. 1) such that 22 SNPs and 289 trios (282 parent-proband trios and 7 parent-affected sibling trios) remained for analysis. The sample pass rates did not differ based on the source of DNA. Of the 22 SNPs analyzed, 20 were HapMap tag SNPs. These 20 tag SNPs tagged 29 of 31 (93%) eligible alleles (MAF>0.05) in the DLGAP3 region and 10kb upstream and downstream of DLGAP3 at r2>0.8 and with a mean max r2=0.987. The 2 remaining common alleles, rs11583978 and rs6682829, were captured at r2 values of 0.605 and 0.743, respectively.
SNP rs11264126, located in the sixth intron of DLGAP3, was nominally associated with TS (p=0.013) with over-transmission of the G allele to TS offspring (Table I & Fig. 1). In haplotype-based analyses, two DLGAP3 haplotypes, containing rs11264126 and rs12141243, were also nominally associated with TS (AT, frequency= 0.406, p=0.026; GT, frequency= 0.449, p=0.025), with over-transmission of the rs11264126 G allele and under-transmission of the A allele. (Table II & Fig. 1). However, none of these findings remained significant following correction for multiple hypothesis testing using permutation (Tables I & II).
TABLE I.
Single marker SNP analysis of DLGAP3 in TS
| SNP | BP | A1 | A2 | MAF | Trans:Untrans | Odds Ratio |
p-value | Permuted p-value | Disorders with reported associations1 |
|---|---|---|---|---|---|---|---|---|---|
| rs14103 | 35093829 | G | T | 0.077 | 36:28 | 1.28 | 0.317 | 0.998 | |
| rs4653107 | 35095090 | A | G | 0.122 | 51:50 | 1.02 | 0.920 | 1 | |
| rs4653108 | 35100619 | A | G | 0.196 | 62:72 | 0.86 | 0.387 | 0.999 | |
| rs4653109 | 35100832 | C | T | 0.375 | 108:100 | 1.08 | 0.579 | 1 | PNB |
| rs1001616 | 35105513 | G | C | 0.297 | 84:91 | 0.92 | 0.596 | 1 | |
| rs11587343 | 35107037 | T | C | 0.006 | 0:2 | 0 | 0.157 | 0.960 | |
| rs4653112 | 35113235 | A | G | 0.069 | 36:22 | 1.63 | 0.066 | 0.665 | |
| rs7541937 | 35114569 | T | G | 0.480 | 105:129 | 0.81 | 0.116 | 0.850 | |
| rs11264126 | 35114682 | A | G | 0.403 | 94:131 | 0.71 | 0.013 | 0.230 | |
| rs12141243 | 35119509 | C | T | 0.143 | 58:60 | 0.96 | 0.853 | 1 | |
| rs11264155 | 35129265 | G | C | 0.473 | 114:121 | 0.94 | 0.647 | 1 | |
| rs6662980 | 35132665 | G | A | 0.330 | 104:92 | 1.13 | 0.391 | 0.999 | TTM |
| rs4259608 | 35137044 | G | T | 0.228 | 78:78 | 1 | 1 | 1 | |
| rs4652867 | 35139877 | T | G | 0.254 | 84:101 | 0.83 | 0.211 | 0.986 | PSP |
| rs11264172 | 35141018 | A | C | 0.433 | 125:110 | 1.13 | 0.327 | 0.998 | |
| rs6686484 | 35141347 | G | A | 0.368 | 137:131 | 1.04 | 0.714 | 1 | |
| rs11264173 | 35141358 | G | A | 0.369 | 103:130 | 0.79 | 0.076 | 0.708 | |
| rs10493064 | 35141601 | A | T | 0.162 | 54:61 | 0.88 | 0.513 | 1 | |
| rs12120523 | 35141857 | G | A | 0.140 | 66:62 | 1.06 | 0.723 | 1 | |
| rs7555884 | 35146465 | G | T | 0.413 | 100:125 | 0.8 | 0.095 | 0.784 | |
| rs16837122 | 35151165 | G | C | 0.204 | 76:92 | 0.82 | 0.217 | 0.990 | |
| rs4652869 | 35151521 | G | T | 0.417 | 120:109 | 1.10 | 0.4673 | 1 | TTM |
Family-based association testing was conducted using the Transmission Disequilibrium Test (TDT) in Plink (Purcell et al., 2007). A1 indicates the minor allele and A2 the major allele. The transmitted to untransmitted ratio is listed in the column labeled Trans:Untrans. The nominally significant SNP association is bolded. Corrected p-values following 10,000 permutations are also indicated.
Bienvenu et al., 2008
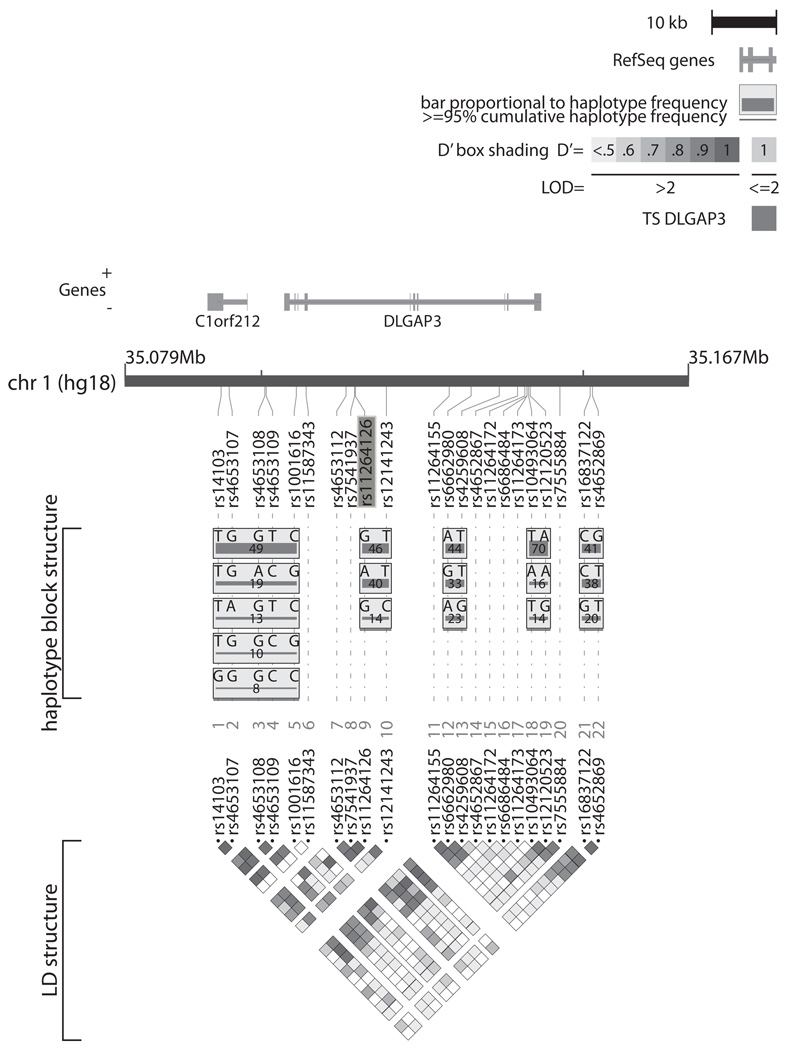
FIGURE 1.
Linkage disequilibrium (LD) map and haplotype structure of the DLGAP3 locus. DLGAP3 and the downstream open reading frame C1orf212 are indicated relative to the positions of the 22 genotyped SNPs in the current study. SNP minor allele frequencies were calculated from non-founders. Haplotype blocks, as defined by the confidence interval classification of Gabriel et al., 2002, are indicated in gray boxes with haplotype frequencies in the study population displayed below each haplotype. The nominally significant SNP rs11264126 is highlighted.
TABLE II.
DLGAP3 haplotype analysis in TS families
| Locus | Haplotype | Frequency | Trans | Untrans | P-value | Permuted P-value |
SNPs in haplotype |
|---|---|---|---|---|---|---|---|
| H1 | TGGTC | 0.4883 | 113.7 | 118 | 0.779 | 1 | rs14103|rs4653107|rs4653108|rs4653109|rs1001616 |
| H1 | TGACG | 0.1905 | 64.51 | 72 | 0.521 | 1 | rs14103|rs4653107|rs4653108|rs4653109|rs1001616 |
| H1 | TAGTC | 0.1203 | 51.55 | 49.3 | 0.822 | 1 | rs14103|rs4653107|rs4653108|rs4653109|rs1001616 |
| H1 | TGGCG | 0.09795 | 41.99 | 36.39 | 0.527 | 1 | rs14103|rs4653107|rs4653108|rs4653109|rs1001616 |
| H1 | GGGCC | 0.07778 | 35.98 | 27 | 0.257 | 0.999 | rs14103|rs4653107|rs4653108|rs4653109|rs1001616 |
| H2 | GT | 0.4492 | 141.1 | 106.1 | 0.025 | 0.656 | rs11264126|rs12141243 |
| H2 | AT | 0.4057 | 94.91 | 127.9 | 0.026 | 0.694 | rs11264126|rs12141243 |
| H2 | GC | 0.1428 | 57.91 | 57.93 | 0.998 | 1 | rs11264126|rs12141243 |
| H3 | AT | 0.4382 | 109 | 126 | 0.267 | 1 | rs6662980|rs4259608 |
| H3 | GT | 0.3319 | 103 | 88 | 0.277 | 0.993 | rs6662980|rs4259608 |
| H3 | AG | 0.2299 | 77 | 75 | 0.871 | 1 | rs6662980|rs4259608 |
| H4 | TA | 0.6932 | 99 | 96.21 | 0.84 | 1 | rs10493064|rs12120523 |
| H4 | AA | 0.1654 | 54 | 59.79 | 0.587 | 1 | rs10493064|rs12120523 |
| H4 | TG | 0.1399 | 63 | 58.79 | 0.702 | 1 | rs10493064|rs12120523 |
| H5 | CG | 0.4128 | 120 | 106 | 0.351 | 1 | rs16837122|rs4652869 |
| H5 | CT | 0.3819 | 123 | 120 | 0.847 | 1 | rs16837122|rs4652869 |
| H5 | GT | 0.2054 | 69 | 86 | 0.172 | 0.995 | rs16837122|rs4652869 |
Haplotypes were defined using the 95% confidence interval classification of Gabriel et al., 2002. Nominally significant haplotype associations with TS are bolded. Corrected p-values following 10,000 permutations are also indicated.
In order to test whether the nominal association between TS and rs11264126 could be explained by the presence of comorbid OCD or TTM in the TS-affected subjects, additional TDT analyses were performed using OCD and TTM as the primary phenotypes. SNP rs11264126 and the haplotypes containing rs11264126 and rs12141243 were not associated with either OCD (126 TS+,OCD+ trios, p=0.477) or TTM (24 TS+, TTM+ trios, p=0.818).
DISCUSSION
Dysfunction of CSTC loops has been implicated in TS, OCD, and OCD-spectrum disorders [Graybiel and Rauch, 2000; Mink, 2006]. Glutamatergic neurotransmission has been identified as an important component of CSTC circuits in OCD through previous positive candidate gene association studies, neuroimaging studies, and recent treatment trials [Arnold et al., 2004; Arnold et al., 2006; Delorme et al., 2004; Dickel et al., 2006; Pittenger et al., 2006; Rosenberg et al., 2004; Shugart et al., 2009; Stewart et al., 2007; Wendland et al., 2009]. SAPAP3/DLGAP3 is highly expressed in the striatum, is part of the CSTC circuit, and interacts with the SAP90/PSD95 and SHANK family proteins to form a postsynaptic anchoring/signaling complex at excitatory glutamatergic synapses [Scannevin and Huganir, 2000; Welch et al., 2007]. Welch and colleagues [2007] recently demonstrated that Sapap3-knockout mice exhibited cortico-striatal synaptic deficits and a compulsive grooming phenotype reminiscent of OCD and TTM in humans. Given these previous findings, the current study investigated DLGAP3 as a candidate TS susceptibility gene.
To the authors’ knowledge, this is the first candidate gene association study of DLGAP3 and TS. This analysis identified a nominally significant association between TS and the rs11264126 G allele as well as two DLGAP3 haplotypes consisting of rs11264126 and rs12141243. The haplotype tests, while not independent of the single marker test, do help to refine localization of a putative TS risk locus to the over-transmitted GT rs11264126-rs1214123 haplotype. Conversely, the AT haplotype was undertransmitted, indicating that it may have a protective effect. Furthermore, none of these associations could be explained by the presence of co-morbid OCD or TTM in the sample. Thus, these results suggest that DLGAP3 may be a candidate TS susceptibility gene, though the findings did not survive correction for multiple hypothesis testing.
The current analysis did not detect an association between TS and the four DLGAP3 SNPs previously reported by Bienvenu and colleagues [2008] to be nominally associated with various grooming disorders (GDs): Pathological nail biting (PNB) with rs4653109; TTM with both rs662980 and rs4652869; and Pathological skin picking (PSP) with rs4652867 (Table 1). Of note, Bienvenu and colleagues [2008] did not screen for rs11264126, since they limited their analyses to SNPs with minor allele frequencies ≥20%. However, it is unlikely that rs11264126 serves as a proxy for any of the previously reported Bienvenu et al. SNPs, since this SNP has a low correlation (r2 <0.5) in the HapMap CEU population with each of the nominally significant SNPs from the prior study. Bienvenu and colleagues also excluded probands with TS from their cohort, which suggests that their findings are not likely to be caused by the presence of comorbid TS in the sample. The differing results between the two studies could potentially be explained by their small sample sizes and the limited power to detect SNPs associated with each of the different disorders. Alternatively, there could be non-overlapping sets of susceptibility loci for TS, OCD and GDs despite their proposed common pathophysiology and genetic overlap.
Limitations of the current study should be acknowledged. First, the overall sample size is unlikely to detect susceptibility genes with small effect sizes. In particular, the small number of TS+,TTM+ trios (n=24) has essentially no power to identify an association between TTM and DLGAP3. Nonetheless, the low rate of TTM comorbidity in the sample and absence of association with rs11264126 in the TS+,TTM+ trios suggest that the reported signal in the overall TS sample is unlikely to be explained by underlying TTM in these families. Second, since there was only a single screening question for TTM, it is possible that TTM was not accurately captured in the secondary analysis using TTM as the phenotype of interest.
Additionally, this study only investigated common variants in DLGAP3 with minor allele frequencies greater than 5%. Thus, further screening for rare variants, similar to the study of Zuchner and colleagues [2009] who recently reported an increased frequency of rare DLGAP3 missense variants in patients with either TTM or OCD compared to controls, may be informative. Given the nominally significant results of the current study and the results of previous studies by Welch et al. [2007], Bienvenu et al. [2008], and Zuchner et al. [2009], further investigation of the associations between DLGAP3 and GDs, OCD, and TS is warranted.
Supplementary Material
Linkage Disequilibrium (LD) map of DLGAP3 in the European HapMap population in Haploview (Barrett et al., 2005). Red shading on the map indicates D’ values; numbers within boxes indicate r2 values. Haplotype blocks were defined using the 95% confidence interval classification of Gabriel et al., 2002. Tag SNPs selected for the current study are circled in red; additional non-HapMap SNPs which were genotyped are illustrated in red-font; SNPs excluded after quality control are crossed out.
ACKNOWLEDGMENTS
Members of the Tourette Syndrome Association International Consortium for Genetics (TSAICG), listed alphabetically by city: D. Cath and P. Heutink, Departments of Psychiatry and Human Genetics, Free University Medical Center, Amsterdam, The Netherlands; M. Grados, H.S. Singer and J.T. Walkup, Departments of Psychiatry and Neurology, Johns Hopkins University School of Medicine, Baltimore, MD; J.M. Scharf, C. Illmann, D. Yu, J. Platko, S. Santangelo, S. E. Stewart, and D.L. Pauls, Psychiatric and Neurodevelopmental Genetics Unit, Center for Human Genetic Research, Massachusetts General Hospital Harvard Medical School, Boston, MA; N.J. Cox, Departments of Medicine and Human Genetics, University of Chicago, Chicago, IL; S. Service, D. Keen-Kim, C. Sabatti and N. Freimer, Departments of Psychiatry, Human Genetics and Statistics, U.C.L.A Medical School, Los Angeles, CA; M.M. Robertson, Department of Mental Health Sciences, University College London, Institute of Neurology, National Hospital for Neurology and Neurosurgery, Queen Square, London, England; G.A. Rouleau, J.-B. Riviere, S. Chouinard, F. Richer, P. Lesperance and Y. Dion, University of Montreal, Montreal, Quebec, Canada; R.A. King, J.R. Kidd, A.J. Pakstis, J.F. Leckman and K.K. Kidd, Department of Genetics and the Child Study Center, Yale University School of Medicine, New Haven, CT; R. Kurlan, P. Como and D. Palumbo, Department of Neurology, University of Rochester School of Medicine, Rochester, NY; A. Verkerk, B.A. Oostra, Department of Clinical Genetics, Erasmus University, Rotterdam, The Netherlands; W. McMahon, M. Leppert and H. Coon, Departments of Psychiatry and Human Genetics, University of Utah School of Medicine, Salt Lake City, UT; C. Mathews, Department of Psychiatry, University of California, San Francisco, San Francisco, CA; P. Sandor and C.L. Barr, Department of Psychiatry, The Toronto Hospital and University of Toronto, Toronto, Ontario, Canada.
The TSAICG is grateful to all the families with Gilles de la Tourette Syndrome who generously agreed to be part of this study. Furthermore, the members of the Consortium are deeply indebted to the Tourette Syndrome Association and in particular to Ms. Judit Ungar, TSA president and Ms. Sue Levi-Pearl, TSA Director of Medical and Scientific Programs. Both have dedicated their professional lives to the understanding and treatment of Tourette Syndrome. Without their support and guidance, this study would not have been possible.
This work was supported by an American Academy of Neurology Foundation grant and NIH grant MH-085057 to JMS as well as NIH grants NS-40024 and NS-16648 to DLP and the TSAICG.
REFERENCES
- American Psychiatric Association. 4th edition-Text Revision (DSM-IV-TR) Washington, DC: American Psychiatric Press; 2000. Diagnostic and Statistical Manual of Mental Disorders; p. 943. [Google Scholar]
- Arnold PD, Rosenberg DR, Mundo E, Tharmalingam S, Kennedy JL, Richter MA. Association of a glutamate (NMDA) subunit receptor gene (GRIN2B) with obsessive-compulsive disorder: a preliminary study. Psychopharmacology (Berl) 2004;174(4):530–538. doi: 10.1007/s00213-004-1847-1. [DOI] [PubMed] [Google Scholar]
- Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL. Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63(7):769–776. doi: 10.1001/archpsyc.63.7.769. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Wang Y, Shugart YY, Welch JM, Grados MA, Fyer AJ, Rauch SL, McCracken JT, Rasmussen SA, Murphy DL, et al. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(5):710–720. doi: 10.1002/ajmg.b.30897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dbSNP. http://www.ncbi.nlm.nih.gov/projects/SNP/.
- de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37(11):1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- Delorme R, Krebs MO, Chabane N, Roy I, Millet B, Mouren-Simeoni MC, Maier W, Bourgeron T, Leboyer M. Frequency and transmission of glutamate receptors GRIK2 and GRIK3 polymorphisms in patients with obsessive compulsive disorder. Neuroreport. 2004;15(4):699–702. doi: 10.1097/00001756-200403220-00025. [DOI] [PubMed] [Google Scholar]
- Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M, Himle JA, Leventhal BL, Cook EH, Jr, Hanna GL. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63(7):778–785. doi: 10.1001/archpsyc.63.7.778. [DOI] [PubMed] [Google Scholar]
- Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, Belmont JW, Boudreau A, Hardenbol P, Leal SM, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449(7164):851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, et al. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28(2):343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- King RA, Scahill L, Vitulano LA, Schwab-Stone M, Tercyak KP, Jr, Riddle MA. Childhood trichotillomania: clinical phenomenology, comorbidity, and family genetics. J Am Acad Child Adolesc Psychiatry. 1995;34(11):1451–1459. doi: 10.1097/00004583-199511000-00011. [DOI] [PubMed] [Google Scholar]
- Lenane MC, Swedo SE, Rapoport JL, Leonard H, Sceery W, Guroff JJ. Rates of Obsessive Compulsive Disorder in first degree relatives of patients with trichotillomania: a research note. J Child Psychol Psychiatry. 1992;33(5):925–933. doi: 10.1111/j.1469-7610.1992.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Mink JW. Neurobiology of basal ganglia and Tourette syndrome: basal ganglia circuits and thalamocortical outputs. Adv Neurol. 2006;99:89–98. [PubMed] [Google Scholar]
- NIMH Genetics Workgroup. Genetics and mental disorders. Rockville, MD: National Institute of Mental Health; 1998. [Google Scholar]
- Novak CE, Keuthen NJ, Stewart SE, Pauls DL. A twin concordance study of trichotillomania. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(7):944–949. doi: 10.1002/ajmg.b.30922. [DOI] [PubMed] [Google Scholar]
- Pauls DL. An update on the genetics of Gilles de la Tourette syndrome. J Psychosom Res. 2003;55(1):7–12. doi: 10.1016/s0022-3999(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Pauls DL, Alsobrook JP, 2nd, Goodman W, Rasmussen S, Leckman JF. A family study of obsessive-compulsive disorder. Am J Psychiatry. 1995;152(1):76–84. doi: 10.1176/ajp.152.1.76. [DOI] [PubMed] [Google Scholar]
- Pauls DL, Raymond CL, Stevenson JM, Leckman JF. A family study of Gilles de la Tourette syndrome. Am J Hum Genet. 1991;48(1):154–163. [PMC free article] [PubMed] [Google Scholar]
- Pauls DL, Towbin KE, Leckman JF, Zahner GE, Cohen DJ. Gilles de la Tourette's syndrome and obsessive-compulsive disorder. Evidence supporting a genetic relationship. Arch Gen Psychiatry. 1986;43(12):1180–1182. doi: 10.1001/archpsyc.1986.01800120066013. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Krystal JH, Coric V. Glutamate-modulating drugs as novel pharmacotherapeutic agents in the treatment of obsessive-compulsive disorder. NeuroRx. 2006;3(1):69–81. doi: 10.1016/j.nurx.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S. PLINK v1.06. http://pngu.mgh.harvard.edu/purcell/plink/.
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva A, Kohane IS. SNPper: retrieval and analysis of human SNPs. Bioinformatics. 2002;18(12):1681–1685. doi: 10.1093/bioinformatics/18.12.1681. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, Bhandari R, Rose M, Ivey J, Boyd C, Moore GJ. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J Am Acad Child Adolesc Psychiatry. 2004;43(9):1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- Scannevin RH, Huganir RL. Postsynaptic organization and regulation of excitatory synapses. Nat Rev Neurosci. 2000;1(2):133–141. doi: 10.1038/35039075. [DOI] [PubMed] [Google Scholar]
- Scharf JM, Pauls DL. Genetics of tic disorders. In: Rimoin DL, Connor JM, Pyeritz RE, Korf BR, editors. Emery and Rimoin's Principles and Practices of Medical Genetics. 5th ed. New York: Elsevier; 2007. pp. 2737–2754. [Google Scholar]
- Sequenom. Sequenom Applications Overview V1.1. 2009:36. [Google Scholar]
- Shugart YY, Wang Y, Samuels JF, Grados MA, Greenberg BD, Knowles JA, McCracken JT, Rauch SL, Murphy DL, Rasmussen SA, et al. A family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder in 378 families. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(6):886–892. doi: 10.1002/ajmg.b.30914. [DOI] [PubMed] [Google Scholar]
- Stewart SE, Fagerness JA, Platko J, Smoller JW, Scharf JM, Illmann C, Jenike E, Chabane N, Leboyer M, Delorme R, et al. Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(8):1027–1033. doi: 10.1002/ajmg.b.30533. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448(7156):894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JM, Wang D, Feng G. Differential mRNA expression and protein localization of the SAP90/PSD-95-associated proteins (SAPAPs) in the nervous system of the mouse. J Comp Neurol. 2004;472(1):24–39. doi: 10.1002/cne.20060. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Moya PR, Timpano KR, Anavitarte AP, Kruse MR, Wheaton MG, Ren-Patterson RF, Murphy DL. A haplotype containing quantitative trait loci for SLC1A1 gene expression and its association with obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66(4):408–416. doi: 10.1001/archgenpsychiatry.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchner S, Wendland JR, Ashley-Koch AE, Collins AL, Tran-Viet KN, Quinn K, Timpano KC, Cuccaro ML, Pericak-Vance MA, Steffens DC, et al. Multiple rare SAPAP3 missense variants in trichotillomania and OCD. Mol Psychiatry. 2009;14(1):6–9. doi: 10.1038/mp.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Linkage Disequilibrium (LD) map of DLGAP3 in the European HapMap population in Haploview (Barrett et al., 2005). Red shading on the map indicates D’ values; numbers within boxes indicate r2 values. Haplotype blocks were defined using the 95% confidence interval classification of Gabriel et al., 2002. Tag SNPs selected for the current study are circled in red; additional non-HapMap SNPs which were genotyped are illustrated in red-font; SNPs excluded after quality control are crossed out.