Abstract
Background and purpose
Emerging data suggests that the molecular cell death pathways triggered by ischemic insults differ in male and female brain. Cell death in males is initiated by poly (ADP-ribose) polymerase-1 (PARP-1) activation; however manipulation of this pathway paradoxically increases ischemic damage in females. In contrast, females are exquisitely sensitive to caspase mediated cell death. The effect of caspase inhibition in PARP-1 knockout mice was evaluated to determine if the detrimental effects of PARP deletion in females was secondary to increased caspase activation.
Methods
Focal stroke was induced by transient or permanent middle cerebral artery occlusion (MCAO) in wild type (WT) and PARP-1−/− mice of both sexes. The pan-caspase inhibitor, quinoline-Val-Asp(Ome)-CH2-O-phenoxy (Q-VD-OPh) was administered 90 minutes after MCAO. Infarct size and neurological sores were assessed. Separate cohorts were utilized for protein analysis for PAR, AIF, caspase-9 and -3.
Results
WT mice of both sexes had increased nuclear AIF after stroke compared to PARP-1−/− mice. PARP-1−/− females had higher mitochondrial cytochrome C, activated caspase-9 and -3 levels than WT female mice. PARP-1−/− females also had an increase in stroke-induced cytosolic C release compared to WT females which was not seen in males. Q-VD-OPh decreased caspase-9 in both males and females but only led to reduction of infarct in females. PARP-1−/− males had smaller infarcts whereas PARP-1−/− females had larger strokes compared to WT. Q-VD-OPh significantly decreased infarct in both WT and PARP-1−/− females in both transient and permanent MCAO models, but had no effect in males.
Conclusions
Deletion of PARP-1 reduces infarct in males, but exacerbates injury in females. PARP−/− females have enhanced caspase activation. The detrimental effects of PARP loss in females can be reversed with caspase inhibition.
Keywords: AIF, caspase, MCAO, PARP, sex differences
Introduction
It is increasingly recognized that stroke is a sexually dimorphic disease both experimentally and clinically, although these sex differences are still poorly understood 1. Although estrogen (E2) has long been suggested to contribute to sex differences in stroke 2, non-hormonal factors are also involved in ischemic sexual dimorphism 3. Accumulating experimental studies have demonstrated sex differences in ischemic cell death in vitro from cultures derived from male vs. female cells 4, 5. Caspase inhibition dramatically reduced injury in female but not male pups 6 whereas PARP deletion protected male pups, but not females 7. We have previously reported similar findings in adult stroke models 8–10. Importantly, in these previous studies PARP inhibition or deletion significantly exacerbated injury in females, suggesting that PARP activation may be protective in the female brain.
In this study, we tested the hypothesis that loss of PARP leads to up-regulation of caspase-mediated pathways in PARP-1 knock out (KO) females, leading to elevated levels of caspases and subsequent enhancement of damage after MCAO. We administered Q-VD-OPh, a novel broad-spectrum caspase inhibitor to determine if the detrimental effects of PARP deletion in females could be ameliorated with caspase inhibition.
Materials and Methods
Animals
PARP-1 KO and corresponding WT (SV-129 Brown) mice were originally purchased from Jackson laboratories (KO) or Taconic (WT) (Germantown, NY USA) and bred in house (F9). All experiments were performed according to NIH guidelines for the care and use of animals in research and under protocols approved by the UCHC Animal Care and Use Committee of the University of Connecticut Health Center. Young mice (19–12 weeks; 21–25 g) of both sexes were utilized. Genotypes were confirmed with PCR.
Ovariectomy and Hormone Measurements
To determine the contribution of estrogen and to normalize infarct size, mice of each genotype were ovariectomized two weeks prior to MCAO. Loss of estrogen was confirmed by ELISA for serum 17β-estradiol levels (IBL HAMBURG, Hamburg, Germany) and uterine weights as previously described 11.
Focal Cerebral Ischemia
Focal transient cerebral ischemia was induced by MCAO (tMCAO) for 90 minutes followed by reperfusion as described previously12. Permanent MCAO (pMCAO) was induced in a similar fashion, but the occluding suture remained in the MCA until sacrifice (48h after stroke). Cerebral blood flow (CBF) was measured by Laser Doppler flowmetry (LDF, Moor Instruments Ltd, England) during MCAO. Neurological deficits (NDS) were scored at 1.5h and 24h post-stroke, as described in 12.
Drug administration
Q-VD-OPh (MP Biomedicals, Aurora, Ohio, USA; 25 mg/kg) was dissolved in DMSO and further diluted with sterile PBS (10% DMSO) and injected intraperitoneally (0.21~0.25 cc total volume based on body weight) at reperfusion or 90 minutes post-occlusion as in 8 in both stroke and surgical shams. Control mice were injected with vehicle alone.
Histological Assessment
24 hours after stroke the mice were euthanized and the brains were stained with 1.5% 2,3,5-triphenyltetrazolium (TTC) solution as previously described 12.
Sub-cellular Fractionation and Western Blotting
Brain samples were obtained by rapid removal of the brain 6h after MCAO and were fractionated into the cytosol, mitochondrial, and nuclear fraction as described in 8. Protein concentration was determined by BCA™ Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL) and subjected to Western Blotting as described 11. Primary antibodies were: PAR polymers (BD Biosciences, 1:1000), AIF (Abcam, 1:500), and caspase 9 (Cell Signaling, 1:1000) and -3 (Santa Cruz Biotechnology, 1:500. Macrophage migration inhibitory factor (MIF) (1:1000; Cell Sciences), COX IV (Abcam, 1:1000), and Histone H3 (1:4000; Abcam). Secondary antibodies were from Santa Cruz: goat anti-rabbit IgG 1:5000, goat anti-mouse IgG 1:2000, donkey anti-goat IgG 1:1000. Densitometry of Western Blots was analyzed with Scion Image as described in 11.
Statistics
Data from individual experiments were presented as Mean ± SEM and analyzed with a t-test for two groups, and two-way analysis of variance (with Turkey post-hoc correction for multiple comparisons where appropriate) for the comparison of the means between the experimental groups. Neurological deficit scores were analyzed with Mann-Whitney U test. P<0.05 was considered statistical significant. All behavioral and histological assessments were performed by an investigator blinded to genotype/drug treatment.
Results
PARP-1 deletion protected males but exacerbated injury in females after MCAO
PARP-1−/− male mice had significantly smaller infarct than WT male mice (KO vs. WT: 19.7±4.5 vs. 38.4±3.8 %; n=9/gp, P<0.05) (Fig. 1A), confirming the protective effect of PARP deletion in males in this model 10. PARP-1−/− ovariectomized female mice exhibited a significant exacerbation in infarct size compared with WT ovariectomized females (KO vs. WT: 52.2±4.8 vs. 36.0±4.5 %; n=12/gp, P<0.05).
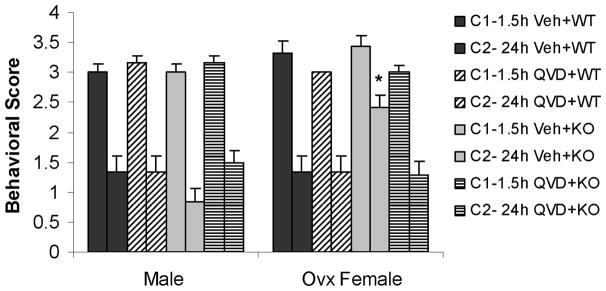
Figure 1. Stroke outcomes in male and female mice after MCAO.
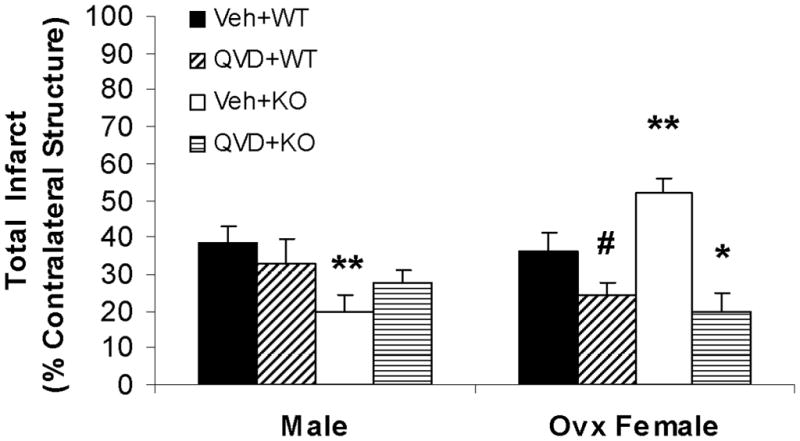
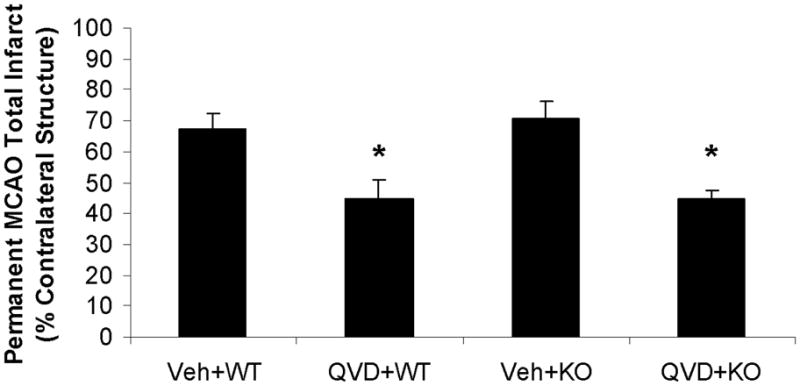
(A) Total infarct (24h after MCAO); percentage of contralateral structure. *P<0.01 versus vehicle treated KO female group; **P<0.05 versus vehicle treated WT mice; #P<0.05 versus vehicle treated WT female group. (B). NDS at 1.5h and 24h of MCAO. The adjacent columns (C1 and C2) of the same pattern indicate the same group at the 1.5h and 24h time points. *P>0.05 versus 1.5h in vehicle treated KO female group, and *P<0.05 versus 24h in other groups. (C). Total infarct after pMCAO. Brains were assessed at 48h of stroke. *P<0.05 versus vehicle treated group. Veh, vehicle; QVD, Q-VD-OPh; WT, wild type; KO, knock out; C, column.
Q-VD-OPh treatment was neuroprotective in female but not male WT and PARP−/− mice
Administration of Q-VD-OPh had no effect on infarct in male WT mice, although there was an apparent increase in damage in PARP-1−/− mice treated with Q-VD-OPh, this trend did not reach statistical significance (Fig. 1A). In females, treatment with Q-VD-OPh significantly decreased infarct volumes in WT ovariectomized mice (vehicle vs. drug: 36.0±4.5 vs. 24.1±3.5 %; n=12/gp, P<0.05). Q-VD-OPh administration significantly reduced infarct size in PARP-1−/− female mice (vehicle vs. drug: 52.2±4.8 vs. 19.9±5.2; n=12/gp %, P<0.01) to levels seen in WT Q-VD-OPh treated mice. All cohorts had improved behavioral deficits at 24h compared to that seen immediately (1.5h) after stroke except PARP-1−/− vehicle-treated females, which had worse deficits at 24h (Fig. 1B). PARP-1−/− males had lower scores than WT males, and PARP-1−/− females had higher scores than WT, an effect that was reversed with Q-VD-OPh treatment.
Q-VD-OPh decreased infarct volumes in OVX females subjected to pMCAO
To test whether the effect of Q-VD-OPh on stroke was model dependent, or dissipated at longer survival endpoints, another cohort of OVX WT and PARP-1 KO female mice were subjected pMCAO with 48 hours survival. Similar to the results seen in temporary/reperfusion models, damage from pMCAO was exacerbated in KO compared to WT littermates. Infarct was more severe in the permanent model in both genotypes compared to transient ischemia models in vehicle treated groups: WT pMCAO vs. tMCAO: 64.3±5.0% vs. 36.0±4.5%, n=7/gp, p<0.01; KO pMCAO vs. tMCAO: 74.86±5.2% (n=6) vs. 52.2±4.8% (n=12), p<0.05 (Fig. 1A&C). Q-VD-OPh treatment reduced infarct damage after pMCAO in both WT and PARP-1 KO female mice (Fig. 1C).
Physiological parameters, serum E2 levels, and uterine weights were not significantly different between WT and KO female mice
No differences in pH, pO2, pCO2, blood glucose, mean arterial pressure (MAP) or LDF were seen between vehicle- and drug-treated mice of either WT (data not shown) or PARP-1−/− mice (Table 1). There were no differenced in estrogen levels between any of the animal groups Figure 2A. All female mice had low uterine weights and atrophy (Fig. 2B) consistent with the loss of end-organ estrogenic effects 11.
Table 1.
Physiological measurements in vehicle- and drug-treated KO mice
| Group | pH | pO2mmHg | pCO2mmHg | Glucose mg/dl | MABP mmHg | LDF(%) ischemia | LDF(%) reperfusion |
|---|---|---|---|---|---|---|---|
| Male-----vehicle | 7.34 ± 0.12 | 86 ± 5 | 38 ± 11 | 170 ± 17 | 61 ± 3 | 11 ± 1 | 87 ± 4 |
| -----drug | 7.28 ± 0.07 | 88 ± 13 | 31 ± 9 | 166 ± 26 | 65 ± 4 | 11 ± 3 | 90 ± 3 |
| Female--vehicle | 7.29 ± 0.07 | 94 ± 11 | 39 ± 5 | 181 ± 16 | 63 ± 3 | 12 ± 1 | 90 ± 2 |
| --drug | 7.26 ± 0.13 | 91 ± 18 | 34 ± 11 | 178 ± 29 | 63 ± 4 | 11 ± 2 | 89 ± 3 |
No differences were seen in physiologic variables between vehicle- and drug-treated mice of either sex prior to (not shown) and 60 minutes after MCAO.
Figure 2.
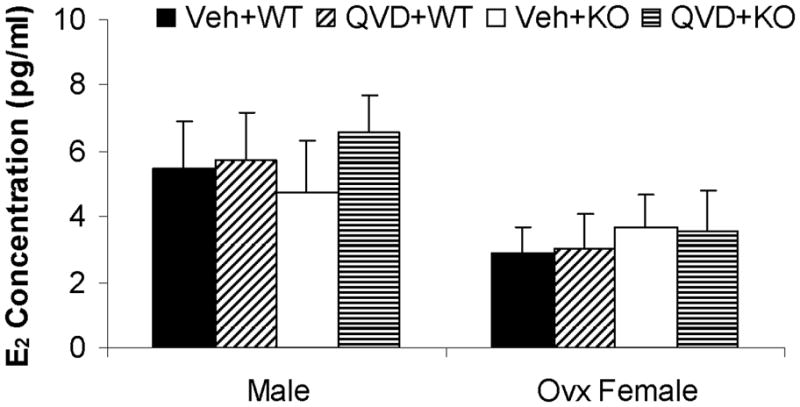
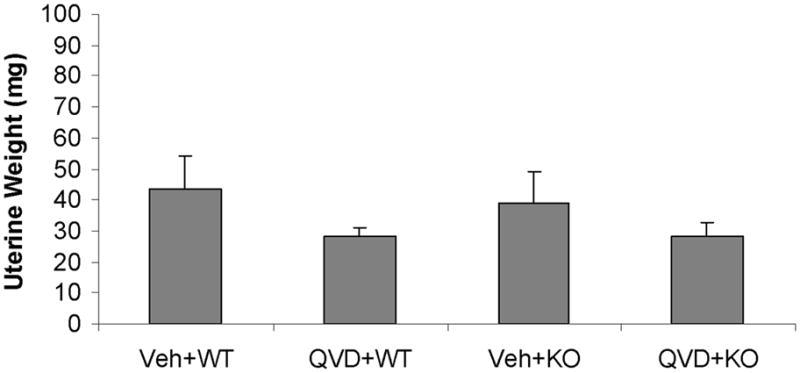
Serum E2 levels (A) and uterine weights (B). No significant were found between groups. Veh, vehicle; QVD, Q-VD-OPh; WT, wild type; KO, knock out.
PARP-1 deletion significantly reduced PAR polymer formation and AIF translocation in both male and female mice
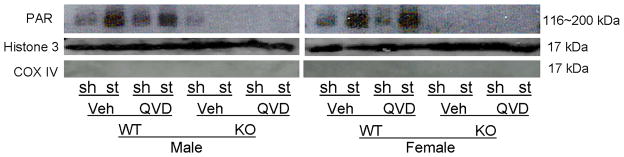
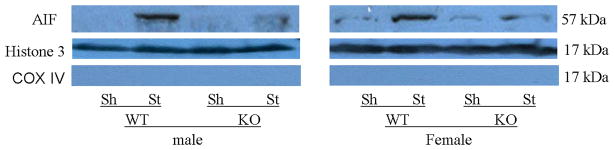
PAR polymer formation and nuclear AIF translocation 6h after tMCAO was examined. In both male and female WT mice, nuclear PAR levels dramatically increased after stroke compared to sham; however, there was no difference in PAR levels between vehicle and drug treated WT mice of either sex (Fig. 3A; n=4/gp). PARP-1−/− mice of both sexes had a dramatic reduction in nuclear PAR polymers as shown in Figure 3A regardless of Q-VD-OPh treatment. As expected, very little nuclear AIF translocation was seen in sham mice and KO mice (Fig. 3B~D).
Figure 3.
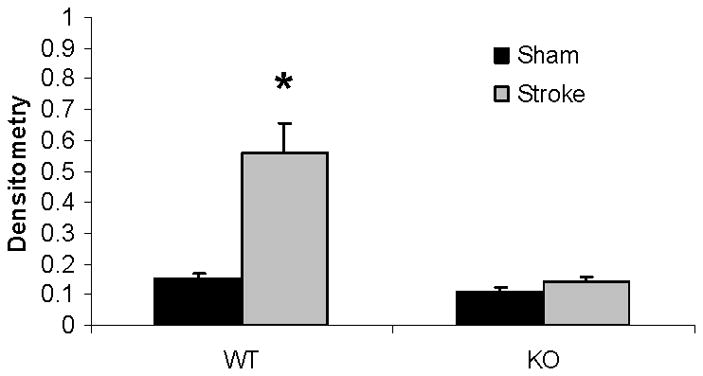
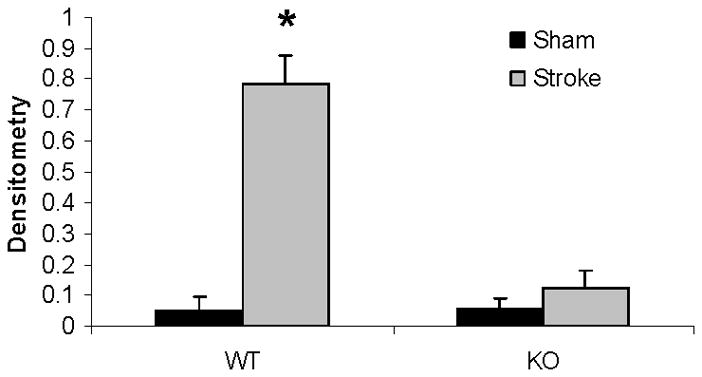
Western blots of key proteins in the PARP-1-AIF cell death pathway. (A). Nuclear PAR in both male and female group (n=4 hemispheres/gp). Each sample had biological triplicates. (B). Nuclear AIF translocation in both male and female group. n=6 animals/gp, repeated in triplicate. Histone 3 served as the loading control and COX IV as the negative control to confirm nuclear fraction purity in both (A) and (B). (C & D).The optical density (OD) was expressed as the ratio of the AIF bands to control bands (histone 3). (C), Male; (D), Female. *P<0.01 versus any other group. Veh, vehicle; QVD, Q-VD-OPh; WT, wild type; KO, knock out; Sh, sham; St, stroke.
PARP-1 deletion had different effects on cytochrome C and caspase-3 levels in male and female mice after tMCAO
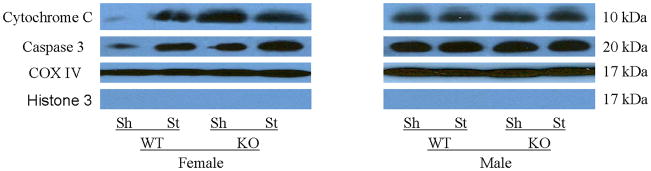
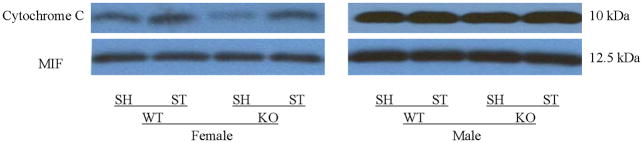
Mitochondrial cytochrome C and caspase-3 levels after tMCAO were higher in PARP-1−/− females compared to WT females; whereas no changes were seen as a consequence of either MCAO or PARP-1 deletion in male mice (Fig. 4A~C). Cytosolic Cytochrome C levels significantly increased after stroke in both WT and KO female mice; however, the ratio of sham to stroke-induced increase in cytosolic cytochrome C was significantly higher in PARP KO females compared to WT females. No differences in cytochrome C levels were seen in males (Fig. 5A~C).
Figure 4.
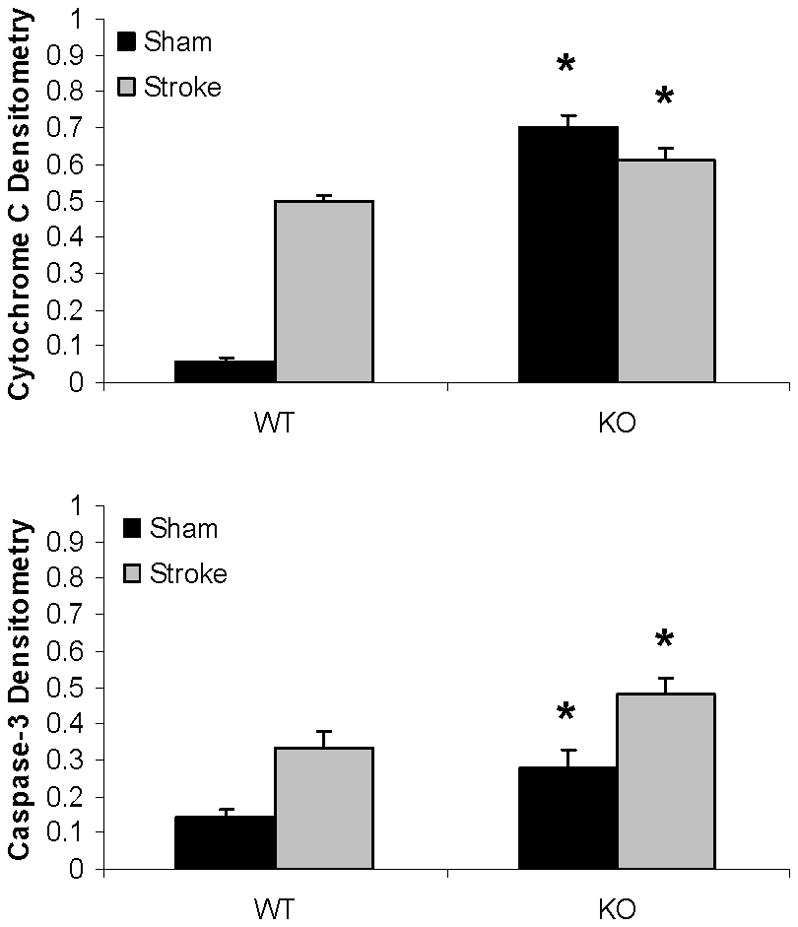
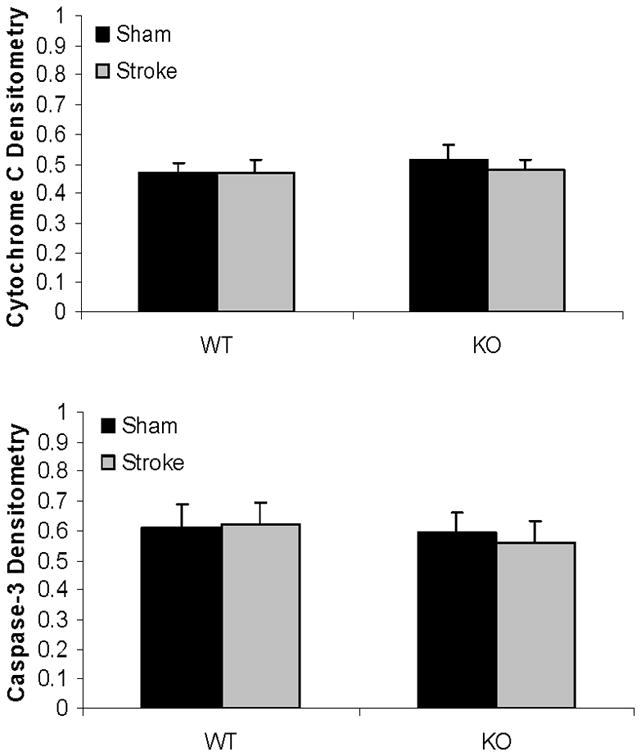
Western blots of key proteins in cytochrome C-caspase cell death pathway in the mitochondrion. (A). Cytochrome C and caspase-3 in both male and female group. COX IV served as the loading control and histone 3 as the negative control to confirm the purity of the mitochondrion. (B & C). OD was expressed as the ratio of the cytochrome C (upper panel) or caspase-3 (lower panel) bands to control bands. (B), Female; (C), Male. *P<0.05 versus WT and drug treated KO counterparts. n=6 animals/gp, samples run in triplicate. Veh, vehicle; QVD, Q-VD-OPh; WT, wild type; KO, knock out; Sh, sham; St, stroke.
Figure 5.
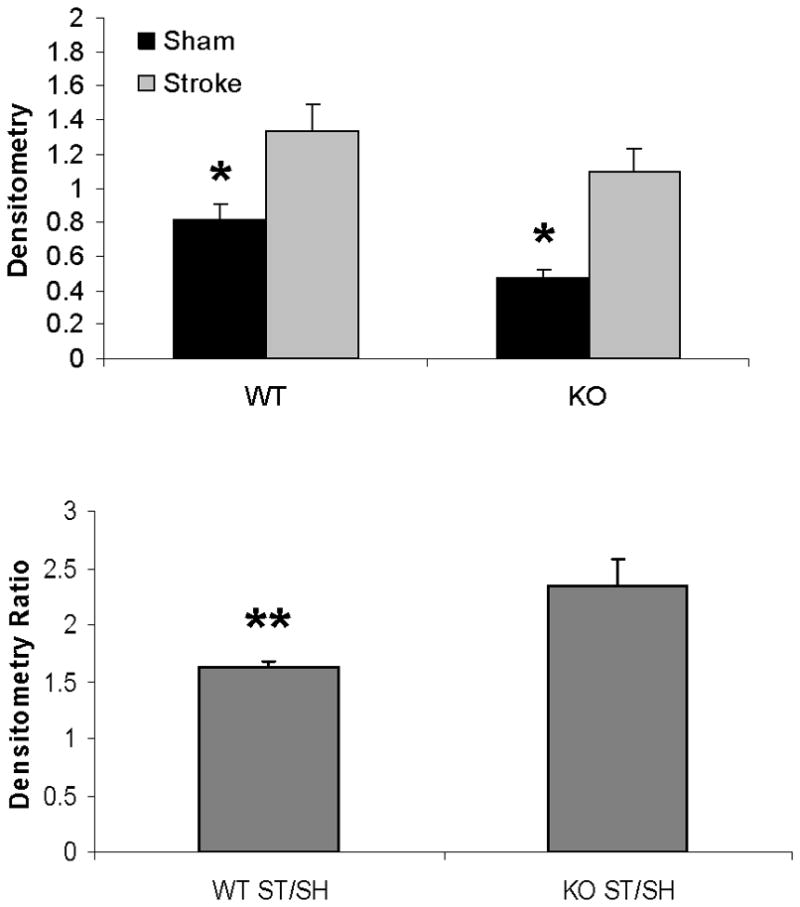
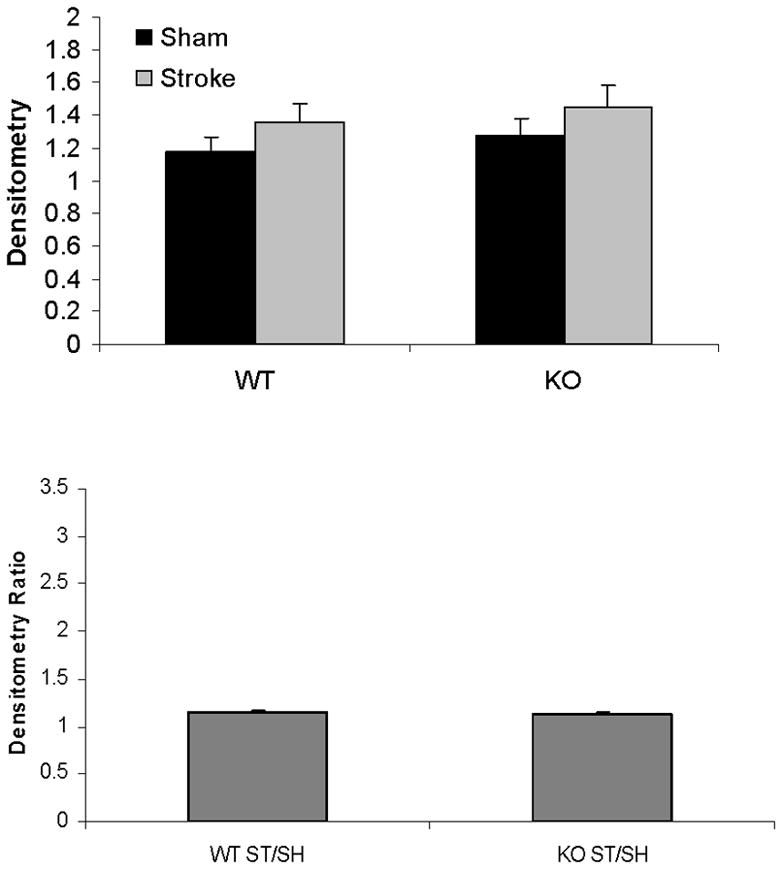
Western blots of cytochrome C in the cytosol. (A). Cytochrome C levels in both male and female group. MIF served as the loading control. (B & C). OD (upper panel) was expressed as the ratio of the cytochrome C bands to control bands. The density ratio (lower panel) was calculated by dividing densitometry of stroke group with that of sham group. (B), Female; (C), Male. *P<0.05 versus stroke group; **P<0.05 versus KO group. n=6 animals/gp, samples run in triplicate. Veh, vehicle; QVD, Q-VD-OPh; WT, wild type; KO, knock out; Sh, sham; St, stroke.
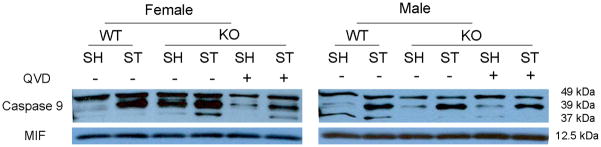
Q-VD-OPh treatment decreased caspase-9 levels in female mice after tMCAO
In order to assess the effect of Q-VD-OPh on caspase expression in PARP-1−/− mice, we administered drug or vehicle to PARP-1−/− male and female mice immediately after reperfusion. Activated (cleaved) caspase-9 levels were low in sham WT mice of both sexes (see lane 1; Fig. 6A). Stroke increased caspase-9 cleavage products in both female and male WT mice (lane 2), with significantly higher levels in females (female: lane 1 vs. lane 2) (Fig. 6B). Interestingly, PARP-1−/− females had higher levels of caspase-9 cleavage products both in sham and after MCAO compared to WT mice (female: lane 1&2 vs. 3&4 respectively). Q-VD-OPh treatment reduced MCAO-induced caspase-9 activation in KO female and male mice after stroke (lane 4 vs. lane 6) and reduced the baseline elevation in caspase activation in sham PARP−/− females (female: lane 3 vs. lane 5). No differences in cleaved caspase-9 were observed between WT and KO vehicle treated male mice (male: lane 1&2 vs. lane 3&4 respectively) (Fig. 6C).
Figure 6.
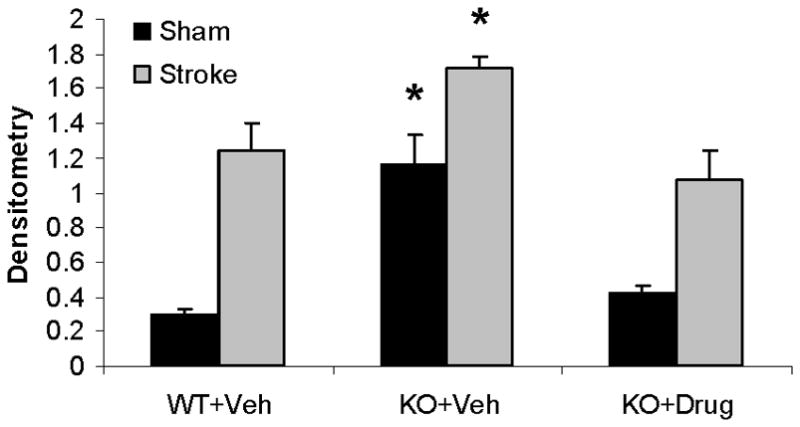
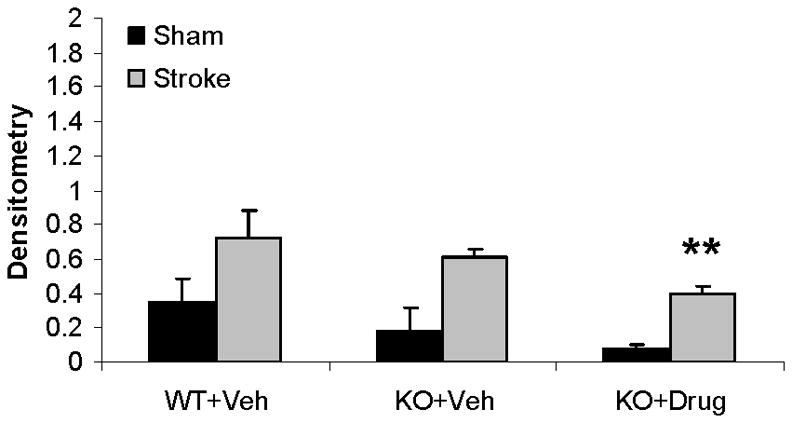
Q-VD-OPh treatment decreases expression of activated Caspase-9 in females. (A). Caspase-9 levels in the cytosol with (+) or without (vehicle −) Q-VD-OPh treatment. MIF served as the loading control. (B & C). OD was expressed as the ratio of cleaved caspase-9 bands (39 and 37 kDa) to control bands. *P<0.05 versus WT and drug treated KO counterparts; **P<0.05 versus vehicle treated KO counterpart. n=6 animals/gp, samples run in triplicate. Veh, vehicle; QVD, Q-VD-OPh; WT, wild type; KO, knock out; Sh, sham; St, stroke.
Discussion
This study examined PARP and caspase activation in cerebral ischemia. There are several important findings. 1). PARP-1 deletion is deleterious in females subjected to ischemic injury, in both reperfusion 10 and permanent MCAO models. 2). Caspase inhibition with Q-VD-OPh protects females but not males. Importantly, Q-VD-OPh decreased infarct volumes in both WT and KO female mice. Caspase inhibition reduced infarct to levels seen in WT Q-VD-OPh mice, rescuing the deleterious PARP KO phenotype. This neuroprotection was independent of acute estrogen levels as all the females were ovariectomized. In contrast, Q-VD-OPh had no effect in either WT or KO males. Taken together, this implies that the effect of caspase inhibition is dependent on sex but independent of PARP. 3). The neuroprotective effect of caspase inhibition was seen in a permanent ischemia model, and was present even after 48 hours. In this model the differences between WT and PARP KO mice were more modest, likely due to a ceiling effect. 4). PAR polymer formation and nuclear AIF translocation were increased after stroke in both male and female WT mice. PARP-1 deletion attenuated both PAR formation and nuclear AIF translocation but this led to different effects depending on the sex of the animal, with neuroprotection in males, and increased infarct in females. 5). PARP-1−/− females had a baseline elevation in mitochondrial cytochrome C, and activated caspase-3 and -9 expression, suggesting that the brain is “primed” to turn on caspase pathways, and does so even without an identified stressor, e.g. ischemia. 6). Stroke induced significantly more cytosolic cytochrome C release and caspase activation (as measured by cleavage products) in PARP-1 KO females compared to either WT females or KO males, showing a further response to this “priming”. Q-VD-OPh ameliorated this abnormal caspase response in KO female mice.
Emerging data provides evidence for striking and previously unsuspected sexual dimorphism in cell death pathways induced by ischemic injury and this study provides further evidence for this concept. In experimental models, males preferentially utilize caspase-independent mechanisms to trigger cell death initiated by PARP activation with subsequent nuclear AIF translocation 9, 10, 13, 14. The smaller infarcts in PARP KO males support the hypothesis that PARP-1 is an important mediator of cell death in male animals 15. Interestingly, these same molecular events occur in females 10 (Figure 3), but do not lead to cell death as evidenced by PARP KO females who have significant reductions in PAR and AIF yet have an exacerbation in stroke damage compared to WT. The significant exacerbation in damage in PARP-1 KO females after MCAO suggested that PARP activation is either 1) a protective in females or 2) that loss of PARP allows for activation of a more deleterious pathway. As female neurons 5, neonates 6, and adults8 are more sensitive to caspase mediated cell death, we first evaluated caspase activation in PARP KO mice. PARP-1−/− females had higher levels of mitochondrial cytochrome C, caspase-9 and -3 than WT mice suggesting that the caspase pathway is induced by the loss of PARP. The importance of changes in mitochondrial levels of cytochrome C is unknown. Traditionally, the triggering factor for caspase-mediated cell death is the mitochondrial release of cytochrome C into the cytosol16, however no previous studies examined what occurs in the female brain in the mitochondrial fraction. WT females have increased cytosolic cytochrome C release with injury compared to males8, and the timing of release differs by sex in neonatal models6. In this study, we saw an increase in cytosolic cytochrome C levels after stroke in both WT and PARP−/− females vs. sham females. No changes were seen in males with stroke at this time-point. Importantly, in agreement with our hypothesis that PARP females have enhanced caspase induced cell death due to the loss of PARP, PARP KO females had significantly more cytosolic cytochrome C release than WT females (expressed as a ration of stroke/sham).
Controversy exists in the literature as to effects of Q-VD-OPh on male animals 6, 17, 18. In this study Q-VD-OPh attenuated the protective effect of PARP-1 deletion in males (Figure 1a), suggesting that caspase activation may either be a protective response in the male brain or alternatively that males are insensitive to downstream events triggered by caspase activation. This could account, in part, for the variable results seen in preclinical stroke models that have examined the effects of caspase inhibition, the vast majority of which were performed exclusively in male animals 19.
Levels of cytochrome C, activated caspase-9 and -3 were significantly higher in both sham and MCAO PARP KO females compared to sex-matched WT. This interaction between caspase dependent and independent pathways has not been previously reported and could explain the severe phenotype seen in PARP−/− females after MCAO. The translational importance of these findings become clear when one considers the pharmacological agents currently being developed for clinical trials, such as Minocycline, which acts as PARP inhibitor 20 and has sex-specific effects in pre-clinical models.
Suggesting that an absolute dichotomy exists in sensitivity to specific cell death pathways is overly simplistic. These two cell death pathways do not act independently, as crosstalk exists through the interaction of AIF and cytochrome C in the mitochondrion 21 and PARP can be directly cleaved by caspase 3 and 7 22. PARP activation enhances formation of PAR polymers that are directly toxic when injected into neurons, leading to apoptosis and nuclear condensation inducing cell death even without loss of NAD+ 23. The downstream nuclear events that trigger the final phase of cell death in females remain unknown.
There are several limitations to this work, and our results must be interpreted with these in mind. Stroke induced changes in caspases and cytochrome C in the mitochondria need to be confirmed. Despite appropriate loading controls, technical issues (ie., cytosolic contamination) could be a factor. We only evaluated a genetic model of PARP deficiency, which have had the loss of PARP-1 throughout development. Findings could differ in pharmacological models, as the enhanced baseline and stroke-induced caspase activation may take time to develop. We specifically did not employ PARP-1 inhibitors secondary to possible interactions with Q-VD-OPh dosing and metabolism. We only examined ovariectomized female mice in this study for several reasons: 1) the ovariectomy model is more translationally relevant to the population at risk for stroke, post-menopausal women; 2) stroke outcomes are more variable in gonadally intact females due to fluctuating levels of estrogen across estrus 24; 3) we were specifically interested in examining sex rather than hormone effects, therefore hormone replacement was not utilized. We recognize that acute ovariectomy cannot ameliorate the many developmental “organizational” effects of steroids25, and the contribution of ovarian steroids vs. sex (XX vs. XY) will be evaluated in future studies utilizing genetic models.
In conclusion, PARP-1 deletion and caspase inhibition have strikingly different effects on stroke outcomes that are dependent on the sex of the animal evaluated. Sensitivity to caspase activation predominates in females whereas stroke-induced cell death in males is mediated by PARP-1-AIF activation. Removal of PARP-1 protects males, but leads to a paradoxical exacerbation of injury in females. This is secondary to enhancement of caspases and can be reversed with caspase inhibition. Understanding the sex specific sensitivity to cell death has important translational relevance for the development of neuroprotective agents.
Acknowledgments
Sources of Funding
This work was supported by the NINDS (LDM NS055215 and NS050505).
Footnotes
Disclosures
None.
References
- 1.Bushnell CD, Hurn P, Colton C, Miller VM, del Zoppo G, Elkind MS, Stern B, Herrington D, Ford-Lynch G, Gorelick P, James A, Brown CM, Choi E, Bray P, Newby LK, Goldstein LB, Simpkins J. Advancing the study of stroke in women: Summary and recommendations for future research from an ninds-sponsored multidisciplinary working group. Stroke. 2006;37:2387–2399. doi: 10.1161/01.STR.0000236053.37695.15. [DOI] [PubMed] [Google Scholar]
- 2.Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29:289–295. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vannucci SJ, Hurn PD. Gender differences in pediatric stroke: Is elevated testosterone a risk factor for boys? Ann Neurol. 2009;66:713–714. doi: 10.1002/ana.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Pin S, Zeng Z, Wang MM, Andreasson KA, McCullough LD. Sex differences in cell death. Ann Neurol. 2005;58:317–321. doi: 10.1002/ana.20538. [DOI] [PubMed] [Google Scholar]
- 5.Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- 6.Renolleau S, Fau S, Goyenvalle C, Joly LM, Chauvier D, Jacotot E, Mariani J, Charriaut-Marlangue C. Specific caspase inhibitor q-vd-oph prevents neonatal stroke in p7 rat: A role for gender. J Neurochem. 2007;100:1062–1071. doi: 10.1111/j.1471-4159.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- 7.Hagberg H, Wilson MA, Matsushita H, Zhu C, Lange M, Gustavsson M, Poitras MF, Dawson TM, Dawson VL, Northington F, Johnston MV. Parp-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke. 2009;40:1842–1848. doi: 10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (adp-ribose) polymerase-1 in cerebral ischemia: Male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 10.Yuan M, Siegel C, Zeng Z, Li J, Liu F, McCullough LD. Sex differences in the response to activation of the poly (adp-ribose) polymerase pathway after experimental stroke. Exp Neurol. 2009;217:210–218. doi: 10.1016/j.expneurol.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu F, Schafer DP, McCullough LD. Ttc, fluoro-jade b and neun staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du L, Zhang X, Han YY, Burke NA, Kochanek PM, Watkins SC, Graham SH, Carcillo JA, Szabo C, Clark RS. Intra-mitochondrial poly(adp-ribosylation) contributes to nad+ depletion and cell death induced by oxidative stress. J Biol Chem. 2003;278:18426–18433. doi: 10.1074/jbc.M301295200. [DOI] [PubMed] [Google Scholar]
- 14.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(adp-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 15.Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL. Poly(adp-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 16.Fujimura M, Morita-Fujimura Y, Murakami K, Kawase M, Chan PH. Cytosolic Redistribution of Cytochrome c After Transient Focal Cerebral Ischemia in Rats. JCBFM. 1998;18:1239–1247. doi: 10.1097/00004647-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Braun JS, Prass K, Dirnagl U, Meisel A, Meisel C. Protection from brain damage and bacterial infection in murine stroke by the novel caspase-inhibitor q-vd-oph. Exp Neurol. 2007;206:183–191. doi: 10.1016/j.expneurol.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol. 2009;66:378–389. doi: 10.1002/ana.21714. [DOI] [PubMed] [Google Scholar]
- 19.Loetscher H, Niederhauser O, Kemp J, Gill R. Is caspase-3 inhibition a valid therapeutic strategy in cerebral ischemia? Drug Discov Today. 2001;6:671–680. doi: 10.1016/s1359-6446(01)01826-8. [DOI] [PubMed] [Google Scholar]
- 20.Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab. 2009;29:670–674. doi: 10.1038/jcbfm.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cregan SP, Dawson VL, Slack RS. Role of aif in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23:2785–2796. doi: 10.1038/sj.onc.1207517. [DOI] [PubMed] [Google Scholar]
- 22.Wang KK. Calpain and caspase: Can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- 23.Heeres JT, Hergenrother PJ. Poly(adp-ribose) makes a date with death. Curr Opin Chem Biol. 2007;11:644–653. doi: 10.1016/j.cbpa.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 24.Horsburgh K, Macrae IM, Carswell H. Estrogen is neuroprotective via an apolipoprotein e-dependent mechanism in a mouse model of global ischemia. J Cereb Blood Flow Metab. 2002;22:1189–1195. doi: 10.1097/01.wcb.0000037991.07114.4e. [DOI] [PubMed] [Google Scholar]
- 25.Siegel C, Turtzo C, McCullough LD. Sex differences in cerebral ischemia: Possible molecular mechanisms. J Neurosci Res. 2010;88:2765–2774. doi: 10.1002/jnr.22406. [DOI] [PubMed] [Google Scholar]