Abstract
Background
Using adult C57BL/6J (B6) mice, we previously developed a procedure that causes a progressive increase in ethanol intake and preference (i.e., alcohol escalation effect) following weekly (intermittent) access to ethanol (Melendez et al. Alcohol Clin Exp Res 30, 2006). A limitation of this procedure is that it requires many weeks of testing, which limits its use to study ethanol escalation (i.e., binge-like drinking) during adolescence. Previous studies have shown that intermittent every-other-day (EOD) access to ethanol is sufficient to induce ethanol escalation in rats. The objective of this study was to verify if EOD access is sufficient to induce escalated levels of ethanol intake and preference in adult and adolescent B6 mice.
Methods
Male B6 mice received free-choice 24 hr access to 15% ethanol and water on an EOD or daily basis for 2 weeks. Food and water was available at all times. Using adult mice, Experiment 1 characterized the induction of ethanol escalation following EOD access at 6 (i.e., drinking in the dark) and 24 hr intervals, whereas Experiment 2 determined if daily drinking reverses escalation induced by EOD drinking. Experiment 3 compared ethanol-drinking capacity following daily versus EOD drinking in adolescent (P30–45) and adult (P70–85) mice.
Results
Experiment 1 revealed that EOD drinking leads to a significant (nearly two-fold) increase in ethanol intake and preference over mice given daily access. Experiment 2 demonstrated that EOD-elicited escalation is blocked and subsequently reversed following daily drinking. Experiment 3 revealed that ethanol drinking was greater in adolescent mice compared to adults following daily drinking and EOD (escalated) drinking. Although the escalated levels of ethanol intake were greater in adolescent mice, the rate or onset of escalation was comparable between both age groups.
Conclusions
This study is the first to demonstrate that EOD drinking leads to escalation of ethanol intake and preference in adolescent and adult mice. Moreover, our results indicate that daily ethanol reverses ethanol escalation induced by intermittent drinking. The study also revealed that adolescent mice have a greater capacity to drink ethanol under both daily (controlled) and EOD (escalated) conditions, which further supports the notion of adolescent’s susceptibility to heavy drinking.
Keywords: ethanol escalation, high alcohol preference, adolescent drinking
Introduction
Alcohol research has benefitted tremendously from selective rodent breeding techniques to evaluate different facets of alcohol-related behaviors and the genetic factors that underlie alcoholism (reviewed by McBride and Li, 1998; Murphy et al., 2002). However, it is becoming clear that genetic manipulation of alcohol preference alone is not sufficient to yield a genotype that will escalate their level of drinking to the point of intoxication. To date, ethanol-drinking levels obtained from limited or continuous daily access conditions in outbred or selectively bred rodents are remarkably stable over long periods of time. Therefore, ongoing attempts have been made to develop models that express well-defined features of the addiction process (i.e., beyond high alcohol preference), with the ultimate objective of identifying neurobiological targets that facilitate the transition to alcohol dependence-addiction.
Since the pioneering observations of Sinclair and Senter (1968) various studies have shown that renewed availability of ethanol following a period of deprivation leads to a pronounce increase in ethanol intake and preference, also known as the alcohol deprivation effect (ADE; reviewed by Rodd et al., 2004; Spanagel 2000). Moreover, studies have shown that factors associated with the ADE could be strengthened by repeated deprivation cycles. Indeed, studies show that ethanol escalation can be obtained following short deprivation periods (e.g., Hargreaves et al., 2009; Simms 2008; Sinclair and Li, 1989; Wise, 1973) and long periods (e.g., McKenzie et al., 1998; Melendez et al., 2006; Rodd et al., 2002). Thus, it is reasonable to conclude that the schedule by which ethanol is offered (i.e., intermittent versus continuous) proves to be an essential factor mediating the expression of ethanol escalation in rodents.
Previously, we have shown that adult B6 mice given 15% ethanol on a weekly (i.e., intermittent) basis will escalate their levels of ethanol intake and preference compared to continuously exposed mice (Melendez et al., 2006). Notably, the transition from stable to escalated drug use has been posited as a model that can differentiate mere drug taking behavior from patterns of drug use characteristic of the addiction process (reviewed by Koob et al., 2004). To our knowledge, ethanol escalation has never been studied in adolescent B6 mice. This is important considering the prevalence of use and misuse (e.g., episodic intake or “binge drinking”) among human adolescents (reviewed by Spear, 2000). Therefore, the present study used the intermittent (EOD) access procedure initially described by Wise (1973) to determine if access to ethanol on alternating days is sufficient to induce ethanol escalation in adolescent and adult B6 mice. It is hypothesized that EOD drinking is sufficient to induce ethanol escalation in adult and adolescent B6 mice.
Methods
Animals
Male B6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and housed in a temperature-controlled AAALAC-approved colony room. Mice were individually housed in ventilated cages fitted with steel lids and filter tops and given ad libitum access to food (Teklad Rodent Chow, Lowell, MA) and water. Experiment 1, mice were placed on a 12 hr reverse light-dark cycle (lights off at 8:00 AM), whereas for Experiments 2 and 3, mice were on a regular 12 hr light-dark cycle (lights on at 8:00 AM). At all times, animals were treated in accordance with the National Institutes of Health, Guide for the Care and Use of Laboratory Animals (Institute of Animal Resources, 1996). The Institutional Animal Care and Use Committee approved all experimental procedures.
Ethanol Drinking Procedures (two-bottle choice test)
Mice received continuous (i.e., daily) or every-other-day (i.e., EOD) access in the home cage to 15% ethanol solution with food and water available at all times. Ethanol and water solutions were contained in 25-ml serological pipettes that were cut at both ends and sealed with a rubber stopper and silicon tube fitted with a stainless steel straight sipper tube. Ethanol solutions were prepared fresh every 48 hr by mixing ethanol with tap water to arrive at the 15% (v/v) ethanol concentration. The left/right position of the tubes was switched daily to control for the possibility of side preference. Body weights of mice were recorded every 72 hr and ethanol and water intake values were recorded every 24 hr (to the nearest 0.2 ml). These data were used to calculate self-administered ethanol dose (i.e., g/kg) and relative preference for ethanol (i.e., 15% ethanol intake/total fluid intake).
Experiment 1
The objective of this experiment was to determine if access to ethanol on alternating days (EOD) will lead to elevated subsequent ethanol intake and preference in adult mice. Mice were obtained at 8 weeks of age (n=16) and acclimated for a week prior to testing. During the first day of testing (i.e., session 1), all mice were 9 weeks of age and were given access to 15% ethanol and water for 24 hr and divided into two groups (equated for session 1 ethanol intake and preference). Immediately thereafter, EOD mice (n=8) received 24 hr access to ethanol on alternating days whereas daily mice (n=8) received ethanol continuously for 2 weeks. To evaluate ethanol drinking in the circadian active dark cycle, ethanol and water intake values were recorded at 6 hr in the dark and 24 hr intervals. Food and water was available at all times.
Experiment 2
The objective of this experiment was to initially induce ethanol escalation with EOD drinking and then determine if daily drinking reverses it. A separate cohort of mice (n=14) were obtained at 8 weeks of age and acclimated for a week prior to testing. During session 1, mice were 9 weeks of age and were given access to 15% ethanol and water for 24 hr and divided into two groups (equated for session 1 intake and preference). The EOD group (n=7) received 24 hr access to ethanol on alternating days (i.e., EOD) whereas the daily group (n=7) received ethanol continuously for 2 weeks. After session 7, EOD and daily mice were offered ethanol on a daily basis for 1 additional week (i.e., sessions 8–14). Ethanol and water intake values were recorded every 24 hr. Food and water was available at all times.
Experiment 3
The objective of this experiment was to evaluate EOD and daily access to ethanol in adolescent compared to adult mice. A single shipment of mice were obtained at 3 and 9 weeks of age (n=16/age group) and acclimated to housing conditions for 1 week. Testing began when mice were at 4 and 10 weeks of age, which approximates human adolescence and adulthood, respectively (Spear, 2000). Adolescent and adult mice were divided into EOD (n=8) and daily (n=8) access groups (equated for session 1 intake and preference) and given access to 15% ethanol for 2 weeks. EOD mice received access to ethanol on alternate days, whereas daily mice received ethanol continuously. Ethanol and water intake values were recorded every 24 hr. Food and water was available at all times.
Data Analysis
Analysis of variance (ANOVA) was performed with SPSS package version 9.0 (SPSS Inc. Chicago, IL). Data analysis for Experiments 1–3 consisted of a two-way factorial ANOVA with repeated measures over time (i.e., access sessions). ANOVAs were followed by post hoc comparisons with Fisher’s LSD and/or one-way ANOVA (i.e., t-tests) to determine the locus of significant interactions and/or main effects. A significance level of p < 0.05 was used for all statistical analysis.
Results
Experiment 1: Rapid Escalation Elicit by EOD Access Conditions
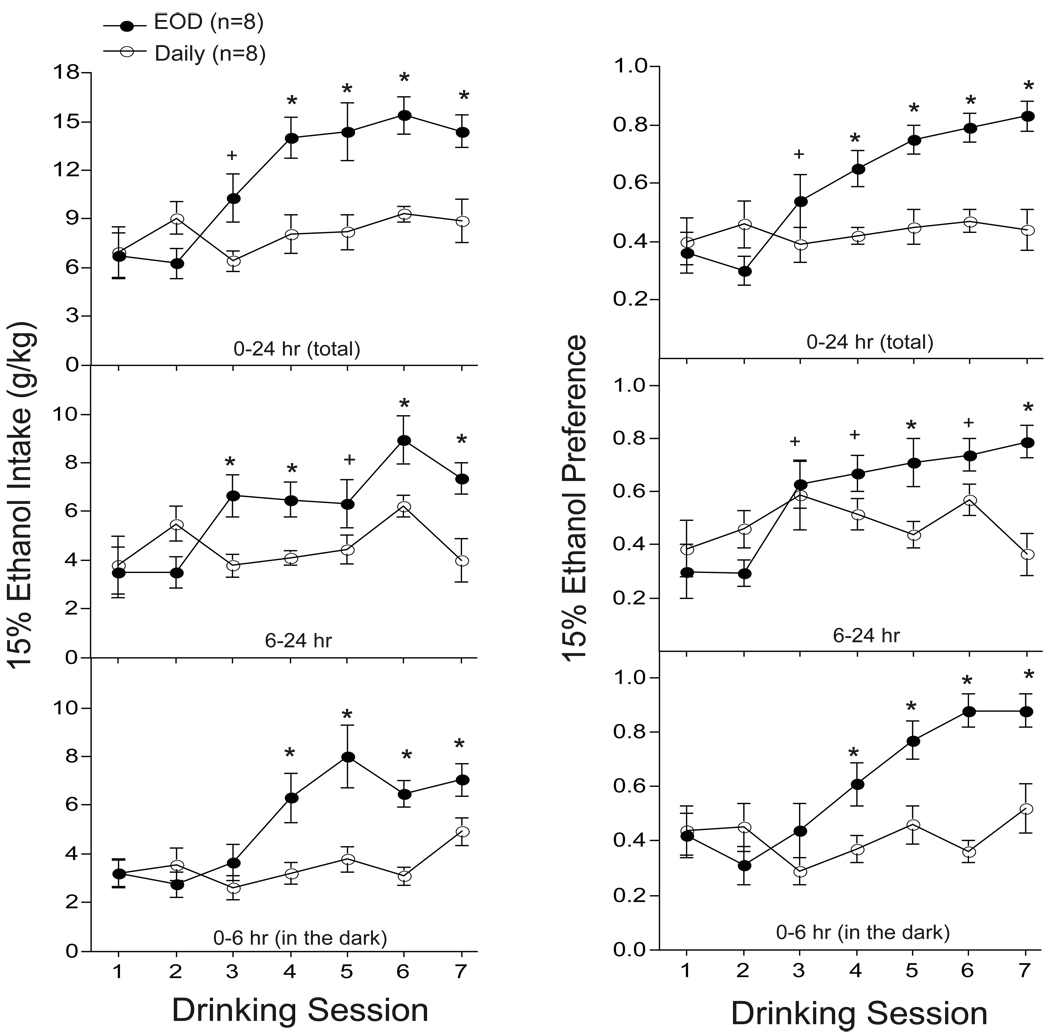
The purpose of this experiment was to determine if EOD access to ethanol causes a progressive increase of ethanol intake and preference in adult mice. Ethanol intake and preference values were analyzed at 6, 6–24, and 24 hr intervals. Analysis of 24 hr (total) intake and preference values (Fig. 1, top panel) revealed that EOD mice showed greater ethanol intake and preference compared to daily mice (group × session: F’s6,84 > 6.84; p < 0.01). EOD mice expressed ethanol escalation as early as the third session of intermittent drinking, which was sustained across sessions 4–7 relative to session 1 (session: F’s6,42 > 17.1; p < 0.01). No significant differences in 24 hr ethanol intake and preference were observed during daily access drinking (session 1–7: F’s6,42 < 1.2; p > 0.34). Analysis of the 6–24 hr drinking interval (Fig. 1, middle panel) also revealed greater levels of ethanol intake and preference in EOD compared to daily mice (group × session: F’s6,84 > 6.88; p < 0.01). EOD-induced escalation of ethanol intake and preference was also observed during session 3 and sustained across sessions 4–7 (session: F’s6,42 > 12.2; p < 0.01). No significant differences in 6–24 hr ethanol intake and preference were observed in daily mice (session 1–7: F’s6,42 < 3.5; p > 0.10). With regards to drinking during the circadian dark cycle (Fig. 1, bottom panel), our analysis revealed that ethanol drinking and preference was greater in EOD compared to daily mice (group × session: F’s6,84 > 3.94; p < 0.01). Compared to session 1, EOD mice escalated their levels of ethanol intake and preference during session 4, which was sustained across session 5–7 (session: F’s6,42 > 7.6; p < 0.01). No significant differences in ethanol drinking and preference were observed at 6 hr in the dark during daily access conditions (session: F’s6,42 < 1.2; p > 0.34). It is interesting to note that nearly half of all ethanol intakes in EOD (~7.5 g/kg) and daily (~5 g/kg) mice took place during the active (0–6 hr) circadian dark cycle.
Fig. 1.
Rapid escalation of ethanol intake and preference following EOD (every-other-day) compared to daily access to ethanol in adult B6 mice. Ethanol intake (g/kg) and preference scores for EOD (n=8) and daily (n=8) mice were recorded for 2 weeks at 24 hr (top panel), 6–24 hr (middle panel), and 0–6 hr (lower panel) intervals. + p < 0.05 compared to session 1; * p < 0.05 compared to session 1 and group.
Experiment 2: Daily Drinking Blocks and Reverses Escalation Induced By EOD Drinking
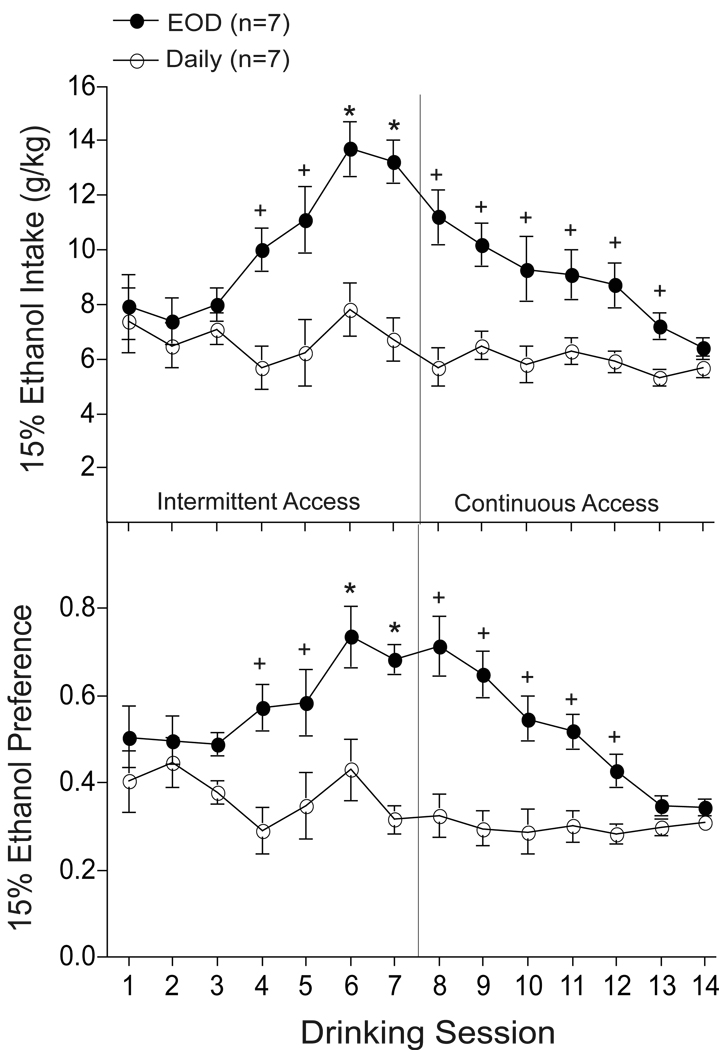
The objective of this experiment was to determine if ethanol escalation induced by EOD is reversed by continuous-daily access to ethanol. Using a separate replicate of adult mice, EOD and daily groups were exposed to ethanol intermittently and daily respectively, for 1 week (sessions 1–7) followed by continuous-daily access only (sessions 8–14). As shown in Fig. 2, our analysis revealed a significant increase in ethanol intake and preference following EOD compared to daily access conditions (session × group interaction: F’s13,156 > 4.13; p < 0.01). Compared to session 1, a significant escalation of ethanol intake and preference was observed in EOD mice during sessions 6–7 (session: F’s13,78 > 8.8; p < 0.01). However, further analysis revealed that ethanol escalation elicited by EOD was blocked by daily drinking during session 8 and further reduced across sessions 9–14 (post hoc LSD, p > 0.05). Notably, persistent excessive ethanol intake and preference levels were observed in EOD mice during sessions 8–13 (post hoc one-way ANOVA, p < 0.05). No significant differences in ethanol intake and preference were observed in daily mice (time: F’s13,78 < 1.4; p > 0.15), which indicates that continuous access to ethanol results in a stable-controlled pattern of ethanol consumption for at least 3 consecutive weeks (i.e., sessions 1–14).
Fig. 2.
Escalated levels of ethanol intake and preference following every-other-day (EOD) access to ethanol were reversed by daily access to ethanol in adult B6 mice. Following 2 weeks of intermittent (EOD; n=7) and continuous (daily; n=7) access to ethanol (i.e., sessions 1–7) EOD and daily mice received ethanol continuously (i.e., session 8–14). + p < 0.05 compared to session 1; * p < 0.05 compared to session 1 and group.
Experiment 3: Greater Daily Drinking and EOD-induced Escalation in Adolescent Mice
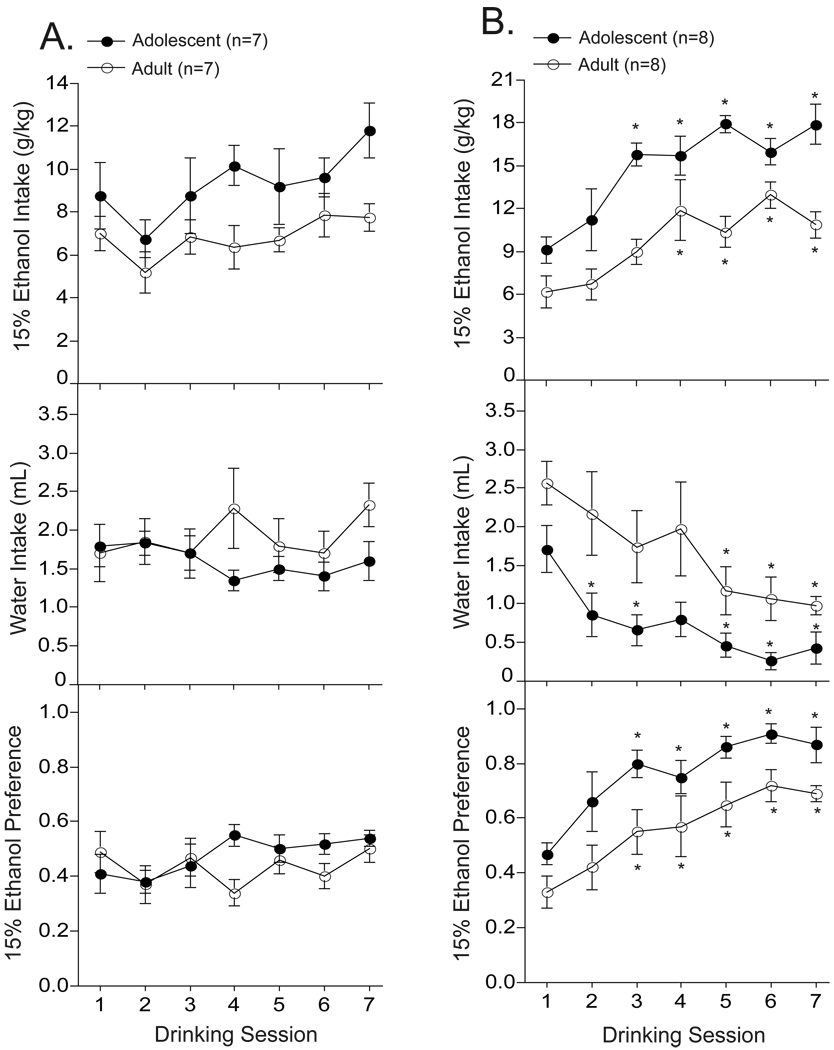
The purpose of this experiment was to evaluate daily and EOD drinking in adolescent and adult mice. As shown in Fig. 3A, our analysis revealed that adolescent mice drank more ethanol on a daily basis (session: F6,84 = 3.17; p < 0.01) compared to adults (age: F1,14 = 5.5; p < 0.05). There were no significant differences in daily water intake or ethanol preference across time (F’s6,84 < 2.1; p > 0.40) or between age-groups (F’s1,14 < 2.9; p > 0.15). Moreover, there were no significant age × time interactions on ethanol intake, water intake, or ethanol preference (F’s6,84 < 1.38; p > 0.12), which suggests that excessive ethanol intake in adolescents were sustained during the adolescent period (i.e., sessions 1–7). Mean (± S.E.M.) ethanol intake and preference scores following 2 weeks of daily access was 9.3 ± 0.9 and 0.48 ± .03 for adolescents and 6.8 ± 0.6 and 0.44 ± .04 for adults, respectively.
Fig. 3.
Adolescent mice have a greater capacity to drink ethanol on a daily and every-other-day (EOD) basis compared to adult B6 mice. Adolescent (P30 at start; n=7–8) and adult (P70 at start; n=7–8) were given daily (Fig. 3A) or EOD (Fig. 3B) access to ethanol for 2 weeks. * p < 0.05 compared to session 1.
With regards to ethanol escalation (Fig. 3B), our analysis revealed that EOD access enhanced ethanol intake and preference in adolescent and adult mice (session: F’s6,72 > 6.9; p < 0.01). Analysis of water intake revealed a significant effect of time (F6,72 = 10.8; p < 0.01) and age (F1,12 = 6.9; p < 0.03) but no time × age interactions (F6,72 = 0.7; p = 0.64). Compared to session 1, EOD mice significantly escalated their levels of ethanol intake and preference during session 3, which was sustained across session 5–7 (session: F’s6,42 > 7.6; p < 0.01). However, the EOD analysis did not reveal a session × age interaction on ethanol intake or preference (F’s6,72 < 1.6; p > 0.15), which suggests that the rate of ethanol escalation was relatively similar between adolescent and adult mice. The average escalated level of ethanol intake and preference was greater during adolescence compared to adulthood (F’s1,12 > 8.1; p < 0.02). Mean (± S.E.M.) ethanol intake and preference scores following 2 weeks of EOD access was 15.3 ± 0.4 and 0.77 ± .03 for adolescents and 11.4 ± 0.8 and 0.57 ± .06 for adults, respectively.
Discussion
The findings of the present study revealed that EOD (intermittent) drinking is sufficient to elevate 15% ethanol intake and preference in B6 mice (Figures 1–3). Interestingly, the expression of EOD-induced escalation (i.e., two-fold increase over daily intake and preference) is similar to what was previously observed following weekly (i.e., intermittent) access to ethanol (Melendez et al., 2006). This study also demonstrated that drinking ethanol daily blocks and reverses the expression of ethanol escalation induced by intermittent-EOD drinking (Figure 2). Moreover, the present study clearly indicated that adolescent mice have a greater capacity to consume 15% ethanol following daily-controlled and EOD-escalated drinking compared to adults (Figure 3). In addition, EOD access elicited a greater increase in ethanol preference in adolescent relative to adult mice. In summary, the EOD mouse model is a simple and time efficient procedure to study escalation of ethanol intake, which is a hallmark in the transition to drug addiction-dependency. Furthermore, the EOD model appears suitable to study adolescent’s vulnerability to excessive drinking, including binge-like consumption.
Previous studies have shown that EOD access to ethanol leads to elevated subsequent ethanol drinking in rats (Simms 2008; Sinclair and Li, 1989; Wise, 1973). This study is the first to show that EOD access induces ethanol escalation in B6 mice (i.e., Figures 1–3). Furthermore, the present study shows that EOD mice consumed a large portion of ethanol during the first 6 hr of the dark phase, when they are normally engaged in their highest level of activity. Notably, a growing number of mouse models have been developed to study the propensity for ethanol drinking in the dark (DID; e.g., Rhodes et al., 2005). In a recent study by Crabbe et al. (2009), selectively bred high DID (HDID) mice consumed on average ~5.0 g/kg ethanol 4 hr into the circadian dark cycle, which is comparable with EOD intake at 6 hr into the dark (~8.0 g/kg; Fig. 1C). However, an important limitation of the HDID model is that mice were water deprived during the limited access test session. Indeed, when water was made available (i.e., two-bottle choice conditions), ethanol intake was significantly reduced to nearly half of the levels obtained with one-bottle DID (Crabbe et al., 2009). Thus, it is possible that daily expression of excessive drinking with two-bottle choice DID may require longer training periods, which may be a potential limitation for modeling excessive drinking in adolescent mice. Notably, one of the major limitations of the present study is that it did not assess blood ethanol concentrations (BEC). Based on previous findings, however, it is reasonable to suspect that EOD mice have BEC well above 0.50 mg/dl (e.g., Crabbe et al., 2008) and are experiencing pharmacological consequences (e.g., Middaugh et al., 2003). Future studies remain to determine whether EOD mice are experiencing signs of intoxication or withdrawal, especially during the circadian dark.
In humans, escalation of drug use is considered a hallmark in the transition to addiction-dependency and has been posited as an animal model of compulsive drug use (Koob et al., 2004). Although intermittent access to ethanol has a dramatic influence on subsequent ethanol intake, the mechanisms remain poorly understood. Historically, escalation of drug use has been interpreted as reflecting the development of tolerance to the drug’s effect (DSM-IV-TR, 2000). However, several lines of evidence would argue that escalation of drug use actually reflects the development of sensitization (reviewed by Zernig et al., 2007). For instance, operant conditioning studies have shown that when scheduled-control access conditions are switched to discrete (intermittent) trails, animals typically self-administer in a binge-like fashion (e.g., Morgan and Roberts, 2004). Moreover, the possibility exists that shifts from continuous to intermittent-access conditions exacerbates novelty-seeking, anticipatory processes, and/or other drug related memories that may sensitize with repeated drug use. This hypothesis is consistent in part with the incentive sensitization theory of addiction (Robinson and Berridge, 2003). Although future studies will be necessary to clarify the mechanisms mediating ethanol escalation, targeting the circuits and neurochemistry involved may ultimately lead to new insights and effective molecular strategies.
A salient feature of the EOD model is that it can be used to detect escalated ‘binge-like’ drinking during adolescence, which is a period of elevated ethanol consumption in humans and rats (reviewed by Spear, 2000). The present study supports these findings by showing that adolescent B6 mice have a greater capacity to drink ethanol under daily (controlled) and intermittent (escalated) conditions (Figure 3). Although few studies exist, Hefner and Holmes (2007) had originally shown no significant differences in continuous-daily 10% ethanol intake between adolescent and adult B6 mice. It is tempting to suggest that less concentrated ethanol solutions (i.e., lower than 15%) are not sufficient to induce excessive (daily) ethanol drinking in adolescent B6 mice. Interestingly, a recent study by Moore et al., (2010) has shown that 20% ethanol DID (albeit one-bottle choice; see limitation above) is greater in adolescent compared to adult B6 mice. Future studies will be needed to address the role of concentrated ethanol solutions on the induction of excessive ethanol intake in adolescent and adult B6 mice.
In the present study, EOD-induced escalation of ethanol intake and preference was clearly greater in adolescents compared to adults. Nevertheless, this study indicated no significant age-differences in the rate of ethanol escalation during 2 weeks of EOD access. Therefore, the mechanisms mediating the expression of ethanol escalation appear to be shared among adolescent and adult B6 mice. It is important to note, however, that the relatively short ethanol exposure period during EOD access (e.g., 7 exposures/14 days) may not provide sufficient information for those studying long-term effects of ethanol consumption. Future work will continue to explore the long-term consequences of EOD-induced escalation, especially as it relates to EOD-drinking during adolescence and subsequent ethanol drinking during adulthood.
To conclude, the present study illustrated that the EOD mouse model possesses many strengths to induce escalated drinking. As presently shown, its greatest strength is that it is simple, fast, economical, and can be used to compare ethanol escalation during critical periods (e.g., adolescence). Most importantly, considering that ethanol escalation is a multifaceted phenomenon, the EOD mouse model may help elucidate the environmental and neurochemical factors involved, with the ultimate goal of developing effective pharmacological treatments for preventing the transition to alcohol addiction-dependency.
Acknowledgments
This work was supported in part by NIH grant R21AA015953 and UPR-SOM Institutional Funds. The authors would like to thank Mildred Tirado and Maria P. Grant for their technical assistance and Natalie Watters for her helpful comments.
References
- Crabbe JC, Metten P, Rhodes JS, Yu CH, Brown LL, Phillips TJ, Finn DA. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. >Biol Psychiatry. 2009;65:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- >Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) 4th ed. Washington: American Psychiatric Association; 2000. [Google Scholar]
- Hargreaves GA, Monds L, Gunasekaran N, Dawson B, McGregor IS. Intermittent access to beer promotes binge-like drinking in adolescent but not adult Wistar rats. >Alcohol. 2009;43:305–314. doi: 10.1016/j.alcohol.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. >Psychopharmacology. 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O'Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. >Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. >Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Murphy JM, Li TK, Lumeng L, McBride WJ. Development of alcohol drinking behavior in rat lines selectively bred for divergent alcohol preference. >Alcohol Clin Exp Res. 1998;22:1584–1590. [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW. Development of an alcohol deprivation and escalation effect in C57BL/6J mice. >Alcohol Clin Exp Res. 2006;30:2017–2025. doi: 10.1111/j.1530-0277.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Szumlinski KK, Van Patten Y, Marlowe AL, Kalivas PW. Chronic ethanol consumption by C57BL/6 mice promotes tolerance to its interoceptive cues and increases extracellular dopamine, an effect blocked by naltrexone. >Alcohol Clin Exp Res. 2003;27:1892–1900. doi: 10.1097/01.ALC.0000099264.36220.48. [DOI] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melón LC, Boehm SL., 2nd Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. >Alcohol Clin Exp Res. 2010;34:734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Roberts DC. Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. >Neurosci Biobehav Rev. 2004;27:803–812. doi: 10.1016/j.neubiorev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. >Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. >Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. >Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. >Pharmacol Biochem Behav. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li TK. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. >Alcohol Clin Exp Res. 2000;24:747–753. [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. >Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, Li TK. Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. >Alcohol. 1989;6:505–509. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. >Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Spanagel R. Recent animal models of alcoholism. >Alcohol Res Health. 2000;24:124–131. [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. >Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. >Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stöckl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. >Pharmacology. 2007;80:65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]