Abstract
The Beclin-1 protein is essential for the initiation of autophagy and recent studies suggest this function may be compromised in Alzheimer’s disease (AD). In addition, in vitro studies have supported a loss of function of Beclin-1 due to proteolytic modification by caspases. In the present study we examined whether caspase-cleavage of Beclin-1 occurs in the AD brain by designing a site-directed caspase-cleavage antibody based upon a known cleavage site within the protein at position D149. We confirmed that Beclin-1 is an excellent substrate for caspase-3 and demonstrate cleavage led to the formation of a 35 kDa C-terminal fragment labeled by our novel antibody following Western blot analysis. Application of this antibody termed Beclin-1 caspase-cleavage product antibody or BeclinCCP in frontal cortex tissue sections revealed strong immunolabeling within astrocytes that localized with plaque-regions and along blood vessels in all AD cases examined. In addition, weaker, more variable BeclinCCP labeling was also observed within neurofibrillary tangles that co-localized with the early tau conformational marker, MC-1 as well as the late tangle marker, PHF-1. Collectively, these data support a depletion of Beclin-1 in AD following caspase-cleavage.
Keywords: Beclin-1, Alzheimer’s disease, Astrocytes, Caspase
Introduction
Autophagy is an important catabolic process involving the removal of long-lived proteins, and organelles by a complex process involving numerous regulatory proteins and the formation of double-membrane vesicles known as autophagosomes, which ultimately fuse with lysosomes for degradation of contents. Because of the central role that autophagy plays in removing unwanted proteins this process may serve a protective role by clearing aggregated disease proteins in certain age-related disorders including Alzheimer’s disease (AD). On the other hand, defects in autophagosome formation may lead to accumulation of toxic protein species thereby promoting neurodegeneration. In AD, neurons contain more autophagosomes than do control patients and tend to accumulate in dystrophic neurites. Thus, impairment of this pathway may contribute to the pathogenesis observed in AD .
A key protein involved in the initiation of autophagy is the Bcl-2-interacting protein-1 (Beclin-1), the mammalian homolog of yeast Atg6. Beclin-1 is a 60 kDa coiled-coil protein that is expressed in neurons and glia. A recent study demonstrated reduced levels of Beclin-1 in early AD . In the same study, the authors generated transgenic mice that were deficient in Beclin-1 and demonstrated a disruption in autophagy, increased neurodegeneration, and Aβ accumulation. These results suggest an important role for autophagy in general and Beclin-1 specifically in clearing Aβ aggregates and preserving neuronal function in AD.
Although decreased expression of the Beclin-1 protein has been demonstrated to occur in AD, the exact mechanism and cellular subtypes involved remains unknown. Because of the well-documented role of caspases in AD, we hypothesize that one mechanism leading to lower levels of Beclin-1 in AD may be proteolytic cleavage by caspases. Indeed, previous in vitro studies have demonstrated that Beclin-1 is a substrate for caspase-3 cleavage, leading to inactivation of autophagy and enhanced apoptosis in Ba/F3 cell lines . To assess whether Beclin-1 is cleaved in the AD brain, we designed a site-directed caspase-cleavage antibody to Beclin-1 based upon a known caspase-3 cleavage consensus site within Beclin-1, DQLD149 Wirawan et al., 2010). In vitro, this novel antibody detected a 35 kDa C-terminal fragment of Beclin-1 following incubation with caspase-3. In addition, immunohistochemical analysis revealed the presence of caspase-cleaved Beclin-1 in the AD brain that was localized primarily within astrocytes within plaque-rich regions and around blood vessels with additional labeling found within tangle-bearing neurons. These results support a mechanism leading to diminished levels of Beclin-1 in the AD brain and suggest that the caspase-cleavage of Beclin-1 may be one mechanism leading to the impairment of autophagy that has been observed in this disease.
Methods and Materials
Materials
The mouse anti-GFAP antibody (MAB3402), and the anti-Aβ mAb 1560 (clone 6E10) was purchased from Invitrogen (Carlsbad, CA). Two rabbit polyclonal Beclin-1 antibodies that detect full-length Beclin-1 near the N-terminus and another directed towards the C-terminus were purchased from ProSci Incorporated (Poway, CA). A rabbit anti-active caspase-3 polyclonal antibody (PAb CM1) was purchased from BD Pharmingen (San Jose, CA). MC-1 and PHF-1 monoclonal, mouse antibodies were generous gifts from Dr. Peter Davies (Albert Einstein College of Medicine, Bronx, NY). Fluoro Jade C was purchased from Millipore (Billerica, MA). The beta-actin rabbit polyclonal antibody and a rabbit polyclonal antibody to full-length Beclin-1 (epitope, C-terminus) were both from AbCam (Cambridge, MA). Active, human recombinant caspase-3 was purchased from Calbiochem (La Jolla, CA). Human, recombinant Beclin-1 protein was purchased from Abnova (Walnut, CA).
Generation of the polyclonal caspase-cleaved Beclin-1 antibody
Polyclonal antibodies were synthesized based upon a putative caspase cleavage consensus site (DQLD149) within Beclin-1. We chose the 10-mer peptide TQLNVTENEC, which represents the C-terminal downstream neoepitope fragment of Beclin-1 that would be generated following cleavage by caspases. Following synthesis, this peptide was coupled to KLH and injected into two different rabbits. The resulting sera (verified by ELISAs) were used to affinity purify antibodies using a sulfolink column coupled with the peptide TQLNVTENEC. For this antibody, synthesis of peptides, injections of immunogens, and collection of antisera were contracted out to Bethyl laboratories (Montgomery, TX).
Fluoro Jade C labeling
To assess for neurodegeneration, the fluorescent dye, Fluoro Jade C was utilized as previously described by Bian et al. . Briefly, fixed brain sections were mounted and pretreated for 5 min in a 80% alcohol solution containing 1% sodium hydroxide, followed by a 70% alcohol and a distilled water wash for 2 min. Sections were then incubated for 10 min in a 0.06% potassium permanganate solution followed by rinsing in distilled water for 2 min. Sections were then transferred into a 0.0001% solution of Fluoro Jade C containing 0.1% acetic acid for 10 min. Following 3 successive washes in distilled water for 1 min, slides were dried, dehydrated and cover-slipped with Depex.
Cell-free proteolytic cleavage of Beclin-1
To examine whether caspase-3 can cleave Beclin-1 (N-terminal GST-tag), 3.6 μg of purified human recombinant Beclin-1 was incubated with active human recombinant caspase-3 in 2x reaction buffer containing 10 mM DTT for 24 hours at 37°C. Reactions were terminated by the addition of 5x sample buffer and stored at −20°C until analyzed.
In vitro transcription/translation
The cDNA encoding the Beclin-1-D133A-D149A mutant was constructed by site-directed PCR mutagenesis as previously described (Wirawan et al., 2010). The plasmids pEF6-Beclin-1-myc-His and pEF6-Beclin-1-D133A/D149A-myc-His were used as a template for in vitro coupled transcription/translation in a rabbit reticulocyte lysate system according to the manufacturer’s instructions (Promega, Madison, WI, USA). Translation reactions (2 μl each) were left untreated or incubated with 300 nM recombinant mouse caspase-3 in 24 μl of cell-free system buffer (10 mM HEPES-NaOH pH 7.4, 220 mM mannitol, 68 mM sucrose, 2 mM NaCl, 2.5 mM KH2PO4, 0.5 mM EGTA, 2 mM MgCl2, 5 mM pyruvate, 0.1 mM PMSF, 200 U/ml aprotinin, 10 mg/ml leupeptin) for 1 h at 37°C. The cleavage products were analyzed by Western blot analysis using the BeclinCCP antibody.
Expression and purification of recombinant Beclin-1-FL, Beclin-1-N and Beclin-1-C
Synthesis of cDNA expression vectors to express Beclin-1-FL, Beclin-1-N (amino acids 1-133) and Beclin-1-C (amino acids 150-450) were cloned and expressed as previously described (Wirawan et al., 2010). Purified proteins were then analyzed by Western blot analysis using the BeclinCCP antibody, or antibodies to the N- and C-terminal regions of Beclin-1.
Western blot analysis
Recombinant human Beclin-1, or human brain lysates were processed for Western blot analysis. Soluble or insoluble human frontal cortex homogenates from control or AD brains were prepared as previously described. Proteins were separated by 12% SDS-PAGE and transferred to nitrocellulose. Transferred slabs were stained in coomassie blue to verify equal loading between samples. Membranes were incubated in the Beclin-1 caspase-cleavage product antibody (BeclinCCP, 1:500) or in separate experiments with an activated caspase-3 antibody (1:1,000) and primary antibodies were visualized using goat anti-rabbit HRP-linked secondary (1:5,000; Jackson s Laboratory, West Grove, PA), followed by ECL detection. All samples were analyzed for protein content using the BCA assay (Pierce) to ensure equal protein loading. In addition, Western blot analysis was performed utilizing a beta-actin antibody (1:400) as a loading control.
Human Subjects
Autopsy brain tissue from the frontal cortex of eleven neuropathologically confirmed AD cases and five nondemented cases diagnosed as normal was studied. Case demographics for samples used for immunohistochemistry are presented in Table 1. Age at death was not significantly different between AD (mean, 74.4 ± 6.40) and controls (mean, 78.6 ± 7.09). Human brain tissues used in this study were provided by the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine.
Table 1.
AD Case Demographics for Immunohistochemistry Studies
| Group | Age (yrs) | Sex | Cause of death |
|---|---|---|---|
| AD | 77 | M | AD |
| AD | 76 | M | AD |
| AD | 71 | M | AD |
| AD | 61 | F | AD |
| AD | 80 | F | Dehydration |
| AD | 83 | F | Cardiac Arrest |
| AD | 75 | F | AD |
| AD | 65 | F | AD |
| AD | 76 | M | AD |
| AD | 77 | F | Cardiac Arrest |
| AD | 77 | F | Sepsis |
| Ctl | 75 | F | Pulmonary Disease |
| Ctl | 81 | F | Cardiac Arrest |
| Ctl | 73 | F | Heart Failure |
| Ctl | 90 | F | Heart Failure |
| Ctl | 74 | F | Cancer |
Immunohistochemistry and Immunofluorescence Microscopy
Free-floating 40 μm-thick serial sections were used for immunohistochemical and immunofluorescence studies as previously described. Antibody dilutions were the following: BeclinCCP (1:100), mAb GFAP (1:400), GFAPccp (in house antibody, 1:100), anti beta-amyloid mAb 1560 clone 6E10 (1:400), full-length Beclin-1 (polyclonal, 1:100), mAb PHF-1 (1:500) and mAb MC-1 (1:500). To visualize beta-amyloid staining, sections were pretreated for 7 minutes in 95% formic acid. Antigen visualization was determined using ABC complex (ABC Elite immunoperoxidase kit, Vector labs), followed by DAB substrate (Vector Labs). For bright-field immunohistochemical double-labeling, primary antibody labeling was detected using the brown DAB substrate, while the second label was visualized using the Blue SG substrate (Vector Labs). For immunofluorescence co-localization studies, antigen visualization was accomplished using an Alexa Fluor 488-labeled tyramide (green, Ex/Em = 495/519) or streptavidin Alexa Fluor 555 (red, Ex/Em = 555/565), both from Invitrogen (Carlsbad, CA).
Confocal microscopy
For confocal immunofluorescence imaging, the primary antibodies were visualized with secondary antibodies tagged with either Alexa Fluor 488 or Alexa Fluor 555 (Invitrogen, Carlsbad, CA.) Images were taken using a Zeiss LSM 510 Meta system combined with the Zeiss Axiovert Observer Z1 inverted microscope and ZEN 2009 imaging software (Carl Zeiss, Inc., Thornwood, NY). Images were acquired utilizing a 63X Plan-Apochromat oil-immersion objective (NA1.4), an Argon laser (488 nm) with an emission band pass of 505-550 nm for the detection Alexa Fluor 488 and HeNe laser (543 nm) with an emission band passes 550-647 nm for the detection of Alexa Fluor 555.
Statistical analysis
Statistical difference between the average numbers of BeclinCCP-positive astrocytes in control (n=5) versus AD (n=11) cases was determined using Student’s two-tailed T-test.
Results
To determine whether Beclin-1 is a target for caspase cleavage in the AD brain, we synthesized a site-directed antibody according to a putative caspase cleavage site (DQLD149) located in the middle of the protein. The antibody was directed towards the downstream neo-fragment that would be generated following cleavage by caspases at this site. An 10-mer peptide was conjugated to KLH (TQLNVTENEC-[KLH]) and injected into two different rabbits. Strong antibody titers were obtained in both rabbits. For example, the dilution of antibody solution that produced an OD of 1.0 in an ELISA assay for rabbit 965 was >1/500,000, while that for rabbit 966 was 1/83,481. Similar results were obtained using affinity-purified antibodies from both rabbits, however, in all studies we chose to use the purified antibody from rabbit 965 due to higher yields following purification from anti-serum.
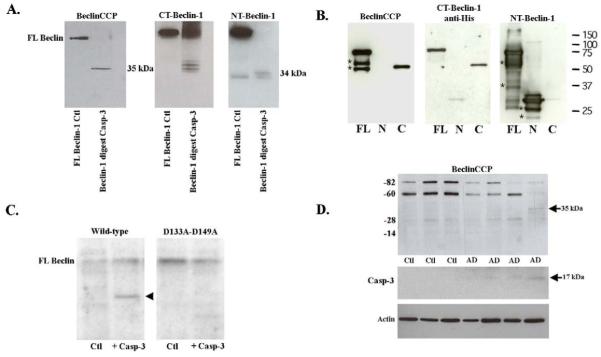
Following affinity purification, we tested the specificity of this antibody (herein termed the Beclin caspase-cleavage product (CCP) antibody) utilizing a cell-free system consisting of purified human recombinant Beclin-1 (with amino-terminal GST-tag, Abnova) incubated with or without human recombinant caspase-3. Following proteolytic cleavage of Beclin-1, samples were separated by SDS-PAGE gel electrophoresis and probed with the BeclinCCP antibody (Fig. 1A). The BeclinCCP antibody recognized the predicted ~35 kDa cleavage fragment following cleavage at D149 (Fig. 1A, left panel). Under identical experimental conditions, a commercial antibody that binds to the C-terminal region of Beclin-1 detected both a 35 kDa as well as a 37 kDa fragment most likely representing cleavage at the nearby upstream caspase-cleavage site within Beclin-1 at position D133 (Wirawan et al., 2010) (Fig. 1A, middle panel). These data suggest the BeclinCCP antibody is specific for the C-terminal fragment generated following cleavage at D149, but not at D133. A similar experiment using an antibody that recognizes the amino-terminus of Beclin-1 revealed two smaller fragments that most likely represent the upstream fragments of Beclin-1 following caspase-3 cleavage at D133 and D149 (Fig. 1A, far right panel). Similar experiments were performed using recombinant Beclin-1 protein fragments corresponding to the upstream N-terminal caspase-cleavage fragment (amino acids 1-133, following cleavage at D133) or the downstream C-terminal fragment following cleavage of Beclin-1 at position D149 (amino acids 150-450). Application of the BeclinCCP antibody confirmed the specificity of the antibody to only the C-terminal fragment (Fig. 1B, left panel). Confirmation of this fragment was accomplished using an antibody specific for the C-terminal region of Beclin-1 (Fig. 1B, middle panel). Mutation of the two potential caspase-cleavage sites located within Beclin-1 at position D133 and D149 has previously been shown to abrogate caspase cleavage and prevent the formation of C-terminal fragments (Wirawan et al., 2010). As a negative control for the specificity of the BeclinCCP antibody, Western blot analysis was performed utilizing this caspase-resistant form of Beclin-1 and the results indicated immunoreactivity to the C-terminal fragment in wild-type Beclin-1, which was absent in the double mutant (Fig. 1C, arrowhead).
Figure 1. Characterization of BeclinCCP antibody by Western blot analysis.
(A): Cell-free incubation of human Beclin-1 by caspase-3 was performed and Western blot analysis was undertaken using three different antibodies as indicated. Panel A, far left depicts undigested Beclin-1 (lane marked Ctl) and Beclin-1 following digest with caspase-3. The BeclinCCP antibody detected the predicted caspase-cleaved C-terminal fragment at 35 kDa. Commercial antibodies to the C-terminal (middle panel) and N-terminal region of Beclin-1 (far right panel) were also tested to confirm the binding pattern of BeclinCCP (see text for details). (B): Recombinant His-tagged Beclin-1-FL, Beclin-1-N and Beclin-1-C were loaded on SDS-PAGE and immunoblotting was performed using the BeclinCCP, anti-pentahis and anti-NT-Beclin-1 antibodies as indicated. Non-specific bands detected on Western are indicated with *. (C): Wild-type Beclin-1 and Beclin-1-D133A-D149A were in vitro transcribed and translated and then left untreated (lane marked Ctl) or incubated with 300 nM of recombinant caspase-3 (lane marked + Casp-3. Cleavage fragments were analyzed by Western blotting using the BeclinCCP antibody. Mutation of these two caspase-cleavage sites prevented proteolytic cleavage and detection of the C-terminal fragment. (D): Western blot analysis with the BeclinCCP antibody or activated capase-3 in soluble human brain extracts. Bottom panel D represents loading control blot following stripping and reprobing using a rabbit antibody to beta-actin (1:400). Data are representative of three independent experiments.
Western blot analysis in human control or AD frontal temporal extracts with the BeclinCCP confirmed the previous finding that Beclin-1 levels are diminished in the AD brain (Fig. 1D). There was also a band running near 82 kDa, which may represent a non-specific band. In addition, detection of the predicted 35 kDa, C-terminal fragment was observed in one AD case (Fig. 1D, , arrow at 35 kDa). Coincidently, tissue sections from this same AD case were available for immunohistochemical analysis and indicated the highest number of labeled BeclinCCP-positive astrocytes of all AD cases examined (see below). Similar results were obtained utilizing insoluble fractions from control or AD brains (data not shown).
Supporting that the 35 kDa band detected in this particular AD case represents a caspase-cleaved fragment of Beclin-1 were data obtained following Western blot analysis using an antibody to active caspase-3 (Fig. 1D). We were only able to detect active caspase-3, albeit faint labeling, in the same AD case that revealed the 35 kDa fragment of Beclin-1 following application of BeclinCCP antibody. The inability to detect this fragment in the other AD cases could be either due to insufficient cleavage by caspase-3 or as a result of inadequate levels of this fragment to be detected by Western blot analysis.
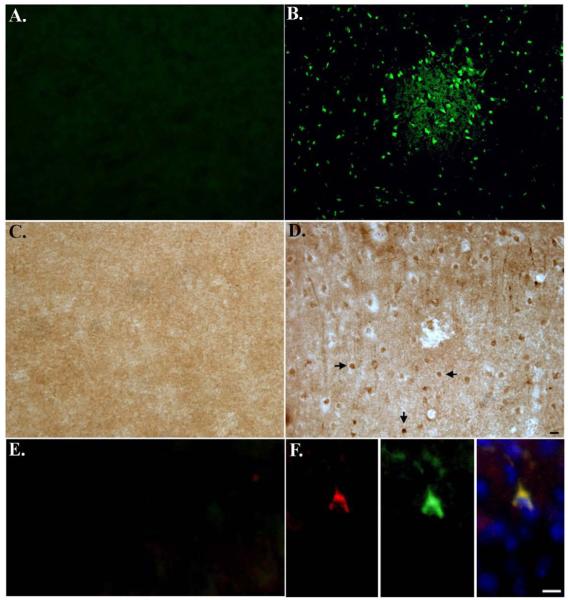
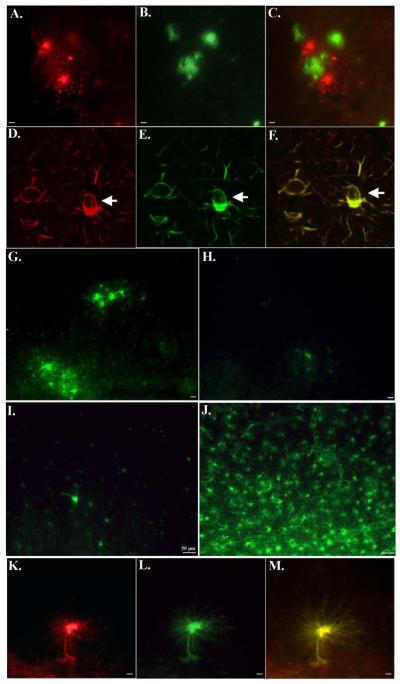
To examine the specificity of BeclinCCP in situ, experiments were undertaken using MCAO mice, an animal model for neurodegeneration. The advantage of this model is that it creates an ischemic infarct confined to one side of the brain while leaving the other hemisphere undamaged, which thus can serve as an internal control. As an initial approach, we first determined whether we could detect neurodegeneration in ischemic infarct regions of MCAO mice. To accomplish this, we employed the use of a specific marker for neurodegeneration, Fluoro Jade C. Fluoro Jade C is a fluorescent dye that detects neuronal degeneration in animal models and has been shown to co-localize with markers for apoptosis. Application of Fluoro Jade C to MCAO brain sections revealed widespread neuronal labeling in ischemic infarct areas (Fig. 2B), while the contralateral side of the brain showed no evidence of labeling (Fig. 2A). Sections were further analyzed for caspase-cleaved Beclin-1 using BeclinCCP and indicated the labeling of neurons in ischemic infarct areas (Fig. 2D). In contrast there was no labeling of any cell types with the BeclinCCP antibody on the contralateral side of the brain indicating a lack of immunoreactivity to full-length Beclin-1, in situ (Fig. 2C). In addition, we demonstrated co-localization between activated caspase-3 and BeclinCCP within neurons in ischemic infarct areas of MCAO mice (Fig. 2F). These data suggest caspase cleavage of Beclin-1 occurs in vivo under conditions known to activate caspases and can be specifically detected using our BeclinCCP antibody.
Figure 2. Characterization of BeclinCCP antibody in an in vivo model of apoptosis.
Mice were subjected to 1 hr MCAO, followed by reperfusion for 24 hr. (A and B): Brain sections incubated with Fluoro Jade C revealed labeling of shrunken, damaged neurons in the cortex of MCAO mice in ischemic infarct areas (B), while no staining with Fluoro Jade C was evident on the contralateral control side of the brain (A). (C and D): Identical to Panels A and B except sections were analyzed for caspase-cleaved beclin-1 using the BeclinCCP antibody. In this case, BeclinCCP labeling was evident in ischemic infarct areas (D) and was absent on the contralateral side of the brain (C). (E and F): Double-label immunofluorescence experiments with the BeclinCCP antibody (red) and an antibody to activated caspase-3 (green) indicated co-localization of these two markers within ischemic infarct areas (F) that was absent on the contralateral control side of the brain (E). Blue labeling in Panel F (far right) depicts labeling of nuclei with Hoechst. Scale bars represent 10μm.
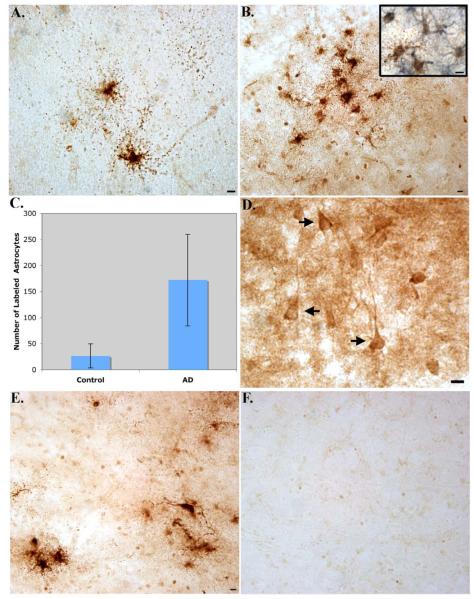
Following characterization of the BeclinCCP antibody, immunohistochemical analysis was performed on post-mortem frontal cortex brain sections from AD subjects. Bright-field analysis revealed a highly specific and consistent staining pattern in degenerated astrocytes of all AD cases examined (n=11, Fig. 3A). Double-label immunohistochemical analysis using a monoclonal antibody to GFAP suggested localization of BeclinCCP labeling within astrocytes (Inset, Fig. 3B). Typically, labeled astrocytes were located within plaque-rich regions (Fig. 3B), and morphologically displayed hallmarks of cells undergoing apoptosis, including cell shrinkage and fragmented processes that were punctated in appearance with the processes reduced to clusters or lines of dots radiating outward from the cell body (Fig. 3A). This staining profile was very similar to labeling we previously observed using a caspase-cleavage antibody to GFAP in the AD brain . Quantification of the number of labeled degenerated astrocytes indicated a significant difference between AD and age-match control cases (p= 0.003), (Fig. 3C). Besides labeling of the BeclinCCP antibody within astrocytes, we also observed staining within apparent neurofibrillary tangles (NFTs), although labeling was less intense (Fig. 3D) and more variable (Table 2). Specificity of the BeclinCCP antibody was confirmed following experiments with pre-adsorbed antibody (Fig. 3E and F). In this manner, there was a complete lack of staining in serial AD sections under conditions whereby purified BeclinCCP was pre-adsorbed with the peptide used as the immunogen (Fig. 3F).
Figure 3. Characterization of BeclinCCP antibody in the human AD brain by immunohistochemistry.
Data are representative staining from human postmortem frontal cortex brain sections following application of the BeclinCCP antibody. (A-B): Application of the BeclinCCP antibody in representative AD cases indicated strong immunolabeling within astrocytes (A) that were localized within plaque-rich regions (B). The inset in Panel B depicts bright-field double-labeling with the BeclinCCP antibody (brown) and a monoclonal antibody to GFAP (blue) (C): Quantification analysis indicated a significant increase in the number of BeclinCCP-positive astrocytes in AD cases (n=11) versus controls (n=5), (p= 0.003). (D): Labeling of the BeclinCCP antibody within apparent NFTs in a representative AD case (arrows, D). (E and F): Serial AD sections were immunolabeled with purified BeclinCCP (1:200) and labeling of astrocytes was observed (E). In contrast, staining with purified BeclinCCP was prevented after preadsorption with free peptide (F). All scale bars represent 10 μm.
Table 2.
Relative distribution of labeling with BeclinCCP in degenerating astrocytes or NFTs in the AD brain
| Case Number | Degenerated Astrocytes | Neurofibrillary Tangles |
|---|---|---|
| #1 | ++++ | − |
| #2 | + | ++ |
| #3 | +++ | + |
| #4 | ++ | +++ |
| #5 | ++++ | + |
| #6 | ++ | − |
| #7 | ++++ | − |
| #8 | +++ | ++ |
| #9 | ++ | +++ |
| #10 | +++ | + |
| #11 | ++ | − |
-denotes absence of any labeling with BeclinCCP
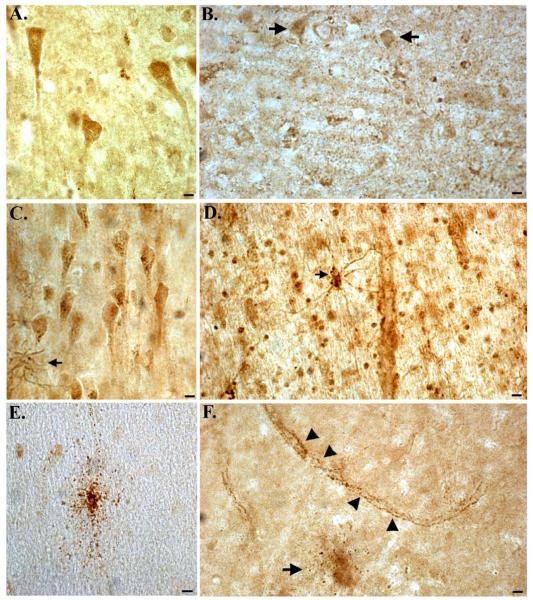
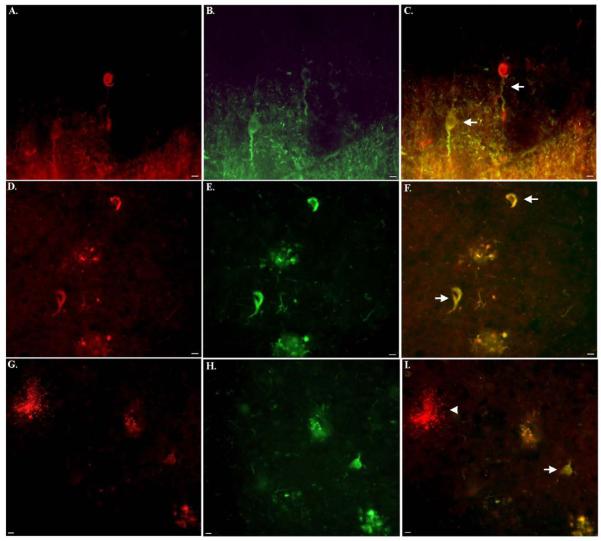
In contrast to Western blot analysis (Fig. 1), in situ experiments in MCAO mice (Fig. 2) or in human brain tissue sections (Fig. 3), indicated a specificity of the BeclinCCP antibody for cleaved Beclin-1 and not the full-length protein. To further confirm the specificity of the BeclinCCP antibody, we compared the staining profile with this antibody in control and AD cases to commercially available antibodies to human Beclin-1 that recognizes full-length Beclin-1 near either the N- or C-terminus. Staining with the N-terminus antibody in control cases revealed labeling of intact, healthy neurons in all control cases examined (Fig. 4A). In contrast, there was a general decrease in staining intensity with this antibody in AD cases compared to age-match controls (arrows, Fig. 4B). Immunohistochemical analysis utilizing the full-length C-terminus antibody to Beclin-1 in control cases revealed a similar staining pattern of healthy neurons with the occasional reactive astrocytes also labeled (Fig. 4C). In AD cases, this antibody labeled numerous astrocytes that appeared to contain cytoplasmic vacuoles (arrow, Fig. 4D), as well as staining of neuronal cell bodies (Fig. 4D). In contrast to the two commercial antibodies, the BeclinCCP antibody did not label neurons in control brains and staining was limited to the rare, degenerated astrocyte (Fig. 4E). In AD cases, BeclinCCP antibody labeled astrocytes (arrow, Fig. 4D), and punctate labeling was also observed along blood vessels (arrowheads, Fig. 4D). There were clear similarities in the staining pattern between the BeclinCCP and the full-length C-terminus antibody except the latter appeared less specific with regards to cell-type and the labeled astrocytes did not appear as damaged. Taken together, these data provide further evidence that in situ, the BeclinCCP antibody is specific for caspase-cleaved Beclin-1.
Figure 4. Comparison of labeling between the BeclinCCP antibody and commercially available antibodies to full-length beclin-1.
(A and B): Representative staining in an age-matched control case (A) or AD case (B) utilizing a commercially available antibody to the N-terminal region of full-length, human beclin-1 revealed labeling of healthy neurons in frontal cortex tissue sections (A), while staining appeared to be diminished in AD (arrows, B). (C and D): Identical to Panels A and B except a commercial antibody to the C-terminal region of beclin-1 was utilized. In this case, labeling was identified within neurons and reactive astrocytes (arrow, C) and in astrocytes along blood vessels in AD cases (D). (E and F): In the same control case, the BeclinCCP antibody only labeled the occasional damaged astrocyte (E), while in AD cases, labeling of astrocytes (arrow, F) and blood vessels (arrowheads, F), was evident. All scare bars represent 10 μm.
Double-label immunofluorescence experiments were undertaken to determine whether BeclinCCP labeling was within astrocytes in plaque-rich regions of the AD brain. BeclinCCP labeling within astrocytes (red, Fig. 5A) was confirmed to be localized in plaque areas using an anti-Aβ antibody (green, Fig. 5B). In this case, the overlap image depicts that although both markers are present in the same field, there is no co-localization of the two antibodies as indicated by the lack of yellow or orange coloring (Fig. 5C). Confocal analysis confirmed the co-localization of BeclinCCP with an antibody to full-length GFAP (Fig. 5F). Moreover, we identified numerous BeclinCCP-positive astrocytes that contained what appeared to be cytoplasmic vacuoles (white arrows, Fig. 5D-F).
Figure 5. Evidence for caspase-cleaved beclin-1 within astrocytes that co-localizes with caspase-cleaved GFAP and are present within plaque-rich regions in the AD brain.
(A-C): Double-label immunofluorescence labeling with BeclinCCP in red (A), anti-Aβ antibody in green (B), and the overlap image of both labels (C). Data support the presence of labeling of astrocytes within plaque regions. (D-F): Double-label confocal immunofluorescence images with BeclinCCP (red, D), full-length GFAP antibody (green, E), and the two images overlapped (F). Arrows denote a vacuole within a BeclinCCP-positive astrocyte. (G-J): Single-label immunofluorescence images depicting the inverse relationship between full-length GFAP and BeclinCCP labeling in AD cases. In cases with strong BeclinCCP immunolabeling (G), there was a corresponding lack of staining with the full-length GFAP antibody (H), whereas cases with weak BeclinCCP staining (I) displayed robust labeling with the full-length antibody (J). (K-M): Co-localization of caspase-cleaved beclin-1 and caspase-cleaved GFAP within astrocytes in the AD brain. Data depict labeling utilizing the BeclinCCP antibody in red (K), an antibody to caspase-cleaved GFAP in green (L), and the two images overlapped (M). All scale bars represent 10 μm except panels I and J, which represent 50 μm.
Single-labeling experiments indicated an inverse relationship between the BeclinCCP antibody and the full-length GFAP antibody. In this regard, we chose two cases that following quantification (Table 2) revealed either a strong presence of BeclinCCP staining (Fig. 5G), or a weak degree of labeling (Fig. 5I). In this manner, staining with the full-length GFAP antibody showed a corresponding paucity of labeling in the same case that exhibited strong BeclinCCP staining (Fig. 5H). Conversely, in serial sections that indicated a relatively weak degree of labeling with the BeclinCCP antibody, there was widespread labeling using the full-length antibody to GFAP (Fig. 5J). Thus, the BeclinCCP antibody may serve as a general marker for overall astrocytic health.
Experiments were performed to assess the possible co-localization between BeclinCCP and an in house antibody designed to detect caspase-cleaved GFAP. Strong co-localization was evident between the BeclinCCP antibody (red, Fig. 5K) and caspase-cleaved GFAP (green, Fig. 5L), further confirming the presence of caspase-cleaved Beclin-1 within astrocytes of the AD brain. To ensure that the BeclinCCP antibody is not cross-reacting with the GFAPccp antibody, we performed ELISAs with the immunogens used to create these two antibodies to determine if, for example, the BeclinCCP antibody cross-reacts with the immunogen used to create the GFAPccp antibody and vice-versa. There was a complete lack of cross-reactivity between the two antibodies and the representative immunogens (data not shown), supporting the conclusion that the antibodies are recognizing distinct epitopes.
Bright-field immunohistochemical analysis indicated possible labeling of BeclinCCP within neurons with apparent tangle morphology. To confirm this finding, double-label immunofluorescence studies were carried out using the late NFT marker, PHF-1, and the early marker, MC-1, an antibody that recognizes an early aberrant folded conformational change in tau. The majority of neurons labeled with PHF-1 did not co-localize with BeclinCCP. However, on rare occasion we did observe the presence of both markers in neurons, although co-localization within neurons was minimal as evidenced by the lack of yellow/orange coloring (arrows, Fig. 6A-C). In addition, although PHF-1 readily labeled ghost tangles, this was not observed with BeclinCCP. In contrast, strong colocalization between MC-1 and BeclinCCP was observed in the AD brain (Fig. D-I). We rarely observed BeclinCCP-positive neurons that did not also show colocalization with MC-1 (data not shown). However, there were many more MC-1 single-labeled neurons that did not contain BeclinCCP, suggesting that caspase-cleaved Beclin-1 is most likely not a cause but effect of tangle evolution. Moreover, as expected, there was no colocalization with MC-1 in BeclinCCP-positive astrocytes (arrowhead, Fig. 6I). These results suggest that the caspase-cleavage of Beclin-1 is a more regular occurrence in early-stage tangles, a result supported by other studies suggesting a relationship between caspase activation and NFTs .
Figure 6. Caspase-cleaved beclin-1 co-localizes with both early and late tangle markers of the Alzheimer’s brain.
(A-C): Double-label immunofluorescence images using the PHF-1 antibody (red, A), BeclinCCP antibody (green, B), and the two images overlapped (C). (D-F): Double-label immunofluorescence images using BeclinCCP antibody (red, D), the early tau conformation marker, MC-1 (green, E) and the two images overlapped (F). Yellow, orange coloring represents areas where markers are overlapping, which in this case was evident (arrows, F). (G-I): Identical to (D-F) showing in this case the lack co-localization within a damaged astrocyte labeled with BeclinCCP antibody (arrowhead, I), whereas co-localization between the two antibodies was evident within an apparent tangle-bearing neuron (arrow, I). All scale bars represent 10 μm.
Discussion
Beclin-1 is a critical protein involved in the initiation of autophagy, an intracellular process designed to remove cytoplasmic constituents including proteins and damaged organelles and to recycle the degraded biomolecules for cellular remodeling Mizushima et al., 2007). A protective role for Beclin-1 has previously been demonstrated in other neurodegenerative diseases including alpha-synucleinopathies. For example, in Parkinson’s disease, Beclin-1 gene transfer activated autophagy and prevented neurodegeneration in mouse models of this disease. In AD, a putative role for autophagy would be the removal of intracellular Aβ aggregates. If autophagy is occurring in too large extent or if the autophagic flux is blocked, it can turn into a degenerative process (Jaeger and Wyss-Coray, 2009). Indeed, autophagic degeneration has been observed in AD and neurons contain more autophagosomes than do control patients and tend to accumulate in dystrophic neurites. Thus, impairment of the autophagic flux resulting in accumulation of autophagosomes may contribute to the pathogenesis observed in AD. In support of a protective role for Beclin-1 in AD comes from a recent study by Pickford et al., who demonstrated a reduced expression of Beclin-1 in early AD and provided supporting evidence for the role of autophagy in AD by generating AD transgenic mice deficient in this protein. Beclin-1 depletion in APP transgenic mice disrupted autophagy, exacerbated Aβ pathology, and promoted neurodegeneration .
One potential mechanism for disruption of Beclin-1 function may be proteolytic modification by caspases. Previous in vitro studies have demonstrated that Beclin-1 is a substrate for caspase-3 modification and caspase-mediated cleavage of Beclin-1 inactivated autophagy and coincidently, enhanced apoptosis in cell lines. In AD, the activation of caspases is a well-documented phenomenon (for review see and therefore, we hypothesize that one mechanism leading to impairment of Beclin-1-induced autophagy could be the caspase-mediated proteolysis. To address this hypothesis in the current study, we designed a site-directed caspase-cleavage antibody to Beclin-1 based upon the known caspase-cleavage sequence within Beclin-1, DQLD149. This neoepitope antibody, which we termed BeclinCCP (caspase-cleavage product antibody) was designed to recognize the 35 kDa, C-terminal fragment that would be generated following cleavage at this site. In vitro characterization of BeclinCCP indicated that it correctly labeled the predicted 35 kDa fragment following caspase-cleavage of Beclin-1 as well as the full-length form of the protein.
In contrast to Western blot analysis, in situ characterization of the BeclinCCP antibody indicated a specificity for the caspase-cleaved fragment of Beclin-1. Supporting this conclusion were data demonstrating that in MCAO mice the application of BeclinCCP labeled apoptotic neurons in ischemic infarct areas, while there was no staining on the contralateral side of the brain despite the fact that full-length Beclin-1 would be expressed throughout neuronal populations in the entire brain section. In addition, in human post mortem brain sections from control and AD subjects, BeclinCCP was highly specific for astrocytes that clearly displayed morphological features of cells undergoing apoptosis. We directly compared the staining profile of BeclinCCP with commercial antibodies known to detect full-length Beclin-1 at either the N- or C-terminus. The results indicated that while the commercial antibodies readily identified healthy neurons, BeclinCCP labeled damaged astrocytes as well as NFTs in the AD brain, but there was a complete absence of staining within healthy neuronal cells. Taken together, these data argue for the specificity of the BeclinCCP for the caspase-cleaved fragment of Beclin-1, in situ, and suggest this is a conformational-specific antibody. Thus, one possible explanation for the lack of immunoreactivity of BeclinCCP for full-length Beclin-1 is that in situ Beclin-1 is properly folded such that the epitope for the BeclinCCP antibody is inaccessible. However, following the caspase-cleavage of the protein, this site is now revealed, allowing for antibody binding and detection. In contrast, when applied in Western blot analysis, because under these conditions, proteins are denatured, the BeclinCCP antibody now is able to recognize and bind to the epitope regardless of whether Beclin-1 has been cleaved or not. Taken together, although our data support a specificity for the BeclinCCP antibody for the caspase-cleaved fragment in vivo. Although the BeclinCCP antibody reacted to both the C-terminal caspase-cleaved fragment as well as to the full-length form of Beclin-1 by Western blot, this was a fortuitous characteristic of the antibody, as it allowed us to confirm the depletion of full-length Beclin-1 in AD samples as compared to age-match controls, a finding that may explain the decrease in Beclin-1 expression in early AD .
Recently, we and others described that the caspase-mediated cleavage of Beclin-1 results in a loss-of-function with respect to its function as an autophagy inducing platform, but the caspase-generated C-terminal fragment would represent a gain-of-function regarding the sensitization of cytochrome c release (Wirawan et al., 2010). The latter would result in an apoptotic auto-amplifying loop promoting cell death. Therefore, the observed accumulation of the BeclinCCP may represent a marker for those astrocytes that have a sensitized cell death pathway.
In situ characterization of BeclinCCP indicated a high degree of specificity of labeling of astrocytes in the AD brain. Although Beclin-1 is known to be expressed in both neuronal and glial cell populations, our findings were somewhat surprising given the number and intensity of observed labeling in astrocytes. Astrocytes labeled with the BeclinCCP antibody displayed morphological features associated with apoptosis; most notably fragmented processes that were reduced to clusters or lines of dots radiating outward from the cell body. There was a significant increase (p=0.003) in the number of labeled astrocytes found in AD versus age-match control cases, and often, damaged astrocytes were located within plaque-rich regions. These data suggest that the toxic properties of Aβ are not exclusive to neuronal populations, but also may damage astrocytes that are localized near plaque regions. Interesting, confocal analysis with the BeclinCCP antibody indicated labeling of astrocytes, some of which appeared to contain cytoplasmic vacuoles. Considering the important role that Beclin-1 plays in the regulation of autophagy, it is not surprising that we identified such vacuoles within astrocytes of the AD brain. Moreover, disruption of autophagy following the caspase-cleavage of Beclin-1 could lead to the demise of astrocytes and contribute to the degeneration of this particular cell type. This idea was experimentally supported by the inverse relationship we observed between the staining of BeclinCCP to that of a full-length antibody to GFAP: in cases with weak BeclinCCP immuno-labeling there was a corresponding strong, widespread staining with the full-length GFAP antibody. Thus, the BeclinCCP antibody appeared to serve as a general marker for overall astrocytic health in the AD brain.
Similar to a previous study utilizing a caspase-cleavage antibody to GFAP, we also observed punctate labeling along blood vessels in the AD brain (Fig. 4D). In many cases labeling of BeclinCCP along blood vessels co-localized with an antibody to full-length GFAP (data not shown), providing support for astrocyte degeneration along blood vessels. Because of the vital role astrocytes play in protecting the CNS from systemic insults, our data suggest but do not directly demonstrate this as one mechanism that may contribute to a compromised blood-brain barrier that has been reported to occur in the AD brain .
In addition to labeling of astrocytes, the BeclinCCP antibody also labeled apparent NFTs in the AD brain, although staining was weaker and more variable (Table 2). Previous studies have linked the activation of caspases to NFT formation in AD, and our findings provide further support for this relationship. In this regard, our data clearly showed a much stronger degree of co-localization with MC-1, an early tangle marker than for PHF-1, a known late-stage tangle marker. However, in general there appeared to be many more labeled neurons with either MC-1 or PHF-1 that did not contain BeclinCCP, suggesting that the caspase-cleavage of Beclin-1 is most likely an effect not a cause of NFT formation in the AD brain.
In conclusion, generation and application of a novel caspase-cleavage site-directed antibody to Beclin-1 demonstrated the caspase-cleavage of Beclin-1 in the AD brain. Caspase-cleaved Beclin-1 was identified consistently within damaged astrocytes, and more variably within NFTs. Due to the critical role that Beclin-1 plays in the process of autophagy, our data provide a mechanism by which this process may be disrupted in AD and represent a condition of sensitized cell death pathways.
Acknowledgments
The authors would like to thank Dr. Sheng T. Hou (Experimental NeuroTherapeutics Laboratory, National Research Council Institute for Biological Sciences, National Research Council Canada, Ottawa, Ontario, Canada) for providing us with tissue sections from MCAO mice. Funded by a grant from the KO Alzheimer’s Disease Foundation (Boise, ID). Human brain tissues used in this study were graciously provided by the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine. Research in the Vandenabeele group is supported by VIB, Ghent University (UGent), Research Foundation Flanders (FWO-Vlaanderen) (3G.0218.06 and G.0226.09), Federal Research Program IAP 6/18, European Research Program FP6 ApopTrain (MRTN-CT-035624) and FP7 Apo-Sys 200767, and the GROUP-ID consortium of the UGent MRP initiative. P.V. holds a Methusalem grant (BOF09/01M00709) from the Flemish Government.
Funded by gift from the KO Alzheimer’s Disease Foundation (Boise, ID)
Abbreviations
- AD
Alzheimer’s disease
- Aβ
beta amyloid
- BeclinCCP
caspase-cleavage products antibody to human beclin-1
- CCPs
caspase-cleavage products
- GFAP
glial fibrillary acidic protein
- MCAO
middle cerebral artery occlusion
- NFTs
neurofibrillary tangles
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bian GL, et al. Fluoro-Jade C can specifically stain the degenerative neurons in the substantia nigra of the 1-methyl-4-phenyl-1,2,3,6-tetrahydro pyridine-treated C57BL/6 mice. Brain Res. 2007;1150:55–61. doi: 10.1016/j.brainres.2007.02.078. [DOI] [PubMed] [Google Scholar]
- Chidlow G, et al. Evaluation of Fluoro-Jade C as a marker of degenerating neurons in the rat retina and optic nerve. Exp Eye Res. 2009;88:426–37. doi: 10.1016/j.exer.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Chun W, Johnson GV. The role of tau phosphorylation and cleavage in neuronal cell death. Front Biosci. 2007;12:733–56. doi: 10.2741/2097. [DOI] [PubMed] [Google Scholar]
- Crews L, et al. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS One. 2010;5:e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue JE, Johanson CE. Apolipoprotein E, amyloid-beta, and blood-brain barrier permeability in Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:261–70. doi: 10.1097/NEN.0b013e31816a0dc8. [DOI] [PubMed] [Google Scholar]
- Dufty BM, et al. Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation. Am J Pathol. 2007;170:1725–38. doi: 10.2353/ajpath.2007.061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finder VH, et al. The recombinant amyloid-beta peptide Abeta1-42 aggregates faster and is more neurotoxic than synthetic Abeta1-42. J Mol Biol. 2010;396:9–18. doi: 10.1016/j.jmb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, et al. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2003;100:10032–7. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Hou ST, et al. Calpain-cleaved collapsin response mediator protein-3 induces neuronal death after glutamate toxicity and cerebral ischemia. J Neurosci. 2006;26:2241–9. doi: 10.1523/JNEUROSCI.4485-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger PA, Wyss-Coray T. All-you-can-eat: autophagy in neurodegeneration and neuroprotection. Mol Neurodegener. 2009;6:16. doi: 10.1186/1750-1326-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jicha GA, et al. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J Neurosci Res. 1997;48:128–32. doi: 10.1002/(sici)1097-4547(19970415)48:2<128::aid-jnr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Komatsu M, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–4. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Larsen KE, Sulzer D. Autophagy in neurons: a review. Histol Histopathol. 2002;17:897–908. doi: 10.14670/HH-17.897. [DOI] [PubMed] [Google Scholar]
- Liang XH, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–6. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Liang XH, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–96. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Rubinsztein DC. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 2010;17:268–77. doi: 10.1038/cdd.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, et al. Immunohistochemical evidence for macroautophagy in neurons and endothelial cells in Alzheimer’s disease. Neuropathol Appl Neurobiol. 2010 doi: 10.1111/j.1365-2990.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;15:2861–73. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Mouser PE, et al. Caspase-mediated cleavage of glial fibrillary acidic protein within degenerating astrocytes of the Alzheimer’s disease brain. Am J Pathol. 2006;168:936–46. doi: 10.2353/ajpath.2006.050798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–22. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- Pickford F, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–9. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami A. Review: autophagy in neurodegeneration: firefighter and/or incendiarist? Neuropathol Appl Neurobiol. 2009;35:449–61. doi: 10.1111/j.1365-2990.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Rissman RA, et al. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest. 2004;114:121–30. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn TT, Head E. Caspase activation in Alzheimer’s disease: early to rise and late to bed. Rev Neurosci. 2008;19:383–93. doi: 10.1515/revneuro.2008.19.6.383. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Head E. Caspases as therapeutic targets in Alzheimer’s disease: is it time to “cut” to the chase? Int J Clin Exp Pathol. 2009;2:108–18. [PMC free article] [PubMed] [Google Scholar]
- Rohn TT, et al. Caspase-9 Activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol Dis. 2002;11:341–354. doi: 10.1006/nbdi.2002.0549. [DOI] [PubMed] [Google Scholar]
- Spencer B, et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 2009;29:13578–88. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann C, et al. Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer’s disease. Evidence for apoptotic cell death. Am J Pathol. 1999;155:1459–66. doi: 10.1016/S0002-9440(10)65460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirawan E, et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death and Disease. 2010;1 doi: 10.1038/cddis.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, et al. Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]