Abstract
Background
Several staging systems have been proposed for hepatocellular carcinoma (HCC), however, none has incorporated circulating angiogenic biomarkers. This study sought to determine whether vascular endothelial growth factor (VEGF) could independently predict overall survival in patients with HCC, and whether adding VEGF level into the Cancer of the Liver Italian Program (CLIP) score could improve patients stratification and prediction of overall survival.
Methods
Between 2001 and 2008, baseline plasma VEGF levels were available from 288 patients and multivariate Cox regression models and median survival (95% confidence intervals) were calculated. Recursive partitioning was used to determine the optimal cut point for VEGF, using 10 repeated training/validation samples, each using 2/3 of the data to determine the best cut point and the remaining 1/3 to validate it. Prognostic ability of CLIP and V-CLIP was compared using C-index.
Results
Plasma VEGF was a significant independent predictor of overall survival, with an optimal VEGF cut point of 450 pg/ml. After CLIP validation in our patients, we added VEGF to the CLIP score and found that the new V-CLIP score better separates patients into homogenous prognostic groups (p-value=0.005).
Conclusion
The assessment of baseline plasma VEGF levels increases the precision of the CLIP scoring system for predicting HCC prognosis, which may assist in equally randomizing patients with HCC in clinical trials. Prospective validation of the V-CLIP scoring system is warranted.
Keywords: Hepatocellular carcinoma, staging, CLIP, VEGF, prognosis
INTRODUCTION
In the United States, the incidence of hepatocellular carcinoma (HCC), the most common form of primary liver cancer, has been steadily rising over the last three decades.1 A recent study indicated that the incidence rate of HCC in the United States tripled from 1975 to 2005.2
The management of patients with advanced, unresectable, HCC presents several challenges, including the need for prognostic staging systems to predict prognosis and stratify patients on clinical trials. Therefore, increasingly specific parameters have been used to evaluate survival and prognosis of HCC patients, starting with the presence of cirrhosis, as the prognosis of HCC depends not only on tumor size but also on underlying liver function. A limitation of the Child-Pugh score, which reflects the degree of hepatic reserve in patients with cirrhosis, is the lack of any parameter that directly pertains to the tumor itself.3 Therefore, the concept of adding more parameters to assess the tumor status was established, and subsequently several clinical staging systems for HCC have been proposed,4-8 including the Cancer of the Liver Italian Program (CLIP),6 and the Barcelona Clinic Liver Cancer (BCLC) staging system.8 Derived from European patients with predominantly hepatitis C- and alcohol-related HCC, the CLIP score has gained wide acceptance among scientists in the Western world. The CLIP score has been compared with another scoring system known as the Chinese University Prognostic Index (CUPI),7 which was derived from Asian patients with predominantly hepatitis B-related HCC. The investigators’ attempt to apply the CLIP scoring system to their population led to false predictions of outcome, suggesting that different scoring systems may apply to different patient populations, most likely related to the different risk factors, disease stage (early versus advanced), and demographics.7
Therefore, among the many and varied systems for HCC staging, the CLIP scoring system is among the most commonly used systems in Europe and the United States to predict prognosis and stratify patients on clinical trials. Furthermore, several groups have validated the CLIP score,9-13 and most recently, a US study evaluated six HCC staging systems for their ability to predict survival by using the concordance index (c-index). The study concluded that CLIP score was among the tops three most informative systems in predicting survival in advanced HCC patients.14 However, since nearly 80% of the patient population is classified as having a CLIP score of 0–3, questions regarding poor stratification ability have been raised. Furthermore, alpha fetoprotein (AFP), one of the CLIP parameters, is detectable in only about 70% of HCC cases, and hence both false-negative and false-positive rates are high with the use of AFP as the serological marker for the detection of HCC.15
HCC is highly vascular and frequently associated with vascular invasion. In fact, angiogenesis is involved in the development of HCC from the initial stage of carcinogenesis to the end stage of metastastatic disease.16 Vascular endothelial growth factor (VEGF) is the major mediator of angiogenesis in HCC, and was found to be correlated with prognosis in several studies.17-20 In addition, the VEGF pathway has been studied extensively as a target for therapy, and recent clinical trial results have validated anti-VEGF or anti-VEGF receptor (VEGFR) directed therapy in HCC.21-26 After an extensive review of the literature, we concluded that there was sufficient evidence to warrant investigating the use of plasma VEGF measured by enzyme-linked immunoassay (ELISA) as a marker of VEGF levels in tumor tissue, and as a prognostic indicator.27
Given the fundamental importance of angiogenesis for HCC tumor growth and progression, and the key role of VEGF in these processes, we chose to study the value of adding the plasma level of VEGF to the CLIP score, after validating it in our patient population, as a prognostic indicator in HCC patients. We sought to determine whether VEGF plasma levels measured at diagnosis could better stratify patients with HCC and independently predict their overall survival.
PATIENTS AND METHODS
Study Population
Using a protocol approved by M. D. Anderson’s Institutional Review Board, we enrolled new patients with histologically confirmed HCC who lived in the United States and were evaluated and treated at the Gastrointestinal Center of The University of Texas M. D. Anderson Cancer Center in Houston. All patients gave written informed consent prior to participation. The inclusion criteria were as follows: pathologically confirmed diagnosis of HCC, U.S. residency, and the ability to communicate in English. The exclusion criteria included the presence of other types of primary liver cancer (such as cholangiocarcinoma or fibrolamellar HCC), or concurrent or past history of other types of cancers. The primary endpoint of the study was evaluation of the correlation between baseline plasma biomarkers and overall survival. From January 2000 through March 2008, from all patients referred to our center, we enrolled 394 eligible patients with HCC. Baseline plasma samples were available for 288 (73%) of the recruited population and missed or insufficient for 106 (27%) HCC patients. While all subjects agreed to participate in the study, the main reason for missing these blood samples was related to insufficient time to obtain blood samples during the initial clinical assessment of HCC patients.
Patient characteristics are shown in table 1. Notably, statistical analyses indicated no difference between recruited subjects with and without blood samples in terms of demographic characteristics (age, sex, race, education level); HCC risk factors (HCV, HBV, diabetes history, alcohol consumption, and cigarette smoking); cirrhosis, child-Pugh classification; pathological tumor differentiation; baseline value of ALT and albumin, or CLIP scoring. However, patients without plasma samples tended to have multinodular tumor, but tumor size <50% of the liver, radiological evidence of portal vein thrombosis, and high baseline AFP.
Table 1.
Patients’ Characteristics
| Variables | N=288 (%) | Variables | N=288 (%) |
|---|---|---|---|
| α-fetoprotein | Nodularity | ||
| <400 | 199 (69.1) | Uni- | 105 (36.5) |
| ≥ 400 | 86 (29.8) | Multi- | 183 (63.5) |
| Missing | 3 (1) | ||
| Differentiation | Lymph Nodes | ||
| Well | 112 (38.9) | Yes | 122 (42.4) |
| Moderate | 95 (33) | No | 166 (57.6) |
| Poor | 50 (17.4) | Bilirubin | |
| Unknown | 31 (10.8) | ≤ 1.6 | 260 (90.3) |
| Tumor size | > 1.6 | 28 (9.7) | |
| ≤ 50% | 191 (66.3) | Cirrhosis | |
| > 50 % | 97 (33.7) | Yes | 173 (60.1) |
| Vascular invasion | No | 115 (39.9) | |
| Yes | 53 (18.4) | Child–Pugh | |
| No | 235 (81.6) | A | 206 (71.5) |
| Metastases | B | 76 (26.4) | |
| Yes | 60 (20.8) | C | 6 (2.1) |
| No | 228 (79.2) |
Baseline plasma VEGF assay
Plasma was prepared from 3-5 ml of peripheral blood collected in ethylenediaminetetraacetic acid (EDTA)-containing tubes through 21-gauge needles. Samples were then centrifuged at 4°C for 15 minutes (3000 rpm), and removed, aliquoted, and snap frozen at −20°C. We measured plasma VEGF-A (the VEGF165 isoform) by ELISA (Quantikine Human VEGF Immunoassay ELISA Kit; R&D Systems, Minneapolis, MN). Each measurement was made in duplicate, and the VEGF level was determined from a standard curve generated for each set of samples assayed.
Statistical Analysis
We used Wilcoxon rank sum test to correlate baseline VEGF levels with various clinical characteristics and staging systems and Cox regression to assess factors associated with overall survival.
To find an optimal VEGF cut point, we randomly split the data into training (2/3) and validation (test) (1/3) sets, and applied recursive partitioning 28 to the training set to find the optimal cut point maximizing the survival difference between the low and high VEGF groups, and then validated that cut point by fitting a Cox regression model to the dichotomized VEGF factor on the test data. We repeated this process for 10 different random splits of the data into training/test sets.
To assess whether VEGF was an independent prognostic factor after adjusting for other known factors, we fit multivariable Cox regression models including VEGF, dichotomized at the optimal cutpoint, and the variables in the CLIP scoring system.
To assess the performance of the scoring systems, we computed the median survival for the patients in each V-CLIP group (0, 1, 2, 3, 4, and 5+) and compared the groups using log-rank tests, and did likewise for the CLIP score. The sign test was used to assess whether the VEGF high groups tended to have shorter median survival within the CLIP groups than VEGF low groups. The prognostic ability of the CLIP, V-CLIP, and BCLC were compared using a C-Index test.
RESULTS
Patient characteristics
The estimated overall median survival duration and 95% confidence interval (CI) of 288 patients was 13.8 months, 95% CI: 11.7–17.3, see Figure 1. A total of 87 patients had HCV infection (30.2%). As shown in Table 2, the hazard ratio (HR, 95%) estimated from Cox regression models indicated that strongest associations were with the tumor parameters; tumor size, nodularity, differentiation, vascular invasion, and AFP; in addition to liver function parameters, such as bilirubin, ALT, and AST.
Figure 1.
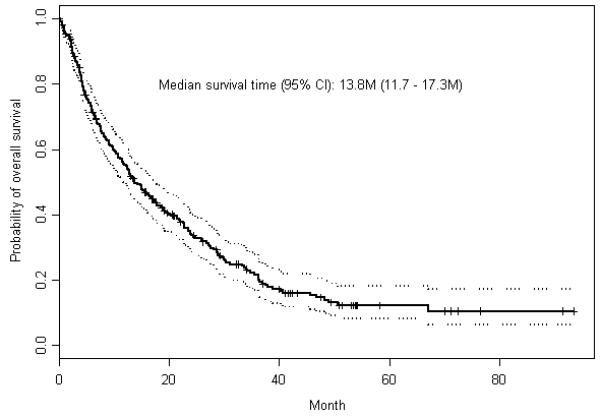
Kaplan Meier estimates of overall survival
Table 2.
Survival predictors: Univariate Cox regression analysis
| Predictor | HR | 95%CI of HR |
P |
|---|---|---|---|
| Age (>60 vs ≤60) | 0.88 | 0.66-1.16 | 0.351 |
| Gender (male vs female) | 1.44 | 1.06-1.96 | 0.018 |
| Race (white vs nonwhite) | 0.75 | 0.56-1.00 | 0.051 |
| Hepatitis Virus infection | |||
| (no infection vs HBV+HCV) | 0.51 | 0.32-0.80 | 0.004 |
| (HBV alone vs HBV+HCV) | 0.76 | 0.44-1.32 | 0.334 |
| (HCV alone vs HBV+HCV) | 0.72 | 0.43-1.18 | 0.192 |
| AFP (≥400 vs <400) | 2.26 | 1.69-3.02 | <0.0001 |
| Tumor differential (poor vs other) | 1.63 | 1.15-2.31 | 0.006 |
| Tumor nodularity (multi vs uni) | 2.28 | 1.68-3.11 | <0.0001 |
| Tumor size (>50% vs ≤50%) | 2.92 | 2.19-3.90 | <0.0001 |
| Vascular invasive (yes vs no) | 2.65 | 1.90-3.70 | <0.0001 |
| Lymph Node involvement (yes vs no) | 1.82 | 1.38-2.40 | <0.0001 |
| Metastasis (yes vs no) | 1.76 | 1.27-2.45 | 0.001 |
| Bilirubin (>1.6 vs ≤1.6) | 2.74 | 1.78-4.22 | <0.0001 |
| Serum ALT (>40 vs ≤40) | 1.77 | 1.34-2.34 | <0.0001 |
| Serum AST (>45 vs ≤45) | 2.17 | 1.57-3.00 | <0.0001 |
| Cirrhosis (yes vs no) | 1.35 | 1.02-1.79 | 0.036 |
| Treatment (chemotherapy vs none) | 0.56 | 0.38-0.84 | 0.0047 |
| (surgery vs none) | 0.19 | 0.12-0.31 | <.0001 |
| (chemoembolization vs none) | 0.38 | 0.22-0.67 | 0.0008 |
| VEGF (100-unit increase) | 1.04 | 1.01-1.07 | 0.007 |
Validation of CLIP scoring system
First we validated the CLIP scoring system by fitting a multivariable Cox regression model to our data including the factors contained in the CLIP score [see Table 3]. Because AFP values were missing from 3 patients, only 285 patients were included.
Table 3.
Multivariable Cox proportional hazards model for CLIP score variables, with p-values, hazard rate (HR) estimates, and 95% confidence intervals (CIs). The hazard rates from the CLIP publication6 are also included for comparison.
| CLIP Score Variables | P | HR | 95% CI of HR | HR from CLIP publication6 |
|
|---|---|---|---|---|---|
| Child-Pugh stage | (B vs A) | 0.0008 | 1.72 | 1.26-2.37 | 1.72 |
| (C vs A) | 0.03 | 3.10 | 1.12-8.58 | 3.92 | |
| Tumor morphology | (1 vs 0) | 0.003 | 1.80 | 1.23-2.65 | 1.74 |
| (2 vs 0) | <0.0001 | 4.28 | 2.87-6.37 | 3.18 | |
| AFP | (≥400 vs <400) | 0.0002 | 1.81 | 1.33-2.46 | 1.79 |
| Portal vein thrombosis (yes vs no) | 0.14 | 1.42 | 0.90-2.25 | 1.58 | |
We found that Child-Pugh score (HR = 1.72 for B vs A, P = 0.0008; HR = 3.10 for C vs A, P = 0.030), tumor morphology (HR = 1.80 for 1 vs 0, P = 0.0027; HR = 4.28 for 2 vs 0, P < 0.0001), and AFP (HR=1.81 for >400 vs ≤400, P = 0.0002) were all highly significant, with HRs very close to those reported in the original CLIP paper.6 While the presence or absence of portal vein thrombosis (HR = 1.42, P = 0.14) was not statistically significant, it nonetheless had an estimated effect size close to that observed in the original description of CLIP.
As expected, we found that the CLIP score separated the patients very effectively into different prognostic groups (P < 0.0001), with median survival durations of 37.0, 23.1, 11.7, 7.6, and 2.5 months for CLIP scores of 0, 1, 2, 3, and 4+, respectively. Note that the HRs for the factors in the CLIP model are all very similar in magnitude, and the HRs for the 3-level factors increase in a roughly linear fashion. These results strongly justify the use of a simple count-based scoring system like the CLIP. Finally, we compared C-index between CLIP score and BCLC staging in our patients. In the C-index analysis: the concordance probabilities for CLIP and BCLC were 0.70 and 0.65, respectively. Using U-statistics the difference was significant and the p-value was 0.007. Our results confirm that CLIP scoring system better predicted patients’ survival than BCLC staging.
High levels of VEGF as an independent prognostic factor
The recursive partitioning was applied to the 10 randomly selected training/test sets to find the optimal single cut point for baseline VEGF in terms of predicting survival, see Table 4. We observed that 5/10 of the training sets found an optimal cut point of roughly 450 ng/ml, and that for 4 of these 5 sets, this split was found to significantly separate low- from high-risk groups for overall survival in the corresponding test sets. This suggests that patients with high VEGF levels (>450 ng/ml) had a worse prognosis. When this factor was considered in a univariate Cox regression model fit to the entire data set, this effect was highly significant (P = 0.0002, HR = 1.89, 95% CI = 1.36–2.65).
Table 4(a).
Summary of search for optimal VEGF cut point.
| VEGF |
Training Set |
Testing Set |
||||||
|---|---|---|---|---|---|---|---|---|
| Resampling | Cutpoint | Strata | N | E | P-training | N | E | P-testing |
| 1 | 450.748 | 0 | 150 | 105 | 0.00024 | 80 | 59 | 0.2720 |
| 1 | 40 | 32 | 16 | 12 | ||||
| 2 | 450.274 | 0 | 150 | 111 | 0.01236 | 80 | 53 | 0.0065 |
| 1 | 40 | 31 | 16 | 13 | ||||
| 3 | 382.7135 | 0 | 144 | 102 | 0.01395 | 74 | 54 | 0.0734 |
| 1 | 46 | 35 | 22 | 17 | ||||
| 4 | 317.8525 | 0 | 129 | 91 | 0.00387 | 72 | 53 | 0.4686 |
| 1 | 61 | 46 | 24 | 18 | ||||
| 5 | 496.0195 | 0 | 157 | 117 | 0.00106 | 81 | 52 | 0.1357 |
| 1 | 33 | 29 | 15 | 10 | ||||
| 6 | 450.274 | 0 | 155 | 108 | 0.03522 | 75 | 56 | 0.0008 |
| 1 | 35 | 26 | 21 | 18 | ||||
| 7 | 450.748 | 0 | 151 | 106 | 0.00361 | 79 | 58 | 0.0184 |
| 1 | 39 | 31 | 17 | 13 | ||||
| 8 | 509.59 | 0 | 161 | 103 | 0.00001 | 80 | 68 | 0.9052 |
| 1 | 29 | 26 | 16 | 11 | ||||
| 9 | 450.748 | 0 | 160 | 114 | 0.00784 | 70 | 50 | 0.0078 |
| 1 | 30 | 22 | 26 | 22 | ||||
| 10 | 60.889 | 0 | 52 | 39 | 0.01768 | 29 | 20 | 0.7040 |
| 1 | 138 | 105 | 67 | 44 | ||||
As shown in the Table 4(b), tumor size, lymph node involvement, extrahepatic metastases, Child-Pugh score, CLIP score, BCLC staging, and ECOG performance status score were all significantly associated with the baseline level of VEGF in plasma. The strongest association was with tumor size (the mean plasma VEGF level for tumors involving <50% of the liver was 218, and the mean level for tumors involving >50% of the liver was 425, P <0.0001).
Since baseline VEGF was correlated with other clinical prognostic factors, we tested whether baseline VEGF was an independent prognostic factor [see Table 5(a)]. We observed that even after adjusting for the Child-Pugh score, tumor morphology, AFP, and portal vein thrombosis, the baseline level of VEGF was a significant independent prognostic factor for overall survival (P = 0.0013, HR = 1.78, 95% CI = 1.25–2.52). Note that even with VEGF incorporation in the model, the HRs for the other CLIP factors did not change much, and all retained the same degree of statistical significance.
Table 4(b).
Correlations between Plasma VEGF Level and Patient Characteristics by the Wilcoxon Rank-Sum Test
| Patient Characteristics | Variable Label | HCC Patients N=288 (%) |
Plasma VEGF Mean |
(pg/ml) ± SE |
P value |
|---|---|---|---|---|---|
| Age (years) | <60 | 111(38.5%) | 284.77 | 390.29 | 0.69 |
| ≥ 60 | 177(61.5%) | 290.44 | 399.55 | ||
| Sex | Male | 199(69.1%) | 285.53 | 412.83 | 0.82 |
| Female | 89(30.9%) | 294.34 | 355.23 | ||
| Race | Non-White | 89(30.9%) | 303.54 | 426.43 | 0.31 |
| white | 199(69.1%) | 281.42 | 381.53 | ||
| Hepatitis Virus Infection | HCV | 60(20.8%) | 284.21 | 341.33 | 0.27 |
| HBV | 38(13.2%) | 375.54 | 478.99 | ||
| HBV and HCV | 27(9.4%) | 220.94 | 318.27 | ||
| None | 163(56.6%) | 280.54 | 403.96 | ||
| Serum α-FP (ng/mL) | <400 | 199(69.1%) | 270.31 | 411.94 | 0.15 |
| ≥ 400 | 86(29.9%) | 333.39 | 358.85 | ||
| unknown | 3(1%) | 184.19 | 138.13 | ||
| Tumor differentiation | Well | 112(38.9%) | 290.83 | 477.27 | 0.19 |
| Moderate | 95(33%) | 280.92 | 336.95 | ||
| Poor | 50(17.4%) | 268.85 | 355.72 | ||
| Unknown | 31(10.8%) | 332.67 | 295.05 | ||
| Tumor nodularity | Uninodular | 105(36.5%) | 261.32 | 433.68 | 0.25 |
| Multinodular | 183(63.5%) | 303.71 | 371.91 | ||
| Tumor size | ≤ 50% | 191(66.3%) | 218.60 | 288.27 | <.0001 |
| > 50% | 97(33.7%) | 425.41 | 523.55 | ||
| Vascula invasion | No | 235(81.6%) | 287.43 | 397.77 | 0.94 |
| Yes | 53(18.4%) | 291.88 | 388.02 | ||
| Lymph node involvement | No | 166(57.6%) | 277.76 | 422.76 | 0.04 |
| Yes | 122(42.4%) | 302.53 | 355.84 | ||
| Metastasis | No | 228(79.2%) | 273.06 | 399.39 | 0.01 |
| Yes | 60(20.8%) | 345.96 | 377.16 | ||
| Bilirubin (mg/Dl) | ≤1.6 | 260(90.3%) | 290.02 | 408.00 | 0.54 |
| > 1.6 | 28(9.7%) | 271.87 | 253.22 | ||
| ALT (U/L) | ≤ 40 | 134(46.5%) | 284.62 | 359.07 | 0.27 |
| > 40 | 153(53.1%) | 286.13 | 421.82 | ||
| unknown | 1(0.3%) | 1099.61 | |||
| AST (U/L) | ≤ 45 | 88(30.6%) | 288.88 | 479.80 | 0.16 |
| > 45 | 179(62.2%) | 276.28 | 346.12 | ||
| unknown | 21(7.3%) | 387.63 | 404.26 | ||
| Cirrhosis | No | 115(39.9%) | 299.46 | 437.41 | 0.88 |
| Yes | 173(60.1%) | 280.80 | 365.83 | ||
| Child-Pugh score | A | 206(71.5%) | 288.35 | 399.99 | 0.05 |
| B | 76(26.4%) | 269.45 | 388.11 | ||
| C | 6(2.1%) | 523.16 | 282.89 | ||
| CLIP Scoring System | 0 | 55(19.2%) | 239.43 | 284.11 | 0.0076 |
| 1 | 75(26.3%) | 213.34 | 307.17 | ||
| 2 | 77(27%) | 304.86 | 494.83 | ||
| 3 | 53(18.5%) | 380.46 | 462.37 | ||
| 4 | 19(6.6%) | 346.42 | 310.68 | ||
| 5 | 6(2.1%) | 529.93 | 288.68 | ||
| BCLC Staging System | 0 | 21(7.3%) | 131.91 | 106.28 | 0.0003 |
| A | 28(9.7%) | 216.35 | 374.48 | ||
| B | 29(10.1%) | 273.49 | 372.76 | ||
| C | 189(65.6%) | 288.30 | 398.10 | ||
| D | 21(7.3%) | 560.38 | 496.84 | ||
| ECOG Performance Status | 0 | 127(44.1%) | 210.87 | 282.83 | 0.0002 |
| 1 | 104(36.1%) | 266.44 | 327.80 | ||
| 2 | 38(13.2%) | 454.75 | 645.24 | ||
| 3 | 15(5.2%) | 486.88 | 364.26 | ||
| 4 | 4(1.4%) | 985.52 | 825.74 |
VEGF separates high- and low-risk groups within each CLIP score
We split out the patients within each CLIP score group according to whether they had low/high VEGF [Table 5(b)]. At each of the five CLIP levels, the estimated median survival for VEGF-high patients was less than the median survival for VEGF-low patients, suggesting that overall, VEGF-high patients had worse prognosis than VEGF-low patients(p=0.031, Sign test). Looking at comparisons of VEGF-high vs. VEGF-low within each specific CLIP group, we found that the VEGF high/low comparison was statistically significant for V-CLIP 3 and 4+ (p=0.05), while the other groups (V-CLIP 0, 1, 2) demonstrated strong trends that were not quite statistically significant. While our overall test assessing the prognostic information of VEGF was significant (p=0.031), it was not too surprising that the specific comparisons were not statistically significant within some of the CLIP groups, given the low power (<0.15) for these subgroup analyses, each of which had relatively small numbers of VEGF-high patients (≤15 subjects). Note that, in most cases, the VEGF-high patients in a particular CLIP group tended to have median survivals more like the next higher CLIP score. Given this observation, and the fact that the magnitude of the effect of high-VEGF in our multivariate Cox regression model (HR = 1.78, P = 0.0013) is as strong as the effect of the other factors in the CLIP score, we devised a new scoring system. Our system, which we term the V-CLIP, adds a high VEGF level (>450 ng/ml) to the factors already included in the CLIP score, resulting in an integer score between 0 and 7 for each patient [see Table 6(a)].
Table 5 (a).
Multivariable Cox proportional hazards model for V-CLIP score variables, with p-values, hazard rate (HR) estimates, and 95% confidence intervals (CIs).
| (a) V-CLIP Score Variables | P | HR | 95%CI of HR | |
|---|---|---|---|---|
| Child-Pugh stage | (B vs A) | 0.0003 | 1.82 | 1.32-2.51 |
| (C vs A) | 0.0767 | 2.54 | 0.91-7.13 | |
| Tumor morphology | (1 vs 0) | 0.0024 | 1.82 | 1.23-2.67 |
| (2 vs 0) | <0.0001 | 4.11 | 2.75-6.14 | |
| AFP (≥400 vs <400) | 0.0002 | 1.80 | 1.32-2.44 | |
| Portal vein thrombosis (yes vs no) | 0.118 | 1.44 | 0.91-2.28 | |
| VEGF (>450 vs ≤450) | 0.0013 | 1.78 | 1.25-2.52 | |
Table 5 (b).
Median survival (95% confidence interval) divided by CLIP score and further by VEGF level (<450 vs. >450). The P-values correspond to a test comparing median survival VEGF-high and VEGF-low patients within the specified CLIP score, and the power indicates power to detect a significant difference given the observed sample sizes, assuming the true difference in median survival was the same as the observed difference in these data. Note how VEGF-high patients in a given CLIP group seem to have median survivals more similar to VEGF-low patients in the next CLIP group than VEGF-low patients in their own CLIP group.
| all | VEGF <450 | VEGF >450 | ||||||
|---|---|---|---|---|---|---|---|---|
| CLIP | Median Survival | Median Survival | Median Survival | |||||
| score | n | (months) | n | (months) | n | (months) | P-value | Power |
| 0 | 55 | 37.0 (24.8-51.3) | 48 | 37.5 (29.4-68.1) | 7 | 19.5 (9.23-na) | 0.10 | 0.14 |
| 1 | 75 | 23.1 (17.5-34.2) | 64 | 23.1 (17.5-34.2) | 11 | 18.1 (13.4-38.4) | 0.50 | 0.08 |
| 2 | 77 | 11.7 (8.3-15.3) | 62 | 12.4 (8.3-17.3) | 15 | 9.6 (4.7-21.7) | 0.23 | 0.10 |
| 3 | 53 | 7.6 (4.6-9.3) | 40 | 7.8 (6.1-11.7) | 13 | 3.6 (2.3-10.6) | 0.05 | 0.48 |
| 4+ | 25 | 2.5 (2.2-3.9) | 15 | 4.4 (2.1-12.6) | 10 | 2.5 (0.9-2.7) | 0.05 | 0.17 |
V-CLIP scoring appears to provide more accurate stratification than CLIP scoring alone does
The V-CLIP score divided patients very well (P < 0.0001), with median survivals of 37.5, 23.1, 14.5, 8.7, 3.6, and 2.5 months for V-CLIP scores of 0, 1, 2, 3, and 4+, respectively [see Table 6(b)]. Based on a C-Index analysis,28 we compared the predictive ability of CLIP versus V-CLIP and found that the V-CLIP index was more able to predict patients’ prognosis than the CLIP index (p=0.005).
Table 6 (a).
V-CLIP Scoring system (0-7)
| V-CLIP Score Variable | Scores |
||
|---|---|---|---|
| 0 | 1 | 2 | |
| Child-Pugh stage | A | B | C |
| Tumor morphology | Uninodular and ≤50% | Multinodular and ≤50% | Massive or >50% |
| AFP | <400 | ≥400 | |
| Portal vein thrombosis | No | Yes | |
| VEGF | ≤450 | >450 | |
Discussion
We validated the CLIP scoring in our patient population with hazard ratios very close to those reported in the original CLIP paper,6 and observed that baseline plasma VEGF, a key mediator of angiogenesis in HCC, was a significant independent predictor of overall survival, with an optimal VEGF cut point of 450 pg/ml. Therefore, we chose to incorporate it into CLIP, one of the most widely accepted prognostic scoring systems for HCC in the Western world. Our newly developed V-CLIP score provides an ordinal score from 0 to 7 for each patient based on their CLIP factors, and their optimal cut point dichotomized VEGF levels. V-CLIP showed better discriminative ability than CLIP for stratifying patients with HCC into different risk groups. This could be because the tumor parameters are not well represented in CLIP, while VEGF correlated significantly with all of them based on our univariate analysis.
We internally validated our results by randomly splitting the data into training (2/3) and validation (test) (1/3) sets, to find the optimal VEGF cut point and then validated that cut point on the test data. We had a successful rate (73%) of accrual of HCC patients to our biomarker study, given the challenge of accruing patients with such a poor prognosis disease. The major aim of our large, single-institution, biomarker study was to create a novel and simple prognostic scoring system that would provide a more precise prediction of overall survival in patients with HCC. Notably, we compared the prognostic ability of CLIP score to BCLC staging system,8 another widely used HCC staging system, using the C-index analysis. Remarkably, we observed that the concordance probabilities for CLIP and BCLC were 0.70 and 0.65, respectively, with a significant p-value of 0.007. Notably, BCLC was designed in its first version (Llovet et al 1999) as a treatment allocation system, not a prognostic system to predict survival and stratify patients for clinical trials. However, after validation, BCLC has been accepted for use as a prognostic system to stratify patients for clinical trials. However, advanced HCC patients who are candidates for systemic therapy clinical trials are grouped in a single category, BCLC-stage C. Furthermore, the BCLC system links the patients’ survival, not only to the liver and the tumor parameters, but also to the type of treatment received. Therefore, both systems are conceptually different, thus, it is challenging to directly compare their respective prognostic abilities. Nevertheless, the CLIP score validation in our patient population was very successful, and so was integrating VEGF into the new V-CLIP system. Therefore, we believe that our approach, after independent prospective validation, may prove very promising in stratifying patients on clinical trials. However, integration of baseline plasma VEGF into other commonly used HCC staging systems is warranted to compare their predictive abilities to that of the V-CLIP.
Importantly, comparing the CLIP and V-CLIP scores, we noted that the key differences were in the moderate risk patients (CLIP 2-3 and V-CLIP 2-4), as the median survival for the lowest risk patients (CLIP 0-1 and V-CLIP 0-1) and highest risk patients (CLIP 4+, V-CLIP 5+) were similar to each other. For the moderate risk patients, the CLIP only separated patients into two groups (CLIP=2 and CLIP=3) with median survivals of 11.7 and 7.6 months, respectively, while the V-CLIP separated these patients into three prognostic groups (V-CLIP 2, 3, 4) with disparate median survivals, 14.5, 8.7, and 3.6 months, respectively. This more precise stratification of the moderate-risk patients is of particular importance in stratifying patients for therapeutic clinical trials, and in predicting the likelihood of patients’ survival at certain time points. Several clinical trials have used and validated CLIP scoring system based on the difference between categories of CLIP ≤ 3 versus CLIP > 3. We see from Table 5(b) that the CLIP 3 group is heterogeneous, with high-VEGF patients having worse median survival (3.6 months) than the low-VEGF patients (7.8 months) (p=0.05). Using V-CLIP, the high-VEGF CLIP 3 patients are stratified with the high-risk group, which appears to be more accurate in terms of predicting survival.
One of the limitations of our study is that our patient population had mainly unresectable disease. However, predicting prognosis of patients with unresectable HCC is critically important for clinical trial stratification and interpretation purposes. Therefore, our single-institution study will benefit from prospective validation, in other patient populations, with difference demographics, risk factors, and stages of disease. To that end, our system to estimate prognosis in patients with HCC is advantageous, since it is simple, based on variables that are easily testable, and therefore can be independently validated. Another limitation of our study was that our patient population tended to be selective of subjects who were able to return to our center to get their blood withdrawn. Therefore, patients who were missed (27%) tended to have more advanced disease. Nevertheless, our results indicated that even in patients with possibly better prognosis, the VEGF level was significantly associated with overall survival and correlated with other features of advanced HCC. However, this further reinforces the need to validate our results prospectively.
Notably, considerable efforts have been made by several groups to obtain a molecular classification of HCC that would reflect the tumor parameters more accurately, but the overwhelming genomic complexity of this disease has rendered this goal challenging. Therefore, a molecular classification of this highly complex disease has remained elusive. Moreover, HCC is a heterogeneous disease, in terms of the risk factors, natural history, and even response to different modalities of therapy. This has become more evident recently, based on the difference in systemic therapy outcome between Western patients on SHARP trial,21 and Eastern patients on the Asia-Pacific trial using the same drug, sorafenib.26
Finally, this research and other work have demonstrated the prognostic importance of pro-angiogenic molecules that are expected to play a role in HCC initiation and progression. Our study also showed that the BCLC staging system, one of the most commonly used systems for stratifying patients with HCC, was significantly associated with the baseline level of VEGF in plasma (p=0.0003), see Table 4. Therefore, future prospective studies in different patient populations will be necessary, not only to validate the V-CLIP scoring system, but also to investigate the utility of integrating VEGF into other staging systems in predicting prognosis and refining stratification of patients with HCC. In addition, other biomarkers involved in hepatocarcinogenesis should also be examined for their effect on prognosis. These emerging molecular approaches to designing newer prognostic systems may prove to be more accurate in predicting prognosis and in stratifying patients with HCC during therapeutic clinical trials, and may also be helpful when used to guide treatment decisions.
Table 6 (b).
Survival by V-CLIP Scoring system
| V-CLIP Score | n | Median Survival (months) |
|---|---|---|
| 0 | 48 | 37.5 (29.4-68.1) |
| 1 | 71 | 23.1 (17.5-31.3) |
| 2 | 73 | 14.5 (10.1-18.0) |
| 3 | 55 | 8.7 (6.3-11.7) |
| 4+ | 38 | 2.7 (2.3-4.1) |
ACKNOWLEDGMENT
The authors would like to thank Dr. Lee M. Ellis at The University of Texas M. D. Anderson Cancer Center for his guidance in this research.
All principal investigators of the above-funded studies had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Research Support: Supported by National Institutes of Health (NIH) RO3 grant ES11481, CA106458-01 (to M.H.), and by philanthropic funds to the Department of Gastrointestinal Medical Oncology.
Footnotes
Conflict of Interest: All authors have no commercial associations (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All authors of this manuscript contributed to the administrative functions of the study without potential competing or financial interests.
References
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–15. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 5.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–28. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 6.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–5. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 7.Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–9. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 9.Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000;31:840–5. doi: 10.1053/he.2000.5628. [DOI] [PubMed] [Google Scholar]
- 10.Farinati F, Rinaldi M, Gianni S, Naccarato R. How should patients with hepatocellular carcinoma be staged? Validation of a new prognostic system. Cancer. 2000;89:2266–73. [PubMed] [Google Scholar]
- 11.Levy I, Sherman M. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut. 2002;50:881–5. doi: 10.1136/gut.50.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, Baba Y, Imamura Y, Aikou T. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001;34:529–34. doi: 10.1053/jhep.2001.27219. [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Bruix J. Prospective validation of the Cancer of the Liver Italian Program (CLIP) score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;32:679–80. doi: 10.1053/jhep.2000.16475. [DOI] [PubMed] [Google Scholar]
- 14.Huitzil-Melendez FD, Capanu M, O’Reilly EM, Duffy A, Gansukh B, Saltz LL, Abou-Alfa GK. Advanced Hepatocellular Carcinoma: Which Staging Systems Best Predict Prognosis? J Clin Oncol. 2010;28:2889–95. doi: 10.1200/JCO.2009.25.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou L, Liu J, Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World J Gastroenterol. 2006;12:1175–81. doi: 10.3748/wjg.v12.i8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poon RT, Fan ST, Wong J. Clinical significance of angiogenesis in gastrointestinal cancers: a target for novel prognostic and therapeutic approaches. Ann Surg. 2003;238:9–28. doi: 10.1097/01.sla.0000075047.47175.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao DF, Wu XH, Zhu Y, Shi GS, Dong ZZ, Yao DB, Wu W, Qiu LW, Meng XY. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2005;4:220–6. [PubMed] [Google Scholar]
- 18.Tseng CS, Lo HW, Chen PH, Chuang WL, Juan CC, Ker CG. Clinical significance of plasma D-dimer levels and serum VEGF levels in patients with hepatocellular carcinoma. Hepatogastroenterology. 2004;51:1454–8. [PubMed] [Google Scholar]
- 19.Poon RT, Ho JW, Tong CS, Lau C, Ng IO, Fan ST. Prognostic significance of serum vascular endothelial growth factor and endostatin in patients with hepatocellular carcinoma. Br J Surg. 2004;91:1354–60. doi: 10.1002/bjs.4594. [DOI] [PubMed] [Google Scholar]
- 20.Kamel L, Nessim I, Abd-el-Hady A, Ghali A, Ismail A. Assessment of the clinical significance of serum vascular endothelial growth factor and matrix metalloproteinase-9 in patients with hepatocellular carcinoma. J Egypt Soc Parasitol. 2005;35:875–90. [PubMed] [Google Scholar]
- 21.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 22.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B, Taylor I, Moscovici M, Saltz LB. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 23.Thomas MB, Morris JS, Chadha R, Iwasaki M, Kaur H, Lin E, Kaseb A, Glover K, Davila M, Abbruzzese J. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009;27:843–50. doi: 10.1200/JCO.2008.18.3301. [DOI] [PubMed] [Google Scholar]
- 24.Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, Chen H, Clark-Garvey S, Weinberg A, Mandeli J, Christos P, Mazumdar M, Popa E, Brown RS, Jr., Rafii S, Schwartz JD. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992–8. doi: 10.1200/JCO.2007.15.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27(18):3027–35. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 27.Kaseb AO, Hanbali A, Cotant M, Hassan MM, Wollner I, Philip PA. Vascular endothelial growth factor in the management of hepatocellular carcinoma: a review of literature. Cancer. 2009;115:4895–906. doi: 10.1002/cncr.24537. [DOI] [PubMed] [Google Scholar]
- 28.Harrell FECR, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]


