Abstract
We measured event-related potentials (ERPs) with a craving manipulation to investigate the neural correlates of drug cue-reactivity in 13 cannabis-dependent (CD) adolescents (ages 14–17). The P300 responses to marijuana (MJ) pictures (MJ-P300) and control pictures (C-P300) were assessed after handling neutral objects and again after handling MJ paraphernalia (MJP). Self-reported drug craving and heart rates also were measured. MJ-P300 were larger than C-P300 (p<0.001) and both the MJ-P300 and craving increased significantly after handling MJP (p=0.002 and p=0.003, respectively), with no association between the magnitude of craving and MJ-P300. Heart rates were not affected by handling MJP. Our results show that CD adolescents have an attentional bias to MJ stimuli that increases after handling marijuana paraphernalia. Generally, our results are consistent with what has been reported for adult heavy chronic cannabis smokers; although there were some differences that require further investigation.
Keywords: Adolescent, Marijuana, Cue-reactivity, Craving, P300
Introduction
Drug-dependent individuals exhibit an excessive attentional bias to drug-related stimuli (Franken, 2003) that has been linked to addictive behaviors, including drug craving and relapse (Carter and Tiffany, 1999). Excessive attention to drug cues, autonomic responses, and self-reported craving are collectively known as cue-reactivity and drug-dependent individuals may experience any combination of these effects at any given time. Drug-dependent adults have been shown to experience cue-reactivity for most drugs of abuse, including nicotine (Warren and McDonough, 1999; Ehrman, Robbins, Bromwell, Lankford, Monteross, et al., 2002), heroin (Marissen, Franken, Waters, Blanken, van den Brink, et al., 2006), alcohol (Field, Mogg & Bradley, 2005), cocaine (Hester, Dixon & Garavan, 2006), and marijuana (Field, Eastwood, Bradley, & Mogg, 2006; Wölfling, Flor, & Grüsser, 2008; Filbey, Schacht, Myers, Chavez & Hutchison, 2009). In addition, there is evidence that drug-dependent adolescents also experience cue-reactivity (Gray, LaRowe, & Upadhyaya, 2008; Tapert, Cheung, Brown, Frank, Paulus, et al., 2003; Upadhyaya, Drobes, & Thomas, 2004; Thomas, Drobes, & Deas, 2005).
Marijuana is the most widely used illicit drug among adolescents and cannabis dependence (CD) is one of the most frequent diagnoses of adolescents entering substance abuse treatment (Crowley, 2006). To our knowledge, there has been only one study of cue-reactivity in CD adolescents that used a laboratory-based cue-reactivity paradigm (Gray et al., 2008). This study found that adolescents with cannabis use disorders experienced subjective (increased craving) and physiological cue reactivity (increased skin conductivity) in response to drug cues. These findings are consistent with studies that have reported alcohol (Tapert et al., 2003; Thomas et al., 2005) and nicotine (Uphadayaya et al., 2004) cue-reactivity in drug-dependent adolescents.
Brain function can be measured with very high temporal resolution from scalp recordings of the electroencephalogram (EEG). Measurements of voltage deflections on millisecond (msec) to second timescales that are linked in time with a particular physical or mental event are referred to as the event-related potential (ERP). The positive-going component of the ERP that occurs approximately 300 msec after the presentation of a target stimulus, known as the P300, and the extended positivity after the P300 (late positive potential, or LPP) are both thought to depend on the salience of the stimulus and have been shown to reflect attentional resource allocation and degree of arousal (Polich and Criado, 2006). Larger P300 and LPP responses to drug pictures compared with neutral pictures have been reported in both drug-dependent adults and in healthy adults for several drugs of abuse, with drug-dependent adults exhibiting much greater responses to drug-related pictures than healthy adults (heroin: Franken, Stam, Hendriks, & van den Brink, 2003; Lubman, Allen, Peters, & Deakin, 2007; cocaine: Franken, Dietvorst, Hesselmans, Franzek, van de Wetering, et al., 2008; marijuana: Wölfling et al., 2008).
Current models predict that increases in craving will increase attentional bias and vise versa (for a review, see Field and Cox, 2008). The aims of the present study were to investigate aspects of cue-reactivity in CD adolescents (1) to determine if changes in self-reported drug craving and attentional bias (measured by the P300 response to drug pictures) could be elicited by exposure to marijuana (MJ) paraphernalia and (2) to assess potential associations between the changes in craving and the P300 responses. We measured P300 responses to MJ-related and control pictures after handling neutral objects, and again after handling MJ paraphernalia. We hypothesized that CD adolescents would have greater P300 responses to MJ pictures than to control pictures and that handling drug paraphernalia would result in increased MJ craving, greater P300 responses to MJ pictures, and increased heart rate. We also hypothesized that increases in craving would be associated with increases in P300 responses.
Methods
Participants
Fifteen Caucasian cannabis-dependent adolescent patients (12 males, age range 14–18 years, mean 16.9 (SD 1.3) years) were recruited from the Adolescent Residential Treatment Program at McLean Hospital. Of those, 13 participants (10 males) successfully completed the study (malfunction with the button press device). The full committee of the Institutional Review Board (IRB) approved the protocol. Informed consent was obtained from participants 18 or older and from parents of participants under 18 (those under 18 gave informed assent). The Structured Clinical Interview–DSM IV was administered to assess substance dependence and psychiatric disorders. All patients met criteria for cannabis (and nicotine) dependence but were abstinent from MJ an average of two weeks prior to this study. All patients were admitted for treatment of cannabis dependence; however, 7/13 patients had co-morbid diagnoses and were taking medication (see Table 1). Exclusionary criteria were: history of substance dependence (except cannabis and nicotine), neurological illness, head trauma, attention deficit hyperactivity disorder (ADHD), and psychotic disorders. Adolescent control populations were not studied due to IRB guidelines that prevented exposing healthy adolescents and/or adolescents with psychiatric illnesses that were not cannabis dependent to cannabis-related cues. The objection in this case was not to the exposure to drug pictures, but to the exposure of the used drug paraphernalia by teens with little or no prior drug experience.
Table 1.
Demographic details of participants that successfully completed the study.
| Subject | Sex | Age in years(months) | Co-Morbid Diaqnosis | Medication |
|---|---|---|---|---|
| 1 | F | 16(10) | None | None |
| 2 | M | 16(6) | ETOH Abuse | None |
| 3 | M | 16(7) | None | None |
| 4 | M | 14(8) | DD | None |
| 5 | M | 18(11) | None | None |
| 6 | F | 18(4) | None | None |
| 7 | M | 17(2) | ETOH Abuse | Remeron |
| 8 | M | 17(3) | Conduct Disorder | Abilify |
| 9 | M | 15(7) | MD | Lithium |
| 10 | F | 15(2) | MD, cocaine abuse | Welbutrin |
| 11 | M | 18(8) | MD | Welbutrin |
| 12 | M | 16(8) | ETOH Abuse | Abilify |
| 13 | M | 17(4) | Opioid abuse, DD | Prozac, Trazadone |
ETOH: Alcohol, MD: Major Depression, DD: Dysthymic Disorder
ERP Recordings
ERPs were recorded from 32 tin electrodes (Electro-Cap International, Eaton OH), and a single electro-oculographic (EOG) electrode placed on the scalp (we were interested in mid-line responses only (Gratton, Coles & Donchin, 1983)). Interelectrode impedances were <10 kilowatt at 30 Hertz (Hz). EPA-6 Electrophysiology Amplifiers (Sensorium, Inc., Charlotte, VT) were set for a wide bandpass filter setting of 0.001 – 100 Hz and a gain of 20,000; all filtering was performed offline. Analog-to-digital conversion was accomplished at 256 Hz. Data were recorded using the bridge of the nose as a reference. A three-stimuli visual oddball paradigm was employed with 50% frequent neutral pictures (mundane household objects), 25% MJ -related (paraphernalia, cannabis, etc), and 25% non-specific arousing (NSA) images (with a negative emotional valence). Although many present-day studies use pictures from the International Affective Picture Set (IAPS, Lang, Bradley, & Cuthbert, 1999), the NSA and neutral picture sets used in this study were created in-house before the extensive validation of the IAPS had occurred. The NSA pictures, similar to IAPS pictures with negative affect, elicit somewhat larger P300-related responses in healthy adult subjects than do the neutral pictures (Nickerson & Lukas, 2004), which is consistent with general findings using the IAPS (Olofsson, Nordin, Sequeira, & Polich, 2008). Pictures were presented in pseudorandom order with repeated images (inter-stimulus interval=1550 msec; duration=750 msec; 80 neutral; 40 of each rare) and no two successive rare events. Participants indicated picture type by button press. ERPs were recorded after handling neutral objects (paper clips, pens, etc) and again after handling MJ paraphernalia (rolled joints, used pipes, etc), with no practice session. Order was not randomized due to possible carryover effects (Carter and Tiffany, 1999; Gray et al., 2008) and the same picture set was used in both blocks.
Behavioral Ratings & Heart Rate Measurements
Questionnaires (developed in-house, 21-items) were completed at baseline, after neutral P300, after drug P300, and end of study, and included ratings for eight questions: “desire to smoke marijuana” and “urge to use [alcohol, cigarettes, opiates, tranquilizers, PCP, LSD or mushrooms, ecstasy]” on a scale of zero (“not at all”) to ten (“most ever”) which were analyzed to demonstrate the specificity of the marijuana cues given that cigarettes and marijuana have potential overlap in cues. Heart rate was recorded continuously (Mini-Logger Series 2000, Mini Mitter Co., Inc., Bend, OR). Measurements were averaged over task time bins: handling neutral objects (7 minutes), first questionnaire, neutral object P300 (4 minutes), relaxation/rest (7 minutes), handling MJ objects (7 minutes), second questionnaire, drug object P300 (4 minutes), and end of study.
Data Analysis
ERPs were analyzed using Brain Vision Analyzer Version 1.05.0004 (Brain Products, GmbH). Processing included filtering (Butterworth zero phase, cutoff = 40 Hz, slope = 48 dB/oct), and ocular correction (Gratton, et al., 1983), automated artifact rejection (maximum voltage step/sampling point = 50 mV, maximum absolute difference between timepoints = 300 mV, and amplitude = −200 mV to +200 mV), and baseline correction to the timepoints 100 msec prior to picture presentation. Correct trials for each picture type were averaged together for each subject. The amplitude and time to peak (TTP) of the P300 waveform were identified for each subject from the largest positive peak from 300–600 msec; all peaks were visually inspected to ensure that in waveforms with broad peaks, the peak was more reflective of the mean value over the width of the peak. Only the P300 amplitudes and TTPs at electrode PZ were subjected to further statistical analyses as inspection of the waveforms showed maximal differences at electrode PZ with decreasing amplitudes from posterior to anterior electrodes and no differences between left and right electrodes (F3 and F4, C3 and C4, and P3 and P4). It should be noted that our set-up was not well-suited for investigating laterality effects. The observed topography in our results is typical of the P300 response seen in general (Duncan, Barry, Conolly, Fischer, Michie, et al., 2009) and in several studies of drug cues (Franken et al., 2003; Lubman et al., 2007; Wölfling et al., 2008; Bartholow, Henry, & Lust, 2007).
Statistical models for each outcome were fit separately. Primary outcomes were self-reported MJ craving and P300 amplitudes. Secondary outcomes were TTPs, reaction times (RTs), and heart rates. Additional post-hoc analyses were performed as described in the results. For each outcome, repeated-measures linear regression models were fit to the data using PROC MIXED in SAS (version 9.1.3; SAS Institute, Cary, NC), which allowed us to test additional covariates, including medication status, craving, object type, picture type, and task type. We considered both main-effects and interaction models. Pairwise comparisons were performed only in the presence of an overall association significant at the 0.05 level. Ninety-five percent confidence intervals are reported in brackets. To simplify the results, we did not report results for interaction models (we found no significant interactions for any model) nor for non-significant pairwise comparisons. As we did not find any evidence of a significant medication effect for any of our outcomes, we did not report results for medication effects.
Results
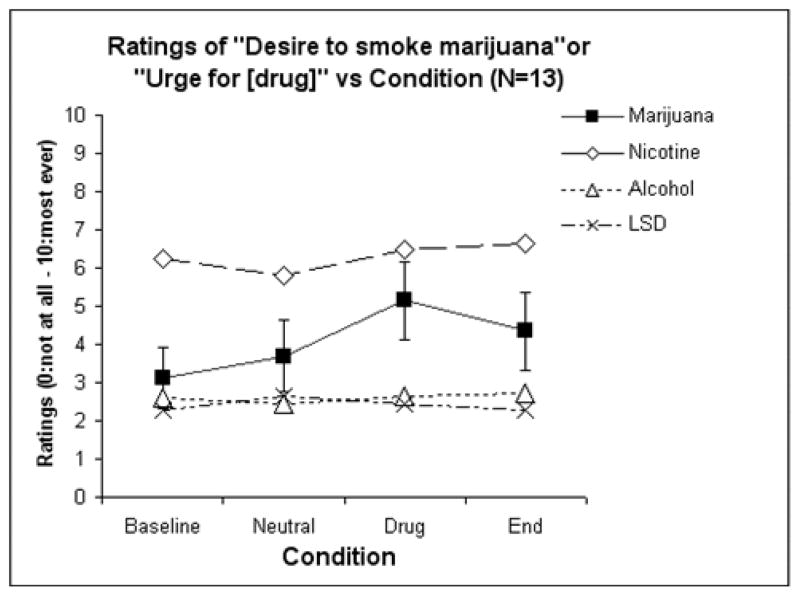
Across all participants, only self-reported MJ craving (Figure 1) was significantly elevated after handling drug objects. (F(2,36)=6.83, p=0.003). Mean MJ craving was higher after handling drug objects than at both baseline (1.81 [0.79–2.82], t(36)=3.62, p<0.001) and after handling neutral objects (1.23 [0.22–2.24], t(36)=2.46, p=0.02). It was also higher at the end of the study than at the baseline (1.15 [0.14–2.17], t(36)=2.31, p=0.03).
Figure 1.
Behavioral ratings for each condition. Standard errors of the mean are shown for marijuana ratings.
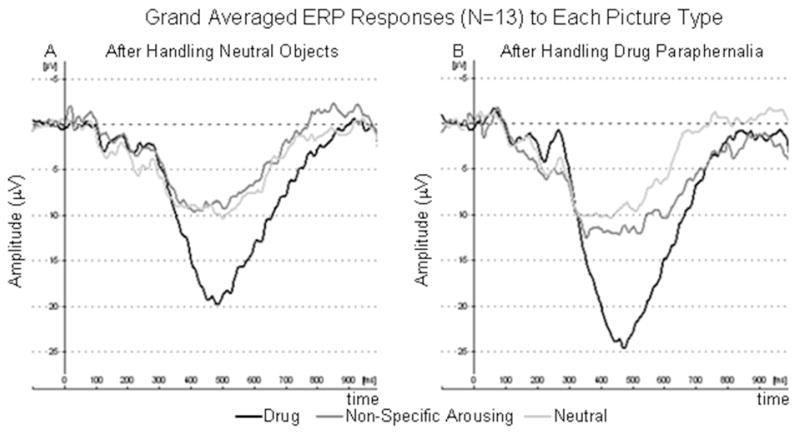
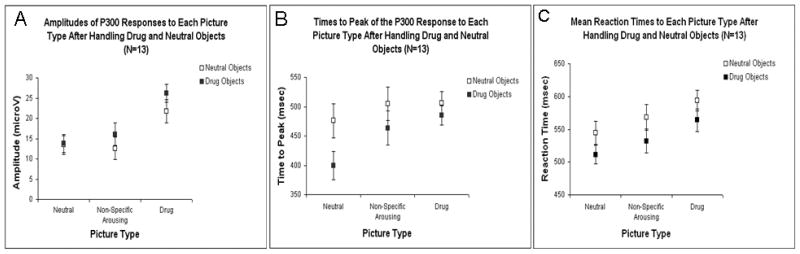
Grand averages across all participants of the responses at electrode PZ to each picture type are shown in Figure 2. Mean amplitudes, TTPs and RTs are shown in Figure 3. Controlling for object type, there was a significant association between P300 amplitudes and picture type (F(2,62)=61.75, p<0.001). Responses to drug pictures were 10.34 [8.23–12.42] mV higher than neutral pictures (t(62)=9.96, p<0.001) and 9.60 [7.52–11.67] mV higher than NSA pictures (t(62)=9.24, p<0.001). Controlling for picture type, P300 amplitudes were also 2.77 [1.07–4.46] mV higher after handling drug paraphernalia than after handling neutral objects (t(62)=3.27, p=0.002). There were no significant associations of P300 responses (or change in P300 responses) with baseline craving (or change from baseline after handling drug objects).
Figure 2.
Grand averages across all subjects of the responses at electrode PZ for each picture type, after handling neutral and drug objects.
Figure 3.
A. Amplitudes, B. Times to Peak, and C. Reaction Times for the P300 responses to each picture type, after handling neutral and drug objects. Error bars reflect standard errors of the means.
Mean TTPs and RTs (Figure 3B and 3C) were longer for drug pictures than for neutral pictures and both decreased after handling drug paraphernalia. Controlling for object type, picture type was significantly associated with both TTP (F(2,62)=3.47, p=0.04) and RT (F(2,62)=14.24, p<0.001). Mean TTP was 58.00 [11.47–104.53] msec longer for drug pictures than neutral pictures (t(62)=2.49, p=0.02). Controlling for picture type, mean TTPs decreased significantly by 45.95 [7.95–83.94] msec after handling drug-related objects (t(62)=−2.42, p=0.02). The mean RT to drug pictures was 50.96 [31.82–70.10] msec longer than to neutral pictures (t(62)=5.32, p<0.001) and 28.78 [9.64–47.92] msec longer than to NSA pictures (t(62)=3.01, p=0.004). The mean RT to NSA pictures was also 22.18 [3.04–41.32] msec longer than the mean RT to neutral pictures (t(62)=2.32, p=0.02). Controlling for picture type, the mean RT decreased by 33.01 [17.38–48.63] msec after handling drug objects (t(62)=−4.22, p<0.001).
There were significant differences in heart rate among tasks (lower during the P300 task than during other tasks) for all participants (F(3,83)=13.32, p<0.001), but no significant effect of handling drug objects after controlling for task type (t(83)=−1.48, p=0.14).
Discussion
We investigated several aspects of cue-reactivity in cannabis-dependent adolescents, including the P300 response as a measure of attentional bias to drug cues, self-reported craving, and heart rate. P300 responses to MJ-related visual stimuli were larger than responses to control stimuli, indicating a greater attentional bias to MJ cues. Handling MJ paraphernalia increased both P300 responses and self-reported MJ craving. Although we expected only the P300 responses to MJ-related pictures to be increased by handling drug paraphernalia, P300s for NSA pictures also were increased relative to neutral pictures, indicative of an overall rise in arousal. Although an increase in heart rate would be consistent with an overall rise in arousal, heart rates were not affected by handling MJ paraphernalia, supporting the general consensus that heart rates are less sensitive to drug-cue manipulations (Carter and Tiffany, 1999).
TTPs and reaction times to all picture types were decreased after handling MJ paraphernalia, consistent with a practice effect; however, decreased P300 responses that are associated with a practice effect when viewing affective stimuli (Codispoti, Ferrari, & Bradley, 2006) were not observed in the current study. Instead, we observed increases in the P300 response that are interpreted as increased attentional bias due to increased craving. However, post-hoc tests for associations between both baseline MJ craving and changes in MJ craving after handling MJ paraphernalia with changes in P300 responses to drug pictures found no evidence of an association between these measures. Although current theories would have predicted the opposite(Field and Cox, 2008), this is consistent with a study in adult chronic heavy cannabis users that found no direct association between self-reported craving and ERP responses to drug stimuli(Wölfling et al., 2008) and with a study by Filbey et al. (2009) in adult MJ users that found no association between urge ratings and brain activity measured using functional magnetic resonance imaging despite the fact that urge ratings during the presentation of MJ paraphernalia were greater than during neutral object handling.
Wölfling et al. (2008) investigated self-reported craving, skin conductance response, and the late positivity of the ERP response to MJ, alcohol, positive affect, negative affect, and neutral pictures in adult chronic heavy MJ smokers (MJS) and healthy adults (HA). Both MJS in their study and CD adolescents in the current study experienced increased craving in response to drug cues and larger P300-related responses to MJ pictures compared to neutral pictures, with no association between the two. However, comparison of Figure 3 in the current report with Figure 1 in Wölfling et al. reveals some differences. First, the responses in CD adolescents to drug pictures are larger than the responses to non-specific arousing (negative affect) pictures, in contrast to the responses in MJS. Second, the responses in CD adolescents to affective pictures were the same as to neutral pictures, unlike responses seen in the MJS and HA in the Wölfling study and in other studies of HA (Schupp et al., 2000; Delplanque et al., 2004). Last, the CD adolescents we studied did not show an elevated positivity following the P300 window used in this report, which is a well-replicated finding in HA (see Oloffson et al., 2008 for a review of findings) and in children (Hajcak & Dennis, 2009). As we did not measure valence and arousal ratings of the stimuli, it is possible that our NSA and neutral pictures were not a robust control picture set. However, results in HA using these same NSA and neutral pictures showed greater responses to the NSA pictures than to the neutral pictures (Nickerson & Lukas, 2004). Another consideration is that while valence and arousal-level of stimuli both modulate the P300 and the LPP, it is currently thought that the LPP to arousing pictures is more related to higher-arousing stimuli rather than affect (Cuthbert, Schupp, Bradley, Birbaumer, & Lange, 2000). The findings of reduced LPPs to NSA and MJ-related pictures in CD adolescents were unexpected and may reflect blunted emotional processing in these participants. This illustrates the need for an adolescent control group, although it does not necessarily impact the present study, as we were interested in differences in P300 due to changes in attention from handling drug paraphernalia.
There are some limitations to our study. First, our group of participants is small, somewhat clinically heterogeneous and mostly Caucasian males, thus we are unable to generalize to all CD adolescents. Due to IRB restrictions, healthy adolescents and adolescents with co-morbid psychiatric illnesses were not studied because the paradigm involved handling used MJ paraphernalia, not just picture viewing. In the only other study of cue-reactivity in young MJ smokers, increased craving and skin conductance were observed during MJ cue presentation, while heart rates were unaffected (Gray et al., 2008). This study also did not include a healthy control group, so although our results are consistent with their results, further work in young healthy subjects is needed to better understand these results. Medication and psychiatric status both may impact P300 responses. We did not find evidence of a medication effect, which is supported by the consistency between our results and the results found in chronic heavy cannabis users discussed above (Wölfling et al., 2008). However, the sample size used in this study may have been too small to detect differences due to medication.
Two additional limitations exist related to our specific study design: we did not have a practice session and we did not use a validated craving questionnaire. Our results suggest a practice effect exists based on the TTPs and reaction times, and it is possible that the P300 responses may have been affected (e.g., increases may have been larger in the absence of a practice effect). However, the increases in the P300 responses we observed still reached statistical significance. Another limitation relates to our craving questionnaire. Although there is an MJ craving questionnaire that has been validated in adults (Heishman et al., 2001), there is no validated MJ craving questionnaire for treatment-seeking CD adolescents. The questionnaire we developed for the current study included several questions to address different aspects of craving (for example, compulsivity), but our primary focus was on the single-item subjective MJ craving question and on questions related to craving for other drugs as we had some preliminary evidence that CD adolescents may experience increased craving to drugs other than just marijuana (Lundahl et al., 2001). Using this questionnaire, we found that cue-induced craving was specific for MJ and that the single-item craving ratings in the participants we studied changed similarly to the ratings from the adolescents studied by Gray et al. (discussed above). However, a multi-dimensional validated questionnaire could have provided additional insight into the nature of the craving experienced by CD adolescents.
In conclusion, we are the first to investigate the association between marijuana craving and brain function in cannabis-dependent adolescents. Our findings provide evidence that CD adolescents experience cue-reactivity, with changes in P300 responses and increased craving similar to those reported in a study by Wölfling et al. (2008) of adults with heavy chronic cannabis use. However, differences in the P300 responses to affective and drug-related pictures between the adults with heavy chronic cannabis use they studied and the adolescents in this study require further work to explain.
Acknowledgments
This work was funded by the National Institutes of Health: K25-DA017712 (Nickerson), R03-DA12582 (Lundahl), T32-DA015036 (Lukas), K05-DA000343 (Lukas).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/adb
Contributor Information
Lisa D. Nickerson, McLean Hospital, Belmont, MA and Harvard Medical School
Caitlin Ravichandran, McLean Hospital, Belmont, MA and Harvard Medical School.
Leslie H. Lundahl, Wayne State Universtity
John Rodolico, McLean Hospital, Belmont, MA.
Steven Dunlap, Genzyme Corporation.
George H. Trksak, McLean Hospital and Harvard Medical School
Scott E. Lukas, McLean Hospital and Harvard Medical School
References
- Bartholow BD, Henry EA, Lust SA. Effects of alcohol sensitivity on P3 event-related potential reactivity to alcohol cues. Psychology of Addictive Behaviors. 2007;21(4):555–563. doi: 10.1037/0893-164X.21.4.555. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. doi: 10.1046/j.1360-0443.1999.9433272.x. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetitive picture processing: Autonomic and cortical correlates. Brain Research. 2006;1068:213–220. doi: 10.1016/j.brainres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Crowley TJ. Adolescents and substance-related disorders: research agenda to guide decisions on Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) Addiction. 2006;101:115–124. doi: 10.1111/j.1360-0443.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/S0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Lavoie ME, Hot P, Solvert L, Sequeira H. Modulation of cognitive processing by emotional valence studied through event-related potentials in humans. Neuroscience Letters. 2004;356:1–4. doi: 10.1016/j.neulet.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Duncan CC, Barry RJ, Connolly JF, Fischer C, Michie PT, Naatanen R, Polich J, Reinvang I, Van Petten C. Event-related potentials in clinical research: Guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clinical Neurophysiology. 2009;120:1883–1908. doi: 10.1016/j.clinph.2009.07.045. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Bromwell MA, Lankford ME, Monteross JR, O’Brien CP. Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug and Alcohol Dependence. 2002;67:185–191. doi: 10.1016/s0376-8716(02)00065-0. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP. Craving and cognitive biases for alcohol cues in social drinkers. Alcohol and Alcoholism. 2005;40:504–510. doi: 10.1093/alcalc/agh213. [DOI] [PubMed] [Google Scholar]
- Field M, Eastwood B, Bradley BP, Mogg K. Selective processing of cannabis cues in regular cannabis users. Drug and Alcohol Dependence. 2006;85:75–82. doi: 10.1016/j.drugalcdep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug and Alcohol Dependence. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proceedings Natl Academy of Science. 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Franken IH, Dietvorst RC, Hesselmans M, Franzek EJ, van de Wetering BJ, Van Strien JW. Cocaine craving is associated with electrophysiological brain responses to cocaine-related stimuli. Addiction Biology. 2008;13:386–392. doi: 10.1111/j.1369-1600.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- Franken IH, Stam CJ, Hendriks VM, van den Brink W. Neurophysiological evidence for abnormal cognitive processing of drug cues in heroin dependence. Psychopharmacology. 2003;170:205–212. doi: 10.1007/s00213-003-1542-7. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gray KM, LaRowe SD, Upadhyaya HP. Cue reactivity in young marijuana smokers: A preliminary investigation. Psychology of Addictive Behaviors. 2008;22(4):582–586. doi: 10.1037/a0012985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Dennis TA. Brain potentials during affective picture processing in children. Biological Psychiatry. 2009;80:333–338. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Liguori A. Marijuana craving questionnaire: development and initial validation of a self-report instrument. Addiction. 2001;96:1023–1034. doi: 10.1080/09652140120053084. [DOI] [PubMed] [Google Scholar]
- Hester R, Dixon V, Garavan H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional stroop task. Drug and Alcohol Dependence. 2006;81:251–257. doi: 10.1016/j.drugalcdep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert B. Technical Report No. A-4. The Center for Research in Psychophysiology, University of Florida; Gainesville, FL: 1999. International Affective Picture System (IAPS): Instruction manual and affective ratings. [Google Scholar]
- Lubman DI, Allen NB, Peters LA, Deakin JF. Electrophysiological evidence of the motivational salience of drug cues in opiate addiction. Psychological Medicine. 2007;37:1203–1209. doi: 10.1017/S0033291707009932. [DOI] [PubMed] [Google Scholar]
- Lundahl LH, Borden KN, Lukas SE. Marihuana cue-induced craving in cannabis dependent adolescents in psychiatric treatment. Presented at College on Problems of Drug Dependence Annual Meeting; Scottsdale, AZ. 2001. [Google Scholar]
- Marissen MA, Franken IH, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–1312. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- Nickerson LD, Lukas SE. Differences in event-related potential responses to cocaine-related drug cues and non-specific arousing stimuli in healthy human subjects. NeuroImage. 2004;22(Suppl 2):e2052. [Google Scholar]
- Oloffson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. Biological Psychiatry. 2008;77(3):247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. International Journal of Psychophysiology. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by emotional relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Drobes DJ, Deas D. Alcohol cue reactivity in alcohol-dependent adolescents. Journal of Studies on Alcohol. 2005;66:354–360. doi: 10.15288/jsa.2005.66.354. [DOI] [PubMed] [Google Scholar]
- Upadhyaya HP, Drobes DJ, Thomas SE. Reactivity to smoking cues in adolescent cigarette smokers. Addictive Behaviors. 2004;29:849–856. doi: 10.1016/j.addbeh.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Warren CA, McDonough BE. Event-related brain potentials as indicators of smoking cue-reactivity. Clinical Neurophysiology. 1999;110:1570–1584. doi: 10.1016/s1388-2457(99)00089-9. [DOI] [PubMed] [Google Scholar]
- Wölfling K, Flor H, Grüsser SM. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. European Journal Neuroscience. 2008;27:976–983. doi: 10.1111/j.1460-9568.2008.06051.x. [DOI] [PubMed] [Google Scholar]