Abstract
Recent studies have identified the leucine rich repeat protein LRRTM2 as a postsynaptic ligand of Neurexins. Neurexins also bind the postsynaptic adhesion molecules, Neuroligins. All three families of genes have been implicated in the etiologies of neurodevelopmental disorders, specifically autism spectrum disorders (ASDs) and schizophrenia. Does the binding promiscuity of Neurexins now suggest complex cooperativity or redundancy at the synapse? While recent studies in primary neuronal cultures and also systematic extracellular protein interaction screens suggest summative effects of these systems, we propose that studying these interactions in the developing zebrafish embryo or larvae may shed more light on their functions during synaptogenesis in vivo. These gene families have recently been extensively characterized in zebrafish, demonstrating high sequence conservation with the human genes. The simpler circuitry of the zebrafish, together with the characterization of the expression patterns down to single, identifiable neurons and the ability to knock-down or overexpress multiple genes in a rapid way lend themselves to dissecting complex interaction pathways. Furthermore, the capability of performing high-throughput drug screens suggests that these small vertebrates may prove extremely useful in identifying pharmacological approaches to treating ASDs.
Keywords: synapse, cell adhesion, development, zebrafish, neurodevelopmental disorder, autism
Introduction
Understanding how the vast complexity of connections between individual neurons within vertebrate nervous systems is generated has fascinated neurobiologists for decades. Making maps of these synaptic connections and elucidating the mechanisms by which they develop holds promise that this will then provide information on how neural circuits process information leading to cellular and mechanistic explanations of higher cognitive functions. Also, many neurological diseases such as schizophrenia and autism spectrum disorders (ASD), whilst not being manifested until later in life, are increasingly viewed as problems resulting from variations in neural circuit formation that occurred at an early stage during the development of the nervous system (Zoghbi 2003, Bourgeron 2009).
The formation of synapses, or synaptogenesis, is initiated by neural cellular recognition events that are mediated by cell surface receptor proteins that make specific extracellular protein interactions with each other (Gerrow & El-Husseini 2006, Dalva et al. 2007, Giagtzoglou et al. 2009). At one level, the wiring of the nervous system could be viewed as the output of the individual adhesive properties of each neuron. The complex wiring of nervous systems, therefore, could naively be viewed as the product of the differential spatiotemporal expression of neural cell adhesion molecules together with their extracellular binding code. Because of this, great efforts have been made to catalogue neural cell adhesion molecules and elucidate their extracellular binding partners, developmental expression patterns and their functional role.
Synapses are asymmetric cellular junctions with a unidirectional flow of information and this polarity must be specified during the formation of a synapse. Because of this, the cell adhesion molecules (CAMs) involved in initial neural recognition are functionally classified into pre- and postsynaptic molecules. While some synaptic CAMs, such as Cadherins (Obst-Pernberg & Redies 1999), appear to be homophilic and are thus present on both sides of the synapse, the Neurexin and Neuroligin families are good examples of a heterophilic pre- and postsynaptic receptor-ligand pair (see below). In addition, there are many other CAMs expressed by neurons, that have no documented extracellular binding partner, but that are likely to have roles in synaptogenesis. Indeed, a recent functional screen for novel synaptogenic CAMs identified only a small proportion of positive clones as belonging to known synaptogenic receptor proteins suggesting that many more remain to be discovered (Linhoff et al. 2009). One particular family that has been the subject of recent studies are cell surface receptor proteins that contain leucine-rich repeat domains (Chen et al. 2006). Recent papers have shown that Neurexins are ligands for LRRTM protein family members (Ko et al. 2009a, de Wit et al. 2009) showing unappreciated links between these two structural families.
Given the vast array of potential synaptic CAMs and their homophilic, heterophilic and promiscuous interactions, it will be important to examine how the various adhesion systems interact with one another in the context of a whole nervous system. The vast majority of studies of synaptogenesis use primary neuronal cultures. Many of these studies are difficult to perform in the rodent brain due to complexity, inaccessibility and inability to modulate many different genes at the same time. Developing zebrafish provide a “simpler” vertebrate nervous system, with fewer neurons, many of which can be reliably identified between individuals. Furthermore, antisense knock-down technologies using morpholine-modified oligonucleotides (morpholinos) allow the manipulation of multiple genes at the same time. Thus, zebrafish may present a system to facilitate progress in understanding the complexity of cell-cell interactions during synaptogenesis.
Inroads have recently been made in this direction. Using a scalable protein interaction technology designed to detect low affinity extracellular protein interactions (Bushell et al. 2008) it was possible to catalogue the extracellular binding specificities for many zebrafish neural cell adhesion molecules from both the immunoglobulin and leucine-rich repeat families (Sollner & Wright 2009). Spatiotemporal expression profiling showed that many of these receptor proteins were expressed in discrete neural subpopulations and by integrating them with the protein interaction network, time-resolved neural receptor recognition maps could be constructed (Martin et al. 2010). Furthermore, a number of CAM gene families, including LRRTMs, Neurexins and Neuroligins have been annotated and their expression patterns described (Rissone et al. 2007, Rissone et al. 2010, Davey et al. 2010), thus facilitating their study in this system. This review will focus on evidence of the interactions of the LRRTM, Neurexin and Neuroligin gene families, how they may work in concert to direct synaptogenesis and the study of these molecules in zebrafish.
Neural LRR proteins
Cell surface receptor proteins that contain the leucine-rich repeat (LRR) are a large family of cell adhesion molecules that have restricted and dynamic expression patterns within developing nervous systems. Although not restricted to vertebrates, they have undergone a significant expansion within the vertebrate lineage. A recent comparative survey (Dolan et al. 2007) showed that mammals contain over 130 extracellular LRR-containing proteins, many of them having no documented function. In many cases, these proteins cluster into discrete subfamilies, each having a characteristic protein domain architecture (Chen et al. 2006). The genes encoding these receptors - even within closely related subfamilies - often have very different expression patterns within the developing and adult central nervous system. This suggests that they have similar but context-dependent functions relating to specific neural subpopulations and/or regions of the brain. Where functional data are available, they have roles in neural development such as axon outgrowth (Aruga & Mikoshiba 2003, Lin et al. 2003, Robinson et al. 2004, Wang et al. 2006), synapse formation (Kim et al. 2006, Ko et al. 2006) and axon fasciculation (Kuja-Panula et al. 2003). Consequently, these genes have been shown to have effects on behaviour such as memory formation (Bando et al. 2005) and implicated in various neurological disorders including schizophrenia (Francks et al. 2007), Tourette’s syndrome (Abelson et al. 2005), epilepsy (Kalachikov et al. 2002) and Alzheimer’s disease (Majercak et al. 2006; see Table 1).
Table 1.
LRR, Neurexins and Neuroligins implicated in human neurological diseases.
| Gene | Associated disease | Reference |
|---|---|---|
| LRRTM1 | Schizophrenia | (Francks et al. 2007) |
| LRRTM3 | Alzheimer’s disease (late onset) | (Majercak et al. 2006) |
| ASD | (Liang et al. 2007) | |
| (Sousa et al. 2010) | ||
| Neurexin1 | ASD | (Kim et al. 2008) |
| (Szatmari et al. 2007) | ||
| Neuroligin1 | ASD | (Glessner et al. 2009) |
| Neuroligin3 | ASD | (Jamain et al. 2003) |
| (Talebizadeh et al. 2006) | ||
| Neuroligin4X | ASD | (Jamain et al. 2003) |
| (Pampanos et al. 2009) | ||
| (Talebizadeh et al. 2006) | ||
| Neuroligin4Y | ASD | (Yan et al. 2008) |
| LGI1 | Autosomal-dominant partial epilepsy with auditory features | (Kalachikov et al. 2002) |
| Autosomal dominant lateral temporal epilepsy | (Morante-Redolat et al. 2002) | |
| LRRN3 | ASD | (Sousa et al. 2010) |
| LRFN5 | ASD | (Wang et al. 2009) |
| LRRN1 | Recessive non-syndromic mental retardation | (Higgins et al. 2004) |
| SLITRK1 | Tourette’s syndrome | (Abelson et al. 2005) |
| SLITRK2 | ASD | (Piton et al. 2010) |
| Bipolar disorder | (Smith et al. 2009) |
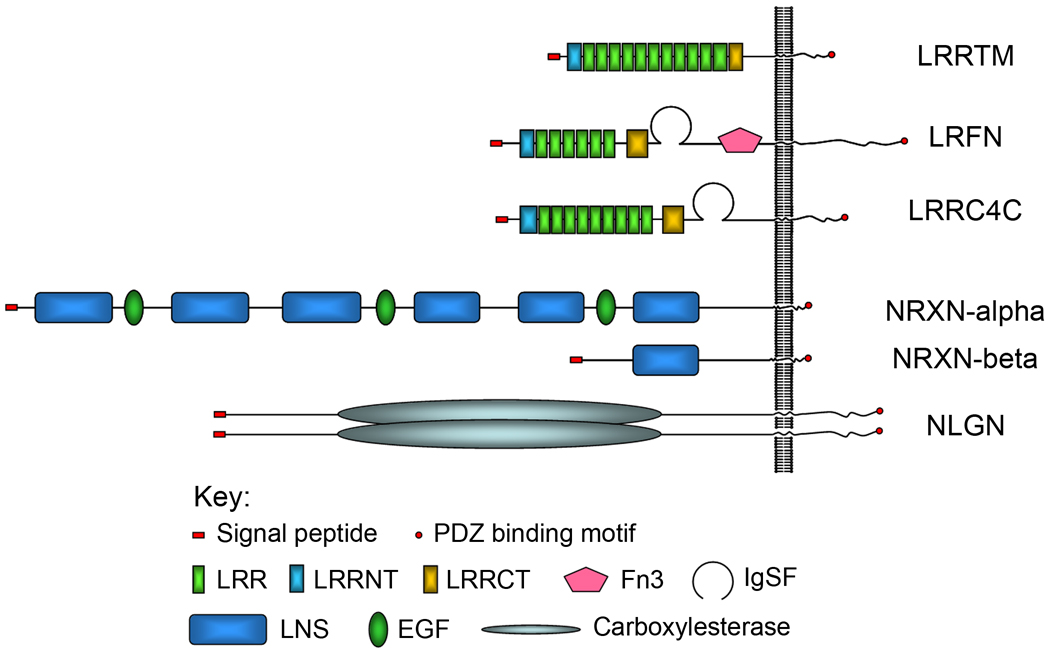
Within this class of proteins are three subfamilies that have been shown to be important in regulating synapse formation, the LRRTMs (de Paiva et al. 1999, Ko et al. 2009b, Linhoff et al. 2009), NGL/LRRC4C (Netrin-G-ligand; Kim et al. 2006) and Lrfn/SALMs (Leucine-rich-repeat and fibronectin III domain-containing/Synaptic adhesion molecule; Ko et al. 2006). These three families are similar in that they all contain a C-terminal PDZ-domain binding motif (Fig. 1) and have been shown to interact with PSD-95, a key postsynaptic density protein (Tallafuss et al. 2010). They have all been shown to be postsynaptic organizers of excitatory synapses.
Figure 1. A schematic showing the domain architecture of the synaptogenic proteins mentioned in this review.
A representative member for each protein family of synaptogenic molecules is drawn to scale showing the number and relative ordering of the domains. Each family is a type I membrane protein containing an N-terminal signal peptide and C-terminal PDZ-domain binding motif. The Neuroligins are shown as a non-covalently-linked dimer.
Neurexins: linchpins in synaptic cell adhesion
Neurexins are single transmembrane proteins that are primarily localized to the presynaptic terminal of synapses (Ushkaryov et al. 1992). They were first identified as receptors for α-latrotoxin from black widow spider venom, which induces synaptic release of neurotransmitter (Ushkaryov et al. 1992). There are three Neurexin genes in mammals, however each gene has two different promoters giving rise to long and short forms of the proteins, called α and β-Neurexins, respectively (Ushkaryov et al. 1994, Ushkaryov et al. 1992).
Neurexins contain a PDZ binding motif in their cytoplasmic region that is known to interact with CASK (Hata et al. 1996; Fig. 1) and thereby associates with a tripartite complex comprising CASK, Veli and Mint1 (Butz et al. 1998; see also Tallafuss et al. 2010). This complex is thought to mediate the recruitment of synaptic vesicles via an interaction between Mint1 and Munc18-1 (Okamoto & Sudhof 1997), a critical component for synaptic vesicle exocytosis (Verhage et al. 2000). Additionally, Neurexins can potentially bind the family of Band 4.1-like proteins through a FERM domain (Biederer & Sudhof 2001). These proteins are known to interact with CAMs at many cell-cell junctions and actively recruit F-actin to these sites (Correas et al. 1986). Thus, Neurexins are in a position to both nucleate actin and recruit synaptic vesicles in the nascent presynaptic terminal, both thought to be key events in synaptogenesis.
This hypothesis was borne out when knock-out mice were generated for all three α-Neurexin genes. The most striking effect of removing α-Neurexins is the reduction in regulated release of synaptic vesicles (Missler et al. 2003). This deficit appears to be due to reduced function of N-type calcium channels at synaptic active zones at both excitatory and inhibitory synapses (Missler et al. 2003). In fact, reduced Ca2+-channel function appears to be a genetic dose-dependent effect: as the number of non-functional α-Neurexin genes is increased by crossing the knock-out mice to each other, the synaptic deficit is enhanced. This results in increased neonatal mortality as a result of decreased breathing efficiency (Missler et al. 2003).
The large extracellular domain of α-Neurexins is made up of six LNS domains, separated by three EGF domains. In contrast, the much shorter β-Neurexins possess only the last LNS domain and EGF domain of α-Neurexins in the extracellular portion of the molecule (Fig. 1). The intracellular tails of α- and β-Neurexins are identical. It is the last LNS domain that is required for physical interaction with its ligands. Neurexins possess 5 sites at which independent, alternative splicing can generate thousands of isoforms (Ullrich et al. 1995). Different isoforms are expressed in distinct cell populations and it has been hypothesized that splicing may represent a mechanism to determine synaptic specificity (Missler & Sudhof 1998). However, this interesting possibility was not substantiated by the knock-out studies described above (Missler et al. 2003).
The first ligand of α- and β-Neurexins to be characterized was Neuroligin1 (Ichtchenko et al. 1995). This interaction is calcium and splicing dependent. Already during the early characterization of this direct interaction it was clear that only splice forms of Neurexin lacking insert 4 would bind to the Neuroligins (Ichtchenko et al. 1996). This suggested that there may be other possible endogenous ligands for the Neurexins. Indeed, recent studies have uncovered other ligands. LRRTMs were found to bind to Neurexins and recruit postsynaptic components around a year ago (Ko et al. 2009a, de Wit et al. 2009), and since then other ligands have been identified. It was noted that overexpression of Neurexins decreased GABAergic transmission (Zhang et al. 2010). This effect on the synaptic strength of GABAergic synapses was attributed to a direct interaction between all Neurexins and the GABA A receptor. This interaction occurs in ‘cis’, i.e. in the postsynaptic plasma membrane via sequences in the extracellular domain of Neurexins (Zhang et al. 2010; see Fig. 3). This conformation goes against the generally assumed localization of Neurexins at the presynaptic terminal. However, Neurexins have been documented to localize to the postsynaptic specialization (Berninghausen et al. 2007), where they might also bind the Neuroligins in a cis conformation (Taniguchi et al. 2007, Fig. 3). Both of the cis interactions of the Neurexins (with the GABA A receptor and Neuroligins) may occur in a developmentally regulated fashion, because Neurexins redistribute from a dendritic localization to the axon shaft in cultured neurons as a result of signaling from astrocytes (Barker et al. 2008).
Figure 3. Protein-protein interactions of Neurexins with other CAMs and receptors.
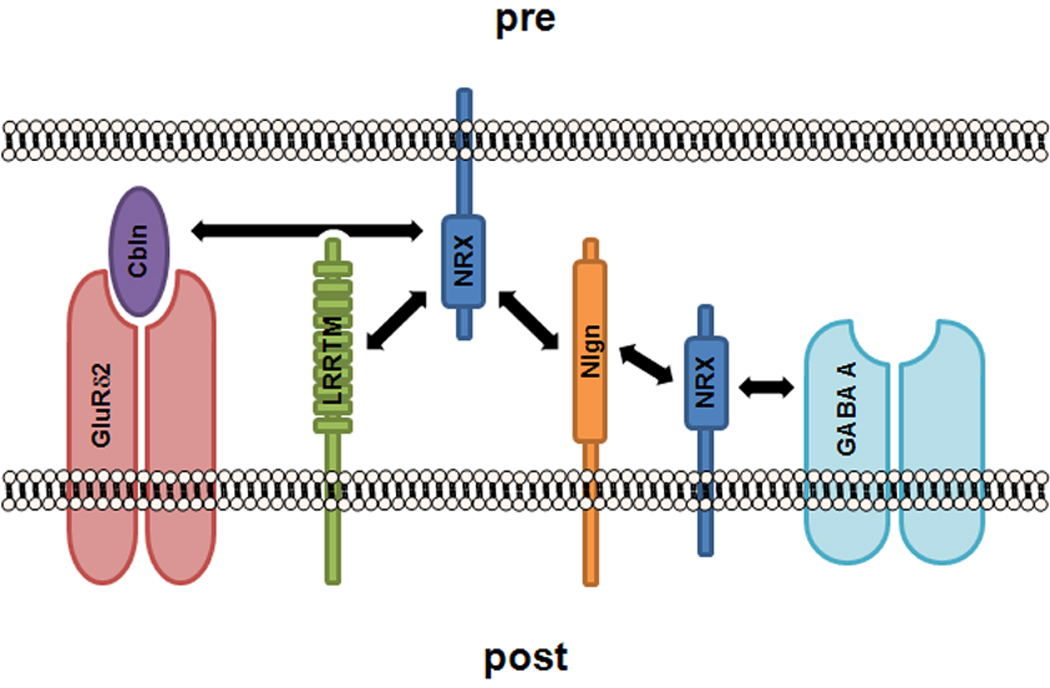
Neurexin (NRX, blue) is generally located in the presynaptic terminal. Neurexins can interact transsynaptically with Neuroligins (Nlgn, orange), LRRTMs (green) and Cerebellin (Cbln, purple), which in turn binds to the orphan glutamate receptor GluRδ2 (red). Neurexins can also localize to dendrites where they are hypothesized to negatively regulate Neuroligins and GABA A receptors (aqua) by direct extracellular interactions.
In addition, Neurexins have been found to bind to the secreted protein Cerebellin (Uemura et al. 2010). Cerebellin, in turn, binds to GluRδ2 (Matsuda et al. 2010). This ternary complex of Neurexins with soluble Cerebellin and the postsynaptic orphan glutamate receptor GluRδ2 (Fig. 3), may represent a specialization within the cerebellum: knock-out mice for both Cerebellin and GluRδ2 form many fewer “parallel fibre to Purkinje cell” synapses (Kashiwabuchi et al. 1995, Hirai et al. 2005). Thus, through multiple sets of protein-protein interactions the Neurexins are placed in a key position to organize and regulate the pre- and postsynaptic compartments of both excitatory and inhibitory synapses.
Neuroligins: the prototypical synaptogenic molecules
Neuroligins are single transmembrane proteins which possess a single large extracellular domain that is derived from an inactive version of cholinesterase (Ichtchenko et al. 1995). It is this cholinesterase domain that binds to Neurexins in a splice-site specific manner (Ichtchenko et al. 1995, Ichtchenko et al. 1996). In the intracellular region of the protein, Neuroligins possess multiple protein binding motifs; the PDZ binding motif has received the most attention (Fig. 1). Indeed, the binding of Neuroligin1 to PSD95 through the PDZ binding domain (Irie et al. 1997) kindled the idea that the Neurexin/Neuroligin1 trans-synaptic adhesion complex could mediate the formation or maintenance of excitatory synapses. This idea was further solidified by the localization of Neuroligin1 to excitatory synapses (Song et al. 1999) and the discovery that expression of Neuroligin1 in non-neuronal cells could drive the formation of presynaptic terminals onto these cells (Scheiffele et al. 2000).
The coculture system, consisting of either HEK293 or COS7 cells cultured with any kind of primary neurons, was originally developed for identifying the synaptogenic activity of Neuroligin1, but has now become the gold standard for the characterization of molecules involved in the formation of synapses (Biederer & Scheiffele 2007). In fact, synapse formation by axons from various neuronal types is so robust onto non-neuronal cells expressing synapse associated cell adhesion molecules, that it is possible to record synaptic vesicle release if neurotransmitter receptors are also introduced into the non-neuronal cells (Biederer et al. 2002, Fu et al. 2003, Hoy et al. 2009). This coculture system was recently used in a large-scale screen to isolate novel postsynaptic cell adhesion molecules with synaptogenic activity (Linhoff et al. 2009).
There are five Neuroligin genes in humans and higher primates, while the rest of mammals possess only 4 Neuroligin genes. This specialization of primates appears to have happened by duplication of the ancestral Neuroligin 4 gene during formation of the sex chromosomes; the two paralogues of this gene are located on the X and Y chromosomes in humans. Presumably, one paralogue was lost in lower mammals during gradual degeneration of the Y chromosome (Graves 2006). Interestingly in mice, the Neuroligin4 gene no longer resides on a sex chromosome, and its sequence has diverged considerably from other mammalian species, resulting in only 60% sequence identity with the human Neuroligin4 proteins (Bolliger et al. 2008). In contrast, the murine and human amino acid sequences of the other Neuroligin proteins are well conserved (80–90% identity).
While Neuroligin1 has been clearly localized to excitatory synapses, other members of the Neuroligin family are associated with different synaptic types. Neuroligin2 localizes to inhibitory GABAergic and glycinergic synapses (Varoqueaux et al. 2004, Graf et al. 2004) and overexpression of this molecule drives the formation of inhibitory synapses (Fu & Vicini 2009, Chih et al. 2005). Neuroligin3 appears to be associated with both excitatory and inhibitory synapses (Chih et al. 2005, Budreck & Scheiffele 2007). It still remains unclear which synapses murine Neuroligin4Y are associated with, while some experiments indicate that human Neuroligin4X may localize to glutamatergic synapses when expressed in cultured neurons from rat hippocampus (Graf et al. 2004).
Some of the Neuroligin (NLGN) genes have been associated with autism spectrum disorders (ASD; Sudhof 2008). Mutations in NLGN3 and NLGN4 (Jamain et al. 2003, Laumonnier et al. 2004, Pampanos et al. 2009, Talebizadeh et al. 2006, Yan et al. 2008, Yan et al. 2005, Zhang et al. 2009) and copy number variations of NLGN1 have been detected in families with ASD (Glessner et al. 2009; see Table 1). The link between the Neuroligin genes and autism-like behavior has been further underlined by the generation of knock-in mice with analogous mutations in the Neuroligin3 and 4 genes as those identified in individuals with autism. For example, mice expressing Nlgn3 with an arginine to cysteine substitution at residue 451 exhibit reduced social interactions with other mice (Tabuchi et al. 2007), although this effect may depend on the background strain (Chadman et al. 2008). In addition, mice with a loss-of-function mutation in the Nlgn4 gene also show reduced social interactions and reduced vocalization of males with females in estrous (Jamain et al. 2008). These studies suggest that mice with deficits in the Neuroligin-Neurexin adhesion complex exhibit anomalous behavior consistent with autism in humans (Sudhof 2008).
The LRRTM family of neural receptor proteins
The LRRTMs (leucine-rich repeat transmembrane neuronal) are a small family of paralogous leucine-rich repeat (LRR) containing cell surface receptors. They were first identified by bioinformatics searches of vertebrate genome sequences looking for novel LRR-domain containing proteins with sequence similarity to the short-range secreted neuronal repellent, Slit (Lauren et al. 2003). In mammals, there are four LRRTM paralogues (LRRTM1 to 4) that are typical of cell surface receptor proteins, containing an N-terminal signal peptide, an extracellular region containing potential N-linked glycosylation sites and a hydrophobic transmembrane region followed by a cytoplasmic domain. No clear orthologues of the LRRTMs are found in invertebrates but LRRTMs have been identified in many other vertebrate species including zebrafish, although there appears to be more than four LRRTM genes (Sollner & Wright 2009).
The LRRTM extracellular region contains 10 LRRs flanked by capping domains that are well conserved across species, followed by a membrane-proximal region which is more divergent. The cytoplasmic region of all LRRTMs terminates in a sequence that bears similarity to a PDZ domain binding motif (ECEV*) and has been shown to interact with the PDZ domain binding protein PSD-95 in vitro (de Wit et al. 2009, Linhoff et al. 2009) consistent with their role in organising postsynaptic terminals on neighbouring neurons (Fig. 1). The gene structures for the LRRTMs are remarkable in that the coding regions contain very few introns: the entire amino acid coding region for LRRTM1 is located on a single exon and just one intron is located within the exons encoding the signal peptide sequence in the LRRTM2 to 4 genes (Lauren et al. 2003). This gene architecture is also a feature of the NGLs (Woo et al. 2009) suggesting that the functional properties of this class of neurogenic receptor proteins are not regulated by alternative splice isoforms.
All the LRRTM family members are dynamically expressed in the developing and adult nervous systems and have strikingly different neural expression patterns (Lauren et al. 2003). In adult mouse brain, for example, each of the four LRRTMs are expressed in the olfactory bulb and cortex, but by neurons located in different laminae. The conserved structural architecture of the LRRTM receptors together with their expression in discrete neural subpopulations suggested a role in neural recognition processes.
The structures of synaptic recognition molecules
A mechanistic explanation of the extracellular recognition processes that are responsible for the correct wiring of the vertebrate nervous system ultimately lie with a structural understanding of how neural cell surface receptors interact. There has been a great deal of recent progress in this area including the structure of neurexin/neuroligin co-complexes which has provided a molecular explanation for the binding specificities within these important synaptogenic protein families.
Neurexins and neuroligins
The structural characterisation of the Neurexins has mainly focused on isolated LNS domains and crystal structures have now been solved for the second (Sheckler et al. 2006), fourth (Shen et al. 2008) and sixth LNS domain of α-Neurexins (Rudenko et al. 1999, Koehnke et al. 2008, Shen et al. 2008); the sixth domain of α-Neurexins corresponds to the entire ectodomain of β-Neurexins. The individual LNS domains adopt a compact structure composed almost entirely of β-sheet that resembles a lectin-like fold and behave as monomers in solution. By using small angle X-ray scattering together with the solved LNS domain structures and homology models, Comoletti and colleagues (Comoletti et al. 2010) were able to construct a model of the entire ectodomain of Neurexin-1α which closely resembled the shape observed by single particle electron microscopy. This revealed that α-Neurexins adopt a “Y”-shaped structure that is 170Å long with the first four LNS domains forming the extended base of the Y and LNS5-6 the two arms.
Structural studies on Neuroligins confirmed that they belong to the α/β-hydrolase fold superfamily which includes catalytically active cholinesterase, lipases and carboxylesterases and non-catalytic adhesion proteins such as Neurotactin (de la Escalera et al. 1990) and Glutactin (Olson et al. 1990); Neuroligins themselves do not possess any catalytic activity. Structures of rodent Nlgn1 (Chen et al. 2008, Arac et al. 2007) and human Nlgn4 (Fabrichny et al. 2007) reveal that Neuroligins form an overall ellipsoid shape composed of a central 12-standed central β-sheet surrounded by 14 α-helices. They form tightly-associated noncovalent dimers. The interaction interface between each monomer is stabilised by a central four helix bundle composed of almost exclusively hydrophobic contacts and covers a large interaction surface (1590Å2) consistent with the high affinity association.
The neurexin-neuroligin interaction interface: structure and regulation
The co-crystallisation of several Neurexin-Neuroligin complexes has not only revealed the nature of how the two proteins interact to instruct synapse formation, but also provides insight into how these interactions are regulated (Fabrichny et al. 2007, Chen et al. 2008, Arac et al. 2007). The Neurexin binding interface on Neuroligins lies away from the dimerisation interface such that each Neuroligin dimer interacts with two monomeric Neurexins leading to a 2:2 binding stoichiometry. The Neuroligin-Neurexin interaction interface is flat and mainly hydrophilic and the total buried surface area is small (1160 Å2) which is consistent with the generally low affinity (KD ~ 0.2 to 0.5 µM) reported for monomeric Neurexin-Neuroligin interactions (Koehnke et al. 2008, Koehnke et al. 2010, Leone et al. 2010). These affinities are typical of cell surface receptor interactions (Wright 2009). The structures of Neurexins are essentially super-imposable in their bound and unbound forms demonstrating that there are no significant structural conformational changes upon binding (Shen et al. 2008, Chen et al. 2008, Arac et al. 2007). Similarly, Nlgn1 showed no structural changes upon binding (Arac et al. 2007) but a recent structure of Nrxn-1β complexed with Nlgn4 revealed conformational changes suggesting the possibility of allosteric regulation in regions of the protein distant from the binding interface (Leone et al. 2010).
All structures revealed the presence of Ca2+ ions at the interaction interface explaining the Ca2+-dependent nature of the binding (Nguyen & Sudhof 1997). Interestingly, while Neurexins directly contribute four of the six Ca2+ coordination sites the remaining two are contributed indirectly by Neuroligin via water-mediated contacts. Consistent with this, isothermal calorimetry measurements showed that the interaction was enthalpy driven due to an unfavourable entropy term, possibly due to the trapping of water at the interface (Chen et al. 2008). The structures also provided insights into how alternative splice isoforms of both Neurexins and Neuroligins might regulate the interaction. Although the regions that contain the alternatively spliced inserted sequences of both Neurexin (SS4) and Neuroligin (SSB) flank the interaction interface, they are only able to moderately influence binding affinities (Koehnke et al. 2010). SSB inserts an eight amino acid sequence in Neuroligins that is just 10Å away from the edge of the interface and its inclusion lowers the binding affinity by a factor of around two. SS4 inserts a larger 30 amino acid sequence in Neurexins and weakens Neurexin1 and 2, but strengthens Neurexin3 binding to Neuroligins. Isothermal titration calorimetry showed that both alternative splice inserts lower binding affinities by a similar mechanism by reducing the enthalpic contribution (although the entropic penalty is lowered) by the loss of a binding contact (Chen et al. 2008). It is important to note, however, that a recent systematic and quantitative binding analysis of all combinations of alternatively spliced β-Neurexins1 to 3 and Neuroligins 1 to 3 concluded that the affinity differences between the isoforms were so subtle that it called into question the significance of alternative splicing in Neurexin/Neuroligin-based synaptic recognition (Koehnke et al. 2010).
Mutations within Neuroligin genes have been correlated with autism spectrum disorders (Table 1). Modeling these mutations (all missense) onto the Neuroligin protein structure has shown that they are located well away from the Neurexin binding interface and are therefore likely to affect other aspects of protein structure such as folding, stability or trafficking (Chen et al. 2008, Fabrichny et al. 2007). Indeed, the mutations in NLGN3 and NLGN4 detected in families with autism lead to intracellular retention of the mutant proteins (Chih et al. 2004), presumably due to misfolding and a block in export from the endoplasmic reticulum (Zhang et al. 2009). Thus, the missense mutations identified in cases of autism lead to a loss of function, as the affected Neuroligin molecules are not delivered to the cell surface.
Extracellular LRR protein structure
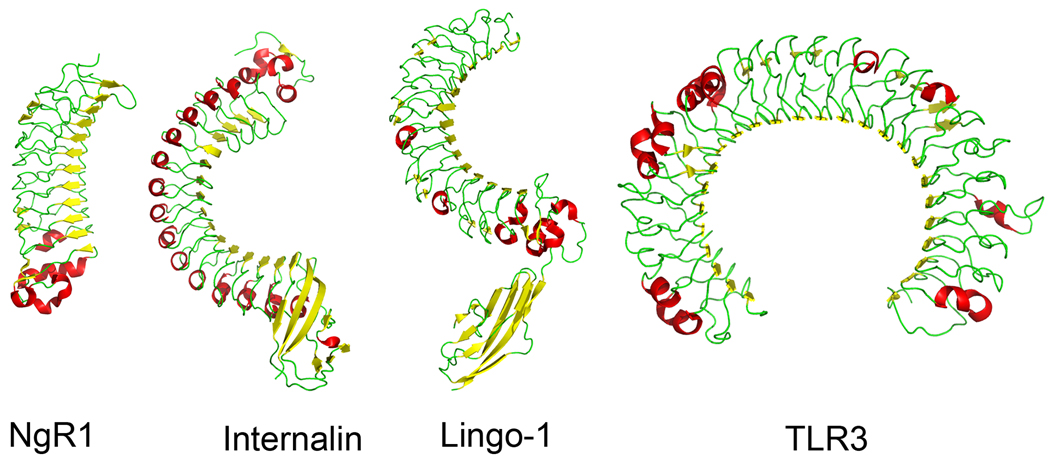
The Leucine-rich repeat (LRR) is defined by a pattern containing four leucine residues and an asparagine (LxxLxLxxNxL - where x represents any amino acid) and is not restricted to extracellular proteins. In fact, proteins containing LRRs have been identified in a wide variety of organisms from bacteria to vertebrates and in proteins which have a variety of functions from nucleic acid processing enzymes (Kobe & Deisenhofer 1993) to exocytosis (Bowser et al. 1992). LRRs are usually grouped in clusters with each cluster containing 2 to over 20 repeats. Around 100 structures of proteins containing LRR domains have been determined and all exhibit a remarkable curved solenoid-like structure with the extent of curvature dependent on the number of LRRs. Extracellular LRR proteins range from shapes that resemble a banana (NgR – 9 LRRs) to a more extended sickle (internalin – 15 LRRs), a question mark (Lingo1 – 12 LRRs, 1 Ig-like), to the 25 domains of TLR3 which has a horseshoe-shaped tertiary structure (Fig. 2). The hydrophobic core of the protein is formed by the aliphatic side chains of the leucine residues and other non-polar R-groups. Each LRR contains an N-terminal region of beta sheet followed by a more variable helical structure which can be either longer stretches of alpha helix or shorter polyproline II or 310 helices. The beta strands from neighboring LRR domains are adjacent to each other in the folded protein to form a continuous layer of parallel beta sheet that forms the concave surface of the curved solenoid, with each LRR domain contributing a single strand within the sheet; the convex side, therefore, is primarily helical (see Fig. 2). In extracellular LRR-containing proteins in particular, the LRRs are usually flanked at both the N and C termini with characteristic sequences that act as “caps” (LRRNT and LRRCT) covering the hydrophobic core of the outer LRRs. Both these capping regions are short (~20 amino acids) and contain a characteristic sequence of four cysteines forming two disulfide bonds (Bella et al. 2008).
Figure 2. Structural diversity of extracellular LRR proteins.
The structures comprising the extracellular regions of human NgR (PDB accession number 1OZN; He et al. 2003), Listeria monocytogenes Internalin (1o6s; Schubert et al. 2002), human Lingo-1 (21D5; Mosyak et al. 2006) and human TLR3 (1ZIW; Choe et al. 2005) illustrating their architectural diversity. Each is drawn in ribbon format with β-sheet drawn in yellow and α-helices in red; the membrane-proximal C-terminus is orientated towards the bottom of the panel in each case. Note that the concave side is almost exclusively composed of parallel β-sheet with a single strand contributed by each LRR domain; the convex side, in contrast is primarily helical.
The curved surface of beta sheet forms a stable scaffold upon which a large variety of different amino acids can be structurally tolerated to create diverse binding sites used for molecular recognition. Indeed, jawless fishes such as lampreys and hagfish, which were once considered to lack an adaptive immune system, use LRR-domain containing proteins as their functional equivalent of antibodies (Pancer et al. 2004). Both the concave and convex surfaces are used by individual LRR proteins to form the interaction surface with their ligands. In the few examples where the ligand has been co-crystalised, it is the concave surface that forms the binding interface with the ligand, which is enclosed by the pincer-like arms of the curved LRR protein. This binding mechanism seems particularly suitable for forming a large number of contact sites to impart a high affinity on the interaction. For example, the angiogenin-placental ribonuclease inhibitor interaction encompasses >2900 Å2 of contact area leading to extraordinarily high interaction affinities of <1fM (Papageorgiou et al. 1997). In structures of extracellular receptor proteins, such as Lingo-1 (Mosyak et al. 2006) and TLR3 (Choe et al. 2005), however, the concave surface is masked by the presence of large hydrophilic N-linked glycan groups suggesting that it is the bald convex surface that is used for forming interaction surfaces. This can then lead to reduced ligand contact areas and consequently lower interaction affinities and indeed the interaction between Lingo-1 and its receptor NgR1 is much lower at 1µM (Mosyak et al. 2006).
The LRR is therefore a highly versatile protein fold that can form a stable scaffold upon which to form a diverse array of different molecular interactions that can range in their binding affinity (covering 9 orders of magnitude) making them particularly useful for mediating a range of intercellular recognition processes.
LRRTMs are synaptogenic receptor proteins
Several recent reports have shown that the LRRTM family of proteins can function as synaptogenic molecules when heterologously expressed by cell lines in a neuron coculture assay (de Wit et al. 2009, Linhoff et al. 2009, Ko et al. 2009b). The LRRTMs can therefore be added to a growing list of neural receptor proteins that have this activity, which include Neuroligins (Ichtchenko et al. 1995, Song et al. 1999, Scheiffele et al. 2000), SynCAM (Biederer et al. 2002), NGLs (Kim et al. 2006) and Ephs (Kayser et al. 2006). This property was initially identified in an unbiased expression screen of rat forebrain cDNA clones that aimed to identify novel synaptogenic cell adhesion molecules (Linhoff et al. 2009). LRRTM receptors expressed on COS cells were able to initiate the formation of excitatory - VGLUT1 positive - presynaptic terminals in co-cultured hippocampal neurons (Linhoff et al. 2009, Ko et al. 2009b). Focusing specifically on LRRTM2, which showed the most potent synaptogenic activity in these assays, LRRTM2 was shown to localise to excitatory synapses (Linhoff et al. 2009). Knocking down the expression of LRRTM2 resulted in a reduction (de Wit et al. 2009) and, conversely, overexpression resulted in an increase of excitatory synapses (Ko et al. 2009b). These effects were shown to be solely dependent on the extracellular regions of LRRTM2 and to occur in trans, suggesting the presence of a presynaptic extracellular ligand for LRRTM2 that was subsequently able to instruct the formation of a presynaptic terminal.
As previously established, the Neurexins are a major family of proteins that promote presynaptic formation and two independent studies (de Wit et al. 2009, Ko et al. 2009b) identified LRRTM2 as a novel Neurexin ligand providing a mechanistic explanation for LRRTM function. By using the recombinant ectodomain regions of LRRTM2 immobilised on a solid substrate, interacting proteins were captured from rat brain lysates and peptides matching Neurexin-family proteins were identified by mass spectrometry. Subsequent binding experiments demonstrate that both Neurexin-1α and Neurexin-1β are receptors for LRRTM2, but not Neurexin-2α or Neurexin-3α, and that all four LRRTMs could bind Neurexin-1β (de Wit et al. 2009, Ko et al. 2009b). Interestingly, the binding of LRRTM2 to Neurexin-1α and Neurexin-1β was only detected when the Neurexins lacked the short 30 amino acid insert at splice site 4 (Ullrich et al. 1995, Boucard et al. 2005). Neurexins containing (and lacking) this insert are, however, able to bind to their other ligands, Neuroligins, provided they too are appropriately spliced (Ko et al. 2009b). This ability to regulate differential binding to Neurexins by both LRRTMs and Neuroligins provides an intriguing mechanism for regulating synapse formation.
Cooperativity or redundancy at synapses
It therefore appears that we are presented with an ever increasing number of molecules that have the potential to induce synapse formation, as defined by the coculture assay. It is now interesting to determine (1) whether these cell adhesion molecules are necessary or sufficient for synaptogenesis and (2) whether the cell adhesion molecules function synergistically or redundantly. Numerous mouse knock-out studies suggest that Neurexins, Neuroligins and LRRTMS are not in themselves necessary for synaptogenesis (Missler et al. 2003, Jamain et al. 2008, Varoqueaux et al. 2006, Linhoff et al. 2009). For example, LRRTM1-deficient mice are viable and have only a subtle detectable phenotype: a mild increase in the size of VGLUT1-positive puncta in some, but not all brain regions (Linhoff et al. 2009). This subtle phenotype may reflect the fact that Neuroligins may compensate for reduced LRRTM function.
Even when the alpha-Neurexins, linchpins in coordinating multiple adhesion events at the synapse, are deleted from the mouse genome, synapses can still form; only synaptic function is compromised (Missler et al. 2003). This may suggest that Neurexins are only presynaptic organizers, and are dispensable for the induction of synapses. It then begs the question as to which molecules do in fact initiate the formation of the presynaptic terminal. Also, to what extent are different CAMs within a protein family or members from different CAM families able to compensate for individual gene deletions? In addition, it is important to ask “what is happening at the postsynaptic side of synapses?” Neurexins can potentially bind not only Neuroligins and LRRTMs, but also GABA A receptors (Zhang et al. 2010) and GluRδ2 through Cerebellin (Matsuda & Yuzaki 2010, Uemura et al. 2010). Are all these adhesion events occurring in the same cells? Possibly - but at different synapses. There is evidence for distinct synaptic subpopulations of glutamatergic synapses within single neurons, such as parallel and climbing fiber synapses in the cerebellum (Kurihara et al. 1997). This diversity may be generated through distinct interactions between Neurexins and various postsynaptic adhesion partners (Uemura et al. 2010).
Alternatively, the multiple partners may function at the same synapses, but they may be required to recruit distinct components to the synapses. Another possibility is that they may cooperate to recruit the same components to the synapse. Recently, this was tested in primary hippocampal cultures by overexpressing both Neuroligins and LRRTMS and examining the effect on glutamatergic synapse formation. Here it appears that Neuroligins and LRRTMs may perform the same function as each other, and that they function in a summative way to enhance the recruitment of the presynaptic proteins Bassoon and Synaptophysin to presynaptic terminals impinging on transfected neurons (Siddiqui et al. 2010).
Study in a reduced in vivo system: zebrafish
While many mouse studies have provided insight into possible roles for individual CAMs in synapse formation, maturation, specificity and maintenance, no studies have yet attempted to answer questions of redundancy or cooperativity of these CAM systems in vivo. We believe that using a simpler and more developmentally accessible system in which the expression of each of the CAMs can be determined and manipulated in identified neurons will be advantageous in characterizing individual contributions or synergistic effects of these molecules on synapse formation.
These kinds of studies are possible in zebrafish. They possess a relatively simple nervous system. Individual neurons, such as the premotor neurons of the caudal hindbrain, can be identified in developing zebrafish embryos and larvae in vivo (Fetcho & Liu 1998) and their physiological functions during the escape response have been well characterized (Liu & Fetcho 1999, Gahtan et al. 2002). Gene expression can be manipulated by injecting embryos with RNA or expression plasmids to drive overexpression in specific neurons or by injecting embryos with morpholinos to block translation or splicing (Eisen & Smith 2008).
Moreover, the genes of LRRTMs, Neuroligins and Neurexins are highly conserved between humans and zebrafish (Rissone et al. 2007, Rissone et al. 2010, Davey et al. 2010). The sequences and genomic structures highly resemble the mammalian orthologs. However, due to a genome duplication event early during the evolution of bony fishes (Postlethwait et al. 2000) many of the lrrtm1, neuroligin and neurexin genes are present as two copies. For example, the zebrafish orthologs of neuroligin 2, 3 and 4 genes are present as duplicates, whereas the zebrafish ortholog of neuroligin1 returned to singleton status by subsequent loss of one of the duplicates (Davey et al. 2010). While it may be that the presence of duplicate genes may increase redundancy, often duplicates end up partitioning functions or expression patterns (Cresko et al. 2003). Thus, knock-down of each of a pair of genes can give distinct, but overlapping phenotypes (Feldman et al. 1998).The expression patterns of the duplicates of nlgn2, 3 and 4 are extremely specific and distinct for each pair of duplicates (Davey et al. 2010), suggesting that the duplicates may have distinct functions or similar functions in distinct cell types.
The lrrtm, neurexin and neuroligin genes are predominantly expressed in the nervous system during development. While the expression patterns for the zebrafish neurexin genes have not been annotated at length, it is possible to compare the expression patterns of the lrrtm and neuroligin genes. The tissue expression profiles of the four lrrtm paralogues in whole embryos show that, each has a unique expression pattern, labeling distinct but overlapping cell populations within the brain, spinal cord and sensory tissues (Charoensawan et al. 2010). Similarly, all of the four lrrtms are expressed within the developing retina, but double labeling showed their expression was mutually exclusive; that is each lrrtm paralogue is expressed by different non-overlapping cell types (Sollner & Wright 2009). The story is similar for the neuroligins, with distinct brain regions showing differential expression levels for the seven neuroligin paralogues (Davey et al. 2010, Rissone et al. 2010).
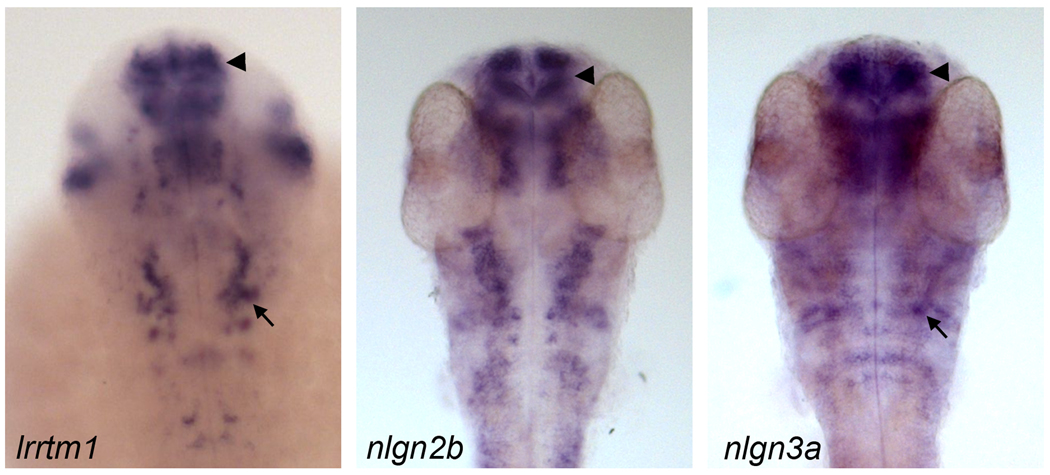
For the purposes of addressing redundancy and/or cooperativity, it is interesting to consider how similar some of individual lrrtm and nlgn expression patterns may be to each other. In a direct comparison of lrrtm1, nlgn2b and nlgn3a expression at 48 hours postfertilization for example (Fig. 4), it is apparent that some regions of the brain show distinct and some show overlapping expression. At this time in development, many of the neurons have been born and are extending axons and are forming synapses, for example in the optic tectum (Burrill & Easter 1995). It is interesting to note that lrrtm1 and nlgn2b show a significant amount of overlap throughout the brain, particularly in the developing forebrain (Fig. 4). This may suggest that these two CAMs may be regulated by similar enhancer elements. Given the presumptive function of lrrtm1 and nlgn2b in excitatory and inhibitory synaptogenesis, respectively, it may be interesting to hypothesize that these expression patterns may reflect a mechanism common to many sets of neurons for setting up glutamatergic and GABAergic inputs.
Figure 4. Examples of CAM expression patterns during zebrafish development.
lrrtm1, nlgn2b and nlgn3a are expressed in overlapping and distinct regions of the zebrafish larval brain at around 48 hours postfertilization as revealed by in situ hybridization. Regions overlap for all three genes include the telencephalon (arrowhead), whereas only lrrtm1 and nlgn3b are expressed together in similar sets of neurons in the caudal hindbrain (arrow). Images courtesy of zfin.org (lrrtm1) and unpublished images (Tallafuss and Washbourne for nlgn2b and nlgn3a).
In contrast, the expression of lrrtm1 and nlgn3a are quite distinct, especially in the mid- and hindbrain regions (Fig. 4). Curiously, however, there appear to be cells in the caudal hindrain with overlapping expression (arrows). Future double in situ hybridization studies will confirm whether these are the exact same cells, and may provide a basis for studying redundant, cooperative or summative interactions between these two genes in establishing glutamatergic inputs onto premotor neurons in the hindbrain. Delving beyond the known functions of these synaptogenic CAMs, it is interesting to note that some of the zebrafish neuroligin and neurexin genes are expressed during the earliest stages of embryonic development (Rissone et al. 2007, Rissone et al. 2010, Davey et al. 2010). This may suggest novel roles for these genes during neuronal specification or migration. Furthermore, the presence of mRNA specific for nrxn1a, nlgn1 and nlgn4a in the fertilized egg suggest that these transcripts are delivered as maternal or paternal contributions (Rissone et al. 2007, Davey et al. 2010). It is unknown whether this early expression may present a specialization of fish, as the presence of mRNA in the early embryo has not been studied in mammals.
Further studies are still required to determine whether the zebrafish orthologs of lrrtms, neurexins and neuroligins possess the same synaptogenic function as in mammals. However, judging by sequence identity it is highly probable that the encoded proteins have the same role. Indeed, the sequence identity for the nlgn4 genes is higher between zebrafish and humans (~80%) than between mice and humans (~60%; Davey et al. 2010). This interesting quirk of evolution may present us with a model system in which to study the molecular and cellular underpinnings of ASDs caused by mutations in Nlgn4 genes.
Given that some of the molecular, cellular and behavioral phenotypes in synaptic CAM knock-out mice are generally quite subtle (Varoqueaux et al. 2006, Linhoff et al. 2009, Missler et al. 2003), it will be interesting to see what consequences knock-down and/or overexpression of these CAMs will have in zebrafish especially given the presence of duplicate genes. To determine physiological consequences of loss-of-function experiments for these synaptic CAMs in zebrafish, it will be necessary to improve phenotyping at the behavioral level. While still in its infancy, analysis of zebrafish behaviors has gained a strong foothold recently (Fetcho & Liu 1998, Guo 2004, Norton & Bally-Cuif 2010). While it may not seem feasible to model neurodevelopmental diseases such as autism in fish, assays studying motor control (Kabashi et al. 2010), memory (Best et al. 2008), aggression (Larson et al. 2006) and even social interaction have been developed (Norton & Bally-Cuif 2010). Given that it is much easier to perform high-throughput small molecule screens in zebrafish (Peterson & Fishman 2004) than it is in mice or rats, one can envisage future experiments to identify drug candidates for synaptic CAM deficits that underlie specific neurodevelopmental disorders (Gerlai 2010).
In addition to the possibility of performing behavioral experiments in fish, the great strengths of the zebrafish lie in cellular imaging in the intact and living animal due to optical clarity of the developing embryos and larvae (McLean & Fetcho 2008). Thus, analysis of the underlying changes in connectivity in the context of loss-of-function experiments for the neurexin, neuroligin and lrrtm genes in a vertebrate, in which many neurons are identifiable in vivo and whose synaptic inputs can be readily analyzed, will be powerful. This can be imagined in the context of a transgenic zebrafish expressing brainbow fluorescent proteins similarly as described in mice (Lichtman et al. 2008), but with the possibility of performing time-lapse imaging of large regions of brain during development.
Conclusions
It is now well established that extracellular recognition events mediated through cell surface adhesion receptors are mechanistically responsible for the construction of neural cellular circuits and synapse formation, which in turn account for the behavioral outputs of nervous systems. It is quite likely that many neurological diseases result from subtle differences in neural connectivity or synaptic patterning; indeed, several genome-wide association studies for these diseases have now implicated neural cell adhesion molecules (Table 1). Understanding how neurons integrate the extracellular cues received by neural receptor proteins to construct and modify nervous systems will be a major challenge, particularly in developmentally inaccessible mammalian models. It is here that we believe the zebrafish model can make an important contribution to the mechanistic understanding of neural circuit and synapse formation and eventually make direct causal links to behaviour.
Acknowledgements
We would like to thank Alexandra Tallafuss for critical reading of the manuscript. Work in the Washbourne laboratory is funded by an R01 federal grant R01NS065795 from the National Institute of Neurological Disorders and Stroke (NINDS). Relevant work in the Wright lab was sponsored by the Wellcome Trust (grant No. 077108/Z/05/Z) and NIH grant RO1NS063400.
Abbreviations
- ASD
Autism Spectrum Disorders
- CAM
cell adhesion molecule
- Eph
ephrin
- FERM
4.1 ezrin radixin moesin
- GluR
glutamate receptor
- Lrfn
leucine rich repeat and fibronectin II domain
- LRR
leucine rich repeat
- NGL
netrin G ligand
- Nlgn
neuroligin
- Nrxn
neurexin
- PDZ
PSD-95 discs large ZO-1
- PSD-95
postsynaptic density 95kD
- SALM
synaptic adhesion-like molecule
Footnotes
(nomenclature for zebrafish genes is in lower case italics)
Competing Interests
The authors declare that they have no competing interests.
References
- Abelson JF, Kwan KY, O'Roak BJ, et al. Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science. 2005;310:317–320. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- Arac D, Boucard AA, Ozkan E, Strop P, Newell E, Sudhof TC, Brunger AT. Structures of neuroligin-1 and the neuroligin-1/neurexin-1 beta complex reveal specific protein-protein and protein-Ca2+ interactions. Neuron. 2007;56:992–1003. doi: 10.1016/j.neuron.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Aruga J, Mikoshiba K. Identification and characterization of Slitrk, a novel neuronal transmembrane protein family controlling neurite outgrowth. Mol Cell Neurosci. 2003;24:117–129. doi: 10.1016/s1044-7431(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Bando T, Sekine K, Kobayashi S, et al. Neuronal leucine-rich repeat protein 4 functions in hippocampus-dependent long-lasting memory. Mol Cell Biol. 2005;25:4166–4175. doi: 10.1128/MCB.25.10.4166-4175.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker AJ, Koch SM, Reed J, Barres BA, Ullian EM. Developmental control of synaptic receptivity. J Neurosci. 2008;28:8150–8160. doi: 10.1523/JNEUROSCI.1744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bella J, Hindle KL, McEwan PA, Lovell SC. The leucine-rich repeat structure. Cell Mol Life Sci. 2008;65:2307–2333. doi: 10.1007/s00018-008-8019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninghausen O, Rahman MA, Silva JP, Davletov B, Hopkins C, Ushkaryov YA. Neurexin Ibeta and neuroligin are localized on opposite membranes in mature central synapses. J Neurochem. 2007;103:1855–1863. doi: 10.1111/j.1471-4159.2007.04918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JD, Berghmans S, Hunt JJ, Clarke SC, Fleming A, Goldsmith P, Roach AG. Non-associative learning in larval zebrafish. Neuropsychopharmacology. 2008;33:1206–1215. doi: 10.1038/sj.npp.1301489. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- Biederer T, Scheiffele P. Mixed-culture assays for analyzing neuronal synapse formation. Nat Protoc. 2007;2:670–676. doi: 10.1038/nprot.2007.92. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sudhof TC. CASK and protein 4.1 support F-actin nucleation on neurexins. J Biol Chem. 2001;276:47869–47876. doi: 10.1074/jbc.M105287200. [DOI] [PubMed] [Google Scholar]
- Bolliger MF, Pei J, Maxeiner S, Boucard AA, Grishin NV, Sudhof TC. Unusually rapid evolution of Neuroligin-4 in mice. Proc Natl Acad Sci U S A. 2008;105:6421–6426. doi: 10.1073/pnas.0801383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Bowser R, Muller H, Govindan B, Novick P. Sec8p and Sec15p are components of a plasma membrane-associated 19.5S particle that may function downstream of Sec4p to control exocytosis. J Cell Biol. 1992;118:1041–1056. doi: 10.1083/jcb.118.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budreck EC, Scheiffele P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur J Neurosci. 2007;26:1738–1748. doi: 10.1111/j.1460-9568.2007.05842.x. [DOI] [PubMed] [Google Scholar]
- Burrill JD, Easter SS., Jr The first retinal axons and their microenvironment in zebrafish: cryptic pioneers and the pretract. J Neurosci. 1995;15:2935–2947. doi: 10.1523/JNEUROSCI.15-04-02935.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushell KM, Sollner C, Schuster-Boeckler B, Bateman A, Wright GJ. Large-scale screening for novel low-affinity extracellular protein interactions. Genome Res. 2008;18:622–630. doi: 10.1101/gr.7187808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz S, Okamoto M, Sudhof TC. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94:773–782. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoensawan V, Adryan B, Martin S, Sollner C, Thisse B, Thisse C, Wright GJ, Teichmann SA. The impact of gene expression regulation on evolution of extracellular signalling pathways. Mol Cell Proteomics. 2010 doi: 10.1074/mcp.M110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liu H, Shim AH, Focia PJ, He X. Structural basis for synaptic adhesion mediated by neuroligin-neurexin interactions. Nat Struct Mol Biol. 2008;15:50–56. doi: 10.1038/nsmb1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Aulia S, Li L, Tang BL. AMIGO and friends: an emerging family of brain-enriched, neuronal growth modulating, type I transmembrane proteins with leucine-rich repeats (LRR) and cell adhesion molecule motifs. Brain Res Rev. 2006;51:265–274. doi: 10.1016/j.brainresrev.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Chih B, Afridi SK, Clark L, Scheiffele P. Disorder-associated mutations lead to functional inactivation of neuroligins. Hum Mol Genet. 2004;13:1471–1477. doi: 10.1093/hmg/ddh158. [DOI] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Choe J, Kelker MS, Wilson IA. Crystal structure of human toll-like receptor 3 (TLR3) ectodomain. Science. 2005;309:581–585. doi: 10.1126/science.1115253. [DOI] [PubMed] [Google Scholar]
- Comoletti D, Miller MT, Jeffries CM, Wilson J, Demeler B, Taylor P, Trewhella J, Nakagawa T. The macromolecular architecture of extracellular domain of alphaNRXN1: domain organization, flexibility, and insights into trans-synaptic disposition. Structure. 2010;18:1044–1053. doi: 10.1016/j.str.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correas I, Speicher DW, Marchesi VT. Structure of the spectrin-actin binding site of erythrocyte protein 4.1. J Biol Chem. 1986;261:13362–13366. [PubMed] [Google Scholar]
- Cresko WA, Yan YL, Baltrus DA, Amores A, Singer A, Rodriguez-Mari A, Postlethwait JH. Genome duplication, subfunction partitioning, and lineage divergence: Sox9 in stickleback and zebrafish. Dev Dyn. 2003;228:480–489. doi: 10.1002/dvdy.10424. [DOI] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey C, Tallafuss A, Washbourne P. Differential expression of neuroligin genes in the nervous system of zebrafish. Dev Dyn. 2010;239:703–714. doi: 10.1002/dvdy.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Escalera S, Bockamp EO, Moya F, Piovant M, Jimenez F. Characterization and gene cloning of neurotactin, a Drosophila transmembrane protein related to cholinesterases. EMBO J. 1990;9:3592–3601. doi: 10.1002/j.1460-2075.1990.tb07570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva A, Meunier FA, Molgo J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci U S A. 1999;96:3200–3205. doi: 10.1073/pnas.96.6.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit J, Sylwestrak E, O'Sullivan ML, et al. LRRTM2 Interacts with Neurexin1 and Regulates Excitatory Synapse Formation. Neuron. 2009;64:799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan J, Walshe K, Alsbury S, Hokamp K, O'Keeffe S, Okafuji T, Miller SF, Tear G, Mitchell KJ. The extracellular Leucine-Rich Repeat superfamily; a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genomics. 2007;8:320. doi: 10.1186/1471-2164-8-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen JS, Smith JC. Controlling morpholino experiments: don't stop making antisense. Development. 2008;135:1735–1743. doi: 10.1242/dev.001115. [DOI] [PubMed] [Google Scholar]
- Fabrichny IP, Leone P, Sulzenbacher G, Comoletti D, Miller MT, Taylor P, Bourne Y, Marchot P. Structural analysis of the synaptic protein neuroligin and its beta-neurexin complex: determinants for folding and cell adhesion. Neuron. 2007;56:979–991. doi: 10.1016/j.neuron.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- Fetcho JR, Liu KS. Zebrafish as a model system for studying neuronal circuits and behavior. Ann N Y Acad Sci. 1998;860:333–345. doi: 10.1111/j.1749-6632.1998.tb09060.x. [DOI] [PubMed] [Google Scholar]
- Francks C, Maegawa S, Lauren J, et al. LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Vicini S. Neuroligin-2 accelerates GABAergic synapse maturation in cerebellar granule cells. Mol Cell Neurosci. 2009;42:45–55. doi: 10.1016/j.mcn.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Washbourne P, Ortinski P, Vicini S. Functional excitatory synapses in HEK293 cells expressing neuroligin and glutamate receptors. J Neurophysiol. 2003;90:3950–3957. doi: 10.1152/jn.00647.2003. [DOI] [PubMed] [Google Scholar]
- Gahtan E, Sankrithi N, Campos JB, O'Malley DM. Evidence for a widespread brain stem escape network in larval zebrafish. J Neurophysiol. 2002;87:608–614. doi: 10.1152/jn.00596.2001. [DOI] [PubMed] [Google Scholar]
- Gerlai R. High-throughput behavioral screens: the first step towards finding genes involved in vertebrate brain function using zebrafish. Molecules. 2010;15:2609–2622. doi: 10.3390/molecules15042609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrow K, El-Husseini A. Cell adhesion molecules at the synapse. Front Biosci. 2006;11:2400–2419. doi: 10.2741/1978. [DOI] [PubMed] [Google Scholar]
- Giagtzoglou N, Ly CV, Bellen HJ. Cell adhesion, the backbone of the synapse: "vertebrate" and "invertebrate" perspectives. Cold Spring Harb Perspect Biol. 2009;1:a003079. doi: 10.1101/cshperspect.a003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glessner JT, Wang K, Cai G, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves JA. Sex chromosome specialization and degeneration in mammals. Cell. 2006;124:901–914. doi: 10.1016/j.cell.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Guo S. Linking genes to brain, behavior and neurological diseases: what can we learn from zebrafish? Genes Brain Behav. 2004;3:63–74. doi: 10.1046/j.1601-183x.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Hata Y, Butz S, Sudhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XL, Bazan JF, McDermott G, Park JB, Wang K, Tessier-Lavigne M, He Z, Garcia KC. Structure of the Nogo receptor ectodomain: a recognition module implicated in myelin inhibition. Neuron. 2003;38:177–185. doi: 10.1016/s0896-6273(03)00232-0. [DOI] [PubMed] [Google Scholar]
- Higgins JJ, Pucilowska J, Lombardi RQ, Rooney JP. Candidate genes for recessive non-syndromic mental retardation on chromosome 3p (MRT2A) Clin Genet. 2004;65:496–500. doi: 10.1111/j.0009-9163.2004.00267.x. [DOI] [PubMed] [Google Scholar]
- Hirai H, Pang Z, Bao D, et al. Cbln1 is essential for synaptic integrity and plasticity in the cerebellum. Nat Neurosci. 2005;8:1534–1541. doi: 10.1038/nn1576. [DOI] [PubMed] [Google Scholar]
- Hoy JL, Constable JR, Vicini S, Fu Z, Washbourne P. SynCAM1 recruits NMDA receptors via protein 4.1B. Mol Cell Neurosci. 2009;42:466–483. doi: 10.1016/j.mcn.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Nguyen T, Sudhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashi E, Lin L, Tradewell ML, et al. Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo. Hum Mol Genet. 2010;19:671–683. doi: 10.1093/hmg/ddp534. [DOI] [PubMed] [Google Scholar]
- Kalachikov S, Evgrafov O, Ross B, et al. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Genet. 2002;30:335–341. doi: 10.1038/ng832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwabuchi N, Ikeda K, Araki K, et al. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci. 2006;26:12152–12164. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, Higgins AW, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Burette A, Chung HS, et al. NGL family PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat Neurosci. 2006;9:1294–1301. doi: 10.1038/nn1763. [DOI] [PubMed] [Google Scholar]
- Ko J, Fuccillo MV, Malenka RC, Sudhof TC. LRRTM2 Functions as a Neurexin Ligand in Promoting Excitatory Synapse Formation. Neuron. 2009a;64:791–798. doi: 10.1016/j.neuron.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Kim S, Chung HS, Kim K, Han K, Kim H, Jun H, Kaang BK, Kim E. SALM synaptic cell adhesion-like molecules regulate the differentiation of excitatory synapses. Neuron. 2006;50:233–245. doi: 10.1016/j.neuron.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Ko J, Zhang C, Arac D, Boucard AA, Brunger AT, Sudhof TC. Neuroligin-1 performs neurexin-dependent and neurexin-independent functions in synapse validation. EMBO J. 2009b;28:3244–3255. doi: 10.1038/emboj.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobe B, Deisenhofer J. Crystal structure of porcine ribonuclease inhibitor, a protein with leucine-rich repeats. Nature. 1993;366:751–756. doi: 10.1038/366751a0. [DOI] [PubMed] [Google Scholar]
- Koehnke J, Jin X, Budreck EC, Posy S, Scheiffele P, Honig B, Shapiro L. Crystal structure of the extracellular cholinesterase-like domain from neuroligin-2. Proc Natl Acad Sci U S A. 2008;105:1873–1878. doi: 10.1073/pnas.0711701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehnke J, Katsamba PS, Ahlsen G, Bahna F, Vendome J, Honig B, Shapiro L, Jin X. Splice form dependence of beta-neurexin/neuroligin binding interactions. Neuron. 2010;67:61–74. doi: 10.1016/j.neuron.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuja-Panula J, Kiiltomaki M, Yamashiro T, Rouhiainen A, Rauvala H. AMIGO, a transmembrane protein implicated in axon tract development, defines a novel protein family with leucine-rich repeats. J Cell Biol. 2003;160:963–973. doi: 10.1083/jcb.200209074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara H, Hashimoto K, Kano M, Takayama C, Sakimura K, Mishina M, Inoue Y, Watanabe M. Impaired parallel fiber-->Purkinje cell synapse stabilization during cerebellar development of mutant mice lacking the glutamate receptor delta2 subunit. J Neurosci. 1997;17:9613–9623. doi: 10.1523/JNEUROSCI.17-24-09613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ET, O'Malley DM, Melloni RH., Jr Aggression and vasotocin are associated with dominant-subordinate relationships in zebrafish. Behav Brain Res. 2006;167:94–102. doi: 10.1016/j.bbr.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J, Airaksinen MS, Saarma M, Timmusk T. A novel gene family encoding leucine-rich repeat transmembrane proteins differentially expressed in the nervous system. Genomics. 2003;81:411–421. doi: 10.1016/s0888-7543(03)00030-2. [DOI] [PubMed] [Google Scholar]
- Leone P, Comoletti D, Ferracci G, Conrod S, Garcia SU, Taylor P, Bourne Y, Marchot P. Structural insights into the exquisite selectivity of neurexin/neuroligin synaptic interactions. EMBO J. 2010;29:2461–2471. doi: 10.1038/emboj.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Martin ER, Schnetz-Boutaud N, et al. Effect of heterogeneity on the chromosome 10 risk in late-onset Alzheimer disease. Hum Mutat. 2007;28:1065–1073. doi: 10.1002/humu.20567. [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Livet J, Sanes JR. A technicolour approach to the connectome. Nat Rev Neurosci. 2008;9:417–422. doi: 10.1038/nrn2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, Ho WH, Gurney A, Rosenthal A. The netrin-G1 ligand NGL-1 promotes the outgrowth of thalamocortical axons. Nat Neurosci. 2003;6:1270–1276. doi: 10.1038/nn1148. [DOI] [PubMed] [Google Scholar]
- Linhoff MW, Lauren J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, Airaksinen MS, Strittmatter SM, Craig AM. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734–749. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron. 1999;23:325–335. doi: 10.1016/s0896-6273(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Majercak J, Ray WJ, Espeseth A, et al. LRRTM3 promotes processing of amyloid-precursor protein by BACE1 and is a positional candidate gene for late-onset Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:17967–17972. doi: 10.1073/pnas.0605461103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Sollner C, Charoensawan V, Adryan B, Thisse B, Thisse C, Teichmann SA, Wright GJ. Construction of a large extracellular protein interaction network and its resolution by spatiotemporal expression profiling. Mol Cell Proteomics. 2010 doi: 10.1074/mcp.M110.004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Miura E, Miyazaki T, et al. Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science. 2010;328:363–368. doi: 10.1126/science.1185152. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Yuzaki M. Cbln1 and the Delta2 Glutamate Receptor-An Orphan Ligand and an Orphan Receptor Find Their Partners. Cerebellum. 2010 doi: 10.1007/s12311-010-0186-5. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Using imaging and genetics in zebrafish to study developing spinal circuits in vivo. Dev Neurobiol. 2008;68:817–834. doi: 10.1002/dneu.20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Sudhof TC. Neurexins: three genes and 1001 products. Trends Genet. 1998;14:20–26. doi: 10.1016/S0168-9525(97)01324-3. [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Morante-Redolat JM, Gorostidi-Pagola A, Piquer-Sirerol S, et al. Mutations in the LGI1/Epitempin gene on 10q24 cause autosomal dominant lateral temporal epilepsy. Hum Mol Genet. 2002;11:1119–1128. doi: 10.1093/hmg/11.9.1119. [DOI] [PubMed] [Google Scholar]
- Mosyak L, Wood A, Dwyer B, et al. The structure of the Lingo-1 ectodomain, a module implicated in central nervous system repair inhibition. J Biol Chem. 2006;281:36378–36390. doi: 10.1074/jbc.M607314200. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sudhof TC. Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules. J Biol Chem. 1997;272:26032–26039. doi: 10.1074/jbc.272.41.26032. [DOI] [PubMed] [Google Scholar]
- Norton W, Bally-Cuif L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010;11:90. doi: 10.1186/1471-2202-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obst-Pernberg K, Redies C. Cadherins and synaptic specificity. J Neurosci Res. 1999;58:130–138. [PubMed] [Google Scholar]
- Okamoto M, Sudhof TC. Mints, Munc18-interacting proteins in synaptic vesicle exocytosis. J Biol Chem. 1997;272:31459–31464. doi: 10.1074/jbc.272.50.31459. [DOI] [PubMed] [Google Scholar]
- Olson PF, Fessler LI, Nelson RE, Sterne RE, Campbell AG, Fessler JH. Glutactin, a novel Drosophila basement membrane-related glycoprotein with sequence similarity to serine esterases. EMBO J. 1990;9:1219–1227. doi: 10.1002/j.1460-2075.1990.tb08229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampanos A, Volaki K, Kanavakis E, Papandreou O, Youroukos S, Thomaidis L, Karkelis S, Tzetis M, Kitsiou-Tzeli S. A substitution involving the NLGN4 gene associated with autistic behavior in the Greek population. Genet Test Mol Biomarkers. 2009;13:611–615. doi: 10.1089/gtmb.2009.0005. [DOI] [PubMed] [Google Scholar]
- Pancer Z, Amemiya CT, Ehrhardt GR, Ceitlin J, Gartland GL, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–180. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- Papageorgiou AC, Shapiro R, Acharya KR. Molecular recognition of human angiogenin by placental ribonuclease inhibitor--an X-ray crystallographic study at 2.0 A resolution. Embo J. 1997;16:5162–5177. doi: 10.1093/emboj/16.17.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RT, Fishman MC. Discovery and use of small molecules for probing biological processes in zebrafish. Methods Cell Biol. 2004;76:569–591. doi: 10.1016/s0091-679x(04)76026-4. [DOI] [PubMed] [Google Scholar]
- Piton A, Gauthier J, Hamdan FF, et al. Systematic resequencing of X-chromosome synaptic genes in autism spectrum disorder and schizophrenia. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwait JH, Woods IG, Ngo-Hazelett P, Yan YL, Kelly PD, Chu F, Huang H, Hill-Force A, Talbot WS. Zebrafish comparative genomics and the origins of vertebrate chromosomes. Genome Res. 2000;10:1890–1902. doi: 10.1101/gr.164800. [DOI] [PubMed] [Google Scholar]
- Rissone A, Monopoli M, Beltrame M, Bussolino F, Cotelli F, Arese M. Comparative genome analysis of the neurexin gene family in Danio rerio: insights into their functions and evolution. Mol Biol Evol. 2007;24:236–252. doi: 10.1093/molbev/msl147. [DOI] [PubMed] [Google Scholar]
- Rissone A, Sangiorgio L, Monopoli M, Beltrame M, Zucchi I, Bussolino F, Arese M, Cotelli F. Characterization of the neuroligin gene family expression and evolution in zebrafish. Dev Dyn. 2010;239:688–702. doi: 10.1002/dvdy.22196. [DOI] [PubMed] [Google Scholar]
- Robinson M, Parsons Perez MC, Tebar L, et al. FLRT3 is expressed in sensory neurons after peripheral nerve injury and regulates neurite outgrowth. Mol Cell Neurosci. 2004;27:202–214. doi: 10.1016/j.mcn.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Rudenko G, Nguyen T, Chelliah Y, Sudhof TC, Deisenhofer J. The structure of the ligand-binding domain of neurexin Ibeta: regulation of LNS domain function by alternative splicing. Cell. 1999;99:93–101. doi: 10.1016/s0092-8674(00)80065-3. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Schubert WD, Urbanke C, Ziehm T, Beier V, Machner MP, Domann E, Wehland J, Chakraborty T, Heinz DW. Structure of internalin, a major invasion protein of Listeria monocytogenes, in complex with its human receptor E-cadherin. Cell. 2002;111:825–836. doi: 10.1016/s0092-8674(02)01136-4. [DOI] [PubMed] [Google Scholar]
- Sheckler LR, Henry L, Sugita S, Sudhof TC, Rudenko G. Crystal structure of the second LNS/LG domain from neurexin 1alpha: Ca2+ binding and the effects of alternative splicing. J Biol Chem. 2006;281:22896–22905. doi: 10.1074/jbc.M603464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KC, Kuczynska DA, Wu IJ, Murray BH, Sheckler LR, Rudenko G. Regulation of neurexin 1beta tertiary structure and ligand binding through alternative splicing. Structure. 2008;16:422–431. doi: 10.1016/j.str.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui TJ, Pancaroglu R, Kang Y, Rooyakkers A, Craig AM. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J Neurosci. 2010;30:7495–7506. doi: 10.1523/JNEUROSCI.0470-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EN, Bloss CS, Badner JA, et al. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry. 2009;14:755–763. doi: 10.1038/mp.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner C, Wright GJ. A cell surface interaction network of neural leucine-rich repeat receptors. Genome Biol. 2009;10:R99. doi: 10.1186/gb-2009-10-9-r99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc. Natl. Acad. Sci. USA. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa I, Clark TG, Holt R, et al. Polymorphisms in leucine-rich repeat genes are associated with autism spectrum disorder susceptibility in populations of European ancestry. Mol Autism. 2010;1:7. doi: 10.1186/2040-2392-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Paterson AD, Zwaigenbaum L, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebizadeh Z, Lam DY, Theodoro MF, Bittel DC, Lushington GH, Butler MG. Novel splice isoforms for NLGN3 and NLGN4 with possible implications in autism. J Med Genet. 2006;43:e21. doi: 10.1136/jmg.2005.036897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallafuss A, Constable JR, Washbourne P. Organization of central synapses by adhesion molecules. Eur J Neurosci. 2010 doi: 10.1111/j.1460-9568.2010.07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Gollan L, Scholl FG, Mahadomrongkul V, Dobler E, Limthong N, Peck M, Aoki C, Scheiffele P. Silencing of neuroligin function by postsynaptic neurexins. J Neurosci. 2007;27:2815–2824. doi: 10.1523/JNEUROSCI.0032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, Taguchi R, Sakimura K, Mishina M. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 2010;141:1068–1079. doi: 10.1016/j.cell.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Ullrich B, Ushkaryov YA, Sudhof TC. Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron. 1995;14:497–507. doi: 10.1016/0896-6273(95)90306-2. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Hata Y, Ichtchenko K, Moomaw C, Afendis S, Slaughter CA, Sudhof TC. Conserved domain structure of beta-neurexins. Unusual cleaved signal sequences in receptor-like neuronal cell-surface proteins. J Biol Chem. 1994;269:11987–11992. [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Sudhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Wang CY, Chang K, Petralia RS, Wang YX, Seabold GK, Wenthold RJ. A novel family of adhesion-like molecules that interacts with the NMDA receptor. J Neurosci. 2006;26:2174–2183. doi: 10.1523/JNEUROSCI.3799-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang H, Ma D, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature. 2009;459:528–533. doi: 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Kwon SK, Kim E. The NGL family of leucine-rich repeat-containing synaptic adhesion molecules. Mol Cell Neurosci. 2009;42:1–10. doi: 10.1016/j.mcn.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Wright GJ. Signal initiation in biological systems: the properties and detection of transient extracellular protein interactions. Mol Biosyst. 2009;5:1405–1412. doi: 10.1039/b903580j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Feng J, Schroer R, Li W, Skinner C, Schwartz CE, Cook EH, Jr, Sommer SS. Analysis of the neuroligin 4Y gene in patients with autism. Psychiatr Genet. 2008;18:204–207. doi: 10.1097/YPG.0b013e3282fb7fe6. [DOI] [PubMed] [Google Scholar]
- Yan J, Oliveira G, Coutinho A, et al. Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol Psychiatry. 2005;10:329–332. doi: 10.1038/sj.mp.4001629. [DOI] [PubMed] [Google Scholar]
- Zhang C, Atasoy D, Arac D, Yang X, Fucillo MV, Robison AJ, Ko J, Brunger AT, Sudhof TC. Neurexins physically and functionally interact with GABA(A) receptors. Neuron. 2010;66:403–416. doi: 10.1016/j.neuron.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Milunsky JM, Newton S, et al. A neuroligin-4 missense mutation associated with autism impairs neuroligin-4 folding and endoplasmic reticulum export. J Neurosci. 2009;29:10843–10854. doi: 10.1523/JNEUROSCI.1248-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse? Science. 2003;302:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]