Abstract
The aim of this study was to compare the safety and efficacy of OsteoSet®2 DBM with autologous cancellous bone in free vascularised fibular grafting for the treatment of large osteonecrotic lesions of the femoral head. Twenty-four patients (30 hips) with large osteonecrotic lesions of the femoral head (stage IIC in six hips, stage IIIC in 14, and stage IVC in ten, according to the classification system of Steinberg et al.) underwent free vascularised fibular grafting with OsteoSet®2 DBM. This group was retrospectively matched to a group of 24 patients (30 hips) who underwent free vascularised fibular grafting with autologous cancellous bone during the same time period according to the aetiology, stage, and size of the lesion and the mean preoperative Harris hip score. A prospective case-controlled study was then performed with a mean follow-up duration of 26 months. The results show no statistically significant differences between the two groups in overall clinical outcome or the radiographic assessment. Furthermore, no adverse events related to the use of the OsteoSet®2 DBM were observed. The results demonstrate that OsteoSet®2 DBM combined with autograft bone performs equally as well as that of autologous bone alone. Therefore, OsteoSet®2 DBM can be used as a safe and effective graft extender in free vascularised fibular grafting for large osteonecrotic lesions of the femoral head.
Introduction
Osteonecrosis of the femoral head (ONFH) is a debilitating disease that usually leads to collapse of the femoral head and destruction of the hip joint. Free vascularised fibular grafting (FVFG) provides the most consistently successful results of any joint-preserving methods, such as core decompression, nonvascularised bone grafting and transtrochanteric rotational osteotomy [1, 6, 7, 12, 16, 21, 23]. After excision of the necrotic bone beneath the weight-bearing region that might inhibit revascularisation of the femoral head, the defect of the femoral head is often filled with the cancellous bone graft to enhance the osteoinductive process [1, 7, 12, 16].
The preferred graft material most commonly consists of autologous cancellous bone (ACB) harvested from the local greater trochanteric region [1, 16, 21] or iliac crest [6, 23] as the graft donor. ACB graft offers a number of advantages, including the excellent success rates and no immune issues or risks for disease transmission. However, there is a substantial incidence of morbidity associated with the harvest procedure. Reported complications of harvest of the iliac crest bone graft include deep infection, osteomyelitis, haematoma, neurological injury, vascular injury, iatrogenic iliac wing or sacroiliac joint injury, persistent pain and cosmetic defects, etc. [4, 15, 22]. In particular, residual pain has been reported to occur in as much as 31% of the cases. Harvest of ACB from the greater trochanteric region may decrease bone strength of the donor site and increase risk of proximal femur fracture, which is a devastating complication for the patients [1, 16, 21]. Aldridge et al. reported that the incidence of subtrochanteric fractures was 1% when ACB was harvested from the greater trochanter. The rate was higher than this when harvested from the greater and lesser trochanters at the same time [1].
In an effort to eliminate or reduce the amount of autologous bone graft needed, considerable research and development activity have been expended to find satisfactory bone substitutes or to produce materials that extend autograft when the supply is limited, as with large osteonecrotic lesions of the femoral head. Demineralised bone matrix (DBM) is one of these because it has been shown that properly demineralised cortical bone preserves the natural capacity of the native bone proteins and growth factors [17, 18]. Clinically, DBM has been shown to be effective in promoting bone-healing and widely used as a graft substitute or as a graft extender in craniofacial reconstructions [9], spinal fusions [2, 8, 10, 14, 19], bone defects [3] and fracture nonunions [5]. To our knowledge, however, few studies have addressed the safety and efficacy of DBM for ONFH.
The purpose of this study was to compare the safety and efficacy of OsteoSet®2 DBM used as a graft extender with that of ACB in FVFG for treatment of large osteonecrotic lesions in two groups of patients who were matched according to aetiology, stage, and extent of the lesion, as well as the preoperative Harris hip score.
Materials and methods
Patient selection
The study was approved by the Committee of Medical Ethics and the Institutional Review Board of our University and performed with the informed consent of each patient and his or her family. For a two-year period beginning in January 2005, 24 patients (30 hips) with a large osteonecrotic lesion of the femoral head underwent FVFG with OsteoSet®2 DBM. These patients were retrospectively matched to 24 patients (30 hips) who had undergone FVFG with ACB during the same period. Matching was based on the stage, extent, and aetiology of the lesion, as well as average age, gender, body mass index, and preoperative Harris hip score (Table 1).
Table 1.
Patient demographics
| Variables | DBM group | ACB group |
|---|---|---|
| Patients/hips (n) | 24/30 | 24/30 |
| Age (years) | 37 (24–52) | 37 (20–55) |
| Male/female (patients) | 18/6 | 18/6 |
| Right/left (hips) | 14/16 | 17/13 |
| Bilateral disease (patients) | 6 | 6 |
| Body mass index (kg/m2) | 22–32 | 21–33 |
| Duration of symptoms (months) | 6.4 ± 6.3 | 7.6 ± 6.4 |
| Mean preop Harris hip score (points) | 70 | 70 |
| Steinberg stage (hips) | ||
| Stage IIC | 6 | 6 |
| Stage IIIC | 14 | 14 |
| Stage IVC | 10 | 10 |
| Medical history (patients/hips) | ||
| Corticosteroids | 11/14 | 11/14 |
| Alcoholism | 6/7 | 6/7 |
| Idiopathic | 7/9 | 7/9 |
| Duration of follow-up (months) | 26 (24–38) | 26 (24–40) |
DBM demineralised bone matrix, ACB autologous cancellous bone
Each patient selected the procedure that he or she preferred after the surgeon (C.Q. Zhang) had explained the potential advantages and disadvantages of the procedure as follows. (1) OsteoSet®2 DBM as a graft substitute has been shown to be effective in promoting bone healing and is widely used in the orthopaedic field, but we do not know the effectiveness of FVFG with OsteoSet®2 DBM for ONFH. (2) The potential advantages of OsteoSet®2 DBM are that it was considered a useful bone substitute and/or enhancer for bone autograft as it has osteoinductive and osteoconductive capacity, can shorten time of operation, and can diminish donor site morbidity such as risk of proximal femur fracture, etc. (3) The potential disadvantages of OsteoSet®2 DBM are that it can evoke the possibility of rejection reaction and increase the risk of infection, etc.
Pretreatment assessments consisted of a complete history and physical examination; laboratory tests, including a complete blood-cell count, a platelet count, measurement of the prothrombin time and partial thromboplastin time, and measurement of the blood urea nitrogen and creatinine levels; an electrocardiogram; and magnetic resonance imaging (MRI) and radiographs of the affected hip. The diagnosis of ONFH was based on the history, thorough clinical evaluation, and imaging modalities including plain anteroposterior and frog-leg lateral radiographs as well as MRI.
Surgical technique
The patient was placed in supine position. Harvest of fibula graft and preparation of the hip were performed successively by the same team of surgeons.
Harvest of fibular graft A straight lateral 12-cm longitudinal incision was made along the fibula. A segment of the fibula (ranging from 6.5–7.5 cm) was exposed and harvested using a wire saw. Once the fibula was cut, two towel clamps were placed both proximally and distally to allow better control and easier rotation. Dissection was performed with a clockwise motion of the left fibula or a counterclockwise motion of the right fibula, with the surgeon standing at the patient’s foot looking down the long axis of the leg. First, the anterolateral muscles were detached from the periosteum, and the interosseous membrane was carefully separated from the fibula attachment. Then, after external rotation of harvested fibula, the deep posterior muscles were detached from the periosteum. The peroneal vessels, posterior tibial vessels and nerve were then exposed. The distal pedicle of the peroneal vessels was identified and ligated. Starting distally, the fibula and adjoining peroneal vessels were dissected and elevated until tethered only by the proximal vascular pedicle. Finally, the proximal vascular pedicle was harvested, as long as could be obtained.
Operative procedure on the hip The operative technique on the hip has been previously described and was originally designed by the senior author (CQ Zhang) [23]. Briefly, an anterior approach of the hip was used. Ascending branches of the lateral femoral circumflex vessels were identified and dissected to serve as recipient vessels. Then the anterior hip capsule was incised longitudinally and a tunnel was made in the anterior aspect of the femoral neck by bone chisel. Cancellous bone taken from the femoral neck was saved for later grafting. With the help of fluoroscopic control, sequential reaming was performed. Necrotic bone was removed as much as possible. Then the graft materials were packed into the femoral head cavity with the aid of a specially-designed impacter, which included ACB taken from the femoral neck combined with OsteoSet®2 DBM pellets (5 cc, 4.8-mm × 3.5-mm-thick per pellet, Wright Medical Technology, Arlington, TN) or a nearly identical average volume of ACB (5 cc) taken from the greater trochanteric region. The grafted fibula was then positioned beneath the subchondral bone of the femoral head. Arterial and venous anastomoses were performed with interrupted 6-0 nylon sutures under the loupe microscope.
Postoperative care
Intravenous antibiotics were used twice a day for three days. Prophylactic anticoagulation included intravenous administration of 500 ml of dextran once a day for five days and oral administration of 300 mg of aspirin twice a day for two weeks, followed by 300 mg a day for six weeks after the operation. The postoperative rehabilitation program was identical for the two groups. Patients were instructed to take postoperative bed rest. Patients were also told to avoid bearing weight on the extremity for three months. During the next three months, the amount of weight bearing was gradually increased to full weight bearing. Each patient was scheduled for follow-up visits at three-, six-, 12-, and 24-month time points following surgery, and on a yearly basis after the 24-month visit. At each visit, a clinical and radiographic assessment was performed.
Clinical outcome
Modified Harris hip scores were used to grade the clinical results. The clinical assessments were evaluated using four classes: excellent, no hip pain and a hip rating more than 90 points; good, mild discomfort or restriction of hip motion and a hip rating of 80 to 89 points; fair, mild or moderate hip pain with a hip rating of 70–79 points and any reduction of hip score after operation; and poor, severe hip pain or grossly limited motion and a hip rating less than 69 points.
Radiographic assessment
Radiographic progression was evaluated regarding the development of anecrotic lesion, a change in the contour of the femoral head, or diminished thickness of the articular cartilage. To circumvent the problem of intra-observer and interobserver variability in assessing radiographic parameters, evaluation of the radiographs was determined by one orthopaedic surgeon and one radiologist independently, blinded to the assigned treatment group of the patients.
Adverse events and complications
Adverse events and complications included all minor and major medical events for which the patient sought medical attention regardless of the nature of the event or its severity. The safety of OsteoSet®2 DBM was evaluated by comparing the nature and frequency of adverse events and complications in the two treatment groups.
Statistical analysis
The Student t test was used to compare the pretreatment and posttreatment values in each group, and the Mann-Whitney U test was used to compare the DBM group and the ACB group. Significance was set at p < 0.05. The end point was conversion to a total hip replacement.
Results
Clinical outcome
The Harris hip scores of FVFG with the DBM and ACB groups are summarised in Table 2. Before treatment, the groups had comparable hip scores (p > 0.05). At the time of the final follow-up, at a mean of 26 months, significant improvement in the mean Harris hip score was noted in both groups (p < 0.001), but there was no significant difference in the mean Harris hip score between the two groups (p > 0.05). With the numbers available (Table 2), there were no significant differences in clinical results according to the aetiology of the osteonecrosis in either group (p > 0.05).
Table 2.
Harris hip score in DBM and ACB groups before and after operation
| Variables | Mean Harris hip score (points) | |||
|---|---|---|---|---|
| Preoperative | Final follow-up | |||
| DBM group | ACB group | DBM group | ACB group | |
| Steinberg stage (n = hips) | ||||
| Stage IIC (n = 6) | 79 (64–90) | 79 (60–89) | 89 (78–100) | 92 (80–99) |
| Stage IIIC (n = 14) | 70 (63–88) | 70 (59–85) | 80 (69–95) | 84 (67–96) |
| Stage IVC (n = 10) | 58 (45–82) | 58 (43–84) | 71 (54–89) | 74 (57–92 ) |
| Medical history (n = hips) | ||||
| Corticosteroids (n = 14) | 72 (46–90) | 73 (48–89) | 84 (64–100) | 85 (65–99) |
| Alcoholism (n = 7) | 68 (45–87) | 70 (45–85) | 74 (54–92) | 78 (58–90) |
| Idiopathic (n = 9) | 70 (54–86) | 67 (43–84) | 76 (60–87) | 80 (57–87) |
| Total (n = 30) | 70 (45–90) | 70 (43–89) | 77 (54–100) | 82 (57–99) |
DBM demineralised bone matrix, ACB autologous cancellous bone
The clinical outcomes in FVFG with the DBM and ACB groups are summarised in Table 3. In the DBM group, the overall result was improved in 21 of 30 hips (70%), whereby eight hips (27%) were rated excellent, 13 (43%) were rated good, five (17%) were rated fair, and four (13%) were rated poor. In the ACB group, the overall result was improved in 23 of the 30 hips (77%), whereby 11 hips (37%) were rated excellent, 12 (40%) were rated good, two (6%) were rated fair, and five (17%) were rated poor. There was no statistically significant difference in the clinical outcomes between two groups (p > 0.05).
Table 3.
Overall clinical outcomes in DBM and ACB groups
| Outcome | Total series | Steinberg stage IIC | Steinberg stage IIIC | Steinberg stage IVC | ||||
|---|---|---|---|---|---|---|---|---|
| DBM group | ACB group | DBM group | ACB group | DBM group | ACB group | DBM group | ACB group | |
| Excellent | 8 | 11 | 3 | 4 | 5 | 6 | 0 | 1 |
| Good | 13 | 12 | 2 | 2 | 7 | 6 | 4 | 4 |
| Fair | 5 | 2 | 1 | 0 | 1 | 1 | 3 | 1 |
| Poor | 4 | 5 | 0 | 0 | 1 | 1 | 3 | 4 |
| Total hips | 30 | 30 | 6 | 6 | 14 | 14 | 10 | 10 |
DBM demineralised bone matrix, ACB autologous cancellous bone
In the ACB group, one hip that had initially been Steinberg stage IVC was converted to a total hip replacement 24 months postoperatively. In the DBM group, no total hip replacement was performed in any of the patients in this study at the latest follow-up.
Radiographic assessment
The radiographic stages of the lesions before and after treatment are summarised in Table 4. In the DBM and ACB groups, 13 of 30 hips (43%) and 12 of 30 hips (40%) were radiological progression or failure, respectively. Of the six stage IIC hips, two in the DBM group versus one in the ACB group were radiological progression or failure. Of the 14 stage IIIC hips, this was the case for four in the DBM group versus five in the ACB group. The remaining stage IIIC hips had apparent arrest of the disease and preservation of the contour of the femoral head (Fig. 1). Of the ten stage IVC hips, seven in the DBM group versus six in the ACB group were radiological progression or failure. But there was no significant difference in the rate of radiographic progression according to the stage between the two groups (p > 0.05). With the numbers available (Table 4), there were no significant differences in the rates of radiographic progression according to the aetiology in either group (p > 0.05).
Table 4.
Radiographic data in DBM and ACB groups before and after operation
| Variables | Radiographic progression | |
|---|---|---|
| DBM group | ACB group | |
| Steinberg stage (n = hips) | ||
| Stage IIC (n = 6) | 2 | 1 |
| Stage IIIC (n = 14) | 4 | 5 |
| Stage IVC (n = 10) | 7 | 6 |
| Medical history (n = hips) | ||
| Corticosteroids (n = 14) | 6 | 5 |
| Alcoholism (n = 7) | 3 | 4 |
| Idiopathic (n = 9) | 4 | 3 |
| Total (n = 30) | 13 | 12 |
DBM demineralised bone matrix, ACB autologous cancellous bone
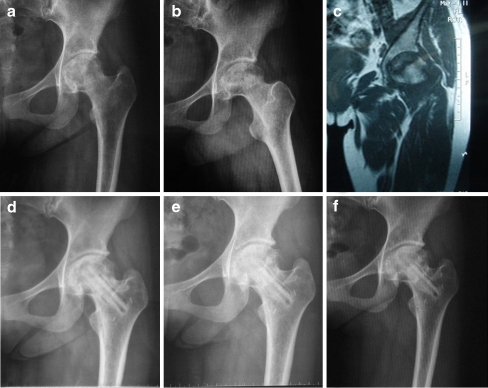
Fig. 1.
a Preoperative anteroposterior radiograph of the left hip in a symptomatic 27-year-old woman with systemic lupus erythematosus (SLE) shows Steinberg stage IIIC lesion in the demineralised bone matrix (DBM) group. b Preoperative frog-leg lateral radiograph of the left hip. c Preoperative MRI of the left hip. d One-year follow-up anteroposterior radiograph of the same hip after free vascularised fibular grafting (FVFG) with OsteoSet®2 DBM. e Two-year follow-up anteroposterior radiograph. f Three-year follow-up anteroposterior radiograph shows stability of the articular cartilage and femoral head
Adverse events and complications
No adverse events or medical complications directly attributed to the use of OsteoSet®2 DBM were noted. The adverse events experienced by the patients enrolled in this study were typical of the operation-related complications. In the DBM group, complications included transient weakness in muscles supplied by the peroneal nerve in one extremity and discomfort in the ankle with activity in one patient. In the ACB group, persistent sensory abnormality in one foot occurred. None of these events affected the results during follow-up. No cases of deep or superficial infections, perforations of the articular cartilage of the femoral head, or graft migration were observed in any of the patients in this study. Contracture of the flexor hallucis longus, proximal femoral fracture, or deep embolism did not take place in either group.
Discussion
This study focussed on the safety and efficacy of OsteoSet®2 DBM used as a graft extender in FVFG for ONFH. We performed a prospective case-controlled study of similar groups of patients. The patient populations were matched as closely as possible on the basis of characteristics such as the stage of the disease, extent of involvement, aetiological factors, and preoperative Harris hip score to allow equal comparison. These parameters have been reported to be important variables in the treatment of ONFH [1, 6, 7, 12, 16, 21, 23]. We used two instruments to assess the outcome in both groups: the Harris hip score and radiographic signs of progression or collapse. Our results showed that there were no statistically significant differences in the Harris hip score, the overall clinical outcomes and radiographic signs of progression or collapse in the mean 26-month follow-up, thus suggesting that OsteoSet®2 DBM provides an effective and reliable alternative to autogenous grafting for ONFH.
DBM is a derivative of allograft bone and the only clinically available material possessing putative biological or osteoinductive activity. It is prepared by pulverisation of allogenic bone to a consistent size, followed by mild acid extraction of the mineralised phase of bone [8, 10]. This process, developed by Urist et al. in 1965 [17], results in a composite of noncollagenous proteins, growth factors, and collagen [18]. DBM is produced as a dry powder; so DBM is often combined with other components intended to make DBM easier to handle by turning it into a putty or paste. These carriers include glycerol, poloxamer, gelatin, calcium sulphate, lecithin, and hyaluronic acid. The amount of osteoinductive ability may rely on its preparation and the type of carrier with which it is combined [3, 13]. As a graft expander, DBM combined with autologous autograft or bone marrow may amplify the volume of bone graft material and aid in the stimulation of bone formation. Cammisa et al. [2] conducted a multicentre prospective study that compared the effectiveness of a Grafton DBM gel composite with an iliac crest autograft in posterolateral spinal fusion. Their findings suggest that DBM gel combined with a small amount of autologous bone can provide a successful fusion with the same frequency as autograft alone. Specifically, fusion rates were the same as autograft when one third the normal amount of autograft was combined with DBM. Vaccaro et al. [19], in a prospective study, also reported that the DBM composite consisting of DBM putty and aspirated bone marrow offer a similar performance as the autograft in posterolateral spinal fusion.
Few studies have addressed the clinical use of DBM in ONFH. Mont et al. [11] reported a series of 19 patients (21 hips) treated with bone allograft with OpteForm (a combination of DBM and a thermoplastic carrier) to avoid donor site morbidity. Eighteen of 21 hips (86%) were clinically successful at a mean four-year follow-up. Wang et al. [20] used a trapdoor to make a window at the head-neck junction to remove necrotic bone and packed the excavated area with an auto-iliac bone (cortical bone and cancellous bone) combination of DBM by gradually compacting. At an average follow-up of two years, overall clinical success rates were 68%, composed of 93.33% in stage IIB and 59.62% in stages IIC and IIIA. Radiological improvement was noted in 100% of patients in stage IIA, 76.67% of patients in stage IIB and 50.96% of patients in stage IIC and IIIA cases. Although clinical results have shown considerable success with the use of DBM, the major limitation of these two studies is lack of a control group, i.e., a group of patients that received only autograft bone.
The material used for grafting in this case was OsteoSet®2 DBM pellets, which are a composite of DBM and OsteoSet®2, medical grade calcium sulphate. A cross-sectional view of an OsteoSet®2 DBM pellet shows DBM particles homogenously dispersed throughout surgical-grade calcium sulphate. We used this material because it has osteoinductive and osteoconductive properties. OsteoSet®2 DBM pellets offer a biological framework into which a patient’s own bone can grow. The pellets are resorbed at a rate consistent with the new bone growth (an average of four to eight weeks). From the results of this study, we concluded that OsteoSet®2 DBM might be used as an ideal bone graft substitute when combined with autologous bone graft for the treatment of ONFH. The clinical benefits of the use of OsteoSet®2 DBM instead of bone graft are evident since similar healing rates and clinical outcomes were found in our two treatment groups. Use of autologous bone graft requires an additional surgical site and increased surgical time, and it is associated with multiple complications that are not seen with the use of DBM. Although this study appears promising, additional follow-up of these patients and further study with larger numbers of patients are required to further evaluate positive and negative predictors of outcome.
Conclusion
This prospective, case-controlled study showed that OsteoSet®2 DBM combined with local autograft bone is as effective as autologous bone for the purposes of grafting in FVFG for ONFH. Thus, potential complications associated with the harvesting of autologous bone may be avoided. The authors therefore think that OsteoSet®2 DBM can be used as a bone graft extender in FVFG for ONFH.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Aldridge JM, 3rd, Urbaniak JR. Avascular necrosis of the femoral head: role of vascularized bone grafts. Orthop Clin North Am. 2007;38:13–22. doi: 10.1016/j.ocl.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Cammisa FP, Jr, Lowery G, Garfin SR, et al. Two-year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side-by-side comparison in the same patient. Spine. 2004;29:660–666. doi: 10.1097/01.BRS.0000116588.17129.B9. [DOI] [PubMed] [Google Scholar]
- 3.Cheung S, Westerheide K, Ziran B. Efficacy of contained metaphyseal and periarticular defects treated with two different demineralized bone matrix allografts. Int Orthop. 2003;27:56–59. doi: 10.1007/s00264-002-0388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goulet JA, Senunas LE, DeSilva GL, et al. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997;339:76–81. doi: 10.1097/00003086-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Hierholzer C, Sama D, Toro JB, et al. Plate fixation of ununited humeral shaft fractures: effect of type of bone graft on healing. J Bone Jt Surg Am. 2006;88:1442–1447. doi: 10.2106/JBJS.E.00332. [DOI] [PubMed] [Google Scholar]
- 6.Judet H, Gilbert A. Long-term results of free vascularized fibular grafting for femoral head necrosis. Clin Orthop Relat Res. 2001;386:114–119. doi: 10.1097/00003086-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Korompilias AV, Lykissas MG, Beris AE, et al. Vascularised fibular graft in the management of femoral head osteonecrosis: twenty years later. J Bone Jt Surg Br. 2009;91:287–293. doi: 10.1302/0301-620X.91B3.21846. [DOI] [PubMed] [Google Scholar]
- 8.Lee KJ, Roper JG, Wang JC. Demineralized bone matrix and spinal arthrodesis. Spine J. 2005;5:S217–S223. doi: 10.1016/j.spinee.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Lye KW, Deatherage JR, Waite PD. The use of demineralized bone matrix for grafting during Le Fort I and chin osteotomies: techniques and complications. J Oral Maxillofac Surg. 2008;66:1580–1585. doi: 10.1016/j.joms.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki M, Tsumura H, Wang JC, et al. An update on bone substitutes for spinal fusion. Eur Spine J. 2009;18:783–799. doi: 10.1007/s00586-009-0924-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mont MA, Etienne G, Ragland PS. Outcome of nonvascularized bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2003;417:84–92. doi: 10.1097/01.blo.0000096826.67494.38. [DOI] [PubMed] [Google Scholar]
- 12.Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Jt Surg Am. 2006;88:1117–1132. doi: 10.2106/JBJS.E.01041. [DOI] [PubMed] [Google Scholar]
- 13.Russell JL, Block JE. Clinical utility of demineralized bone matrix for osseous defects, arthrodesis, and reconstruction: impact of processing techniques and study methodology. Orthopedics. 2004;22:524–531. [PubMed] [Google Scholar]
- 14.Schizas C, Triantafyllopoulos D, Kosmopoulos V, et al. Posterolateral lumbar spine fusion using a novel demineralized bone matrix: a controlled case pilot study. Arch Orthop Trauma Surg. 2008;128:621–625. doi: 10.1007/s00402-007-0495-4. [DOI] [PubMed] [Google Scholar]
- 15.Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury. 2007;38:S75–S80. doi: 10.1016/j.injury.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Urbaniak JR, Coogan PG, Gunneson EB, et al. Treatment of osteonecrosis of the femoral head with free vascularized fibular grafting. A long term follow-up study of one hundred and three hips. J Bone Jt Surg Am. 1995;77:681–694. doi: 10.2106/00004623-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 18.Urist MR, Huo YK, Brownell AG, et al. Purification of bovine bone morphogenetic protein by hydroxyapatite chromatography. Proc Natl Acad Sci USA. 1984;81:371–375. doi: 10.1073/pnas.81.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaccaro AR, Stubbs HA, Block JE. Demineralized bone matrix composite grafting for posterolateral spinal fusion. Orthopedics. 2007;30:567–570. doi: 10.3928/01477447-20070701-06. [DOI] [PubMed] [Google Scholar]
- 20.Wang BL, Sun W, Shi ZC, et al. Treatment of nontraumatic osteonecrosis of the femoral head using bone impaction grafting through a femoral neck window. Int Orthop. 2009 doi: 10.1007/s00264-009-0822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo MC, Kim KI, Hahn CS. Long-term follow up of vascularized fibular grafting for femoral head necrosis. Clin Orthop Relat Res. 2009;466:1133–1140. doi: 10.1007/s11999-008-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Zhang CQ, Zeng BF, Xu ZY, et al. Treatment of femoral head necrosis with free vascularized fibula grafting: a preliminary report. Microsurgery. 2005;25:305–309. doi: 10.1002/micr.20118. [DOI] [PubMed] [Google Scholar]