Abstract
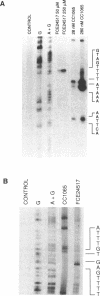
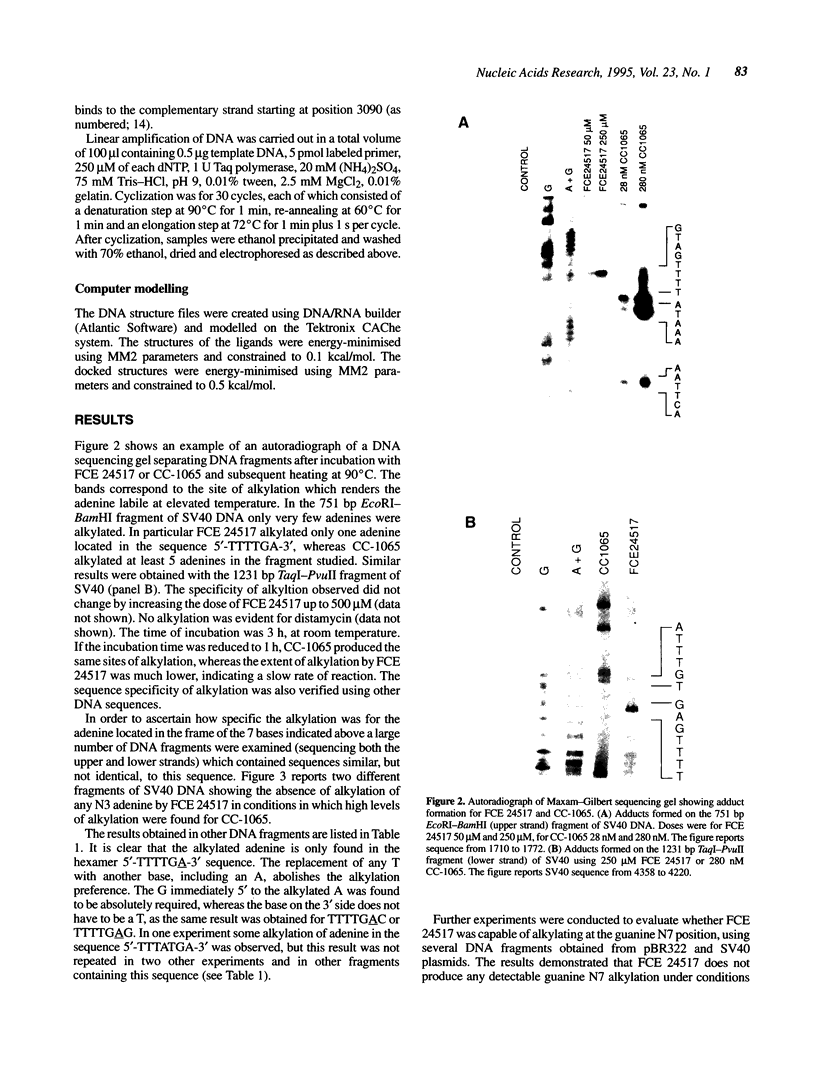
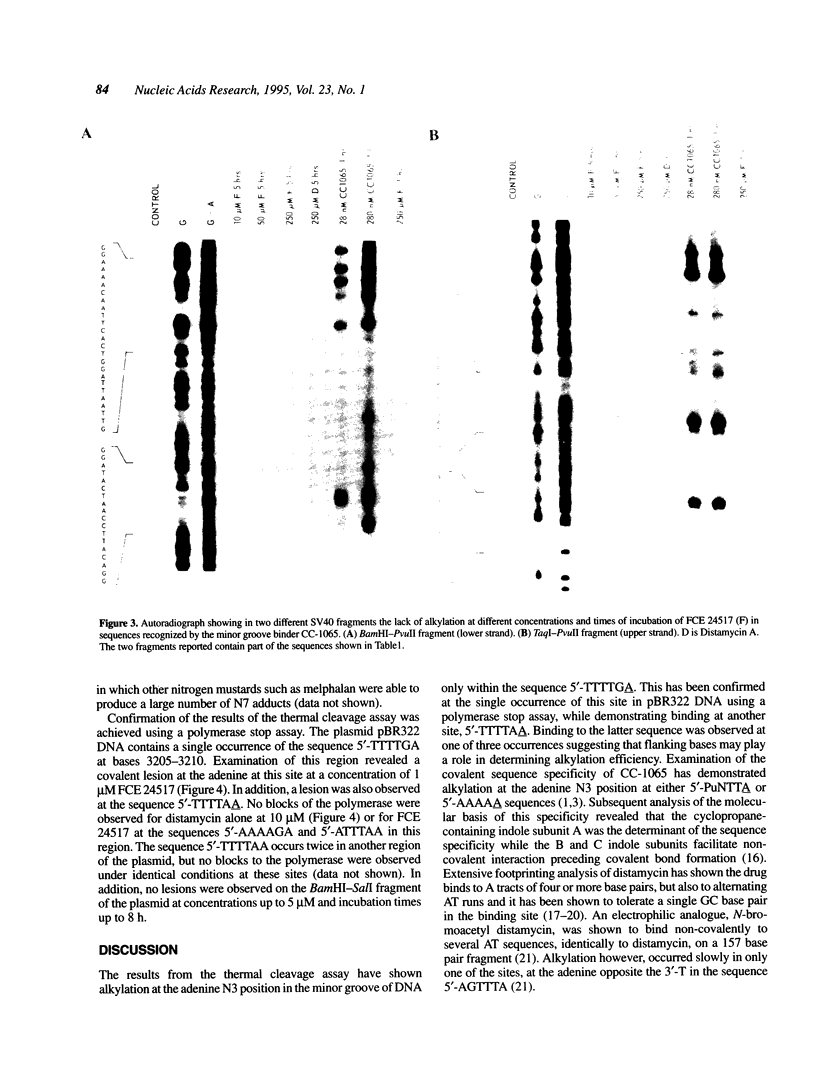
FCE 24517, a novel distamycin derivative possessing potent antitumor activity, is under initial clinical investigation in Europe. In spite of the presence of a benzoyl nitrogen mustard group this compound fails to alkylate the N7 position of guanine, the major site of alkylation by conventional nitrogen mustards. Characterisation of DNA-drug adducts revealed only a very low level of adenine adduct formation. Using a modified Maxam-Gilbert sequencing method the consensus sequence for FCE 24517-adenine adduct formation was found to be 5'-TTTTGA-3'. A single base modification in the hexamer completely abolishes the alkylation of adenine. Using a Taq polymerase stop assay alkylations were confirmed at the A present in the hexamer TTTTGA and, in addition, in one out of three TTTTAA sequences present in the plasmid utilized. The sequence specificity of alkylation by FCE 24517 is therefore the most striking yet observed for an alkylating agent of small molecular weight.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcamone F. M., Animati F., Barbieri B., Configliacchi E., D'Alessio R., Geroni C., Giuliani F. C., Lazzari E., Menozzi M., Mongelli N. Synthesis, DNA-binding properties, and antitumor activity of novel distamycin derivatives. J Med Chem. 1989 Apr;32(4):774–778. doi: 10.1021/jm00124a008. [DOI] [PubMed] [Google Scholar]
- Bhuyan B. K., Newell K. A., Crampton S. L., Von Hoff D. D. CC-1065 (NSC 298223), a most potent antitumor agent: kinetics of inhibition of growth, DNA synthesis, and cell survival. Cancer Res. 1982 Sep;42(9):3532–3537. [PubMed] [Google Scholar]
- Broggini M., Erba E., Ponti M., Ballinari D., Geroni C., Spreafico F., D'Incalci M. Selective DNA interaction of the novel distamycin derivative FCE 24517. Cancer Res. 1991 Jan 1;51(1):199–204. [PubMed] [Google Scholar]
- Calladine C. R. Mechanics of sequence-dependent stacking of bases in B-DNA. J Mol Biol. 1982 Oct 25;161(2):343–352. doi: 10.1016/0022-2836(82)90157-7. [DOI] [PubMed] [Google Scholar]
- Coll M., Frederick C. A., Wang A. H., Rich A. A bifurcated hydrogen-bonded conformation in the d(A.T) base pairs of the DNA dodecamer d(CGCAAATTTGCG) and its complex with distamycin. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8385–8389. doi: 10.1073/pnas.84.23.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Incalci M., Torti L., Damia G., Erba E., Morasca L., Garattini S. Ovarian reticular cell sarcoma of the mouse (M5076) made resistant to cyclophosphamide. Cancer Res. 1983 Dec;43(12 Pt 1):5674–5680. [PubMed] [Google Scholar]
- Dickerson R. E. Base sequence and helix structure variation in B and A DNA. J Mol Biol. 1983 May 25;166(3):419–441. doi: 10.1016/s0022-2836(83)80093-x. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Goodsell D. S., Neidle S. "...the tyranny of the lattice...". Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3579–3583. doi: 10.1073/pnas.91.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. R., Waring M. J. DNA structural variations produced by actinomycin and distamycin as revealed by DNAase I footprinting. Nucleic Acids Res. 1984 Dec 21;12(24):9271–9285. doi: 10.1093/nar/12.24.9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman K. D., Dervan P. B. Molecular recognition of B-DNA by Hoechst 33258. Nucleic Acids Res. 1985 Jul 11;13(13):4825–4835. doi: 10.1093/nar/13.13.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley L. H., Boyd F. L. DNA as a target for drug action. Trends Pharmacol Sci. 1988 Nov;9(11):402–407. doi: 10.1016/0165-6147(88)90067-3. [DOI] [PubMed] [Google Scholar]
- Hurley L. H., Reynolds V. L., Swenson D. H., Petzold G. L., Scahill T. A. Reaction of the antitumor antibiotic CC-1065 with DNA: structure of a DNA adduct with DNA sequence specificity. Science. 1984 Nov 16;226(4676):843–844. doi: 10.1126/science.6494915. [DOI] [PubMed] [Google Scholar]
- Katahira M., Sugeta H., Kyogoku Y. A new model for the bending of DNAs containing the oligo(dA) tracts based on NMR observations. Nucleic Acids Res. 1990 Feb 11;18(3):613–618. doi: 10.1093/nar/18.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopka M. L., Yoon C., Goodsell D., Pjura P., Dickerson R. E. The molecular origin of DNA-drug specificity in netropsin and distamycin. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1376–1380. doi: 10.1073/pnas.82.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishan A., Paika K., Frei E., 3rd Cytofluorometric studies on the action of podophyllotoxin and epipodophyllotoxins (VM-26, VP-16-213) on the cell cycle traverse of human lymphoblasts. J Cell Biol. 1975 Sep;66(3):521–530. doi: 10.1083/jcb.66.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. S., Gibson N. W. Nucleotide preferences for DNA interstrand cross-linking induced by the cyclopropylpyrroloindole analogue U-77,779. Biochemistry. 1993 Mar 16;32(10):2592–2600. doi: 10.1021/bi00061a017. [DOI] [PubMed] [Google Scholar]
- Li L. H., Kelly R. C., Warpehoski M. A., McGovren J. P., Gebhard I., DeKoning T. F. Adozelesin, a selected lead among cyclopropylpyrroloindole analogs of the DNA-binding antibiotic, CC-1065. Invest New Drugs. 1991 May;9(2):137–148. doi: 10.1007/BF00175081. [DOI] [PubMed] [Google Scholar]
- Li L. H., Swenson D. H., Schpok S. L., Kuentzel S. L., Dayton B. D., Krueger W. C. CC-1065 (NSC 298223), a novel antitumor agent that interacts strongly with double-stranded DNA. Cancer Res. 1982 Mar;42(3):999–1004. [PubMed] [Google Scholar]
- Mahon F. X., Belloc F., Reiffers J. Antisense oligomers in chronic myeloid leukaemia. Lancet. 1993 Feb 27;341(8844):566–566. doi: 10.1016/0140-6736(93)90336-f. [DOI] [PubMed] [Google Scholar]
- Neidle S. Minor-groove width and accessibility in B-DNA drug and protein complexes. FEBS Lett. 1992 Feb 17;298(1):97–99. doi: 10.1016/0014-5793(92)80030-k. [DOI] [PubMed] [Google Scholar]
- Nelson H. C., Finch J. T., Luisi B. F., Klug A. The structure of an oligo(dA).oligo(dT) tract and its biological implications. Nature. 1987 Nov 19;330(6145):221–226. doi: 10.1038/330221a0. [DOI] [PubMed] [Google Scholar]
- Pezzoni G., Grandi M., Biasoli G., Capolongo L., Ballinari D., Giuliani F. C., Barbieri B., Pastori A., Pesenti E., Mongelli N. Biological profile of FCE 24517, a novel benzoyl mustard analogue of distamycin A. Br J Cancer. 1991 Dec;64(6):1047–1050. doi: 10.1038/bjc.1991.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti M., Forrow S. M., Souhami R. L., D'Incalci M., Hartley J. A. Measurement of the sequence specificity of covalent DNA modification by antineoplastic agents using Taq DNA polymerase. Nucleic Acids Res. 1991 Jun 11;19(11):2929–2933. doi: 10.1093/nar/19.11.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal J., Waring M. J. Hydroxyl radical footprinting of the sequence-selective binding of netropsin and distamycin to DNA. FEBS Lett. 1987 Dec 10;225(1-2):195–200. doi: 10.1016/0014-5793(87)81156-0. [DOI] [PubMed] [Google Scholar]
- Reynolds V. L., Molineux I. J., Kaplan D. J., Swenson D. H., Hurley L. H. Reaction of the antitumor antibiotic CC-1065 with DNA. Location of the site of thermally induced strand breakage and analysis of DNA sequence specificity. Biochemistry. 1985 Oct 22;24(22):6228–6237. doi: 10.1021/bi00343a029. [DOI] [PubMed] [Google Scholar]
- Searle M. S., Embrey K. J. Sequence-specific interaction of Hoechst 33258 with the minor groove of an adenine-tract DNA duplex studied in solution by 1H NMR spectroscopy. Nucleic Acids Res. 1990 Jul 11;18(13):3753–3762. doi: 10.1093/nar/18.13.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczylik C., Skorski T., Nicolaides N. C., Manzella L., Malaguarnera L., Venturelli D., Gewirtz A. M., Calabretta B. Selective inhibition of leukemia cell proliferation by BCR-ABL antisense oligodeoxynucleotides. Science. 1991 Aug 2;253(5019):562–565. doi: 10.1126/science.1857987. [DOI] [PubMed] [Google Scholar]
- Tobey R. A. Different drugs arrest cells at a number of distinct stages in G2. Nature. 1975 Mar 20;254(5497):245–247. doi: 10.1038/254245a0. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. W., Hertzberg R. P., Dervan P. B. Map of distamycin, netropsin, and actinomycin binding sites on heterogeneous DNA: DNA cleavage-inhibition patterns with methidiumpropyl-EDTA.Fe(II). Proc Natl Acad Sci U S A. 1982 Sep;79(18):5470–5474. doi: 10.1073/pnas.79.18.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland K. L., Dooley T. P. In vitro and in vivo DNA bonding by the CC-1065 analogue U-73975. Biochemistry. 1991 Jul 30;30(30):7559–7565. doi: 10.1021/bi00244a027. [DOI] [PubMed] [Google Scholar]
- Yoon C., Privé G. G., Goodsell D. S., Dickerson R. E. Structure of an alternating-B DNA helix and its relationship to A-tract DNA. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6332–6336. doi: 10.1073/pnas.85.17.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]