Abstract
Trauma-associated acute compartment syndrome (ACS) of the extremities is a well-known complication in adults. There are only a handful of articles that describe the symptoms, the diagnostic procedure and treatment of ACS in children. The aim of this study was to analyse the diagnostic procedures in children compared to adolescents with ACS to obtain evidence for the diagnosis, treatment and outcome of children with ACS. Twenty-four children and adolescents with ACS have been treated at the Department of Trauma Surgery of the Medical University of Vienna, Austria. Two age-related groups were investigated to compare the diagnostic and therapeutic algorithm: group A comprising children aged 2–14 years (n = 12) and group B comprising adolescents aged 15–18 years (n = 12). Patient characteristics, diagnosis and therapy-associated data, complications and clinical outcome were analysed. In both groups we found fractures in most of our patients (n = 19) followed by contusion of the soft tissues (n = 3). In group A most of our patients were injured as pedestrians in car accidents (n = 5) followed by low-energy blunt trauma (n = 3). The most common region of injury and traumatic ACS was the lower leg (n = 7) followed by the feet (n = 3). For fracture stabilisation most of the patients (n = 6) received an external fixator. The mean time from admission to the fasciotomy was 27.9 hours. In four patients a compartment pressure measurement was performed with pressure levels from 30 to 75 mmHg. A histological examination of soft tissue was performed in five patients. From fasciotomy to definitive wound closure 2.4 operations were necessary. The mean hospital stay was 18.9 days. In group B most of our patients had a motorcycle accident (n = 5). The most common region for traumatic ACS in this group was also the lower leg (n = 9). In most of the patients (n = 6) intramedullary nails could be implanted. The mean time from admission to the fasciotomy was 27.1 hours. In six patients a compartment pressure measurement was performed with pressures from 25 to 90 mmHg. In five patients a histological examination was performed. From fasciotomy to definitive wound closure 2.3 operations were necessary. The mean hospital stay was 18.4 days. Secondary fasciotomy closure was performed in all cases. A split-skin graft was only necessary in three patients (13%). We avoided primary closure in the same setting when the fasciotomy was performed. Thus, we found no difference between the two groups in the diagnostic procedures, the indication for fasciotomy, the number of operations needed from fasciotomy to definitive wound closure, time of hospitalisation and clinical outcome. The rate of permanent complications was 4.2% (one patient from group A), which means that nearly all patients experienced full recovery after fasciotomy. ACS represents a surgical emergency and the indication should be determined early even in doubtful cases to avoid complications.
Introduction
Acute compartment syndrome (ACS) has been recognised since 1881 when Volkmann first described the contracture of the hand caused by compartment syndrome [1]. The first reported treatment of acute limb compartment syndrome was by Petersen in 1888 [2]. The diagnosis and management of ACS in the adult population is well known, but only a few authors have reported on the paediatric population [3]. The changing life situation with other injury mechanisms and the changed anatomical situation of adults and adolescents on the one hand (more high-energy trauma, closed epiphyses) and of children on the other hand allow alternative forms of fracture stabilisation (intramedullary nailing, external fixator) with different risk levels of developing an ACS.
ACS is a diagnostic dilemma in small children, who may not have the cognitive and verbal ability to provide clinical information particularly in an extraordinary situation, resulting in delays in diagnosis and adequate therapy. However, early diagnosis is pivotal for the outcome of ACS and is determined by the duration of ischaemia and the pressure in the osseofascial compartment. Immediate and adequate treatment can lead to healing with good functional and cosmetic results. If operative treatment is delayed or inadequate, excessive tissue necrosis may lead to severe local and systemic complications, such as reversible or irreversible muscle and neurovascular damage with functional loss of the extremity or myoglobinaemia with subsequent acute renal failure.
The aim of the study was to analyse the diagnostic procedure for compartment syndrome in children from arrival at the hospital until discharge to obtain better evidence for the diagnosis, treatment and outcome of children with ACS.
Patients and methods
Nearly 144,000 children and adolescents between 0 and 18 years of age were treated between January 1992 and January 2010 at our Level I Trauma Centre. Twenty-four patients (0.02%) who developed ACS in any anatomical region were included in this retrospective study (21 male and three female subjects). The mean age was 12.9 years (range: 2–18 years). Patients included were classified into two groups according to their age: group A (age 2–14) and group B (age 15–18). The group cut-off at the age of 14 was chosen because closure of the epiphyses happens in most humans between the ages of 12–16 years.
Patients’ records were analysed for the demographic data (sex, age, admission periods and cause of the compartment syndrome), information about diagnostic procedures, interventions and other therapeutic procedures (tissue pressure measurements, time from injury to fasciotomy, wound management, number of operations until definitive closure of fasciotomy), complications and clinical outcome. Intracompartmental pressure (ICP) measurement was performed invasively with the Stryker® S.T.I.C. Pressure Monitor System (Stryker Corporation, Kalamazoo, MI, USA). The average follow-up was 11.1 months (range: 1–43 months).
Results
Overall, 24 patients (male n = 21, female n = 3) were included in the study and divided into two age-related groups: group A comprising children aged 2–14 years (n = 12) and group B comprising adolescents aged 15–18 years (n = 12).
Demographics
Group A included (Table 1) 12 children aged 2–14 years (mean age: 9.8 years), three girls with a mean age of 5.3 years (range: 2–11 years) and nine boys with a mean age of 10.2 years (range: 5–14 years). Five patients were injured as pedestrians in car accidents, three patients suffered from blunt trauma (trapped after an earthquake between ruins, jammed between cars at the autodrome and in the baking oven door), two children fell from a height, one patient suffered a sports trauma (sled) and one child had a laceration with severe vascular damage (complete severance of the tibialis posterior artery). The most common region of injury and traumatic ACS was the lower leg (seven patients), followed by the feet (three patients), forearms and hands (two patients each) and the thigh (one patient). One patient developed ACS in four regions at the same time (in both forearms and lower legs).
Table 1.
Group A (patients aged 2–14 years, n = 12): clinical data at presentation after injury, diagnosis, treatment and complications
| Patient (age, years/sex) | Mechanism of injury | Diagnostics | Time from admission to the fasciotomy (h) | Type of fasciotomy | Operations to definitive wound closure (n) | Complications |
|---|---|---|---|---|---|---|
| 1 (3/F) | Pedestrian in car accident | Clinical | 62 | SIF | 3 | 0 |
| 2 (2/F) | Jammed in baking oven door | Clinical | 16.5 | SIF | 2 | 0 |
| 3 (11/F) | Fall from bike | Clinical | 15 | SIF | 4 | 0 |
| 4 (5/M) | Pedestrian in car accident | ICP 75 mmHg intraoperative | 2.5 | SIF | 1 | Delayed bone healing |
| 5 (8/M) | Sports trauma (sled) | ICP 44 mmHg intraoperative | 14.5 | SIF | 2 | 0 |
| 6 (8/M) | Fall from height | Clinical | 14 | SIF | 5 | Volkmann’s contracture |
| 7 (12/M) | Jammed in autodrome | Clinical | 3 | SIF | 2 | 0 |
| 8 (11/M) | Pedestrian in car accident | Clinical | 65 | SIF | 2 | 0 |
| 9 (10/M) | Pedestrian in car accident | Clinical | 46.5 | SIF | 2 | 0 |
| 10 (10/M) | Trapped between ruins | ICP 30 mmHg preoperative | 25 | SIF | 6 | Peripheral nerve damage |
| 11 (14/M) | Fall through glass door | ICP 50 mmHg intraoperative | 66 | SIF | 4 | 0 |
| 12 (14/M) | Pedestrian in car accident | Clinical | 4.5 | SIF | 2 | 0 |
SIF single-incision fasciotomy, DIF double-incision fasciotomy
Group B (Table 2) consisted of 12 boys aged 15–18 years (mean age: 16.8 years). Five patients had a motorcycle accident, two patients were run over by a car, two patients suffered a sports trauma, one patient fell from a height, one patient was injured in a car accident as a passenger and one was injured in an affray. The most common region for traumatic ACS was the lower leg in nine patients, the forearm in one patient and the thigh in another one. One polytrauma patient developed an abdominal compartment syndrome.
Table 2.
Group B (patients aged 15–18 years, n = 12): clinical data at presentation after injury, diagnosis, treatment and complications
| Patient (age, years/sex) | Mechanism of injury | Diagnostics | Time from admission to the fasciotomy (h) | Type of fasciotomy | Operations to definitive wound closure (n) | Complications |
|---|---|---|---|---|---|---|
| 1 (17/M) | Pedestrian in car accident | ICP 60 mmHg intraoperative | 10.5 | SIF | 2 | Delayed bone healing |
| 2 (16/M) | Sports trauma (handball) | Clinical | 3 | SIF | 2 | 0 |
| 3 (16/M) | Affray | ICP 40 mmHg preoperative | 19 | SIF | 1 | 0 |
| 4 (16/M) | Motorcycle | ICP 37 mmHg preoperative | 4 | SIF | 8 | 0 |
| 5 (15/M) | Fall from height | ICP 30 mmHg intraoperative | 9 | SIF | 1 | 0 |
| 6 (18/M) | Pedestrian in car accident | Clinical | 11 | SIF | 4 | 0 |
| 7 (17/M) | Motorcycle | Clinical | 64 | SIF | 3 | 0 |
| 8 (18/M) | Motorcycle | Clinical | 7 | SIF | 2 | 0 |
| 9 (16/M) | Car accident | ICP 90 mmHg preoperative | 11 | SIF | 1 | 0 |
| 10 (17/M) | Motorcycle | ICP 25 mmHg intraoperative | 99 | SIF | 2 | 0 |
| 11 (17/M) | Motorcycle | Clinical | 66 | SIF | 3 | 0 |
| 12 (18/M) | Sports trauma (soccer) | Clinical | 22 | DIF | 1 | Peripheral neurological deficits |
SIF single-incision fasciotomy, DIF double-incision fasciotomy
The most common cause of ACS in both groups was one or multiple fractures of long bones followed by contusion of the soft tissues.
Diagnostic procedures, treatment and clinical course
In group A the mean time from admission to the fasciotomy was 27.9 hours (range: 2.5–66 hours). For fracture stabilisation six patients received an external fixator and one K-wire fixation; four patients were treated conservatively. The number of operations from fasciotomy to definitive wound closure was 2.9 operations (range: 1–6). The mean hospital stay was 18.9 days (range: 6.5–28.5).
In group B the mean time from admission to the fasciotomy was 27.1 hours (range: 3–99 hours). To stabilise the fracture six patients received intramedullary nails, two patients an external fixator and in two patients plates were implanted, while conservative fracture treatment could be implemented in only two patients. The number of operations from fasciotomy to definitive wound closure was 2.4 operations (range: 1–8). The hospital stay was 18.4 days (mean) in group B (range: 10.9–35 days).
There was no significant difference between the groups regarding the diagnostic procedures, the indication for fasciotomy, the number of operation needed from fasciotomy to definitive wound closure, time of hospitalisation and clinical outcome.
To diagnose ACS, ICP measurement was performed preoperatively or intraoperatively in ten patients (four in group A and six in group B). In six patients (three patients in each group) the mean ICP was 60 mmHg (range: 40–90 mmHg) and in the other patients 30 mmHg (range: 25–37 mmHg) (Tables 1 and 2). Antibiotic prophylaxis was used in all patients. Histological examination of tissue from the injured anatomical region and the most suspicious location was performed in ten patients. In three patients necrotic tissue and in another three patients partially necrotic tissue could be confirmed. In four patients the tissue specimen was vital.
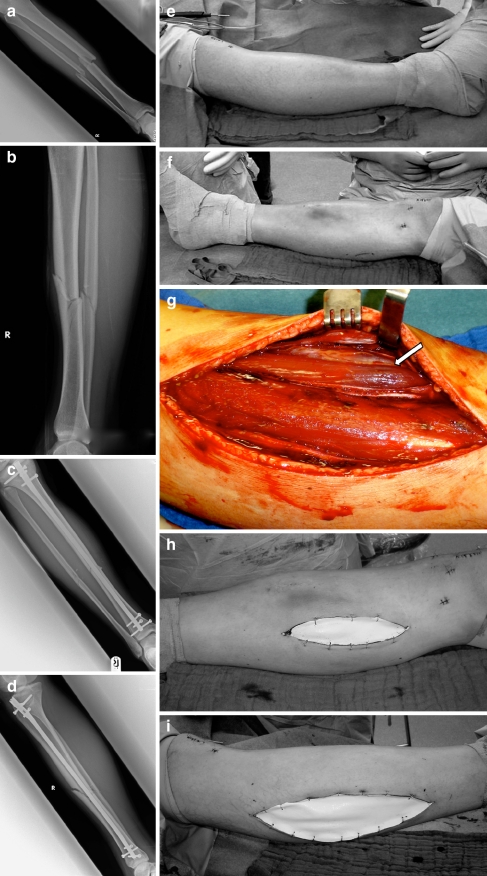
Surgical treatment, i.e. fasciotomy, needed to be performed in all of the patients. Fasciotomies were all performed under sterile conditions in the operating theatre under general anaesthesia (Fig. 1). Only in one patient was it necessary to perform a double-incision fasciotomy (lateral and medial incision) because of clinical evidence of residual tightness of the compartments after the lateral incision. In the other patients a single-incision fasciotomy (lateral incision) was adequate. Overall, 64 surgical interventions (median: 2.7, range: 1–8) were performed in all patients. Three patients (two boys, eight and ten years old, and an adolescent, 16 years old) had more than five surgical interventions (five to eight interventions, median: six). Secondary fasciotomy closure was performed in all cases. Skin wound management in 21 cases (group A: 11 patients, group B: 10 patients) involved temporary closure with Epigard® Synthetic Skin Substitute (The Clinipad Corporation, Norwich, CT, USA) (Fig. 1h, i) combined with Berman’s shoelace technique, requiring definitive closure at a later stage. A split-skin graft was only necessary in three of 24 patients (12.5%). Primary closure in the same setting with fasciotomy was avoided.
Fig. 1.
Case study: 18-year-old boy injured by playing soccer. ACS 16 h after intramedullary nailing. a, b Preoperative X-rays. c, d Postoperative X-rays. e, f Clinical picture before fasciotomy (16 h after fracture stabilisation). g Vital and partially non-vital muscle (arrow) in the fasciotomy wound. h, i Medial and lateral fasciotomy temporarily closed with Epigard®
Overall, the complication rate was 4.2%. Delayed bone healing was observed in two patients (one in each group) and two patients had a peripheral neurological deficit in the lower extremity (one patient in each group). All but one patient experienced full remission. An eight-year-old boy developed permanent contractures of the upper extremity after conservative treatment of epiphysiolysis of the distal radius. In this patient ACS was only diagnosed 12 hours after cast fixation, when the patient returned for the first follow up visit.
Discussion
The most common cause of ACS is trauma, usually after fractures [4]. Following trauma, bleeding or oedema in a closed osseofascial compartment causes an increased ICP with ischaemia [5]. Ischaemia results in tissue membrane damage and leakage of fluid through capillary and muscle membranes. With arterial reperfusion the damaged membranes continue to leak, and the rise in the hydrostatic force results in further increase of ICP thus developing a vicious cycle [6]. The capillary perfusion decreases below a level necessary for tissue viability [7] compromising the circulation and function of the tissues leading to muscle and nerve ischaemia with muscle infarction and nerve damage.
In the early stage of ACS Hoffmeyer et al. [8] found marked perifascicular and intrafascicular oedema with dissociation of the muscle fibres and necrosis in the tissue sections. In the late stages atrophy and hypertrophy of muscle fibres with lipid globules appear in the tissue examined. In our study only six of ten patients, in whom tissue sections were performed, showed necrotic or partially necrotic tissue in the histological examination.
Thus, early treatment by fasciotomy is pivotal. The mean time from admission to fasciotomy was 27.9 hours (range: 2.5–66 hours) in group A and 27.1 hours (range: 3–99 hours) in group B. This means a difference of 0.8 hours (2.9%) between groups A and B. The exact time of appearance of the first symptoms of the ACS could not be evaluated retrospectively.
Diagnosis on clinical grounds is not easy. Pain (severe and out of proportion to the apparent injury) [9], pallor, paraesthesia, paralysis and pulselessness (five Ps) [10] are the relatively unreliable cardinal symptoms of ACS. In small children pain is hard to evaluate. Pulselessness is noted only at a late stage [11]. Ulmer [12] examined the clinical signs and symptoms of compartment syndrome and reported that the false-positive rate was high in relation to the true-positive rate. Thus, clinical findings of ACS were more likely to be present in patients who do not have ACS than in those who do. This implies that other diagnostic devices should be used when in doubt to establish the diagnosis of ACS, although the clinical presentation is the most important indication for fasciotomy. The combination of a relevant trauma, tight extremities, excessive pain sensations, pain on passive stretching of the muscle, sensory loss and an increasing analgesia requirement should give rise to a high suspicion. A high level of serum creatine phosphokinase (CPK) may be another supportive indicator for diagnosing ACS.
Using the ICP for the diagnosis of ACS and a presumptive critical cut-off pressure are still controversial. The discussion regarding a critical pressure to diagnose compartment syndrome is ongoing. Mubarak et al. [13] used an ICP of more than 30 mmHg as the reference value for performing fasciotomy. Whitesides et al. [14] used the difference between the diastolic pressure and the ICP. Other authors evaluated the difference between the mean arterial pressure (MAP) and the ICP [15] to calculate the critical pressure for indicating fasciotomy. Schmidt [16], like Whitesides et al., describes the perfusion pressure (P = RRdia-ICP) as the safest method for diagnosing compartment syndrome. Using this definition, fasciotomy should be considered whenever P is less than 30 mmHg. The normal compartment pressures in the lower leg of healthy children (13–16 mmHg) were significantly higher than those of adults (0–10 mmHg) [17], because children are in a stage of muscle growth and this increasing volume due to muscle hypertrophy may press against the surrounding fascia [18]. ICP measurement is recommended in young children, unconscious patients, patients with regional nerve blocks and when the clinical signs are equivocal. It may not be necessary if the diagnosis is clinically evident [6]. Some authors [19, 20] warn in their case reports against using epidural anaesthesia or nerve blocks in patients at risk of developing an ACS to avoid delay in the diagnostic procedure of ACS. Johnson and Chalkiadis [21] reviewed cases of paediatric patients with working epidural anaesthesia and found no clear evidence that this kind of regional anaesthesia had delayed the diagnosis of an ACS. Compartment pressure measurement can be made for example with the wick catheter technique modified by Mubarak et al. [22], the simple needle manometry by Whitesides et al. [23], infusion technique by Matsen et al. [24], slit catheter technique modified by Barnes et al. [25], side ported needles [26] etc. A single measurement in one compartment seems to adequately estimate pressures in the compartments at least of the lower leg [18].
Near infrared spectroscopy (NIRS) as a non-invasive continuous method seems to be perfect for monitoring the compartment pressures in children. It measures tissue oxygenation or hypoxia by measuring the muscle oxyhaemoglobin [27]. Muscle oxyhaemoglobin levels strongly reflect compartment pressure, perfusion pressure and loss of myoneural function [28]. The problem is that it is an expensive method that measures only to a limited depth. In ten patients included in our study invasive compartment pressure measurement was performed. Six of ten patients showed a compartment pressure over 40 mmHg. In the other four patients the tissue pressure was at a lower level. Even if ICP measurement is helpful, it is primarily the clinical signs that lead to the decision to perform fasciotomy.
The adequate therapy of ACS, after removing any external sources of compression (circumferential dressings, casts etc.), is the decompression of all involved compartments by an open technique. The skin incision alone reduces the ICP by 5–9 mmHg [29]. The subcutaneous technique, even successfully used in chronic compartment syndrome, appears to be insufficient in ACS [30], especially in younger patients with an intact and firm skin. When in doubt about the viability of soft tissue, tissue that is not obviously necrotic should be left in place at the initial fasciotomy because of the high potential for tissue and muscle function recovery in children (Fig. 1g, arrow). The skin defect in open fasciotomy should not be closed in the same session. To avoid skin grafts, Berman’s shoelace technique for secondary closure can be used successfully [31], but healing by secondary intention, secondary skin closure, skin grafting or flap coverage are possible. In our patients in nearly all cases a direct secondary fasciotomy closure could be performed (87.5%); a split-skin graft was only necessary in three patients (12.5%).
Complications occur particularly after late fasciotomies [32]. Local complications are soft tissue and bone infections leading to amputation, nerve damage etc. [33]. We observed delayed bone healing in two patients and a completely reversible neurological deficit in two patients. One patient developed Volkmann’s contracture. Thus the total permanent complication rate in our series was 4.2%. We did not observe any systemic complications in our patients, such as renal insufficiency caused by rhabdomyolysis and myoglobulinaemia induced by the high ICP.
Conclusion
The diagnosis of ACS in children can be challenging because of the difficulty with co-operation. The classic symptoms of compartment syndrome (five Ps) may be unobtainable. Adjunctive diagnostic tests such as invasive ICP measurement may help guide treatment. However, the entire clinical picture must be considered especially in infants. Early fasciotomy is pivotal. In our study, patients underwent fasciotomy within a median of 27.5 hours (range: 2.5–99). Persistent contractures as a post-traumatic complication occurred in one of 23 patients (4.2%). Thus, when ACS is present, the indication for open fasciotomy is clear even in infants. It is a surgical emergency where the surgeons must make rapid treatment decisions including when to perform fasciotomy. Thanks to a timely and adequate treatment in nearly all cases complications may be avoided.
Acknowledgments
Conflict of interest No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1.Volkmann R. Die ischaemischen Muskellähmungen und Kontrakturen. Centralbl Chir. 1881;8:801–803. [Google Scholar]
- 2.Petersen F. Ueber ischämische Muskellähmung. Arch Klin Chir. 1888;37:675–677. [Google Scholar]
- 3.Paletta CE, Dehghan K. Compartment syndrome in children. Ann Plast Surg. 1994;32:141–144. doi: 10.1097/00000637-199402000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85:625–632. [PubMed] [Google Scholar]
- 5.Martin JT. Compartment syndromes: concepts and perspectives for the anesthesiologist. Anesth Analg. 1992;75:275–283. doi: 10.1213/00000539-199208000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Lagerstrom CF, Reed RL, 2nd, Rowlands BJ, Fischer RP. Early fasciotomy for acute clinically evident posttraumatic compartment syndrome. Am J Surg. 1989;158:36–39. doi: 10.1016/0002-9610(89)90312-7. [DOI] [PubMed] [Google Scholar]
- 7.Mubarak SJ, Hargens AR. Acute compartment syndromes. Surg Clin North Am. 1983;63:539–565. doi: 10.1016/s0039-6109(16)43030-6. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmeyer P, Cox JN, Fritschy D. Ultrastructural modifications of muscle in three types of compartment syndrome. Int Orthop. 1987;11:53–59. doi: 10.1007/BF00266058. [DOI] [PubMed] [Google Scholar]
- 9.Rorabeck CH. The treatment of compartment syndromes of the leg. J Bone Joint Surg Br. 1984;66:93–97. doi: 10.1302/0301-620X.66B1.6693486. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths D. Volkmann’s ischaemic contracture. J Bone Joint Surg Br. 1951;33:299–300. doi: 10.1302/0301-620X.33B3.336. [DOI] [PubMed] [Google Scholar]
- 11.Tiwari A, Haq AI, Myint F, Hamilton G. Acute compartment syndromes. Br J Surg. 2002;89:397–412. doi: 10.1046/j.0007-1323.2002.02063.x. [DOI] [PubMed] [Google Scholar]
- 12.Ulmer T. The clinical diagnosis of compartment syndrome of the lower leg: are clinical findings predictive of the disorder? J Orthop Trauma. 2002;16:572–577. doi: 10.1097/00005131-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Mubarak SJ, Owen CA, Hargens AR, Garetto LP, Akeson WH. Acute compartment syndromes: diagnosis and treatment with the aid of the wick catheter. J Bone Joint Surg Am. 1978;60:1091–1095. [PubMed] [Google Scholar]
- 14.Whitesides TE, Haney TC, Morimoto K, Harada H. Tissue pressure measurements as a determinant for the need of fasciotomy. Clin Orthop Relat Res. 1975;113:43–51. doi: 10.1097/00003086-197511000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Matsen FA, 3rd, Winquist RA, Krugmire RB., Jr Diagnosis and management of compartmental syndromes. J Bone Joint Surg Am. 1980;62:286–291. [PubMed] [Google Scholar]
- 16.Schmidt AH (2007) Acute compartment syndrome. In: Stannard J (ed) Surgical treatment of orthopaedic trauma. Thieme, New York, pp 44–57
- 17.Gershuni DH, Yaru NC, Hargens AR, Lieber RL, O’Hara RC, Akeson WH. Ankle and knee position as a factor modifying intracompartmental pressure in the human leg. J Bone Joint Surg Am. 1984;66:1415–1420. [PubMed] [Google Scholar]
- 18.Staudt JM, Smeulders MJ, Horst CM. Normal compartment pressures of the lower leg in children. J Bone Joint Surg Br. 2008;90:215–219. doi: 10.1302/0301-620X.90B2.19678. [DOI] [PubMed] [Google Scholar]
- 19.Strecker WB, Wood MB, Bieber EJ. Compartment syndrome masked by epidural anesthesia for postoperative pain. Report of a case. J Bone Joint Surg Am. 1986;68:1447–1448. [PubMed] [Google Scholar]
- 20.Morrow BC, Mawhinney IN, Elliott JR. Tibial compartment syndrome complicating closed femoral nailing: diagnosis delayed by an epidural analgesic technique–case report. J Trauma. 1994;37:867–868. doi: 10.1097/00005373-199411000-00030. [DOI] [PubMed] [Google Scholar]
- 21.Johnson DJ, Chalkiadis GA. Does epidural analgesia delay the diagnosis of lower limb compartment syndrome in children? Paediatr Anaesth. 2009;19:83–91. doi: 10.1111/j.1460-9592.2008.02894.x. [DOI] [PubMed] [Google Scholar]
- 22.Mubarak SJ, Hargens AR, Owen CA, Garetto LP, Akeson WH. The wick catheter technique for measurement of intramuscular pressure. A new research and clinical tool. J Bone Joint Surg Am. 1976;58:1016–1020. [PubMed] [Google Scholar]
- 23.Whitesides TE, Jr, Haney TC, Harada H, Holmes HE, Morimoto K. A simple method for tissue pressure determination. Arch Surg. 1975;110:1311–1313. doi: 10.1001/archsurg.1975.01360170051006. [DOI] [PubMed] [Google Scholar]
- 24.Matsen FA, 3rd, Mayo KA, Sheridan GW, Krugmire RB., Jr Monitoring of intramuscular pressure. Surgery. 1976;79:702–709. [PubMed] [Google Scholar]
- 25.Barnes MR, Gibson MJ, Scott J, Bentley S, Allen MJ. A technique for the long term measurement of intra-compartmental pressure in the lower leg. J Biomed Eng. 1985;7:35–39. doi: 10.1016/0141-5425(85)90006-8. [DOI] [PubMed] [Google Scholar]
- 26.Awbrey BJ, Sienkiewicz PS, Mankin HJ. Chronic exercise-induced compartment pressure elevation measured with a miniaturized fluid pressure monitor. A laboratory and clinical study. Am J Sports Med. 1988;16:610–615. doi: 10.1177/036354658801600610. [DOI] [PubMed] [Google Scholar]
- 27.Gentilello LM, Sanzone A, Wang L, Liu PY, Robinson L. Near-infrared spectroscopy versus compartment pressure for the diagnosis of lower extremity compartmental syndrome using electromyography-determined measurements of neuromuscular function. J Trauma. 2001;51:1–8. doi: 10.1097/00005373-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Garr JL, Gentilello LM, Cole PA, Mock CN, Matsen FA., 3rd Monitoring for compartmental syndrome using near-infrared spectroscopy: a noninvasive, continuous, transcutaneous monitoring technique. J Trauma. 1999;46:613–616. doi: 10.1097/00005373-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Mubarak SJ, Owen CA. Double-incision fasciotomy of the leg for decompression in compartment syndromes. J Bone Joint Surg Am. 1977;59:184–187. [PubMed] [Google Scholar]
- 30.Due J, Jr, Nordstrand K. A simple technique for subcutaneous fasciotomy. Acta Chir Scand. 1987;153:521–522. [PubMed] [Google Scholar]
- 31.Berman SS, Schilling JD, McIntyre KE, Hunter GC, Bernhard VM. Shoelace technique for delayed primary closure of fasciotomies. Am J Surg. 1994;167:435–436. doi: 10.1016/0002-9610(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 32.Sheridan GW, Matsen FA., 3rd Fasciotomy in the treatment of the acute compartment syndrome. J Bone Joint Surg Am. 1976;58:112–115. [PubMed] [Google Scholar]
- 33.Rush DS, Frame SB, Bell RM, Berg EE, Kerstein MD, Haynes JL. Does open fasciotomy contribute to morbidity and mortality after acute lower extremity ischemia and revascularization? J Vasc Surg. 1989;10:343–350. doi: 10.1067/mva.1989.14338. [DOI] [PubMed] [Google Scholar]