Abstract
Bone extract from reindeer induces new ectopic bone formation (BF) in muscle pouches, but its feasibility in experimental bone lesions has not been evaluated. We investigated the effects of implants, containing 2, 5, 15, 20 or 50 mg of reindeer bone extract in a collagen carrier, on the healing of 8-mm femur defects in 78 rats. We used 30 µg of recombinant human bone morphogenetic protein-2 (rhBMP-2) in a collagen carrier, collagen and untreated defects as controls. Bone healing was evaluated with radiographs, peripheral quantitative computed tomography (pQCT), biomechanics and histology. In comparison with empty defects, the groups receiving bone extracts showed more BF at three weeks and had better bone union (BU), larger mean cross-sectional bone area at the defect site in groups receiving higher doses of extract, showed greater torsional stiffness of the bones and higher maximum breaking load of bones at six weeks. In comparison to all other groups, in the rhBMP-2 group, BF and BU were best at the three- and six-week follow-up, bone area was largest and mechanical test results were best. Although rhBMP-2 is superior for new bone regeneration, native reindeer bone extract is also effective in the six-week follow-up period.
Introduction
Bone morphogenetic proteins (BMPs) are probably the most important growth factors in regeneration of bone and cartilage [1]. Bone extracts containing the growth factor fractions of native bone proteins include most of these BMPs. Thus, studies that aim to develop safe and effective products from native bone extracts can be justified, although favourable results in preclinical and clinical trials of products based on supraphysiological doses of a single recombinant BMP have been achieved [2–4].
We have shown previously that bone extract purified from reindeer bones effectively induces ectopic new bone formation (BF) in vivo [5, 6]. The native reindeer bone extract has proved to possess better osteoinductivity than bovine or ostrich bone extracts, and it has been suggested that this is due to the fact that reindeers renew their antlers annually [7, 8]. Furthermore, it has been suggested that more of the protein material extracted from the reindeer bone is in monocomponent form compared to that from other species such as cattle, sheep or pigs [7].
Despite modern surgical techniques, segmental long bone defects are often difficult to manage. For this reason, animals with segmental long bone defects similar to clinical defects have been used as models for assessing the efficacy of BMPs and carrier materials. The bone healing properties of reindeer bone extract have been previously evaluated in the rabbit radius [9]. However, the time- and dose-dependent effects of reindeer bone extract have not been evaluated previously. Our hypothesis was that reindeer bone extract is capable of healing segmental bone defects in an animal model. We used the commercial recombinant human BMP-2 (rhBMP-2) product as a reference because its bone healing capacity with different scaffold materials is well known [3, 10–12]. We therefore addressed the following questions: (1) What is the effective dose of reindeer bone extract in a collagen carrier? (2) How much time is needed to heal critical-size bone defects (CSDs) in the rat femur with reindeer bone extract?
Materials and methods
The study protocol was accepted by the Institutional Ethics Committee on Animal Experiments.
Reconstitution of implants
A dose of 2, 5, 15, 20 or 50 mg of lyophilised reindeer bone extract (BBS Ltd, Oulu, Finland) was sprinkled onto a collagen sponge (35 × 8 mm for small amounts and 25 × 25 mm for large amounts, Lyostypt compress from a native collagen of bovine origin, mainly type IV of bovine origin, B. Braun, Tuttlingen, Germany). The collagen sponge was soaked in 200 μl sterile water and the sponge with extract was bundled up to form an implant. The doses were chosen according to our previous studies [5, 6, 9]. The InductOs™ kit (Wyeth Europa, Maidenhead/Berkshire, UK) for the implant contained 12 mg of rhBMP-2 and solvent. The solvent was made according to the manufacturer’s instructions just before the operation. A total of 20 μl of solvent, containing 30 μg of rhBMP-2, was pipetted onto the collagen sponge (Lyostypt) to form the implant. The dose of rhBMP-2 was determined according to the literature [2, 4, 10–12]. Control implants were constructed in an identical fashion, but they contained only the collagen carrier (Lyostypt).
Animals and study groups
One hundred and nine 2.5-month-old male Sprague-Dawley rats were used. Each animal was unilaterally treated with an implant, randomised into the following groups: (1) collagen + 2 mg bone extract, (2) collagen + 5 mg bone extract, (3) collagen + 15 mg bone extract, (4) collagen + 20 mg bone extract, (5) collagen + 50 mg bone extract, (6) collagen + 30 µg rhBMP-2, (7) collagen (the “collagen” group) and (8) no implant (the “untreated” group).
Twelve rats died on the night after the operation and two rats later on. No obvious cause for the deaths and no relation to the reindeer bone extract were found, as some deaths occurred in the control groups. Seventeen rats had to be killed due to the failure of fixation before the study endpoint. Thus, 31 rats were excluded, and 78 rats (eight rats in the group of 50 mg of bone extract and ten rats in each of the other groups) survived until the end of the study and were considered in the results.
Operation method
Surgery was performed under general anaesthesia with a blend of fentanyl citrate (80 µg/kg)-fluanisone (2.5 mg/kg) (Hypnorm®, Janssen Pharmaceutica, Inc., Beerse, Belgium) and midazolam (1.25 mg/kg) (Dormicum®, Roche, Basel, Switzerland). The animals were administered cefuroxime (Zinacef®, 20 mg/kg, GlaxoSmithKline Manufacturing S.p.A., Verona, Italy) subcutaneously preoperatively. The pain medication after the operation consisted of buprenorphine (Temgesic®, Reckitt & Colman Pharmaceuticals, Inc., Richmond, UK) at 0.01–0.05 mg/kg subcutaneously. In cases with respiratory problems, animals were given 1 mg of furosemide (Furesis® Orion, Espoo, Finland). Eye gel (Viscotears®, Novartis Healthcare, Copenhagen, Denmark) was applied to avoid dehydration of eyes during anaesthesia. A transverse skin incision was made over the posterior aspect of the thigh after shaving the hair around the left hind limb. The muscles were elevated circumferentially from the femoral diaphysis. A 23 mm × 4 mm × 2.5 mm polyacetyl plaster plate (POM, Vink Finland Ltd., Kerava, Finland) was placed on the surface of the femur. The plate was secured with a stainless steel holder, which had been designed for this operation (Technical Services Unit, University of Oulu). Four holes were predrilled for the plate and the femur through both cortices using a 0.7-mm hard metal bit. After drilling each pilot hole, a 0.8-mm Kirschner wire with a 5- mm threaded end (Synthes Oy, Helsinki, Finland) was inserted through the plate and both cortices. The part of the Kirschner wire that remained outside of the bone and the plate was snapped with the side-cutting pliers. Stability of the fixation was enhanced because some rats had problems with the failure of fixation after three weeks in groups 1, 2, 7 and 8. Thus, the plate length was extended by 2 mm, and six Kirschner wires were used in groups 3–6.
An 8-mm diaphyseal CSD was created using the stainless steel mini burr bit attached to the hand piece of the drill. After a thorough wash with saline, the implant was applied to the defect, or the defect was left empty for controls. The muscles and skin were closed in two layers, using a sterile synthetic absorbable surgical suture, 4.0 Dexon. The animals were allowed full activity in their cages postoperatively. After six weeks, the rats were killed in a chamber with carbon dioxide (CO2).
Radiographic evaluation of BF
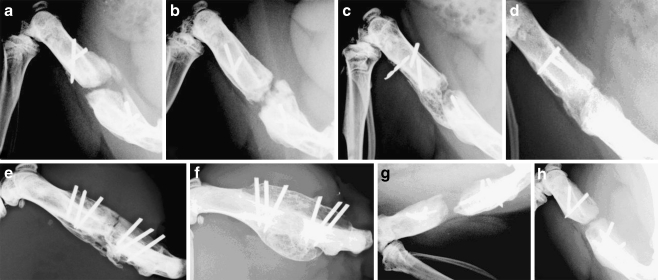
Radiographs of the left femur (26 kV, 9.00 mAs, 0.09 s/exposure, Mamex dc® ami, Orion Ltd., Soredex, Helsinki, Finland) were taken postoperatively and at three and six weeks (Fig. 2a–h) after the operation under neuroleptic analgesia (Hypnorm-Dormicum®, 0.7 ml). These time points were based on the results of a study demonstrating increasing BF with BMP-7 [13]. Radiographs were evaluated with Osiris 4.19 software (Digital Imaging Unit, Geneva, Switzerland). The percentage of orthotopic new BF and the development of union or non-union of the bone (BU) were estimated as previously described [9] according to the scoring system presented in the study of Sciadini et al. [14].
Fig. 2.
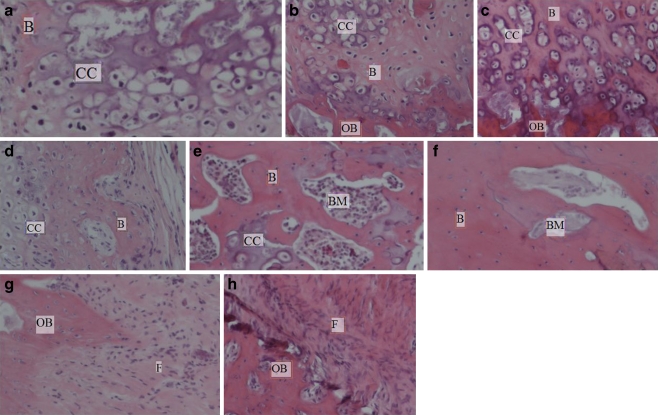
Histological examination showing quality of new bone induced by different amounts of reindeer bone extract in the rat femur after 6 weeks follow-up. a 2 mg of bone extract, b 5 mg of bone extract, c 15 mg of bone extract, d 20 mg of bone extract, e 50 mg of bone extract, f 30 µg of rhBMP-2, g collagen, h untreated defect. OB old bone, F fibroblastic tissue, CC zone of calcified cartilage, B newly formed bone trabeculae, BM bone marrow. (Original magnification ×10, figures are representative images)
Computed tomography
After the soft tissues were removed from the bone, all left femurs were scanned using a peripheral quantitative computed tomography (pQCT) device (Stratec XCT 960 A, 5.21 software version, Norland Stratec Medizintechnik GmbH, Birkenfeld, Germany). A voxel size of 0.148 mm × 0.148 mm × 1 mm was used. One cross-sectional slice from the middle of the defect in each sample was scanned in two directions: with the fixation system above the bone and turned 90° axially. Average values of the two scans were used in the statistical analysis. Cross-sectional bone areas (mm2) from the scanned slices of the samples were recorded, using the pQCT software, with a threshold of 169 mg/cm3 to distinguish bone from surrounding soft tissue and a threshold of 464 mg/cm3 for the inner surface of the bone.
Mechanical tests
After pQCT imaging, the fixation system was removed from the samples. The bone ends were embedded into the moulds of the sleeves with dental stone (GC Fujirock, Improved Dental Stone, G-C Dental Industrial Corp., Tokyo, Japan). The torsional shaft was adjusted to 2 cm. After the cast hardened, the samples were placed in the torque machine and torsionally loaded at a constant angular speed of 6°/s until failure. Maximum breaking load (Nm) and torsional stiffness (Nm/°) were recorded [15]. Mechanically unstable bones were not tested, and their values were considered to be zero in the statistical analysis.
Histological examination
After mechanical tests two samples of every group were prepared for histology. The specimens were fixed in 10% neutral buffered formalin, decalcified in ethylenediaminetetraacetate (EDTA)-formalin solution (pH 7), processed in a tissue processor and finally embedded in paraffin. Slices with a thickness of 4.5 μm were prepared using a microtome and stained with haematoxylin and eosin. The quality of new bone and inflammatory response in the defect site were evaluated in histological analysis by light microscopy (Nikon Eclipse E200, Tokyo, Japan).
Statistics
Statistical analysis was performed using the SPSS for Windows statistical package (SPSS Inc., version 15.0). The non-parametric Kruskal-Wallis test was used to evaluate the statistical differences between the groups. The Mann-Whitney test was used for pairwise comparison between extract and rhBMP-2 groups and the collagen and untreated groups. Values of p < 0.05 were considered statistically significant. Results of pQCT and mechanical testing are given as medians and quartiles. Results of radiographic assessment are given as cross tabulations.
Results
Collagen is widely used in rhBMP products and is also a promising carrier alternative for reindeer bone extract. Typical radiographs showing new BF in the different study groups after six weeks are shown in Fig. 1. BF at the defect site was greater at three and six weeks in all bone extract groups (p < 0.05) when compared to the untreated and collagen groups, (except for the group of 2 mg bone extract) in comparison with the untreated control at three weeks (Table 1). In this study BF showed a clear dose dependency, but only the difference between the groups with 2 and 20 mg of bone extract reached the level of significance (p = 0.030) (Table 1). BU in three weeks was better in the groups receiving 5, 20 or 50 mg of extract than in the untreated or collagen groups (p < 0.05). In six weeks, BU was better in the groups with 5 or 50 mg of bone extract than in the untreated group (p < 0.05), and also better in the groups with 5, 15 or 50 mg of bone extract than in the collagen group (p < 0.05) (Table 2). There were no significant differences in BU between the groups receiving various amounts of bone extract. BF and BU were better in the commercial rhBMP-2 group than in any of the other groups both three and six weeks after the operation (p < 0.001) (Tables 1 and 2).
Fig. 1.
Radiographs showing new BF induced by different amounts of reindeer bone extract in the rat femur after 6 weeks follow-up. a 2 mg of bone extract, b 5 mg of bone extract, c 15 mg of bone extract, d 20 mg of bone extract, e 50 mg of bone extract, f 30 µg of rhBMP-2, g collagen, h untreated defect
Table 1.
BF in the defect area of rat femur 3 and 6 weeks after the operation evaluated by radiographic analysis using the scoring system presented in the study of Sciadini et al. [14]
| Group | n | BF score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 weeks | p | 6 weeks | p | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | ||||
| 2 mg | 10 | 5 | 3 | 2 | 0 | 0 | d | 0 | 5 | 3 | 0 | 0 | b, e |
| 5 mg | 10 | 1 | 4 | 4 | 1 | 0 | a, e | 0 | 1 | 4 | 1 | 1 | c, e |
| 15 mg | 10 | 1 | 8 | 1 | 0 | 0 | a, e | 0 | 2 | 5 | 0 | 0 | c, e |
| 20 mg | 10 | 2 | 4 | 3 | 1 | 0 | a, e | 0 | 2 | 1 | 1 | 3 | c, e, f |
| 50 mg | 8 | 1 | 5 | 1 | 1 | 0 | a, e | 0 | 2 | 3 | 1 | 1 | a, e |
| rhBMP-2 | 10 | 0 | 0 | 0 | 0 | 10 | g | 0 | 0 | 0 | 0 | 10 | g |
| Collagen | 10 | 10 | 0 | 0 | 0 | 0 | 8 | 2 | 0 | 0 | 0 | ||
| Untreated | 10 | 8 | 2 | 0 | 0 | 0 | 5 | 4 | 1 | 0 | 0 | ||
ap < 0.005, bp = 0.007, cp < 0.001 vs untreated
dp = 0.013, ep < 0.001 vs collagen
fp < 0.05 vs 2 mg of bone extract
gp < 0.01 vs all other groups
Table 2.
BU in the defect area of rat femur 3 and 6 weeks after the operation evaluated by radiographic analysis using the scoring system presented in the study of Sciadini et al. [14]
| Group | n | BU score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 weeks | p | 6 weeks | p | ||||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||||
| 2 mg | 10 | 9 | 1 | 0 | 0 | 5 | 2 | 2 | 1 | ||
| 5 mg | 10 | 6 | 4 | 0 | 0 | a, b | 2 | 4 | 3 | 1 | a, b |
| 15 mg | 10 | 8 | 1 | 1 | 0 | 4 | 3 | 2 | 1 | b | |
| 20 mg | 10 | 6 | 3 | 1 | 0 | a, b | 5 | 1 | 1 | 3 | |
| 50 mg | 8 | 5 | 2 | 1 | 0 | a, b | 3 | 2 | 2 | 1 | a, b |
| rhBMP-2 | 10 | 0 | 0 | 0 | 10 | c | 0 | 0 | 0 | 10 | c |
| Collagen | 10 | 10 | 0 | 0 | 0 | 8 | 2 | 0 | 0 | ||
| Untreated | 10 | 10 | 0 | 0 | 0 | 8 | 1 | 1 | 0 | ||
ap < 0.03 vs untreated
bp < 0.05 vs collagen
cp < 0.001 vs all other groups
In biomechanical testing maximum torsional load of the bones (Nm) was significantly higher in the groups receiving 2, 20 or 50 mg of bone extract than in the collagen group (p < 0.05) (Table 3). Stiffness of the bones (Nm/°) was significantly higher in the groups receiving 2, 15, 20 or 50 mg of bone extract than in the collagen group (p = 0.046 to p = 0.004) (Table 3). Maximum torsional load in the rhBMP-2 group was the highest of all groups (p < 0.05). Stiffness of the bones in the rhBMP-2 group was higher than in the control groups and most bone extract groups (p < 0.05) (Table 3).
Table 3.
Bone healing in the defect area of rat femur after 6 weeks as evaluated by pQCT densitometry and mechanical testing
| Group | n | pQCT analysis | Mechanical tests | |
|---|---|---|---|---|
| Cross-sectional area (mm2) | Breaking load (Nm) | Stiffness (Nm/°) | ||
| 2 mg of extract | 10 | 24.65 (8.47–49.41) | 0.089 (0.073–0.101)b | 0.0021 (0.0015–0.0033)b |
| 5 mg of extract | 10 | 3.70 (2.98–44.97) | 0.092 (0.000–0.129) | 0.0017 (0.0000–0.0046) |
| 15 mg of extract | 10 | 35.50 (28.80–40.73)a, b | 0.072 (0.039–0.081) | 0.0017 (0.0010–0.0022)b |
| 20 mg of extract | 10 | 40.70 (33.64–53.91)a, c | 0.095 (0.045–0.153)b | 0.0017 (0.0010–0.0032)b |
| 50 mg of extract | 8 | 45.14 (31.82–53.16)a, b | 0.086 (0.049–0.186)b | 0.0031 (0.0011–0.0031)b |
| rhBMP-2 | 10 | 110.29 (65.48–136.57)f | 0.232 (0.106–0.429)a, c, d | 0.0108 (0.0019–0.0159)c, e |
| Collagen | 10 | 10.21 (3.88–31.13) | 0.000 (0.000–0.076) | 0.0000 (0.0000–0.0019) |
| Untreated | 10 | 6.92 (4.77–31.48) | 0.037 (0.000–0.168) | 0.0006 (0.0000–0.0043) |
Median (quartile) values
ap < 0.02 vs untreated
bp < 0.05, cp < 0.01 vs collagen
dp < 0.05 vs 2, 5, 15, 20, 50 mg of extract
ep < 0.05 vs 5, 15, 20 mg of extract
fp < 0.001 vs all other groups
pQCT showed cross-sectional bone areas to be greater in the groups that received 15 to 50 mg extract than in the negative control groups after 6 weeks (p = 0.019 to p < 0.001) (Table 3). Cross-sectional bone area in the rhBMP-2 group was the greatest compared to the other groups (p < 0.001) (Table 3).
Descriptive histology examination showed no new BF, only fibrotic tissue, in the specimens of the negative control groups. New BF was seen in the specimens of the groups receiving reindeer bone extract or rhBMP. The most abundant BF and BU were seen in the specimens of groups receiving 20 to 50 mg extract and in rhBMP groups which showed hypertrophied and calcified chondrocytes, remodelling ectopic trabecular woven bone and bone marrow. No inflammatory reaction was seen (Fig. 2).
Discussion
In this preliminary study, we investigated the time- and dose-dependent effects of reindeer bone extract and compared the results with rhBMP-2, as a positive control, and untreated or collagen treatment as negative controls. Reindeer native bone extract was able to heal the critical-size segmental bone defect in six weeks, and significant BF was observed already after three weeks.
This study shows the healing capacity of bone extract in the segmental bone defects in the rat. In this study, we used different amounts of native reindeer bone extract (2–50 mg). BF capacity was superior with as little as 2 mg bone extract, compared to the negative control groups. Pekkarinen et al. used 5 and 10 mg of non-sterilised reindeer bone extract in rabbit radius. These amounts healed the defect much better than the controls after eight weeks follow-up. The 10 mg of bone extract group had significantly higher mean stiffness than the collagen group [9]. Our results confirm previous results, which showed that reindeer bone extract has good osteoconductivity both in the muscle pouch model of mice and in the defect model of rabbit radius [5, 6, 9]. It is known that the animal and fracture models, delivery systems and route of administration influence the dose response in bone healing studies [16]. Above a certain threshold, BF cannot be further enhanced as was seen in this study. The minimally effective doses of native reindeer bone extract appear to be between 2 and 5 mg in this study and with this scaffold model. New BF was seen in all specimens containing reindeer bone extract or rhBMP, but the most abundant BF and BU were seen in the specimens of 15 to 50 mg extract. Furthermore, the area of healed new bone at the defect site increased at the doses of 15 mg of extract or more, which might support the use of the higher doses.
rhBMP-2 was included in the comparisons because the bone healing capacity of rhBMP-2 has been well documented and it has received approval from the US Food and Drug Administration (FDA) [10–12]. The BF capacity of rhBMP-2 in the rat femur segmental defect model was superior compared to the negative control groups and to the reindeer bone extract groups. This was seen in both radiographic assessment and cross-sectional bone area evaluated by pQCT. In most cases of bone treated by 30 µg rhBMP-2, the dose was too high, causing new bone area to overgrow. The minimum effective dose of rhBMP-2 in rat femur bone healing was difficult to predict because the amounts of rhBMP-2 used in rat studies with collagen as a carrier have ranged from 0.5 to 200 µg [2, 10, 17–19].
The doses of native bone extracts used are milligrams compared to recombinant products that are in micrograms. This is because the bone extracts and demineralised bone matrix products involve a wide spectrum of all bone proteins and collagens, while recombinants have only one specific protein with a specific task in bone regeneration [20]. The difference in the response of recombinant and native reindeer extract might partly be explained by the different effective doses, and more studies on the optimal dosing are needed. Reindeer bone protein extract as an animal-derived bone tissue extract is very similar in composition, method of manufacture, and intended use and application. The closest comparable products are Colloss® and Colloss® E, demineralised bone extracts made from bovine and equine bone, and human demineralised bone matrix (DBM) products [20]. These could act as better and more closely related reference material than rhBMPs.
The strength of long bones can be measured by torsional testing. This test is suitable because it loads the bone equally in every section along its length and it is not critically dependent on bone geometry. Maximum breaking load (Nm) and stiffness (Nm/°) of the bones were significantly higher in most groups receiving extract than in the collagen group. The group receiving 30 µg of rhBMP-2 had better results on mechanical tests than the negative controls or the bone extract groups. We suppose that six weeks follow-up was not sufficient for complete healing with the native reindeer extract. In the bone extract groups, there was new BF on the bone ends, but BU in the radiographs and preparations of the bones was insufficient and this has an effect on pQCT and mechanical stiffness results. Even those cases that received rhBMP-2 had not fully healed in six weeks, because the bone bridges over the defect were outside it. This was seen in both macroscopic evaluation and histological examination. A longer follow-up could more clearly show this effect.
Surprisingly, the effect of native reindeer bone extract in the rat femur defect model was not as good as in the rabbit ulnar defect model owing to differences in animal species and experimental model [9]. It could also imply that native reindeer bone protein extract is immunogenic for the rat model [11, 21, 22]. In some studies nude rats have been used to obviate problems of immunogenic reactions to xenografts [23, 24].
In our study, despite a well-planned fixation model, the fixation was not sufficiently mechanically stable in some cases. In these cases of groups 1, 2, 7 and 8, the fixation broke during the first three weeks of treatment. However, there were no more fixation breakages six weeks after the operation. The object was that fixation stayed stable during follow-up. Thus, fixation in groups 3–6 was obtained by increasing the length of the polyacetyl plate by 2 mm and fixing the plate to the bone with six, rather than four, Kirschner wires. However, we do not consider that this modification significantly changed our results. Rats with fixation problems had to be killed before the study period was completed for humane reasons. In this study, many rats also died after the operation without any obvious cause, and no relation to bone extract could be inferred because such deaths also occurred among the controls. The deaths may have been reactions to anaesthesia, although the anaesthetic medicine and method we used are accepted in animal testing.
More than 20 BMPs have now been described and about ten of them have functions in different phases of bone healing [1]. Thus, it is possible that using a combination of different BMPs is the most desirable approach in clinical applications [25]. Because reindeer bone extract is a mixture of different BMPs, growth factors and other bone forming proteins, its bone healing capacity is ideal. In future our aim is to produce a formulation of native reindeer bone extract that could be used in clinical treatment of bone defects and injuries.
In conclusion, although rhBMP-2 has superior results in new bone regeneration, native reindeer bone extract is also effective in healing the CSD in the six-week follow-up period. More dose time and formulation studies of the native reindeer bone extract are required before the clinically optimal dosing can be determined.
Acknowledgement
The authors wish to thank the Synthes Company and Mr. Antero Enqvist for providing Kirschner wires for this study, and Bioactive Bone Substitutes – BBS Ltd for providing the reindeer bone extract. We are grateful to Mrs. Merja Kreivi-Kauppinen, Ms. Marja Juustila, Ms. Heli Korkala, and Elli Birr, Ph.D. for their kind assistance with the preparations of the implants, and for their surgical and result analysis assistance. The study is partly supported by Academy of Finland and National Graduate School of Musculoskeletal Disorders and Biomaterials (TBGS, Finland).
Conflict of interest We wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest and to significant financial contributions to this work. Hanna Tölli, M.Sc. (Eng.), is an employee at Bioactive Bone Substitutes – BBS Ltd. Professor Pekka Jalovaara (M.D., Ph.D.) is a major stakeholder in this same company. We state that we have full control of all primary data and we agree to allow the journal to review the data if requested.
References
- 1.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Yasko AW, Lane JM, Fellinger EJ, Rosen V, Wozney JM, Wang EA. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg Am. 1992;74(5):659–670. [PubMed] [Google Scholar]
- 3.Bong MR, Capla EL, Egol KA, Sorkin AT, Distefano M, Buckle R, Chandler RW, Koval KJ. Osteogenic protein-1 (bone morphogenic protein-7) combined with various adjuncts in the treatment of humeral diaphyseal nonunions. Bull Hosp Jt Dis. 2005;63(1–2):20–23. [PubMed] [Google Scholar]
- 4.Vögelin E, Jones NF, Huang JI, Brekke JH, Lieberman JR. Healing of a critical-sized defect in the rat femur with use of a vascularized periosteal flap, a biodegradable matrix, and bone morphogenetic protein. J Bone Joint Surg Am. 2005;87(6):1323–1331. doi: 10.2106/JBJS.C.00913. [DOI] [PubMed] [Google Scholar]
- 5.Pekkarinen T, Lindholm TS, Hietala O, Jalovaara P. New bone formation induced by injection of native reindeer bone morphogenetic protein extract. Scand J Surg. 2003;92:227–230. doi: 10.1177/145749690309200312. [DOI] [PubMed] [Google Scholar]
- 6.Pekkarinen T, Lindholm TS, Hietala O, Jalovaara P. The effect of different mineral frames on the ectopic bone formation in mouse hind leg muscles induced by native reindeer bone morphogenetic protein. Arch Orthop Trauma Surg. 2005;125(1):10–15. doi: 10.1007/s00402-004-0761-7. [DOI] [PubMed] [Google Scholar]
- 7.Jortikka L, Marttinen A, Lindholm TS. Partially purified reindeer (Rangifer tarandus) bone morphogenetic protein has a high bone-forming activity compared with some other artiodactyls. Clin Orthop Relat Res. 1993;297:33–37. [PubMed] [Google Scholar]
- 8.Ulmanen MS, Pekkarinen T, Hietala OA, Birr EA, Jalovaara P. Osteoinductivity of partially purified native ostrich (Struthio camelus) bone morphogenetic protein: comparison with mammalian species. Life Sci. 2005;77(19):2425–2437. doi: 10.1016/j.lfs.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Pekkarinen T, Jämsä T, Määttä M, Hietala O, Jalovaara P. Reindeer BMP extract in the healing of critical-size bone defects in the radius of the rabbit. Acta Orthop. 2006;77(6):952–959. doi: 10.1080/17453670610013286. [DOI] [PubMed] [Google Scholar]
- 10.Kim CS, Kim JI, Kim J, Choi SH, Chai JK, Kim CK, Cho KS. Ectopic bone formations associated with recombinant human bone morphogenetic proteins-2 using absorbable collagen sponge and beta tricalcium phosphate as carriers. Biomaterials. 2005;26(15):2501–2507. doi: 10.1016/j.biomaterials.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Bessho K, Kusumoto K, Fujimura K, Konishi Y, Ogawa Y, Tani Y, Iizuka T. Comparison of recombinant and purified human bone morphogenetic protein. Br J Oral Maxillofac Surg. 1999;37(1):2–5. doi: 10.1054/bjom.1998.0379. [DOI] [PubMed] [Google Scholar]
- 12.Alam I, Asahina I, Ohmamiuda K, Takahashi K, Yokota S, Enomoto S. Evaluation of ceramics composed of different hydroxyapatite to tricalcium phosphate ratios as carriers for rhBMP-2. Biomaterials. 2001;22(12):1643–1651. doi: 10.1016/S0142-9612(00)00322-7. [DOI] [PubMed] [Google Scholar]
- 13.Hak DJ, Makino T, Niikura T, Hazelwood SJ, Curtiss S, Reddi AH. Recombinant human BMP-7 effectively prevents non-union in both young and old rats. J Orthop Res. 2006;24(1):11–20. doi: 10.1002/jor.20022. [DOI] [PubMed] [Google Scholar]
- 14.Sciadini MF, Dawson JM, Johnson KD. Bovine-derived bone protein as a bone graft substitute in a canine segmental defect model. J Orthop Trauma. 1997;11(7):496–508. doi: 10.1097/00005131-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Jämsä T, Jalovaara P. A cost-effective, accurate machine for testing the torsional strength of sheep long bones. Med Eng Phys. 1996;18(5):433–435. doi: 10.1016/1350-4533(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 16.Nunamaker DM. Experimental models of fracture repair. Clin Orthop Relat Res. 1998;355 Suppl:S56–S65. doi: 10.1097/00003086-199810001-00007. [DOI] [PubMed] [Google Scholar]
- 17.Fujimura K, Bessho K, Kusumoto K, Ogawa Y, Iizuka T. Experimental studies on bone inducing activity of composites of atelopeptide type I collagen as a carrier for ectopic osteoinduction by rhBMP-2. Biochem Biophys Res Commun. 1995;208(1):316–322. doi: 10.1006/bbrc.1995.1340. [DOI] [PubMed] [Google Scholar]
- 18.King GN, King N, Hughes FJ. Effect of two delivery systems for recombinant human bone morphogenetic protein-2 on periodontal regeneration in vivo. J Periodontal Res. 1998;33(4):226–236. doi: 10.1111/j.1600-0765.1998.tb02194.x. [DOI] [PubMed] [Google Scholar]
- 19.Hyun SJ, Han DK, Choi SH, Chai JK, Cho KS, Kim CK, Kim CS. Effect of recombinant human bone morphogenetic protein-2, -4, and -7 on bone formation in rat calvarial defects. J Periodontol. 2005;76(10):1667–1674. doi: 10.1902/jop.2005.76.10.1667. [DOI] [PubMed] [Google Scholar]
- 20.Huffer WE, Benedict JJ, Turner AS, Briest A, Rettenmaier R, Springer M, Walboomers XF. Repair of sheep long bone cortical defects filled with COLLOSS, COLLOSS E, OSSAPLAST, and fresh iliac crest autograft. J Biomed Mater Res B Appl Biomater. 2007;82:460–470. doi: 10.1002/jbm.b.30751. [DOI] [PubMed] [Google Scholar]
- 21.Sampath TK, Reddi AH. Homology of bone-inductive proteins from human, monkey, bovine, and rat extracellular matrix. Proc Natl Acad Sci U S A. 1983;80(21):6591–6595. doi: 10.1073/pnas.80.21.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viljanen VV, Lindholm TS. Heterotopic osteoinduction in a rat membrane-isolated latissimus dorsi island flap. A pilot study. Ann Chir Gynaecol Suppl. 1993;207:55–62. [PubMed] [Google Scholar]
- 23.Ripamonti U, Magan A, Ma S, Heever B, Moehl T, Reddi AH. Xenogeneic osteogenin, a bone morphogenetic protein, and demineralized bone matrices, including human, induce bone differentiation in athymic rats and baboons. Matrix. 1991;11(6):404–411. doi: 10.1016/s0934-8832(11)80195-2. [DOI] [PubMed] [Google Scholar]
- 24.Edwards JT, Diegmann MH, Scarborough NL. Osteoinduction of human demineralized bone: characterization in a rat model. Clin Orthop Relat Res. 1998;357:219–228. doi: 10.1097/00003086-199812000-00028. [DOI] [PubMed] [Google Scholar]
- 25.Westerhuis RJ, Bezooijen RL, Kloen P. Use of bone morphogenetic proteins in traumatology. Injury. 2005;36(12):1405–1412. doi: 10.1016/j.injury.2005.02.047. [DOI] [PubMed] [Google Scholar]