Abstract
Transdermal delivery of peptides and proteins avoids the disadvantages associated with the invasive parenteral route of administration and other alternative routes such as the pulmonary and nasal routes. Since proteins have a large size and are hydrophilic in nature, they cannot permeate passively across the skin due to the stratum corneum which allows the transport of only small lipophilic drug molecules. Enhancement techniques such as chemical enhancers, iontophoresis, microneedles, electroporation, sonophoresis, thermal ablation, laser ablation, radiofrequency ablation and noninvasive jet injectors aid in the delivery of proteins by overcoming the skin barrier in different ways. In this review, these enhancement techniques that can enable the transdermal delivery of proteins are discussed, including a discussion of mechanisms, sterility requirements, and commercial development of products. Combination of enhancement techniques may result in a synergistic effect allowing increased protein delivery and these are also discussed.
KEY WORDS: iontophoresis, microneedles, phonophoresis, protein delivery, transdermal delivery
INTRODUCTION
Protein-based drugs are now very important for the treatment of several conditions such as diabetes, osteoporosis, cancer, and so on; made possible due to the developments in recombinant DNA technology which have allowed commercially viable production of pure recombinant proteins. Proteins are complex molecules with large molecular weights, acid–base side chains and are polar in nature. Stability issues, along with their complex nature, make proteins difficult drug candidates for delivery. Currently, proteins are being predominantly administered by the parenteral route. However, since most proteins have short half-lives, this route has the disadvantage of the requirement for repeated administrations and low patient compliance. Other routes such as the oral, pulmonary and nasal routes have also been investigated as alternatives and some products have been launched. However, these routes have limitations such as gastrointestinal degradation, low bioavailability, and local irritation (1).
The skin, the most accessible organ of the body with a large surface area, offers an appealing alternative for delivering proteins into the systemic circulation. However, stratum corneum, the outermost barrier of skin which is made up of dead keratinocytes, acts as a rate limiting barrier. This lipophilic layer allows only small, potent and moderately lipophilic molecules to partition across it passively, into the deeper layers of the skin. This limits the delivery of proteins which have a large molecular weight and are hydrophilic in nature. So the key for delivering proteins is to overcome this barrier after which the proteins can diffuse past the viable epidermis which is comparatively more hydrophilic in nature and into the systemic circulation via the capillaries present in the dermis.
To overcome the stratum corneum barrier, several enhancement techniques have been investigated, some of the major ones being chemical enhancers, iontophoresis, microneedles, sonophoresis, laser ablation, thermal ablation, radiofrequency ablation, jet injectors and electroporation (2). Iontophoresis and electroporation are electrically assisted enhancement techniques where current is applied to increase drug delivery through the skin. On the other hand, microneedle technology is a physical enhancement method which is a bridge between conventional hypodermic needles and passive transdermal patches. Micron-sized needles porate skin in a minimally invasive manner to enable delivery of proteins. Similarly, all the enhancement methods enable delivery of drugs in different ways and bioavailabilites of ≥50% may be possible (3).
After overcoming the stratum corneum barrier and permeating into the deeper layers of skin, proteins need to be intact to exert their pharmacological effect. Skin has proteolytic enzymes which can degrade the proteins in the skin. However, it has been reported that skin has relatively low proteolytic activity as compared with mucosal routes, thereby reducing the amount of protein degradation (4). Therefore, transdermal route may result in a comparatively higher bioavailability as compared with the mucosal routes (5). This route of administration may also be cost-effective compared with the parenteral route as the patients can self-administer the transdermal patches while the latter may require a visit to the clinic.
In this review, principles behind the enhancement techniques, their advantages, and limitations have been discussed with respect to transdermal delivery of peptides and proteins. Products being developed with these technologies are listed in Table I.
Table I.
Transdermal Enhancement Technologies/Products Being Developed by Companies and Key Discontinued Products
| Enhancement method | Company | Product/technology |
|---|---|---|
| Iontophoresis | Alza (former) | E-TRANS®, IONSYS® (withdrawn) |
| Iomed (now Chattanooga) | Chattanooga Ionto™ (Phoresor®); Companion80™, Numbystuff®/Iontocaine® | |
| Vyteris | LidoSite® | |
| Travanti Pharma (now Teikoku Pharma USA) | WEDD®, Iontoptach® | |
| Transport Pharmaceuticals (former) | SoloVir™ (discontinued) | |
| Mattioli engineering | Transderm® Ionto system | |
| Empi | Empi Action patch™ | |
| Dharma Therapeutics (a subsidiary of Transcu Group Ltd) | Iontophoretic drug delivery system (IDDS) | |
| ActivaTek™ | Trivarion® | |
| Isis Biopolymer | IsisIQ™ | |
| Nupathe® | SmartRelief Technology | |
| Animas® corporation (part of Johnson and Johnson) | GlucoWatch® (discontinued) | |
| Microneedles | 3M | Solid Microstructured Transdermal System (sMTS) and Hollow Microstructured Transdermal System (hMTS) technology |
| Zosano | MacroFlux® | |
| Becton Dickinson | BD Soluvia™ | |
| Corium | MicroCor™ | |
| DermaRoller | DermaRoller® | |
| Elegaphy | Soluble microneedles | |
| Kumetrix | Silicon microneedles | |
| NanoPass and Silex Microsystems | MicronJet needle (Hollow); Micropyramid technology (Solid) | |
| Norwood Abbey | Microneedle technology | |
| TheraJect | TheraJectMAT and VaxMAT | |
| Valeritas | Micro-Trans™ | |
| Thermal ablation | Altea Therpeutics | PassPort® Patch |
| Radiofrequency ablation | TransPharma Medical | ViaDorTM |
| Laser-assisted delivery | Pantech Biosolutions AG | P.L.E.A.S.E.® |
| Norwood Abbey | Epiture Easytouch™ | |
| Sonophoresis | Echo Therapeutics (formerly Sontra Medical Corporation) | SonoPrep® system (withdrawn) |
| Jet systems | Valeritas | Mini-Ject™ |
| Developed by PowderJect® pharmaceuticals and now owned by Novartis vaccines and diagnostics | PowderJect® technology |
FORMULATION APPROACHES
Chemical enhancers alter the lipid structure of the stratum corneum thereby reducing its barrier properties and increasing its permeability for drugs which would not pass through the skin passively. For example, Magnusson and Runn (6) reported that transdermal delivery of an analog of thyrotropin releasing hormone increased 2-fold in the presence of ethanol. Peptide delivery increased further in the presence of ethanol and cineole. When employing chemical enhancers in the formulation, care must be taken to choose nonirritating excipients that will not dentaure the peptide.
Coadministration of certain peptide sequences, acting as peptide facilitators, has also been reported to increase peptide delivery. Chen et al. have reported that coadministration of a short synthetic peptide increased passive delivery of insulin and human growth hormone. Fluorescent labeling indicated that the main route of transport for delivery is through the transfollicular route (7). In another similar study by Frankenburg et al. (8), a short peptide was incorporated into a recombinant melanoma protein targeted for vaccination, resulting in a high antibody titre as compared with the control. This method of administration may not be effective and reliable as the follicular density and surface area is small compared with the rest of the skin and will have variation from site to site and person to person. Chemical modification of peptides has also been tried to enhance drug delivery. Lipophilic derivatives of peptides have been reported to have better skin permeability (9,10). However, modification of the peptide might affect its pharmacological activity and hence must be investigated.
Encapsulation of peptides has also been reported to increase peptide delivery across skin. Different types of particles such as liposomes, niosomes, ethosomes, and transfersomes have been developed and investigated. The surfactants in the particles aid in local fluidization of the lipids which then allows for the particles to sit in the upper layers of the stratum corneum where they form a depot for a prolonged effect. Transfersomes are more elastic in nature which has been claimed to allow them to squeeze their way through the pores on the surface of skin into the deeper layers (11).
Enhancement of transdermal delivery of peptides due to incorporation of penetration enhancers, addition of facilitating peptide sequences, chemical modification of the peptide itself or encapsulation, is limited to only a narrow range of small peptides. Therefore, different enhancement methods need to be employed for larger proteins. These methods also offer other advantages as will be discussed in this review.
IONTOPHORESIS MEDIATED TRANSDERMAL DELIVERY OF PROTEINS
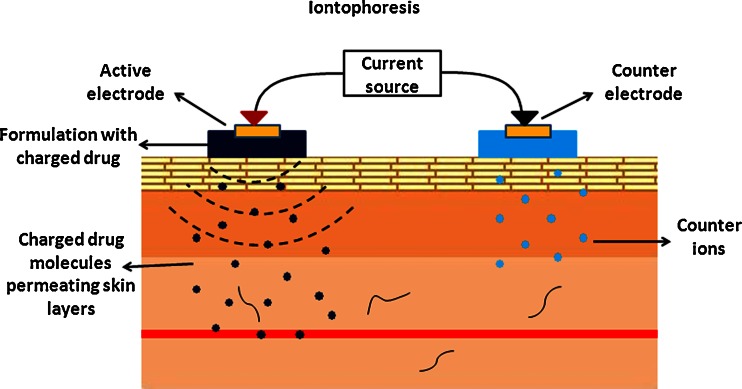
Iontophoresis is one of the more successful techniques and has been extensively used for localized drug delivery in physical therapy clinics and is being investigated more recently to enhance transdermal delivery of drugs for systemic administration. This technique involves the application of mild current (typically, <0.5 mA/cm2 of skin) in a pulsatile or continuous fashion in order to propel charged drug molecules into the skin; in this case, proteins (Fig. 1). The charged protein is placed under an electrode with the same polarity. Therefore, when current is applied, following the rule of like repels like, the protein is propelled away from the electrode into the layers of the skin.
Fig. 1.
Schematic of principle behind iontophoresis. Charged drug molecules are propelled into the skin due to the electrorepulsive forces from electrodes of similar polarity
The two main mechanisms of transport governing iontophoretic delivery are electromigration (EM; for charged molecules) and electroosmosis (EO; for neutral molecules). Electromigration/electrorepulsion is the repulsion of the charged drug by an electrode with the same polarity and this is the predominant mechanism of transport for most proteins. Electroosmosis on the other hand is the flow of solvent from anode to cathode via which neutral water soluble molecules can also be transported into the skin. Therefore, the total flux of proteins during iontophoretic delivery is: JTOT = JEM + JEO. Abla et al. (12) studied the contributions of each mechanism on the transdermal delivery of d-(Arg)-kyotorphin, a model peptide. They reported that electromigration accounted for ~70% of the delivery. However, when permselectivity of the skin was altered by tape stripping, the amount of total peptide delivered remained the same, but contributions of each mechanism changed significantly. Electroosmosis was absent, effect of electromigration decreased and passive delivery increased due to the altered barrier properties (12). Iontophoretic delivery follows Faraday’s law as stated below:
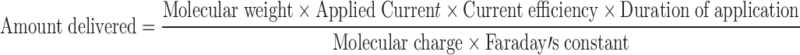 |
As can be referred from the above equation (13), flux is directly proportional to the applied current. However, iontophoretic delivery is not dependent on current alone. It depends on a variety of parameters including the physicochemical properties of the protein (size, overall charge, structure, and lipophilicity) and the experimental parameters employed (current density, duration of application, electrodes employed). Therefore, it is possible to individualize therapy by adjusting the various parameters that affect drug delivery.
Effect of Physicochemical Properties of Proteins
Iontophoretic delivery of proteins is restricted to molecules with a molecular weight of ~10–15 kDa. Therefore, this technique is effective for peptides and proteins that fall within this size restriction. For the peptides that do fit within this size limit, the charge of the protein determines the polarity of the electrodes to be used (anodal/cathodal). Anodal iontophoresis is more effective than cathodal iontophoresis owing to the negatively charged surface of skin and the bulk solvent flow from anode to cathode. Therefore, this enhancement method may be more effective for molecules with a high isoelectric point (pI) as they are positively charged in the formulation. The high pI also avoids precipitation of the protein within the skin by retaining its original charge (2). Molecular size and hydrodynamic radius are related to the molecular weight of a peptide and they play a role in the passage of the peptide through the skin as well.
Effect of Experimental Parameters—Current, Duration of Application, and Electrodes
As can be inferred from Faraday’s law, flux is directly proportional to the applied current. Therefore, drug delivery increases as a function of the applied current (14). This is the main advantage of iontophoretic drug delivery as it allows controlled delivery of drugs. However, it has been reported in the literature that this increase in delivery as a function of the applied current might be nonlinear in certain cases (15,16) or may plateau after a certain point (17). An example of the nonlinear dependence of flux on current density is the delivery of human parathyroid hormome (hPTH). Medi and Singh (15) have indicated that current densities of 0.2, 0.3, and 0.4 mA/cm2 did not have any significant effect on hPTH delivery. However, at 0.5 mA/cm2, hPTH delivery increased significantly. Traditionally, the acceptable maximum value for current for in vivo applications, as reported in the literature, is ~0.5 mA/cm2 (18). Higher current densities can result in burns/complications and hence must be optimized. Investigators have also studied delivery profiles upon application of current in a continuous manner as compared with a pulsatile fashion. In some cases, continuous delivery can result in de-sensitization of the related receptors in the body which will affect further drug delivery (13).
Duration of current application is another important parameter which has to be practical in order to avoid burns/complications and at the same time effective enough to deliver therapeutic levels of proteins. For some proteins, application times can be as short as 15 min., while for others, iontophoretic applications for 24 h may be required.
Iontophoresis also provides programmability of delivery as the drug flux changes with changes in current. In some unique cases, the therapeutic effect may change depending on how the drug is administered. For example, luteinizing hormone releasing hormone (LHRH) is used in the treatment of both prostate cancer and infertility. While for the former disease, it is required to deliver LHRH in a continuous manner, pulsatile delivery is more effective for treatment of infertility (19).
The type of electrodes to be employed in the iontophoretic patch or system is also important for optimal delivery. Platinum electrodes and silver–silver chloride electrodes have been used by several research groups in their studies. The silver–silver chloride electrode system seems to be a better electrode system as it avoids sharp changes in formulation pH due to electrolysis of water. Even, even though some earlier literature have indicated the use of platinum electrodes for protein delivery, it is not advisable as peptides and proteins containing disulfide bridges have been reported to degrade at platinum surfaces (20).
Formulation Considerations—Donor Concentration and Formulation pH
Concentration of peptide in the formulation is an important factor that may affect its delivery. Several researchers have reported in the literature that, for small molecules, as the concentration of the drug goes up, amount of drug delivered also increases. While peptides and proteins might be more complex compared with small molecules, they have also been shown to typically follow a similar trend (21). For example, Langkjaer et al. (22) have indicated that the amount of insulin delivered at concentrations of 1–3 mM were 30-fold higher than the amount delivered at 0.1 mM. Exceptions to this trend have also been reported for certain analogs of LHRH (23). However, as proteins are expensive, it is logical to keep the concentration at the lower limit where pharmacological effects are observed.
As already discussed, proteins have a pI and therefore, the final pH of the formulation will have an effect on the overall charge of the protein. Since anodal iontophoresis is more effective than cathodal iontophoresis, proteins with a high pI value are ideal for iontophoretic delivery as they will be positively charged at physiological pH and also in the skin which has a surface pH of 4–6 (24). Insulin is a classic example of the importance of pH/pI for iontophoretic delivery. Insulin exists as a hexamer and has a pI of 5.3 which makes it a difficult molecule to deliver iontophoretically. The final pH of the formulation has to be in the lower pH range in order to keep the insulin positively charged and this can result in irritation of the skin. Once it reaches in the skin, insulin will lose its charge and reside as a neutral molecule which can lead to formation of a depot. Therefore, it is important to consider these aspects while formulating a peptide/protein formulation for application with iontophoresis.
Limitations of Iontophoretic Delivery
The main limitation of this technique is the restriction on the size limit to ~10–15 kDa. This, therefore, limits the use of this technique to peptides and small proteins. Furthermore, the molecular size of the protein is also important as it could lead to aggregation, resulting in unsuccessful delivery. Potential malfunction of the device itself and the possibility of dose dumping also need to be considered.
MICRONEEDLES MEDIATED DELIVERY OF PROTEINS
Microneedle technology is a minimally invasive technique that has received a lot of attention in the past decade. In this technique, needles of micron-sized dimensions are employed to create micron-sized channels in the skin to overcome the stratum corneum barrier. Proteins can then pass through these channels, typically into the lower epidermis and thereby diffuse into the capillaries located in the dermal layers. Since the created channels are micron-sized in dimensions, there is no size restriction on drug molecules to be delivered via this technique. Microparticles with a size of 2 μm and higher have also been shown to be delivered via microchannels created by microneedles (25,26).
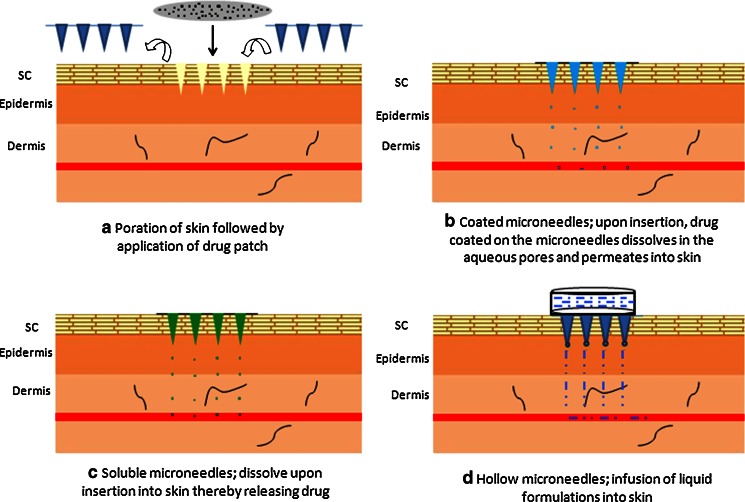
Microneedles can be broadly classified into solid microneedles and hollow microneedles. Solid microneedles are solid structures protruding out of plane while hollow microneedles have a hollow core to aid diffusion of liquid formulation (26). They can be applied in different application modes: porate the skin and place a drug-loaded patch; coat solid microneedles with the protein and porate the skin; encapsulate the protein in a biodegradable microneedle and insert into skin; or infuse a liquid formulation of the protein via hollow microneedles (Fig. 2). The application mode will depend on the objective of the study and the target therapeutic levels. For example, if high amounts of protein need to be delivered, then infusing a high concentration liquid formulation via hollow microneedles may be more effective than coated microneedles as the latter has a maximum limit of ~1 mg of drug for coating.
Fig. 2.
Different application modes of microneedle technology. a Poration of the skin with solid microneedles followed by application of drug-loaded patch; b insertion of drug-coated solid microneedles; c insertion of drug-encapsulated soluble microneedles; and d insertion of hollow microneedles for infusion of liquid formulations
Microneedle Technology—Different Modes of Application
The common mode of microneedle application is microporation followed by application of a drug-loaded patch or a liquid formulation. The aqueous pores in the skin then allow diffusion of drug from the patch into the deeper layers of the skin. Li et al. (27) employed soluble maltose microneedles and metal microneedles to permeabilize the skin and enhance the delivery of human immunoglobulin (IgG). They reported that both types of microneedles enabled IgG delivery as compared with the undetectable passive control. Several others have reported similar increases in delivery of small and macromolecules after microneedle treatment (28–33).
Coating solid microneedles with a protein/drug formulation has been reported to give encouraging results (34–38). The most common coating process is a dip-coating method where the solid microneedles are dipped in the protein formulation and air-dried. Jet-coating the formulation onto microneedles has also been tried as an alternative method. Cormier et al. (35) coated microneedles with desmopressin using the dip-coating method which resulted in therapeutic levels in hairless guinea pigs with a bioavailability of ~85%. In a different study, Widera et al. (36) studied the effect of varying coating thickness on drug delivery. Increasing amounts of ovalbumin (OVA) was coated onto titanium microneedles via the dip-coating process which resulted in a corresponding increase in ovalbumin delivery. However, as mentioned earlier, this application mode is restricted by the amount of protein/drug that can be coated onto the microneedles. It is generally considered that a maximum amount of ~1 mg can be coated on a microneedle array. Also, the formulation needs to be optimized for viscosity and protein concentration avoiding problems such as aggregation.
More recently, soluble microneedles with drug encapsulated in them are also being studied (39–42). For example, Lee et al. (42) prepared carboxymethylcellulose microneedles and encapsulated bovine serum albumin and lysozyme in them. In another study, Park et al. (41) prepared microneedles in a double-encapsulation procedure where the active was first encapsulated into soluble microparticles which were then encapsulated into soluble microneedles. This procedure allows for prolonged release of the drug. A limitation to this approach is that the amount of protein that can be loaded in the microneedles is typically less than 1–2 mg, even with having several hundreds of microneedles in a single patch (42). Increasing the drug load might in turn compromise the mechanical strength of the needles in some cases. This can perhaps be minimized by decreasing the drug load in the microneedle and coating the microneedles with some more drug. A critical point to be noted regarding drug encapsulation in soluble microneedles is the high temperatures involved in the manufacturing process. The micromoulding process usually involves high temperatures for heating the polymers/sugars in order to create a melt to be cast in the micromoulds. These high temperatures could result in denaturing of proteins. It is also important to use generally regarded as safe listed excipients while preparing soluble microneedles to ensure safety and to make regulatory approval easier.
Infusion of liquid formulations of proteins is a promising feature of this technology. In this application mode, hollow microneedles are placed on the skin to create micropathways in the skin, following which liquid formulation from a reservoir can be infused into the deeper layers of the skin. Various research groups have come up with different devices where a drug reservoir was attached to the back of an array of hollow microneedles and they differ in the mechanism by which the formulation is delivered from the reservoir, through the microneedles and into the skin (43–47). This mode is limited by the volume of formulation that can be infused as there is a back pressure from the densely packed layers of skin (48). To overcome this limitation, partial retraction of the needle following insertion has been suggested and this resulted in increased diffusion volumes (25,45). Recent literature suggests that volumes of up to ~1 mL can be infused into the skin over a few minutes with minimal or no leakage concerns. However, aggregation and syringeability are concerns for highly concentrated formulations. Also, the practicality of this approach can be challenging in terms of storage and stability of protein formulations after manufacturing and leakage issues from the reservoir.
Concerns of Microneedle Technology—Sterility and Pore Closure
Sterility of microneedles for clinical applications is a valid concern which has become a topic of discussion. Some speculate that since microneedles perturb only the superficial layers of the skin, sterility is not a mandatory requirement as they are not more invasive than a scratch on the skin. However, if a microneedle product seeks approval from FDA, sterility of the microneedles will most likely be required for regulatory approval. This can be achieved by ethylene oxide gas sterilization or by another method. This approach was used by Wermeling et al. (30) where, while assembling their stainless steel microneedles into a patch, sterility precautions were taken by assembling in a laminar flow hood followed by ethylene oxide sterilization. In another study, Ameri et al. (49) compared the effects of aseptic manufacturing and terminal sterilization on the stability of a parathyroid hormone (PTH) (1–34) patch with coated microneedles. They reported that, terminal sterilization with γ-irradiation or e-beam resulted in increased oxidation of the active in the product which could be reduced by optimizing the irradiation dose and temperature for minimal oxidation. On the other hand, the aseptic process was also found to be challenging as a component of the device, an irradiated adhesive, was incompatible with the active, thereby resulting in the need to find an alternative adhesive for the product. The authors suggest that the aseptic manufacturing approach is the effective way of assembling a sterile product (49). Alternatively, if sterility is not required, perhaps a simple dipping in ethanol for disinfection will help. Sterilization of microneedles and related products is an important issue which needs to be investigated further.
Following microporation, duration of time taken for the pores to close is also an important concern as it may potentially result in complications such as irritation and infection at the site, affecting delivery. Recent literature has tried to address this problem in several ways. Kalluri and Banga studied pore closure in a hairless rat model following treatment with soluble microneedles. They reported that while skin barrier function restored within 3–4 h after poration, complete closure of pores was not observed until 15 h in vivo. They also reported that occlusion with a plastic film or any solution delayed pore closure for up to 72 h in vivo (50). Haq et al. (51) and Gupta (52) have also studied pore closure in humans using staining and skin impendance measurements respectively. Haq et al. compared the healing kinetics of skin after insertion of microneedles to a 25 G hypodermic needle insertion. While the microneedle treated skin showed signs of healing 8–24 h after poration, disruption caused by the hypodermic needle was significantly more even at 24 h postinsertion (51). This reversible nature of microchannel closure is a desirable feature of this technology as it is required for the microchannels to be open for the duration of patch application. Following removal of the patch, skin will regain its barrier function at a faster rate since occlusive conditions have been removed.
To study the safety of this technology in terms of infections, Donnelly et al. studied the permeation efficiencies of three strains: Gram positive (S. epidermidis), Gram negative (P. aeruginosa), and fungi (C. albicans), across microporated silicone membrane and excised porcine skin. All three strains had permeation levels less than the control, a single 21 G hypodermic needle puncture (53). This permeation of different strains can further be controlled or reduced by cleaning the application site with 70% isopropanol before poration, as used by Cormier et al. (35). However, application of alcohol to skin may affect its barrier properties to a certain degree.
Considering the advantages and safety of this technology, microneedles mediated transdermal delivery is an exciting area for delivering peptides and proteins.
ELECTROPORATION
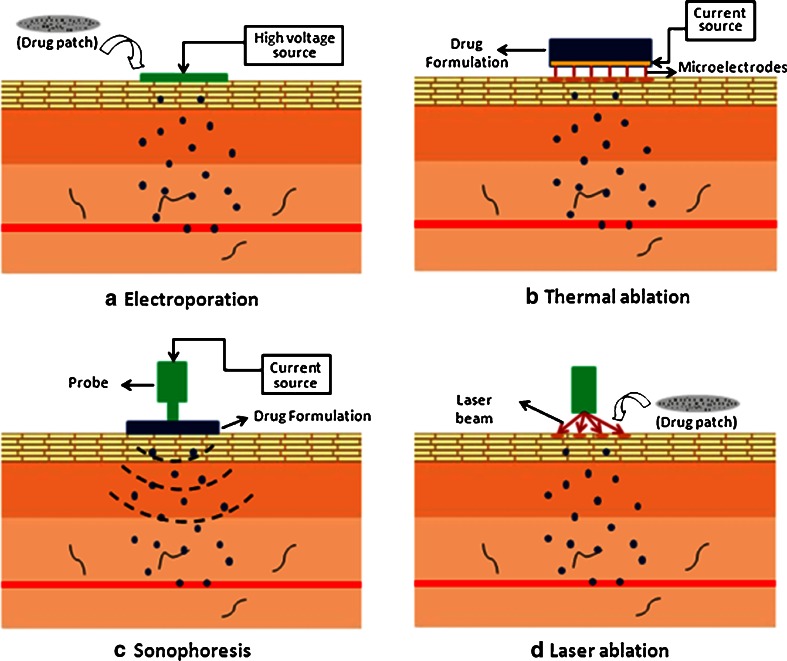
Electroporation was originally used to transfect cells with macromolecules such as DNA by altering their cell membranes in a reversible manner (2). It involves application of high voltage in the form of pulses for short durations of time, typically fractions of a second (~1–100 ms) which tend to increase the permeability of cell membranes by opening the aqueous pores in a reversible manner (Fig. 3a). This technique was then investigated for application in transdermal drug delivery for delivering a wide range of therapeutics including macromolecules such as peptides and proteins. Electroporation of skin requires higher voltages in the order of greater than 50 V (54,55). Even though it involves the application of an electric field similar to iontophoresis, they differ in principle. While iontophoresis directly acts on the drug molecule itself to propel it into the skin, electroporation acts mainly on the skin to alter its permeability in order to enhance protein delivery.
Fig. 3.
Schematic showing the principles of a electroporation, b thermal ablation, c sonophoresis, and d laser ablation. a Electroporation: application of high voltage alters the permeability of the skin and enhances drug delivery; b thermal ablation: an electric pulse is converted to thermal energy on the microfilaments, thereby causing localized high temperature on the stratum corneum surface which vaporizes a localized region to create a micropore; c sonophoresis: activation of the probe transmits low-frequency waves which alter the permeability of the skin by cavitation and local heating; d laser ablation: activation of the probe transmits a laser beam which heats the skin surface causing the water molecules to evaporate rapidly thereby creating microchannels in the epidermis
Studies have been reported indicating the effectiveness of electroporation for transdermal delivery of proteins. For example, Zhao et al. (56) have shown that electroporation enabled delivery of therapeutic levels of a model peptide in a mouse model which elicited the desired immune response as observed by the increase in peptide-specific cytotoxic T lymphocytes. This response was comparable to that of its intradermal injection control. They have also reported that pulsing alone (without the active drug) enhanced migration of the epidermal Langerhans cells which could further aid in vaccination (56). In another study, Chang et al. (57) investigated the effects of different electroporation parameters on salmon calcitonin delivery in the presence of a constant electric field (iontophoresis). It was reported that an increase in the applied voltage from 60 to 100 V significantly increased calcitonin levels. In a similar study by Medi and Singh (15), application of pulses at 100, 200, and 300 V increased delivery of hPTH by 6.9-, 16.5-, and 20.4-fold respectively, as compared with the passive control. However, it is to be noted that skin properties can be altered at such high voltages which may result in nonlinear dependence of flux on voltage in some cases. In most cases, these changes are reversible but the time taken to recover may vary significantly (58–61). Chang et al. (57) also reported that changing the pattern of pulsing, keeping the total number of pulses constant, did not have an effect on calcitonin delivery.
Combination of this technique with other enhancement methods such as iontophoresis has been reported to deliver significantly high levels of several proteins and peptides. This has been discussed later in the paper. Therefore, electroporation has the potential to deliver peptides and proteins. However, it still remains to be seen if this technology makes it to the market as the voltages used are generally very high for skin tolerability. This technique may have more applications for electrochemotherapy, which is outside the scope of this review.
THERMAL AND RADIOFREQUENCY ABLATION
Thermal ablation enhances drug delivery by ablation of the stratum corneum upon application of high temperature for short periods of time (fractions of a second). Altea therapeutics (Atlanta, GA) has developed a patented technology, the PassPort® patch, which consists of metallic filaments. When an electric pulse is applied on the filaments, it is converted to thermal energy, thereby causing localized high temperature on the stratum corneum surface which vaporizes the region (62) (Fig. 3b). This process creates microchannels through which a wide range of drug molecules can pass into the deeper layers of the skin. The created microchannels have been reported to be about 50–200 μm in width and 30–50 μm in depth. Therefore, the ablation process does not cause any damage to the deeper layers of the skin (63). After ablation, skin starts its healing process and the stratum corneum layer regenerates over a period of time. However, the time taken for this has not yet been reported in the literature. Altea therapeutics has reported preclinical data for several molecules including parathyroid hormone, interferon-α and hepatitis B antigen. Currently, clinical trials are also underway for the delivery of insulin and some other drug moieties (63). This technology has the potential for delivering biopharmaceuticals.
Radiofrequency ablation also involves creation of aqueous micropathways in the skin. This technology is being developed by TransPharma Medical™ Ltd (Lod, Israel). The handheld applicator device, ViaDorTM, consists of closely spaced microelectrodes (1 cm2 array) and a drug-loaded patch. Upon application, radiofrequency waves (100–500 kHz) cause the microelectrodes to vibrate on the skin surface, resulting in localized heating and ablation of the stratum corneum in those regions. It has been reported that this process creates about 102 micropathways/cm2 of skin which are ~50 μm deep and ~30–50 μm wide (63). Human studies have indicated the safety of this device with minimal or no irritation (64). Radiofrequency ablation has been reported to enhance delivery of human growth hormone in rats and guinea pigs with bioavailabilities of 75% and 33%, respectively (65). TransPharma is currently developing a patch for human growth hormone with Teva Pharmaceuticals (Israel) (63). It is also co-developing a product for delivering hPTH (1–34) for treatment of osteoporosis in postmenopausal women, in collaboration with Eli Lilly. TransPharma has recently reported the successful completion of Phase II clinical trials for this product and has also indicated that they are currently in Phase I clinical trials for delivering two additional peptide drugs via the ViaDorTM system (64).
SONOPHORESIS/PHONOPHORESIS
Sonophoresis involves the application of low-frequency (20 kHz) ultrasound waves which induces the air pockets present in the stratum corneum layer to grow and oscillate, thereby disrupting the lipid bilayers and creating cavities which then increase permeability of skin (Fig. 3c). This process is known as cavitation. However, these structural changes are limited to only the superficial layers of the skin (66) and barrier properties of skin are restored after a period of time. Sonophoresis has been used for a while in physical therapy clinics for the localized treatment of pain and other indications. Several research articles have also indicated the effectiveness of this technique to deliver peptides and proteins such as insulin (67–71), heparin (72), γ-interferon and erythropoietin (70) along with other hormones (73) and oligoneucleotides (74). However, the degree of enhancement in drug delivery is highly dependent on the physicochemical properties of the drug (75). This technique has also been shown to have applications in noninvasive sampling of biological fluids such as glucose.
Echo therapeutics (formerly Sontra Medical Corporation) developed the SonoPrep® system which emits ultrasound waves of 55 kHz. This device uses short bursts of waves for short periods of time which causes cavitation in the skin. Application of sonophoresis using the SonoPrep® system increased insulin delivery in a porcine model resulting in low blood glucose levels (76). Marketing of this device is currently discontinued.
Even though this method enhanced delivery of a wide range of therapeutic compounds, the practicality of its administration for self use is limited at this time.
LASER-ASSISTED DELIVERY OF PROTEINS
This technique involves abrasion of the stratum corneum layer by the application of a laser beam which heats the skin surface causing the water molecules to evaporate rapidly thereby creating microchannels in the epidermis (Fig. 3a). Pantech Biosolutions AG (Ruggell, Liechtenstein) has developed Painless Laser Epidermal System technology. It is a handheld device equipped with an erbium-doped yttrium aluminum garnet (Er/YAG) laser which emits light at a wavelength of 2,940 nm (63) that results in the creation of microchannels with a diameter of ~200 μm. The depth of microchannels can be controlled over a range of 20–150 μm and the number of microchannels created can also be controlled by varying the application parameters (77). A human study with 12 volunteers was conducted to test the safety of this device. It was reported that >90% of the subjects reported no or minimal discomfort. Slight redness was observed which disappeared within a few hours per day. Skin biopsies and histological examination confirmed that the procedure did not damage the surrounding tissue (77).
Norwood Abbey (Melbourne, Australia) uses a laser-assisted drug delivery technology named Epiture Easytouch™ which has an Er/YAG laser. Clinical studies are currently underway for delivering lidocaine for local anesthesia (63,78,79). This procedure has also been reported to be safe with low incidence of adverse effects of minor nature (78). A few subjects reported hyperpignmentation at the application site which resolved within a few weeks (79). This device has the potential for delivering peptides and proteins.
JET INJECTORS
Jet injection uses the principle of application of high velocities such that the drug formulation is propelled from the reservoir at a high speed, bombarding the skin surface and abrading it simultaneously thereby creating superficial micropathways in the skin. This breach of stratum corneum layer aids in delivery of powder and liquid formulations (80–83).
PowderJect® technology, developed by PowderJect® pharmaceuticals and now owned by other companies, uses helium gas for the ballistic delivery of powder formulations coated onto gold particles or embedded in particles (82). Osorio et al. (83) used the PowderJect® NDI system to deliver powder vaccines into the epidermis. They reported that the response achieved via this jet system approach was comparable to the intramuscular injection, if not better. Dean et al. (81) have also reported the efficacy of this approach to delivery protein vaccines and DNA into the epidermis. Kendall et al. (84) further reported that the relative humidity and temperature of the environment have a significant effect on delivery. An increase in these parameters resulted in a corresponding increase in particulate delivery by 1.8- and 2-fold, respectively. Needle-free liquid jet injectors have also been used to deliver macromolecules such as insulin, human growth hormone and vaccines along with several other small molecules (80).
This needle-free approach seems promising and has been used extensively for delivering vaccines in the past. However, its practical application for treatment of diseases which require repeated administrations (such as diabetes) may not be as promising as compared to some other emerging technologies due to compliance issues involving pain and redness (80).
COMBINATION STRATEGIES
Enhancement techniques can be combined in order to achieve a synergistic effect on drug delivery. The enhancement techniques discussed in this review have been investigated in different combinations such as iontophoresis and microneedles, iontophoresis and chemical enhancers, iontophoresis and electroporation, microneedles, and sonophoresis and so on. Some of these studies are briefly discussed in this section. Pretreatment with chemical enhancers compromises the barrier properties of the skin and further application of iontophoresis enhances the movement of peptides into the skin. This combination treatment has been investigated by Pillai et al. (85). Rat skin was pretreated with two different chemical enhancers, menthone or linoleic acid, followed by iontophoretic treatment. Both combinations results in the highest delivery of insulin as compared with their respective controls.
Poration of skin followed by application of iontoporesis is another effective combination approach. Following pretreatment with microneedles, the diffusion path of the drug molecules is significantly reduced as the microchannels reach into the upper layers of the dermis in most cases. Application of iontophoresis on pretreated skin further pushes the charged molecules down these pathways and into the deeper layers of the skin. This combination approach has been reported to increase drug delivery by several fold (29,86–88). For example, Daniplestim, a peptide with a molecular weight of 13 kDa was shown to have higher delivery levels after the combination treatment as compared with iontophoresis alone (87). In another study by Vemulapalli et al. (88), combination treatment resulted in a 25-fold increase in methotrexate delivery, in vivo.
Pretreatment with electroporation, followed by iontophoresis also results in an enhancement. This combination approach has been reported to be more effective than either enhancement method alone (15,57,89,90). As mentioned earlier, Chang et al. (57) have reported that combination therapy resulted in a 17-fold increase in the delivery of PTH as compared with iontophoresis alone. In another study, combination treatment gave a 5- to 10-fold increase in LHRH flux through human skin (89).
Combination of sonophoresis with chemical enhancers (91), electroporation (92), and iontophoresis (93) has also been reported to enhance drug delivery.
CONCLUSIONS
Transdermal route for delivering protein therapeutics is actively being pursued with several new emerging technologies that can now enable delivery of hydrophilic macromolecules across the skin. Some active/physical enhancement products are currently available in the market and more products are in clinical trials/developmental stages.
REFERENCES
- 1.Antosova Z, Mackova M, Kral V, Macek T. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 2009;27(11):628–635. doi: 10.1016/j.tibtech.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Weert M, Jorgensen L, Horn Moeller E, Frokjaer S. Factors of importance for a successful delivery system for proteins. Expert Opin Drug Deliv. 2005;2(6):1029–1037. doi: 10.1517/17425247.2.6.1029. [DOI] [PubMed] [Google Scholar]
- 4.Steinstrasser I, Merkle HP. Dermal metabolism of topically applied drugs: pathways and models reconsidered. Pharm Acta Helv. 1995;70(1):3–24. doi: 10.1016/0031-6865(94)00047-Y. [DOI] [PubMed] [Google Scholar]
- 5.Tauber U. Drug metabolism in the skin: advantages and disadvantages. In: Hadgraft J, Guy RH, editors. Transdermal drug delivery: developmental issues and research initiatives. New York: Marcel Dekker; 1989. pp. 99–112. [Google Scholar]
- 6.Magnusson BM, Runn P. Effect of penetration enhancers on the permeation of the thyrotropin releasing hormone analogue pGlu-3-methyl-His-Pro amide through human epidermis. Int J Pharm. 1999;178(2):149–159. doi: 10.1016/S0378-5173(98)00316-0. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Shen Y, Guo X, Zhang C, Yang W, Ma M, et al. Transdermal protein delivery by a coadministered peptide identified via phage display. Nat Biotechnol. 2006;24(4):455–460. doi: 10.1038/nbt1193. [DOI] [PubMed] [Google Scholar]
- 8.Frankenburg S, Grinberg I, Bazak Z, Fingerut L, Pitcovski J, Gorodetsky R, et al. Immunological activation following transcutaneous delivery of HR-gp100 protein. Vaccine. 2007;25(23):4564–4570. doi: 10.1016/j.vaccine.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foldvari M, Baca-Estrada ME, He Z, Hu J, Attah-Poku S, King M. Dermal and transdermal delivery of protein pharmaceuticals: lipid-based delivery systems for interferon alpha. Biotechnol Appl Biochem. 1999;30(Pt 2):129–137. [PubMed] [Google Scholar]
- 10.Caccetta R, Blanchfield JT, Harrison J, Toth I, Benson HAE. Epidermal penetration of a therapeutic peptide by lipid conjugation; stereo-selective peptide availability of a topical distereomeric lipopeptide. Int J Pept Res Ther. 2006;12:327–333. doi: 10.1007/s10989-006-9024-5. [DOI] [Google Scholar]
- 11.Jain S, Jain P, Umamaheshwari RB, Jain NK. Transfersomes—a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation. Drug Dev Ind Pharm. 2003;29(9):1013–1026. doi: 10.1081/DDC-120025458. [DOI] [PubMed] [Google Scholar]
- 12.Abla N, Naik A, Guy RH, Kalia YN. Contributions of electromigration and electroosmosis to peptide iontophoresis across intact and impaired skin. J Control Release. 2005;108(2–3):319–330. doi: 10.1016/j.jconrel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Green PG. Iontophoretic delivery of peptide drugs. J Control Release. 1996;41:33–48. doi: 10.1016/0168-3659(96)01354-5. [DOI] [Google Scholar]
- 14.Lau DT, Sharkey JW, Petryk L, Mancuso FA, Yu Z, Tse FL. Effect of current magnitude and drug concentration on iontophoretic delivery of octreotide acetate (Sandostatin) in the rabbit. Pharm Res. 1994;11(12):1742–1746. doi: 10.1023/A:1018963300092. [DOI] [PubMed] [Google Scholar]
- 15.Medi BM, Singh J. Electronically facilitated transdermal delivery of human parathyroid hormone (1–34) Int J Pharm. 2003;263(1–2):25–33. doi: 10.1016/S0378-5173(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 16.Prausnitz MR, Lee CR, Liu CH, Pang JC, Singh TP, Langer R, et al. Transdermal transport efficiency during skin electroporation and iontophoresis. J Control Release. 1996;38:205–217. doi: 10.1016/0168-3659(95)00121-2. [DOI] [Google Scholar]
- 17.Miller LL, Kolaskie CJ, Smith GA, Rivier J. Transdermal iontophoresis of gonadotropin releasing hormone (LHRH) and two analogues. J Pharm Sci. 1990;79(6):490–493. doi: 10.1002/jps.2600790607. [DOI] [PubMed] [Google Scholar]
- 18.Schuetz YB, Naik A, Guy RH, Vuaridel E, Kalia YN. Transdermal iontophoretic delivery of triptorelin in vitro. J Pharm Sci. 2005;94(10):2175–2182. doi: 10.1002/jps.20433. [DOI] [PubMed] [Google Scholar]
- 19.Nestor JJ, Jr, Ho TL, Simpson RA, Horner BL, Jones GH, McRae GI, et al. Synthesis and biological activity of some very hydrophobic superagonist analogues of luteinizing hormone-releasing hormone. J Med Chem. 1982;25(7):795–801. doi: 10.1021/jm00349a006. [DOI] [PubMed] [Google Scholar]
- 20.Pikal MJ. Penetration enhancement of peptide and protein drugs by electrochemical means: transdermal iontophoresis. In: Lee VHL, Hashida M, Mizushima Y, editors. Trends and future perspectives in peptide and protein drug delivery. Chur: Harwood Academic Publishers GmbH; 1995. [Google Scholar]
- 21.Vemulapalli V, Banga AK, Friden PM. Optimization of iontophoretic parameters for the transdermal delivery of methotrexate. Drug Deliv. 2008;15(7):437–442. doi: 10.1080/10717540802035145. [DOI] [PubMed] [Google Scholar]
- 22.Langkjaer L, Brange J, Grodsky GM, Guy RH. Iontophoresis of monomeric insulin analogues in vitro: effects of insulin charge and skin pretreatment. J Control Release. 1998;51(1):47–56. doi: 10.1016/S0168-3659(97)00155-7. [DOI] [PubMed] [Google Scholar]
- 23.Hoogstraate AJ, Srinivasan V, Sims SM, Higuchi WI. Iontophoretic enhancement of peptides: behaviour of leuprolide versus model permeants. J Control Release. 1994;31:41–47. doi: 10.1016/0168-3659(94)90249-6. [DOI] [Google Scholar]
- 24.Banga AK. Therapeutic peptides and proteins. 2. New York: Taylor & Francis; 2006. [Google Scholar]
- 25.Wang PM, Cornwell M, Hill J, Prausnitz MR. Precise microinjection into skin using hollow microneedles. J Invest Dermatol. 2006;126(5):1080–1087. doi: 10.1038/sj.jid.5700150. [DOI] [PubMed] [Google Scholar]
- 26.Kalluri H, Banga AK. Microneedles and transdermal drug delivery. J Drug Del Sci Tech. 2009;19(5):303–310. [Google Scholar]
- 27.Li G, Badkar A, Kalluri H, Banga AK. Microchannels created by sugar and metal microneedles: characterization by microscopy, macromolecular flux and other techniques. J Pharm Sci. 2010;99(4):1931–1941. doi: 10.1002/jps.21981. [DOI] [PubMed] [Google Scholar]
- 28.Verbaan FJ, Bal SM, van den Berg DJ, Groenink WH, Verpoorten H, Luttge R, et al. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J Control Release. 2007;117(2):238–245. doi: 10.1016/j.jconrel.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Wu XM, Todo H, Sugibayashi K. Enhancement of skin permeation of high molecular compounds by a combination of microneedle pretreatment and iontophoresis. J Control Release. 2007;118(2):189–195. doi: 10.1016/j.jconrel.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Wermeling DP, Banks SL, Hudson DA, Gill HS, Gupta J, Prausnitz MR, et al. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci USA. 2008;105(6):2058–2063. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin W, Cormier M, Samiee A, Griffin A, Johnson B, Teng CL, et al. Transdermal delivery of antisense oligonucleotides with microprojection patch (Macroflux) technology. Pharm Res. 2001;18(12):1789–1793. doi: 10.1023/A:1013395102049. [DOI] [PubMed] [Google Scholar]
- 32.Kolli CS, Banga AK. Characterization of solid maltose microneedles and their use for transdermal delivery. Pharm Res. 2008;25(1):104–113. doi: 10.1007/s11095-007-9350-0. [DOI] [PubMed] [Google Scholar]
- 33.Chabri F, Bouris K, Jones T, Barrow D, Hann A, Allender C, et al. Microfabricated silicon microneedles for nonviral cutaneous gene delivery. Br J Dermatol. 2004;150(5):869–877. doi: 10.1111/j.1365-2133.2004.05921.x. [DOI] [PubMed] [Google Scholar]
- 34.Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24(7):1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]
- 35.Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD, et al. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97(3):503–511. doi: 10.1016/j.jconrel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24(10):1653–1664. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 37.Xie Y, Xu B, Gao Y. Controlled transdermal delivery of model drug compounds by MEMS microneedle array. Nanomedicine. 2005;1(2):184–190. doi: 10.1016/j.nano.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19(1):63–70. doi: 10.1023/A:1013607400040. [DOI] [PubMed] [Google Scholar]
- 39.Ito Y, Hagiwara E, Saeki A, Sugioka N, Takada K. Feasibility of microneedles for percutaneous absorption of insulin. Eur J Pharm Sci. 2006;29(1):82–88. doi: 10.1016/j.ejps.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan SP, Murthy N, Prausnitz MR. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Adv Mater. 2008;20:933–938. doi: 10.1002/adma.200701205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park JH, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharm Res. 2006;23(5):1008–1019. doi: 10.1007/s11095-006-0028-9. [DOI] [PubMed] [Google Scholar]
- 42.Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29(13):2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roxhed N, Griss P, Stemme G. Membrane-sealed hollow microneedles and related administration schemes for transdermal drug delivery. Biomed Microdevices. 2008;10(2):271–279. doi: 10.1007/s10544-007-9133-8. [DOI] [PubMed] [Google Scholar]
- 44.Paik SJ, Byun S, Lim JM, Park Y, Lee A, Chung S, et al. In-plane single-crystal-silicon microneedles for minimally invasive microfluid systems. Sens Actuators A. 2004;114:276–284. doi: 10.1016/j.sna.2003.12.029. [DOI] [Google Scholar]
- 45.Martanto W, Moore JS, Kashlan O, Kamath R, Wang PM, O’Neal JM, et al. Microinfusion using hollow microneedles. Pharm Res. 2006;23(1):104–113. doi: 10.1007/s11095-005-8498-8. [DOI] [PubMed] [Google Scholar]
- 46.Nordquist L, Roxhed N, Griss P, Stemme G. Novel microneedle patches for active insulin delivery are efficient in maintaining glycaemic control: an initial comparison with subcutaneous administration. Pharm Res. 2007;24(7):1381–1388. doi: 10.1007/s11095-007-9256-x. [DOI] [PubMed] [Google Scholar]
- 47.Teo MA, Shearwood C, Ng KC, Lu J, Moochhala S. In vitro and in vivo characterization of MEMS microneedles. Biomed Microdevices. 2005;7(1):47–52. doi: 10.1007/s10544-005-6171-y. [DOI] [PubMed] [Google Scholar]
- 48.Martanto W, Moore JS, Couse T, Prausnitz MR. Mechanism of fluid infusion during microneedle insertion and retraction. J Control Release. 2006;112(3):357–361. doi: 10.1016/j.jconrel.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 49.Ameri M, Wang X, Maa YF. Effect of irradiation on parathyroid hormone PTH(1–34) coated on a novel transdermal microprojection delivery system to produce a sterile product—adhesive compatibility. J Pharm Sci. 2010;99(4):2123–2134. doi: 10.1002/jps.21985. [DOI] [PubMed] [Google Scholar]
- 50.Kalluri H, Banga AK. Formation and Closure of Microchannels in Skin Following Microporation. Pharm Res [serial on the Internet]. 2010: Available from: http://www.ncbi.nlm.nih.gov/pubmed/20354766. [DOI] [PubMed]
- 51.Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices. 2009;11(1):35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 52.Gupta J. Microneedles for transdermal drug delivery in human subjects. Atlanta: Georgia Institute of Technology; 2009. [Google Scholar]
- 53.Donnelly RF, Singh TR, Tunney MM, Morrow DI, McCarron PA, O’Mahony C, et al. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm Res. 2009;26(11):2513–2522. doi: 10.1007/s11095-009-9967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prausnitz MR. Do high-voltage pulses cause changes in skin structure? J Control Release. 1996;40:321–326. doi: 10.1016/0168-3659(95)00199-9. [DOI] [Google Scholar]
- 55.Edwards DA, Prausnitz MR, Langer R, Weaver JC. Analysis of enhanced transdermal transport by skin electroporation. J Control Release. 1995;34:211–221. doi: 10.1016/0168-3659(94)00132-E. [DOI] [Google Scholar]
- 56.Zhao YL, Murthy SN, Manjili MH, Guan LJ, Sen A, Hui SW. Induction of cytotoxic T-lymphocytes by electroporation-enhanced needle-free skin immunization. Vaccine. 2006;24(9):1282–1290. doi: 10.1016/j.vaccine.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 57.Chang SL, Hofmann GA, Zhang L, Deftos LJ, Banga AK. The effect of electroporation on iontophoretic transdermal delivery of calcium regulating hormones. J Control Release. 2000;66(2–3):127–133. doi: 10.1016/S0168-3659(99)00262-X. [DOI] [PubMed] [Google Scholar]
- 58.Prausnitz MR, Pliquett U, Langer R, Weaver JC. Rapid temporal control of transdermal drug delivery by electroporation. Pharm Res. 1994;11(12):1834–1837. doi: 10.1023/A:1018944223290. [DOI] [PubMed] [Google Scholar]
- 59.Vanbever R, Lecouturier N, Preat V. Transdermal delivery of metoprolol by electroporation. Pharm Res. 1994;11(11):1657–1662. doi: 10.1023/A:1018930425591. [DOI] [PubMed] [Google Scholar]
- 60.Prausnitz MR, Edelman ER, Gimm JA, Langer R, Weaver JC. Transdermal delivery of heparin by skin electroporation. Biotechnology (NY) 1995;13(11):1205–1209. doi: 10.1038/nbt1195-1205. [DOI] [PubMed] [Google Scholar]
- 61.Prausnitz MR, Bose VG, Langer R, Weaver JC. Electroporation of mammalian skin: a mechanism to enhance transdermal drug delivery. Proc Natl Acad Sci USA. 1993;90(22):10504–10508. doi: 10.1073/pnas.90.22.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Therapeutics A. PassPort Patch. 2010; Available from: http://www.alteatherapeutics.com/. Accessed June 2010
- 63.Banga AK. Microporation applications for enhancing drug delivery. Expert Opin Drug Deliv. 2009;6(4):343–354. doi: 10.1517/17425240902841935. [DOI] [PubMed] [Google Scholar]
- 64.TransPharma. ViaDerm system. 2010; Available from: http://www.transpharma-medical.com/viaderm_system.html. Accessed June 2010
- 65.Levin G, Gershonowitz A, Sacks H, Stern M, Sherman A, Rudaev S, et al. Transdermal delivery of human growth hormone through RF-microchannels. Pharm Res. 2005;22(4):550–555. doi: 10.1007/s11095-005-2498-6. [DOI] [PubMed] [Google Scholar]
- 66.Mitragotri S, Kost J. Low-frequency sonophoresis: a review. Adv Drug Deliv Rev. 2004;56(5):589–601. doi: 10.1016/j.addr.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 67.Luis J, Park EJ, Meyer RJ, Smith NB. Rectangular cymbal arrays for improved ultrasonic transdermal insulin delivery. J Acoust Soc Am. 2007;122(4):2022–2030. doi: 10.1121/1.2769980. [DOI] [PubMed] [Google Scholar]
- 68.Lee S, Snyder B, Newnham RE, Smith NB. Noninvasive ultrasonic transdermal insulin delivery in rabbits using the light-weight cymbal array. Diabetes Technol Ther. 2004;6(6):808–815. doi: 10.1089/dia.2004.6.808. [DOI] [PubMed] [Google Scholar]
- 69.Smith NB, Lee S, Shung KK. Ultrasound-mediated transdermal in vivo transport of insulin with low-profile cymbal arrays. Ultrasound Med Biol. 2003;29(8):1205–1210. doi: 10.1016/S0301-5629(03)00908-6. [DOI] [PubMed] [Google Scholar]
- 70.Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995;269(5225):850–853. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- 71.Tachibana K, Tachibana S. Use of ultrasound to enhance the local anesthetic effect of topically applied aqueous lidocaine. Anesthesiology. 1993;78:1091–1096. doi: 10.1097/00000542-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 72.Mitragotri S, Kost J. Transdermal delivery of heparin and low-molecular weight heparin using low-frequency ultrasound. Pharm Res. 2001;18(8):1151–1156. doi: 10.1023/A:1010979010907. [DOI] [PubMed] [Google Scholar]
- 73.El-Kamel AH, Al-Fagih IM, Alsarra IA. Effect of sonophoresis and chemical enhancers on testosterone transdermal delivery from solid lipid microparticles: an in vitro study. Curr Drug Deliv. 2008;5(1):20–26. doi: 10.2174/156720108783331014. [DOI] [PubMed] [Google Scholar]
- 74.Tezel A, Dokka S, Kelly S, Hardee GE, Mitragotri S. Topical delivery of anti-sense oligonucleotides using low-frequency sonophoresis. Pharm Res. 2004;21(12):2219–2225. doi: 10.1007/s11095-004-7674-6. [DOI] [PubMed] [Google Scholar]
- 75.Mitragotri S, Blankschtein D, Langer R. An explanation for the variation of the sonophoretic transdermal transport enhancement from drug to drug. J Pharm Sci. 1997;86(10):1190–1192. doi: 10.1021/js960528v. [DOI] [PubMed] [Google Scholar]
- 76.Banga AK. New technologies to allow transdermal delivery of therapeutic proteins and small water-soluble drugs. Am J Drug Deliv. 2006;4(4):221–230. doi: 10.2165/00137696-200604040-00005. [DOI] [Google Scholar]
- 77.Biosolutions P. P.L.E.A.S.E. platform. 2010; Available from: http://www.pantec-biosolutions.com/. Accessed June 2010
- 78.Abbey N. Laser Assisted Drug Delivery (LAD). 2010; Available from: www.norwoodabbey.com. Accessed June 2010
- 79.Koh JL, Harrison D, Swanson V, Norvell DC, Coomber DC. A comparison of laser-assisted drug delivery at two output energies for enhancing the delivery of topically applied LMX-4 cream prior to venipuncture. Anesth Analg. 2007;104(4):847–849. doi: 10.1213/01.ane.0000257925.36641.9e. [DOI] [PubMed] [Google Scholar]
- 80.Mitragotri S. Current status and future prospects of needle-free liquid jet injectors. Nat Rev Drug Discov. 2006;5(7):543–548. doi: 10.1038/nrd2076. [DOI] [PubMed] [Google Scholar]
- 81.Dean HJ, Fuller D, Osorio JE. Powder and particle-mediated approaches for delivery of DNA and protein vaccines into the epidermis. Comp Immunol Microbiol Infect Dis. 2003;26(5–6):373–388. doi: 10.1016/S0147-9571(03)00021-3. [DOI] [PubMed] [Google Scholar]
- 82.Chen D, Payne LG. Targeting epidermal Langerhans cells by epidermal powder immunization. Cell Res. 2002;12(2):97–104. doi: 10.1038/sj.cr.7290115. [DOI] [PubMed] [Google Scholar]
- 83.Osorio JE, Zuleger CL, Burger M, Chu Q, Payne LG, Chen D. Immune responses to hepatitis B surface antigen following epidermal powder immunization. Immunol Cell Biol. 2003;81(1):52–58. doi: 10.1046/j.1440-1711.2003.01134.x. [DOI] [PubMed] [Google Scholar]
- 84.Kendall M, Rishworth S, Carter F, Mitchell T. Effects of relative humidity and ambient temperature on the ballistic delivery of micro-particles to excised porcine skin. J Invest Dermatol. 2004;122(3):739–746. doi: 10.1111/j.0022-202X.2004.22320.x. [DOI] [PubMed] [Google Scholar]
- 85.Pillai O, Panchagnula R. Transdermal delivery of insulin from poloxamer gel: ex vivo and in vivo skin permeation studies in rat using iontophoresis and chemical enhancers. J Control Release. 2003;89(1):127–140. doi: 10.1016/S0168-3659(03)00094-4. [DOI] [PubMed] [Google Scholar]
- 86.Badkar AV, Smith AM, Eppstein JA, Banga AK. Transdermal delivery of interferon alpha-2B using microporation and iontophoresis in hairless rats. Pharm Res. 2007;24(7):1389–1395. doi: 10.1007/s11095-007-9308-2. [DOI] [PubMed] [Google Scholar]
- 87.Katikaneni S, Badkar A, Nema S, Banga AK. Molecular charge mediated transport of a 13 kD protein across microporated skin. Int J Pharm. 2009;378(1–2):93–100. doi: 10.1016/j.ijpharm.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 88.Vemulapalli V, Yang Y, Friden PM, Banga AK. Synergistic effect of iontophoresis and soluble microneedles for transdermal delivery of methotrexate. J Pharm Pharmacol. 2008;60(1):27–33. doi: 10.1211/jpp.60.1.0004. [DOI] [PubMed] [Google Scholar]
- 89.Bommannan DB, Tamada J, Leung L, Potts RO. Effect of electroporation on transdermal iontophoretic delivery of luteinizing hormone releasing hormone (LHRH) in vitro. Pharm Res. 1994;11(12):1809–1814. doi: 10.1023/A:1018983804635. [DOI] [PubMed] [Google Scholar]
- 90.Riviere JE, Monteiro-Riviere NA, Rogers RA, Bommannan D, Tamada JA, Potts RO. Pulsatile transdermal delivery of LHRH using electroporation: drug delivery and skin toxicology. J Control Release. 1995;36:229–233. doi: 10.1016/0168-3659(95)00036-8. [DOI] [Google Scholar]
- 91.Mutalik S, Parekh HS, Davies NM, Udupa N. A combined approach of chemical enhancers and sonophoresis for the transdermal delivery of tizanidine hydrochloride. Drug Deliv. 2009;16(2):82–91. doi: 10.1080/10717540802605053. [DOI] [PubMed] [Google Scholar]
- 92.Kost J, Pliquett U, Mitragotri S, Yamamoto A, Langer R, Weaver J. Synergistic effect of electric field and ultrasound on transdermal transport. Pharm Res. 1996;13(4):633–638. doi: 10.1023/A:1016070710397. [DOI] [PubMed] [Google Scholar]
- 93.Le L, Kost J, Mitragotri S. Combined effect of low-frequency ultrasound and iontophoresis: applications for transdermal heparin delivery. Pharm Res. 2000;17(9):1151–1154. doi: 10.1023/A:1026426317413. [DOI] [PubMed] [Google Scholar]