Abstract
Bilayer nicotine mucoadhesive patches were prepared and evaluated to determine the feasibility of the formulation as a nicotine replacement product to aid in smoking cessation. Nicotine patches were prepared using xanthan gum or carbopol 934 as a mucoadhesive polymers and ethyl cellulose as a backing layer. The patches were evaluated for their thickness, weight and content uniformity, swelling behavior, drug–polymers interaction, adhesive properties, and drug release. The physicochemical interactions between nicotine and the polymers were investigated by Fourier transform infrared (FTIR) spectroscopy. Mucoadhesion was assessed using two-arm balance method, and the in vitro release was studied using the Franz cell. FTIR revealed that there was an acid base interaction between nicotine and carbopol as well as nicotine and xanthan. Interestingly, the mucoadhesion and in vitro release studies indicated that this interaction was strong between the drug and carbopol whereas it was weak between the drug and xanthan. Loading nicotine concentration to non-medicated patches showed a significant decrease in the mucoadhesion strength of carbopol patches and no significant effect on the mucoadhesion strength of xanthan patches. In vitro release studies of the xanthan patches showed a reasonable fast initial release profile followed by controlled drug release over a 10-h period.
KEY WORDS: carbopol, FTIR, nicotine, Patches, Xanthan
INTRODUCTION
Nicotine is the primary component of tobacco, and it has a number of complex and sometimes unpredictable effects on the brain. It is recognized as one of the most frequently used addictive drugs, and it is very important for tobacco users to quit (1). Nicotine replacement therapy (NRT) has become the standard of care for the treatment of tobacco dependence to facilitate smoking cessation.
Different types of NRT are available, including nicotine gum, nicotine nasal spray, nicotine inhaler, and the transdermal patch (2,3). Each of these products delivers clinically relevant levels of nicotine to the plasma with modest efficacy. However, each product has disadvantages that limit its use. Nicotine gum may not provide an adequate level of nicotine due to improper chewing techniques or an inability to chew gum for 5–7 h everyday (2). Nasal spray delivery is associated with intense nasal irritation (2,3). Nicotine inhalers are inconvenient because they require frequent use, and 58% of users have reported that the use of this product is embarrassing (4). The transdermal patch provides a constant level of nicotine in the blood stream to suppress the need for tobacco smoke inhalation over a longer period. However, the maximum concentration in the blood stream is achieved after 4–9 h, and therefore, the initial nicotine craving is not satisfied (2). Park et al. (5) formulated nicotine mucoadhesive tablets as a biphasic system to satisfy the initial craving; however, the drug release was studied for only 4 h.
Nicotine can cross biological membranes, and once absorbed, it is metabolized extensively in the liver into a number of major and minor metabolites. However, buccal drug delivery offers unique and compelling benefits because it overcomes the first pass effect of the liver (6–10). One of the major challenges associated with buccal drug delivery is retention of the formulation at the mucosa. The use of excellent buccoadhesive polymers such as xanthan gum and carbopol provides a great opportunity to overcome this challenge (11–13). Xanthan gum is a polysaccharide with a β-d-glucose backbone like cellulose, but every second glucose unit is attached to a trisaccharide that consists of mannose, glucuronic acid and mannose (14). Carbopol 934 has an acrylic acid backbone, which is a water-swellable polymer with a high molecular weight, and it forms hydrogels in aqueous solutions. Carbopol demonstrated numerous key benefits in many studied bioadhesive formulations (15–19).
The main objective of the present study was to study the feasibility of formulating mucoadhesive buccal patches that demonstrate a fast initial release and a lateral, prolonged controlled release that satisfy the needs for smoking cessation.
MATERIALS AND METHODS
Materials
Nicotine and glycerol with a reagent grade of 99.5% were supplied by Sharlau, Barcelona, Spain. Carbopol 934 (no. 1001333) was kindly donated by APM, Salt, Jordan. Xanthan gum (no. 250451) supplied by Jangbazler was kindly donated by JPM, Amman, Jordan. Ethyl cellulose (no. 9004-57-3) was supplied by Sigma-Aldrich. All of the chemicals were used as supplied.
Methods
Preparation of Mucoadhesive Patches
Two types of bilayered mucoadhesive patches of nicotine were prepared using a solvent casting technique. Xanthan gum and carbopol 934 were used as the mucoadhesive polymers. The xanthan gum solution was prepared by dissolving 10 mg xanthan in 100 ml of water using glycerol as a plasticizer, whereas the carbopol polymeric solution was prepared by dissolving 2 g carbopol in 100 ml of hydroalcoholic solution (30% alcohol) using glycerol as a plasticizer. The backing layer was prepared by casting 10 ml of a solution comprising 4.5 g of ethyl cellulose in 150 ml chloroform and using 1.35 g of propylene glycol (30% w/w of polymer content) as a plasticizer. A weighed amount of nicotine was dispersed in each of the polymeric mucoadhesive solutions with stirring to ensure a uniform distribution of the drug. A measured volume of each of the polymeric solutions (20 ml) was poured on top of the pre-cast dried ethyl cellulose layer and dried at room temperature for 48 h. The dried films were peeled from the Petri dishes and prepared for the release study. They were cut into a circular shape with a smaller size (radius = 1 cm; surface area = 3.14 cm2) and stored at 20 ± 1°C in a desiccator containing a saturated solution of sodium dichromate (Na2Cr2O7), which provided environmental conditions of 55% relative humidity, for at least 2 days before testing. Patches with any imperfections, entrapped air, or with mean thickness variations of greater than ±5% were excluded from the analysis.
Physicochemical Characterization of the Buccal Patches: Thickness Uniformity
The thickness of the patches was measured using a micrometer at three different locations in ten different patches (cast volume = 20 ml; diameter = 1 cm).
Weight Uniformity and Drug Content
A weight variation test was conducted for ten patches. The patches were weighed individually, and their average weights and standard deviations were calculated and reported. Drug content was determined by placing the medicated patch in 100 ml of phosphate buffer saline (PBS) solution and shaken for 5 h at 100 rpm. The complete solution was filtered and then analyzed by UV analysis using a spectrophotometer (Shimadzu, SPD-10 A VP) at a wavelength of 259 nm. For content uniformity, the patch was divided into four quadrants of 3.14 cm2 each for the drug content analysis.
Swelling Study
Circular patches (n = 3) with a diameter of 1 cm were cut, weighed and placed in pre-weighed supports made of stainless steel and with a sieve opening of approximately 800 μm. The supports with the patches were introduced into a beaker containing 15 ml of PBS solution.
The beakers were placed in a water bath at 37°C. At regular intervals of 10 min from 0 to 100 min, the supports were removed from the medium using a pair of forceps, blotted with filter paper to remove excess water and immediately weighed. The percentage of water uptake was calculated from the relative weight change (swelling index) according to Eq. 1 (20):
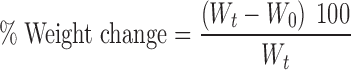 |
1 |
where Wt is the weight at time t and W0 is the initial weight. Each measurement was obtained in triplicate, and the mean and standard deviation were calculated.
Fourier Transform Infrared Analysis
The Fourier transform infrared spectra of medicated and non-medicated carbopol 943 and xanthan films were measured using a Nicolet Avatar 5.1 ESP 360 spectrometer (Nicolet Instrument Corporation).
Evaluation of Mucoadhesion
Mucoadhesion is considered as the ability of a mucoadhesive material to adhere to a mucosal surface. The adhesive force was measured to indicate the strength of the adhesive bond between the film and the biological tissue, which is considered a function of both the interaction energy between the adhesive and the mucosa and the viscoelastic properties of the formulation (21). The mucoadhesive strengths of the medicated and non-medicated buccal films were determined at room temperature using the two-arm balance method reported by Parodi and Emami et al. (22,23) with minor modifications. Fresh sheep buccal mucosa was obtained from a local slaughterhouse and used within 2 h of slaughter. The mucosal membrane was separated by removal of the underlying fat and loose tissues to obtain a thickness of 2 mm. The membrane was washed with PBS solution and fixed to the bottom of a smaller beaker attached to a larger beaker. PBS solution was then added to the beaker up to the upper surface of the mucosa. The film was attached to the upper clamp, and the platform was slowly raised until the film surface contacted the mucosa. After a preload time of 5 min, specific pre-weighed loads were added until the film detached from the buccal mucosa. The total weight was calculated and expressed as the force required for detachment (24,25). At least three replicates of each formulation were analyzed at room temperature, and in each case, a fresh sample was used. The mean and standard deviations were calculated, and the effects of drug loading and patch thickness on the mucoadhesive strength were evaluated.
In Vitro Release
The in vitro drug release studies were conducted using Franz diffusion cells (orifice surface area = 3.14 cm2) at 37 ± 1°C with a receptor compartment containing 15.2 ml of PBS solution at pH 7.0. The patches were placed on a dialysis membrane (MWCO of 25 kDa, previously soaked in buffer solution for 12 h). The membrane was mounted between the two compartments of the diffusion cell such that the backing layer faced the donor compartment and the adhesive film faced the receiver compartment, and fastened with an O-ring. Three-hundred-microliter samples were collected periodically through the sampling port of the receiver cell at predetermined time intervals (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, and 24 h) and replaced with an equal volume of fresh receptor solution. The drug content of the samples was analyzed using a spectrophotometer at a wavelength of 259 nm. All of the release experiments were performed in triplicate, and the effects of drug loading and film thickness on the release profile were assessed.
Unidirectional release across the backing layer was studied by obtaining one sample (1 ml) from the donor compartment 24 h after starting the experiment. The percentage of drug released was calculated by dividing the total amount of released drug in the donor cell by the initial amount of loaded drug in the mucoadhesive film.
RESULTS AND DISCUSSION
Preparation of the Mucoadhesive Patches
Flexible, transparent mucoadhesive patches were obtained using the described casting process. The development of patches is preferred over that of mucoadhesive tablets. Patches are more flexible, comfortable, and effective in supplying the drug across the oral mucosa (26,27).
Physicochemical Characterization
Thickness, Weight, and Drug Content Uniformity
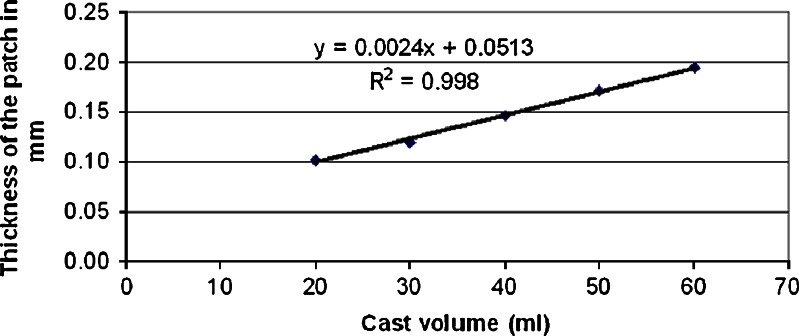
The prepared patches demonstrated an excellent uniformity in thickness, weight and drug content. The inter-batch patch thickness and weight uniformity of the xanthan patches (n = 10) were 0.10 ± 0.08 mm and 92.34 ± 5.09 mg, respectively. For the carbopol patches, the values were 0.14 ± 0.01 mm and 67.3 ± 8.39 mg, respectively. A linear relationship (r2 = 0.998) was observed between the thickness of the patch and the cast volume of the xanthan gum solution, as shown in Fig. 1. The Y-intercept value of 0.05 mm, which is equivalent to a casting volume of zero, revealed the thickness of the backing layer.
Fig. 1.
The relationship between the cast volume of the xanthan gum solution and the thickness of the patch
Swelling Studies
Polymer swelling is an essential stage in the formation of a mucoadhesive bond between hydrophilic matrix formulations and the mucosa. In vitro swelling studies were performed to investigate the performance of the dosage form, swelling capacities and patch integrity after swelling.
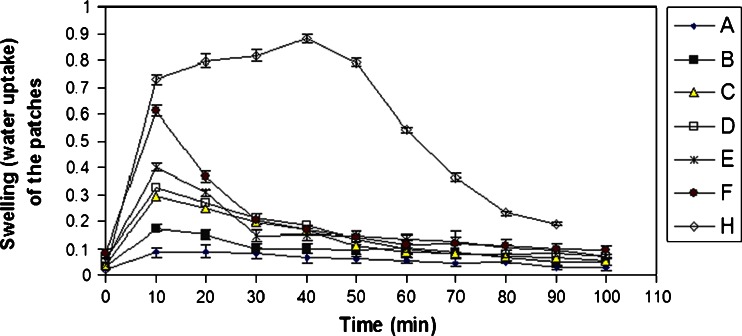
Figure 2 shows that the rate and percentage of water uptake for non-medicated carbopol patches (H) were higher compared to those determined for the non-medicated xanthan patches (E), whereas the percentage of water uptake for the medicated carbopol patches (A) was less than that observed for medicated xanthan patches (D). A maximum water uptake of 86.68% was attained after 10 min for medicated xanthan patches (cast volume = 30 ml, drug content = 2 mg/cm2), after which it leveled off. Different xanthan patch thicknesses (cast volumes of 20, 30 and 40 ml) showed a linear relationship (r2 = 0.9876) with the percentage of water uptake; the thicker the patch, the greater was the water uptake.
Fig. 2.
Water uptake of medicated and non-medicated xanthan and carbopol patches. a Medicated carbopol film (cast volume = 30 ml, nicotine = 2 mg/cm2); b non-medicated xanthan film (cast volume = 20 ml); c medicated xanthan film (cast volume = 30 ml, nicotine = 3.75 mg/cm2); d medicated xanthan film (cast volume = 30 ml, nicotine = 2 mg/cm2); e non-medicated xanthan film (cast volume = 30 ml); f non-medicated xanthan film (cast volume = 40 ml); h non-medicated carbopol film (cast volume = 30 ml)
A maximum water uptake of 97.27% was determined for non-medicated carbopol patches (cast volume = 30 ml) after 40 min and was followed by a stabilization of the change in weight. These results suggested a complete hydration of the patch, whereas the maximum water uptake of the medicated patches (cast volume = 30 ml; drug content = 2 mg/cm2) was 72.4%.
The interaction between the carboxylate groups of carbopol with nicotine likely diminished the number of opportunities for hydrogen bond formation, which would lower the potential for hydration. This interaction shielded the negative charge of the carboxylic group, decreased the repulsive forces between the carboxylic groups, led to recoiling of the carbopol on itself and consequently resulted in difficulties in the formation of secondary chemical bonds with water, which would lead to a sharp decrease in the hydration process. Interestingly, the concentration of nicotine and consequently the nicotine-polymer interaction affected the hydration process. The loading of different nicotine concentrations (2 and 3.75 mg/cm2) into xanthan gum patches (cast volume = 30 ml) had insignificant effects on the percentage of water uptake whereas loading of the same concentration of nicotine onto carbopol patches decreased the hydration process. Based on the results of the swelling studies, the nicotine–xanthan interaction can be considered weak and the nicotine–carbopol interaction strong. In addition, the patch integrity was maintained during the study period.
Fourier Transform Infrared
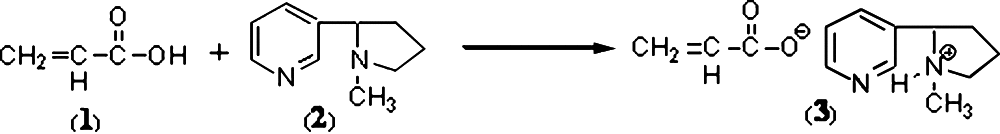
To explicate the interaction between the two polymers and nicotine, Fourier transform infrared (FTIR) studies were conducted. In previous studies, the interaction between the carboxylate of carbopol and the lidocaine base (28) has been considered as a salt formation. Similarly, Eq. 2 shows the suggested acid–base reaction that likely occurs between the organic acid carbopol (1) and the organic base nicotine (2), which leads to the formation of a hybrid (3). The presence of two different basic N atoms in (2) enhances this type of reaction; N-methylpyridine is a preferred base over N-methylpyrrolidine according to their pKb values.
 |
2 |
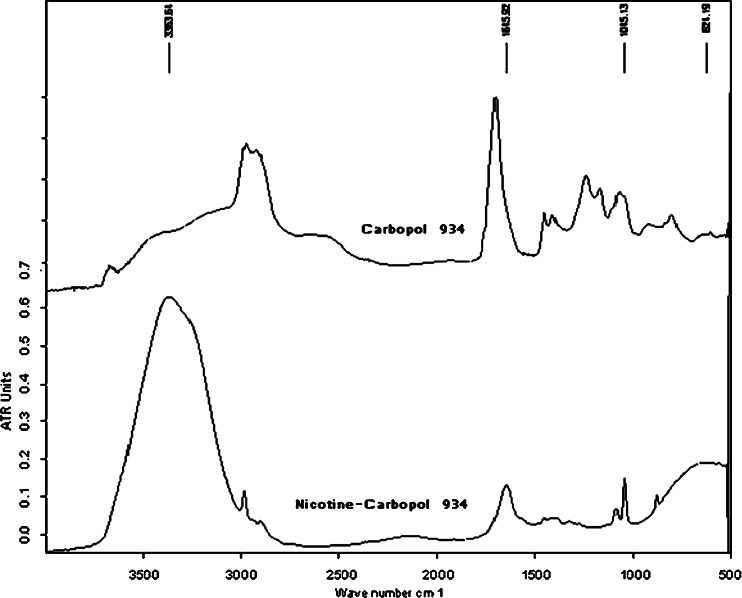
The FTIR spectra of (1) and (3) shown in Fig. 3 support this suggestion, where a lower shift of the carbonyl group (C = O) band from 1,702.4 cm−1 in (1) to 1,645.9 cm−1 in (3) is evident. This shift is likely due to the bond order of (C = O) in the hybrid structure of the carboxylate anion in (3), which is lower than its corresponding value in (1). The other IR bands in (3) are located close to their respective positions in (1) and (2).
Fig. 3.
FTIR of carbopol 934 and nicotine–carbopol 934
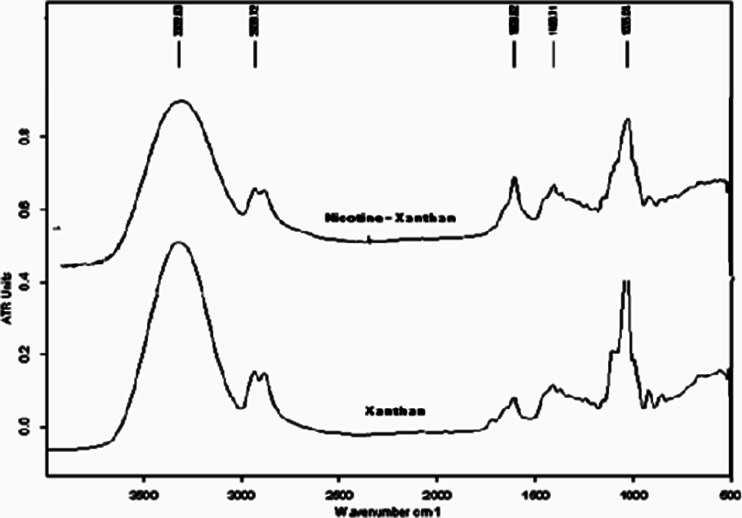
Despite the complexity of the xanthan structure (a β-(1,4)-d-glucopyranose glucan backbone with side chains of (3,1)-α-linked-a-d-mannopyranose-(2,1)-β-d-glucuronic acid-(4,1)-β-d-mannopyranose on alternating residues relative to carbopol), its reaction with nicotine is classified as an acid–base-type reaction. This result can be clearly observed by comparing the IR spectra of the xanthan and xanthan–nicotine samples presented in Fig. 4, in particular the (C = O) IR bands at 1,733 and 1,740 cm−1. These bands disappeared in the nicotine–xanthan sample, which suggested that they could have appeared as a shifted single band at a lower wave number of 1,606.4 cm−1.
Fig. 4.
FTIR of xanthan and nicotine–xanthan
Adhesion Study
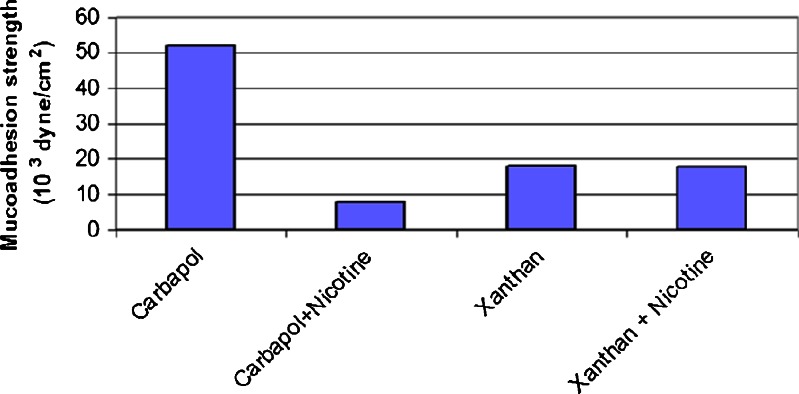
Figure 5 shows that the bioadhesive strength of the carbopol film was significantly (P < 0.05) higher than that of the xanthan film using the same casting volume. The xanthan molecule has a helical form consisting of a backbone of β-1,4-linked d-glucose residues. Side chains containing one glucuronic acid unit between two mannose units are linked to every other glucose unit.
Fig. 5.
Mucoadhesion strength of medicated and non-medicated carbopol and xanthan mucoadhesive patches [cast volume = 20 ml, drug content = 2 mg/cm2)
The steric hindrance of these side chains prevents access to the charged mucin groups in the mucoadhesion interaction (29), as previously proposed by Harding and Ceulemans et al. (30).
Effect of Drug Loading
In the mucoadhesion process, several well-defined events have been identified (31). Any factor that affects these events, whether it is a polymer-related or an environmental factor, will affect the mucoadhesive force (32).
Figure 5 shows that the addition of 2 mg/cm2 of nicotine to a 20 ml carbopol cast volume decreased the mucoadhesive strength significantly (P < 0.05), whereas the addition of the same or a greater amount of nicotine to the same cast volume of xanthan did not affect the mucoadhesive strength significantly (P > 0.05). This phenomenon could be due to the presence of a strong chemical interaction between the functional groups that are responsible for mucoadhesion, the acidic carboxylate group of carbopol, and nicotine (a basic drug), in contrast to the weak interaction that occurs between xanthan and nicotine. This interaction again shielded the negative charge of the carboxylic group, led to recoiling of the carbopol on itself and consequently resulted in difficulties in diffusion, biological tissue penetration and formation of secondary chemical bonds with the biological tissues (mucin). This interaction was observed visually during the casting process, during which a viscous solution was formed upon the addition of nicotine to the carbopol solution, and it is supported by the swelling and FTIR studies. The results of a previous work support our findings. In that study, the addition of a lidocaine base to carbopol led to a decrease in the force of mucoadhesion due to the interaction between the drug and carbopol (28).
Effect of Film Thickness
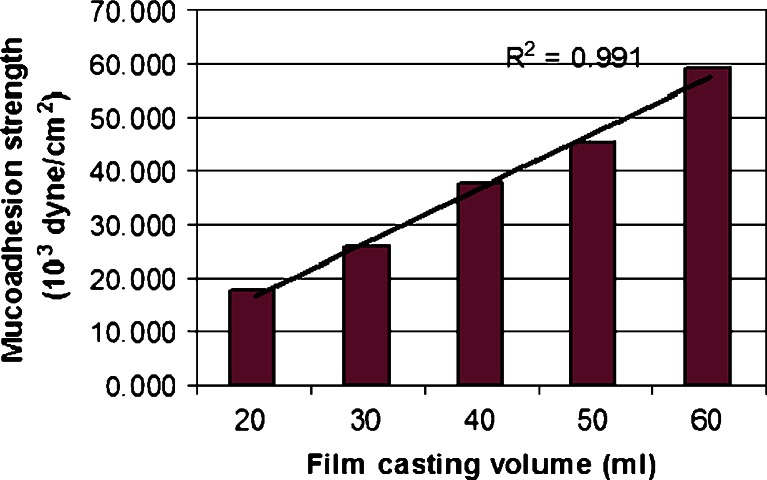
Figure 6 shows that the force of adhesion increased linearly (r2 = 0.991) as the thickness of the xanthan film increased. As the concentration gradient of free polymer chains across the interface increased, the diffusion of free chains across the interface increased, which resulted in stronger mucoadhesion. Guo (33) observed that the average peeling strength of carbopol 934P/polyisobutylene/polyisoprene patches using Instron increased with increasing patch thicknesses.
Fig. 6.
Mucoadhesion strength of different film thickness of medicated xanthan patches (20 ml ≈ 0.101 mm, 30 ml ≈ 0.120 mm, 40 ml ≈ 0.147 mm, 50 ml ≈ 0.172 mm, and 60 ml ≈ 0.195 mm)
In Vitro Release
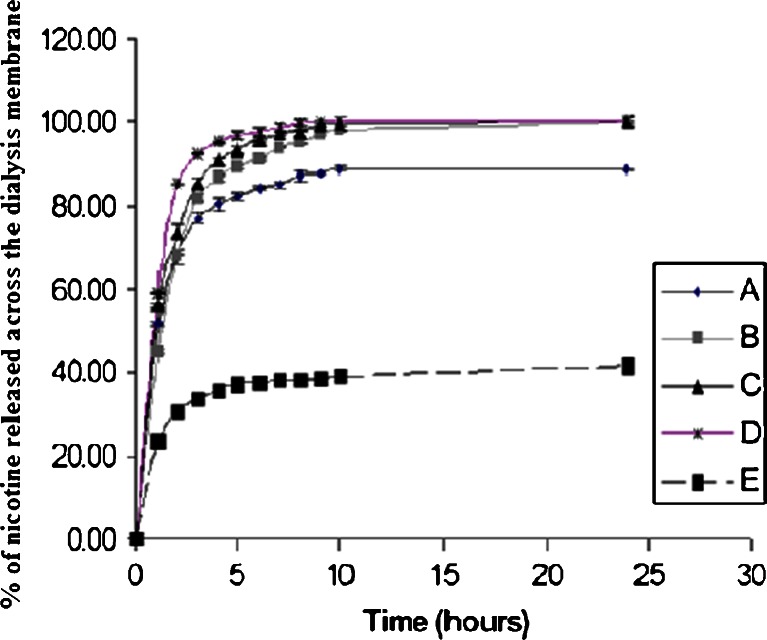
In general, regardless of the polymer type, the rate and extent of drug release decreased significantly with the patch formulation compared to the linear control solution (r2 = 0.9973). Figure 7 shows the in vitro release profiles of nicotine from carbopol and from xanthan gum patches. A fast initial release profile followed by controlled drug release was successfully observed for the xanthan patches over a 10-h period, and complete release occurred after approximately 8–10 h. However, an incomplete release profile of nicotine from carbopol patches was observed; only 39% of the nicotine was released after 10 h. In contrast, 98% of the nicotine was released from the xanthan gum patches, which had the same thickness and drug content.
Fig. 7.
Nicotine release profile across dialysis membrane from carbopol and xanthan gum patches having different drug loading concentration; a xanthan gum loaded with 1 mg/cm2 nicotine, b xanthan gum loaded with 2 mg/cm2 nicotine, c xanthan gum loaded with 2.75 mg/cm2 nicotine, d xanthan gum loaded with 3.75 mg/cm2 nicotine, and e carbopol loaded with 2 mg/cm2 nicotine
The incomplete release profile of carbopol patches was due to a stronger acid–base reaction of the nicotine with carbopol, as compared to its reaction with xanthan gum. This reaction restricted the water uptake process and consequently the swelling process and drug release. The strong acid–base interaction was observed visually by loading higher concentrations of nicotine onto the carbopol patches. The viscosity of the solution increased with the loading of greater amounts of nicotine, and a loss of uniformity in the weight and thickness of the patches was observed.
The controlled release of nicotine from xanthan patches was clearly evident due to the formation of a gel-like structure. Intermolecular associations such as hydrogen bonding and electrostatic and hydrophobic interactions play a crucial role in the changes observed in the rheological properties of xanthan solutions (34). Due to the presence of glucuronic acid and pyruvate in its side chains, xanthan is considered an anionic polyelectrolyte. The negatively charged carboxyl groups on the side chains force the molecules to form very viscous fluids in the presence of water. However, the response of xanthan patches to increasing nicotine concentrations differs from those of carbopol patches; xanthan patches maintain a high structure viscosity due to the increase in their hydrodynamic radius (34). In addition, a greater cumulative amount of drug release was observed from patches that had been loaded with a greater amount of nicotine.
The release of nicotine from xanthan patches loaded with 3.75 mg/cm2 was significantly higher than that released from those loaded with 2 mg/cm2 and 2.75 mg/cm2. These findings support the previous results and demonstrate that the acid–base interaction of xanthan–nicotine is much weaker than that of carbopol–nicotine.
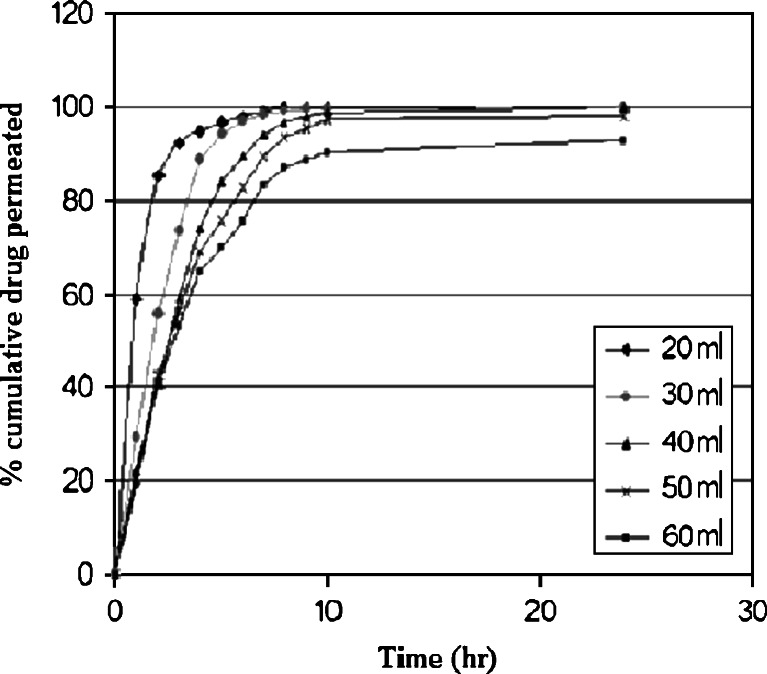
Based on the plots shown in Fig. 8, it is clear that the drug release was governed by the polymer content. No lag time was observed because the patch was exposed directly to the dissolution medium. An increase in polymer content was associated with a decrease in the drug release rate due to an increasing hindrance of the drug diffusion.
Fig. 8.
In vitro release profile of nicotine (2 mg/cm2) using different cast volume of xanthan gum to attain different medicated film thickness (20 ml, 0.101 mm; 30 ml, 0.120 mm; 40 ml, 0.147 mm; 50 ml, 0.172 mm; and 60 ml, 0.195 mm)
The unidirectional drug release of nicotine through the backing layer of ethyl cellulose was assured, where the amount of nicotine in the donor compartment after 24 h of release was insignificant. This result is consistent with a previous study reported by Lopez et al. (35) in which the backing layer capability of ethyl cellulose was assured.
The stored patches did not exhibit changes in color, thickness, weight or drug content, as shown in Table I, which suggested a satisfactory stability of the drug and the patches.
Table I.
Stability Data of Content Uniformity, Thickness, and Weight Variation for Xanthan-Medicated Patches Over 6 Months
| Time (month) | Content uniformity (%; ±STD) | Thickness (mm; ±STD) | Weight variation (mg; ±STD) |
|---|---|---|---|
| 1 | 98% (±0.6) | 0.17 (±0.02) | 0.4 (±0.01) |
| 2 | 99% (±0.5) | 0.16 (±0.02) | 0.04 (±0.01) |
| 3 | 98% (±0.7) | 0.15 (±0.02) | 0.03 (±0.02) |
| 4 | 97% (±0.9) | 0.16 (±0.01) | 0.05 (±0.01) |
| 5 | 97% (±0.8) | 0.14 (±0.01) | 0.04 (±0.01) |
| 6 | 98% (±0.7) | 0.14 (±0.01) | 0.03 (±0.02) |
CONCLUSIONS
The results of the present study demonstrated that xanthan mucoadhesive buccal patches are potential candidates for controlled biphasic nicotine delivery. The promising fast initial drug release followed by a controlled release over a 10-h period and the content uniformity, thickness, swelling and mucoadhesive strength, advocate the use of such a system as a potential candidate for future in vivo studies of the permeation and retention of nicotine in the oral mucosa. These studies can evaluate the suitability of these patches for systemic administration as an aid in smoking cessation.
Interestingly, the release profile, mucoadhesion and swelling studies indicated that the acid–base reaction of nicotine with carbopol is relatively stronger than its reaction with xanthan gum, which restricts the suitability of medicated carbopol patches for controlled drug delivery.
REFERENCES
- 1.Yildiz D. Nicotine, its metabolism and an overview of its biological effects. (Review) Toxicon. 2004;43:619–632. doi: 10.1016/j.toxicon.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Galanti L. Tobacco smoking cessation management: integrating varenicline in current practice. Vasc Health Risk Manag. 2008;4(4):837–845. doi: 10.2147/vhrm.s3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Orlando KJ, Fox BS. Tolerability and pharmacokinetics of single and repeated doses of nicotine with the straw, a novel nicotine replacement product. Nicotine Tob Res. 2004;6(1):63–70. doi: 10.1080/14622200310001656876. [DOI] [PubMed] [Google Scholar]
- 4.Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray and an inhaler. Arch Intern Med. 1999;159:2033–2038. doi: 10.1001/archinte.159.17.2033. [DOI] [PubMed] [Google Scholar]
- 5.Park CR, Munday DL. Development and evaluation of a biphasic buccal adhesive tablet for nicotine replacement therapy. Int J Pharmaceut. 2002;237:215–226. doi: 10.1016/S0378-5173(02)00041-8. [DOI] [PubMed] [Google Scholar]
- 6.Hao J, Heng P. Buccal delivery systems. Drug Dev Ind Pharm. 2003;29(8):821–832. doi: 10.1081/DDC-120024178. [DOI] [PubMed] [Google Scholar]
- 7.Shojaei A. Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Pharm Sci. 1998;1(1):15–30. [PubMed] [Google Scholar]
- 8.Xiang J, Fang X, Li X. Transbuccal delivery of 2′, 3′-dideoxycytidine: in vitro permeation study and histological investigation. Int J Pharmaceut. 2002;231:57–66. doi: 10.1016/S0378-5173(01)00865-1. [DOI] [PubMed] [Google Scholar]
- 9.Veuillez F, Kalia Y, Jacques Y, Deshusses J, Buri P. Factors and strategies for improving buccal absorption of peptides. Eur J Pharm Biopharm. 2001;51:93–109. doi: 10.1016/S0939-6411(00)00144-2. [DOI] [PubMed] [Google Scholar]
- 10.Swarbrick A. Oral mucosal drug delivery. New York: Marcel Dekker; 1996. [Google Scholar]
- 11.Shin S, Kim J, Oh I. Mucoadhesive and physicochemical characterization of carbopol-poloxamer gels containing triamcinolone acetonide. Drug Dev Ind Pharm. 2000;26(3):307–312. doi: 10.1081/DDC-100100358. [DOI] [PubMed] [Google Scholar]
- 12.Park CR, Munday DL. Evaluation of selected Polysaccharide Excipients in buccoadhesive tablets for sustained release of nicotine. Drug Dev Ind Pharm. 2004;30(6):609–617. doi: 10.1081/DDC-120037492. [DOI] [PubMed] [Google Scholar]
- 13.Smart JD. Recent developments in the use of bioadhesive systems for delivery of drugs to the oral cavity. Crit Rev Ther Drug Carrier Syst. 2004;21:319–344. doi: 10.1615/CritRevTherDrugCarrierSyst.v21.i4.20. [DOI] [PubMed] [Google Scholar]
- 14.Roubroeks JP, Andersson R, Mastromauro DI, Christensen BE, Aman P. Molecular weight, structure and shape of oat (1 → 3), (1 → 4)-b-d-glucan fractions obtained by enzymatic degradation with (1 → 4)-b-d-glucan 4-glucanohydrolase from Trichoderma reesei. Carbohydr Polym. 2001;46:275–285. doi: 10.1016/S0144-8617(00)00329-5. [DOI] [Google Scholar]
- 15.Perez-Marcos B, Gutierrez C, Gomez-Amoza JL, Martinez-Pacheco R, Souto C, Concheiro A. Usefulness of certain varieties of Carbopol in the formulation of hydrophilic furosemide matrices. Int J Pharmaceut. 1991;67(2):113–121. doi: 10.1016/0378-5173(91)90423-L. [DOI] [Google Scholar]
- 16.Lee J, Park J, Robinson R. Bioadhesive-based dosage forms; the next generation. J Pharm Sci. 2000;89:850–866. doi: 10.1002/1520-6017(200007)89:7<850::AID-JPS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 17.Hao J, Paul Heng WS. Buccal delivery systems. Drug Dev Ind Pharm. 2003;29(8):821–832. doi: 10.1081/DDC-120024178. [DOI] [PubMed] [Google Scholar]
- 18.Majithiya RJ, Ghosh PK, Umrethia ML, Murthy RS. Thermoreversible-mucoadhesive gel for nasal delivery of sumatriptan. AAPS PharmSciTech. 2006;7(3):67. doi: 10.1208/pt070367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Huwaij R, Assaf S, Salem M, Sallam A. Potential mucoadhesive dosage form of lidocaine hydrochloride: II. In vitro and in vivo evaluation. Drug Dev Ind Pharm. 2007;33(4):437–448. doi: 10.1080/03639040601150211. [DOI] [PubMed] [Google Scholar]
- 20.Patel VM, Prajapati BG, Patel HV, Patel KM. Mucoadhesive bilayer tablets of propranolol hydrochloride. AAPS PharmSciTech. 2007;8(3):77. doi: 10.1208/pt0803077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamburic S, Craig D. A comparison of different in vitro methods for measuring mucoadhesive performance. Eur J Pharm Biopharm. 1997;44:159–167. doi: 10.1016/S0939-6411(97)00073-8. [DOI] [Google Scholar]
- 22.Parodi B, Russo E, Caviglioli G, Cafaggi S, Bigbardi G. Development and characterization of a buccoadhesive dosage form of oxycodane hydrochloride. Drug Dev Ind Pharm. 1996;22(5):445–450. doi: 10.3109/03639049609069353. [DOI] [Google Scholar]
- 23.Emami J, Varshosaz J, Saljoughian N. Development and evaluation of controlledrelease buccoadhesive verapamil hydrochloride tablets. DARU. 2008;16(2):60–69. [Google Scholar]
- 24.Desai KGH, Kumar TMP. Preparation and evaluation of a novel buccal adhesive system. AAP PharmSciTech. 2004;5(3):35. doi: 10.1208/pt050335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal V, Mishra B. Design, development, and biopharmaceutical properties of buccoadhesive compacts of pentazocine. Drug Dev Ind Pharm. 1999;25(6):701–709. doi: 10.1081/DDC-100102229. [DOI] [PubMed] [Google Scholar]
- 26.Perioli L, Ambrogi V, Angelici A, Ricci M. Development of mucoadhesive patches for buccal administration of ibuprofen. J Control Release. 2004;99(1):73–82. doi: 10.1016/j.jconrel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto H, Nakamori T, Arakawa Y, Iida K, Danjo K. Development of polymer film dosage forms of lidocaine for buccal administration: II. Comparison of preparation methods. J Pharm Sci. 2002;91(11):2424–2432. doi: 10.1002/jps.10228. [DOI] [PubMed] [Google Scholar]
- 28.Abu-Huwaij R, Assaf S, Salem M, Sallam A. Potential mucoadhesive dosage form of lidocaine hydrochloride: I. Mucoadhesive and physicochemical characterization. Drug Dev Ind Pharm. 2007;33(8):855–864. doi: 10.1080/03639040701377516. [DOI] [PubMed] [Google Scholar]
- 29.Harding S. Mucoadhesive interactions. Biochem Soc Trans. 2003;31(5):1036–1042. doi: 10.1042/BST0311036. [DOI] [PubMed] [Google Scholar]
- 30.Ceulemans J, Vinckier I, Ludwig A. The use of xanthan gum in an ophthalmic liquid dosage form: rheological characterization of the interaction with mucin. J Pharm Sci. 2002;91(4):1117–1127. doi: 10.1002/jps.10106. [DOI] [PubMed] [Google Scholar]
- 31.Liabot J, Manzo R, Allemandi D. Double layered mucoadhesive tablets containing nystatin. AAPS PharmSciTech. 2002;3(3):22. doi: 10.1208/pt030322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57:1666–1691. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Guo J. Bioadhesive polymer buccal patches for buprenorphine controlled delivery: formulation, in vitro adhesion and release properties. Drug Dev Ind Pharm. 1994;20(18):2809–2821. doi: 10.3109/03639049409042682. [DOI] [Google Scholar]
- 34.Richardson RK, Ross-Murphy SB. Non-linear viscoelasticity of polysaccharide solutions. 2: xanthan polysaccharide solutions. Int J Biol Macromol. 1987;9:257–264. doi: 10.1016/0141-8130(87)90063-8. [DOI] [Google Scholar]
- 35.Lopez C, Portero A, Jato J, Alonso M. Design and evaluation of chitosan/ethylcellulose mucoadhesive bilayered devices for buccal drug delivery. J Control Release. 1998;55:143–152. doi: 10.1016/S0168-3659(98)00044-3. [DOI] [PubMed] [Google Scholar]