Abstract
Two Mg(2+)-dependent DNA endonucleases have been isolated from mammalian cells which have a strong preference to nick within long tracts of guanine residues in vitro. One endonuclease activity is mitochondrial (mt). The other endonuclease, called Endonuclease G, is associated with isolated nuclei, and is released when the nuclear chromatin is exposed to moderate ionic strength. Our laboratory has previously purified the mt endonuclease to near homogeneity from mitochondria of bovine heart and reported the enzyme to be a homodimer of a approximately 29 kDa polypeptide [Cummings, O. W. et al. (1987) J. Biol. Chem., 262, 2005-2015]. Although the purified mt endonuclease will extensively fragment M13 viral ssDNA and plasmid dsDNAs in vitro, the enzyme displays an unusually strong preference to nick within a (dG)12:(dC)12 sequence tract which resides just upstream from the origin of DNA replication in the mitochondrial genome. The nuclear Endonuclease G first identified from its selective targeting of several (dG)n:(dC)n tracts in vitro (where N = 3-29), was subsequently purified from calf thymus nuclei and shown to be a homodimer of a approximately 26-kDa polypeptide [Côté, J. et al. (1989) J. Biol. Chem., 264, 3301-3310]. In the present study, we find that Endonuclease G partially purified from calf thymus nuclei will extensively degrade both viral ss- and dsDNAs in vitro, and that the enzyme possesses biochemical properties and specificity for nucleotide sequences in DNA that are strongly related or identical to those of the mt endonuclease. These findings and the discovery of sequence identity between the proteins strengthen the conclusion that the nuclear Endonuclease G is the same enzyme as the mt endonuclease.
Full text
PDF

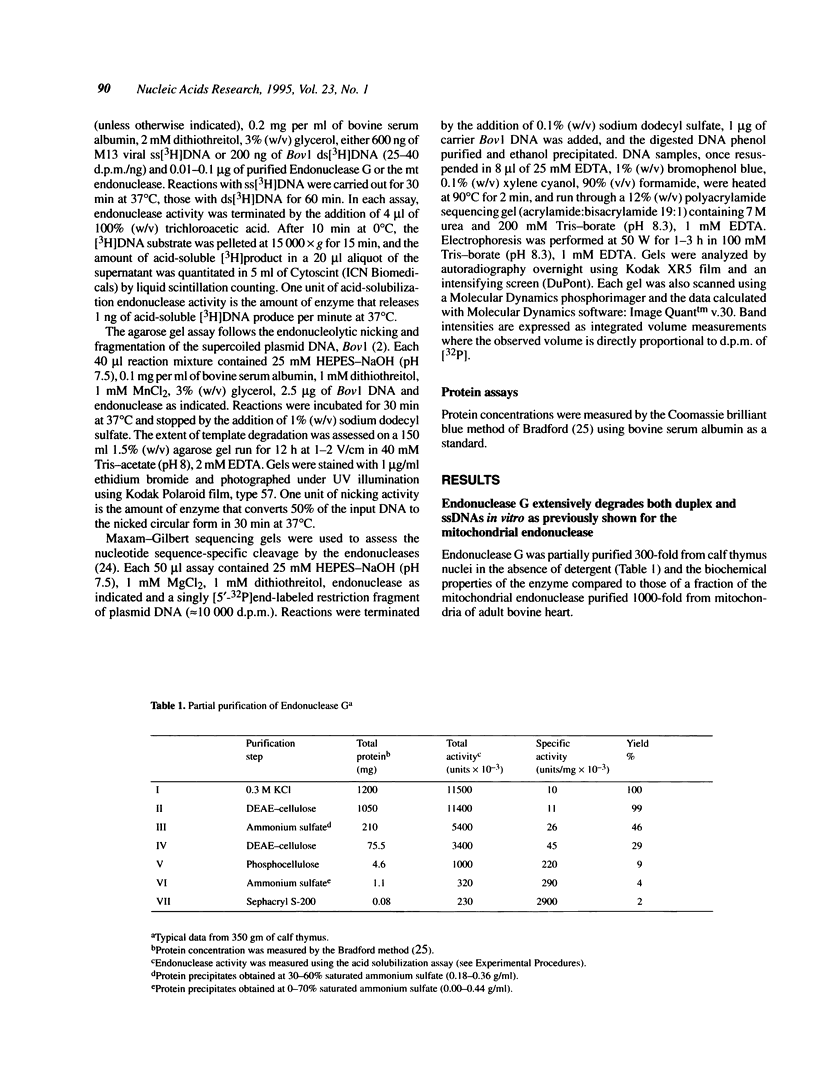
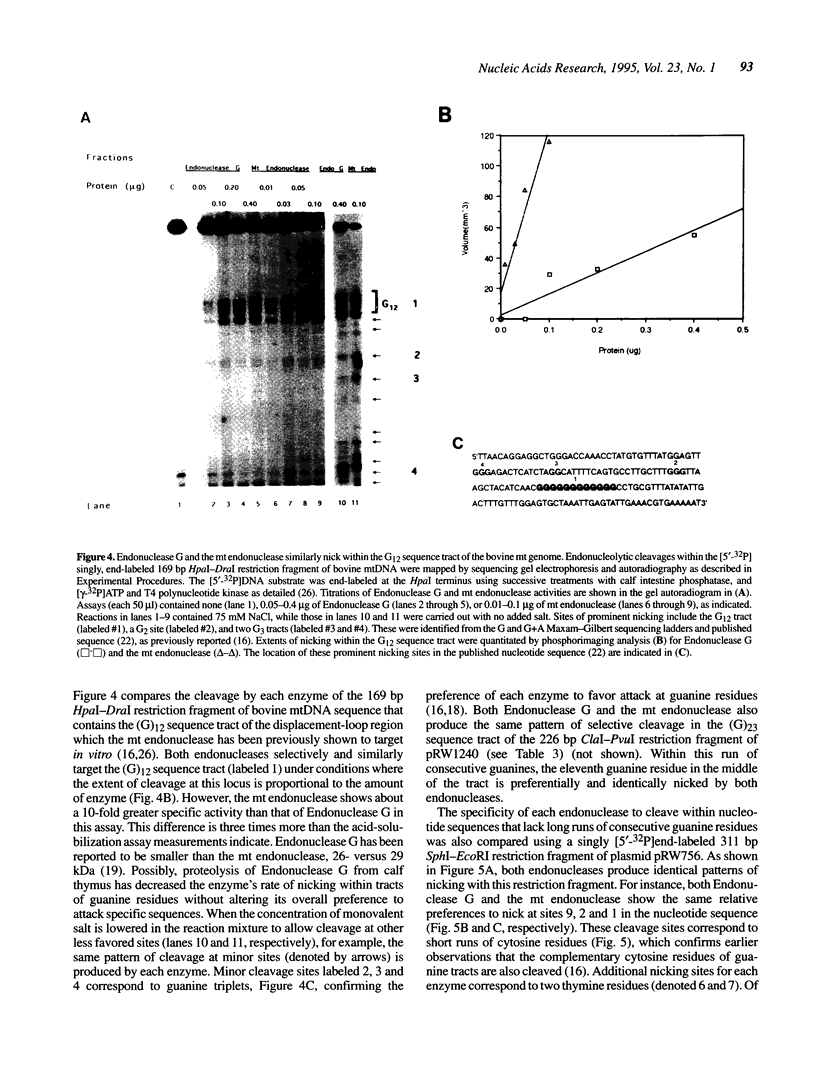
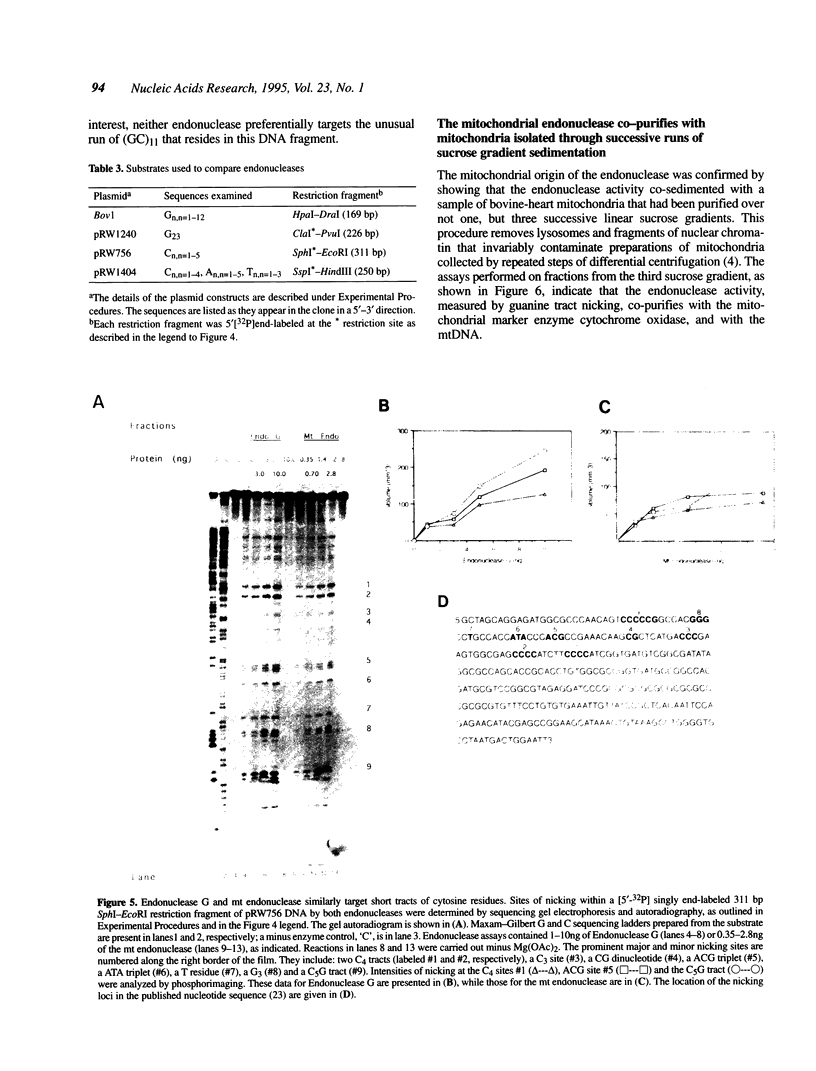
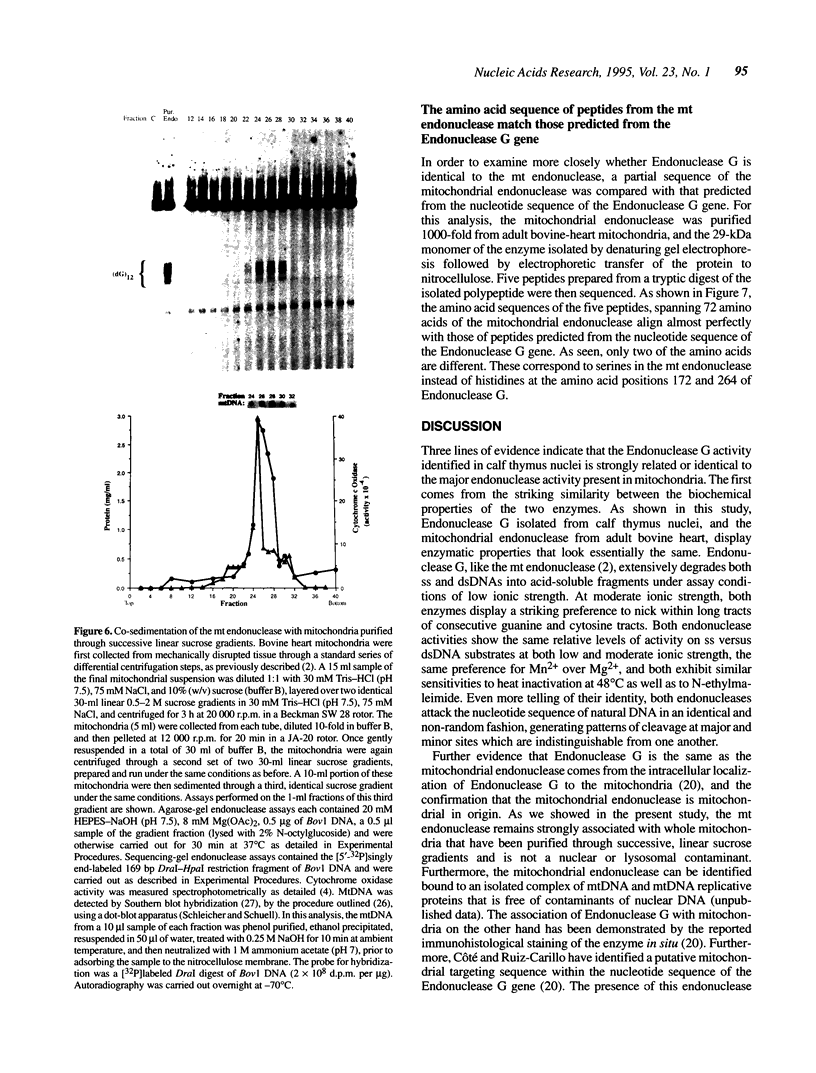


Images in this article
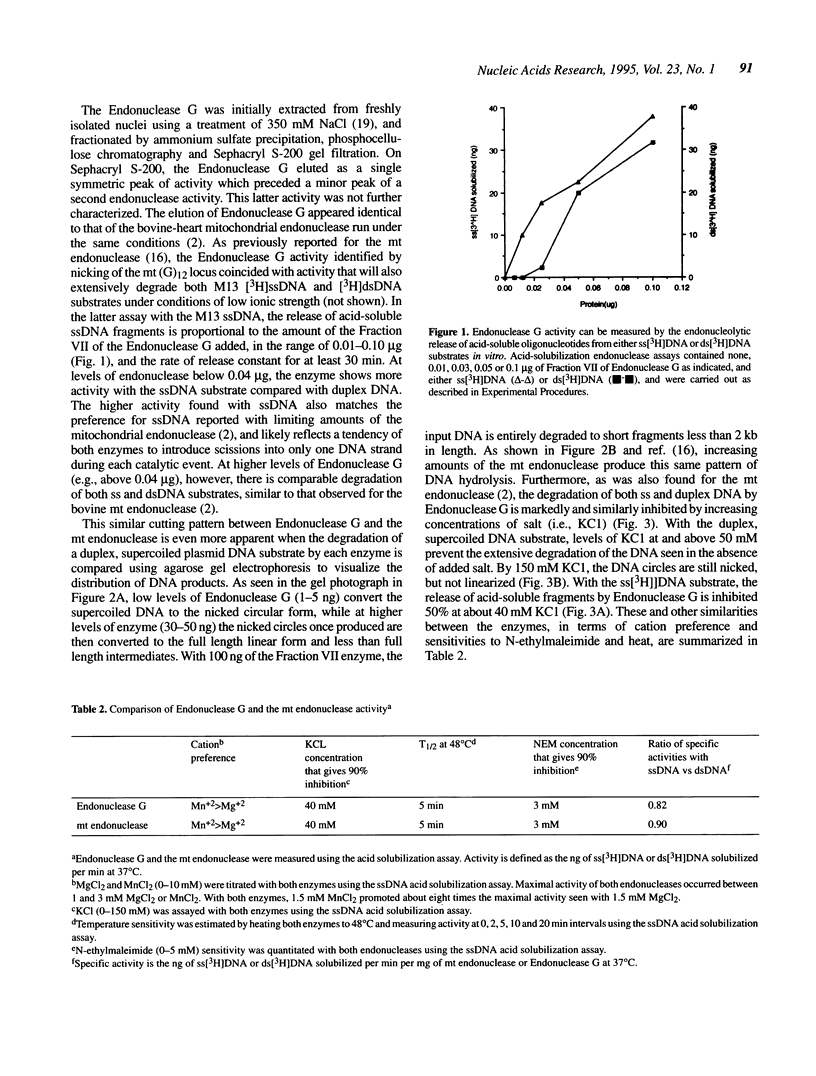
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Boyd J. B., Sakaguchi K., Harris P. V. mus308 mutants of Drosophila exhibit hypersensitivity to DNA cross-linking agents and are defective in a deoxyribonuclease. Genetics. 1990 Aug;125(4):813–819. doi: 10.1093/genetics/125.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
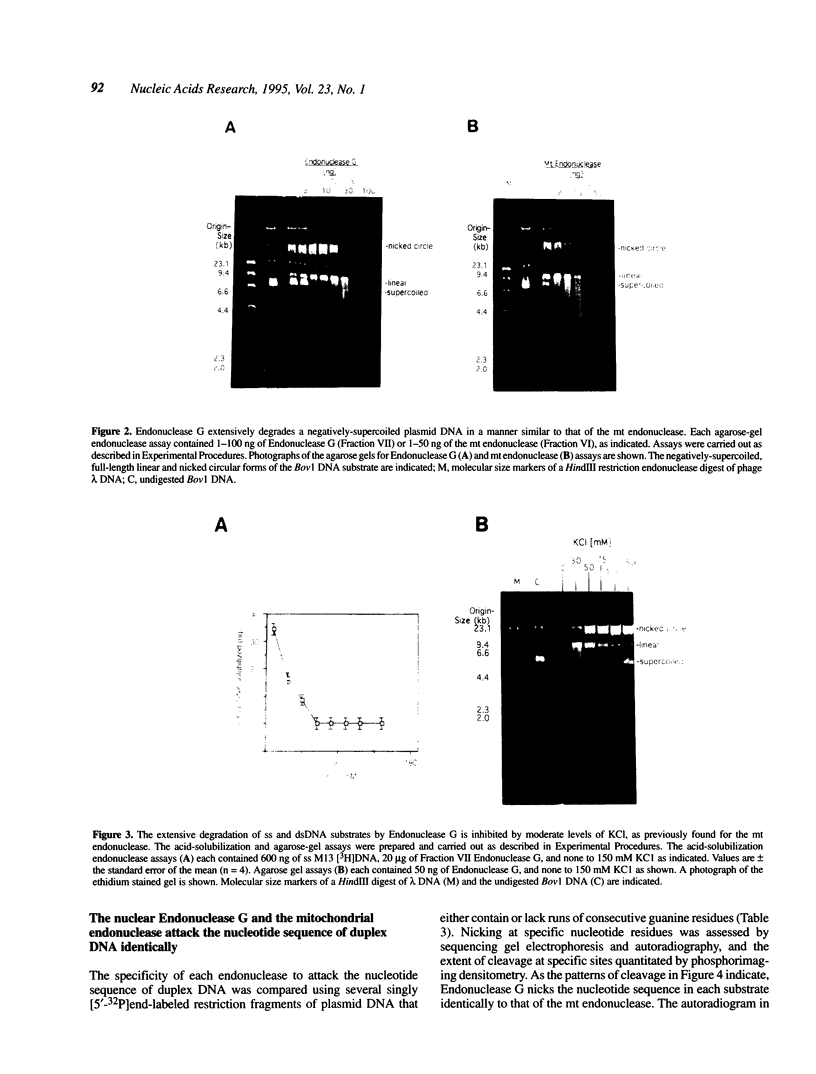
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. A novel endoribonuclease cleaves at a priming site of mouse mitochondrial DNA replication. EMBO J. 1987 Feb;6(2):409–417. doi: 10.1002/j.1460-2075.1987.tb04770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomyn A., Mariottini P., Cleeter M. W., Ragan C. I., Matsuno-Yagi A., Hatefi Y., Doolittle R. F., Attardi G. Six unidentified reading frames of human mitochondrial DNA encode components of the respiratory-chain NADH dehydrogenase. Nature. 1985 Apr 18;314(6012):592–597. doi: 10.1038/314592a0. [DOI] [PubMed] [Google Scholar]
- Chow T. Y., Fraser M. J. Purification and properties of single strand DNA-binding endo-exonuclease of Neurospora crassa. J Biol Chem. 1983 Oct 10;258(19):12010–12018. [PubMed] [Google Scholar]
- Cummings O. W., King T. C., Holden J. A., Low R. L. Purification and characterization of the potent endonuclease in extracts of bovine heart mitochondria. J Biol Chem. 1987 Feb 15;262(5):2005–2015. [PubMed] [Google Scholar]
- Curtis P. J., Burdon M. G., Smellie R. M. The purification from rat liver of a nuclease hydrolysing ribonucleic acid and deoxyribonucleic acid. Biochem J. 1966 Mar;98(3):813–817. doi: 10.1042/bj0980813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté J., Renaud J., Ruiz-Carrillo A. Recognition of (dG)n.(dC)n sequences by endonuclease G. Characterization of the calf thymus nuclease. J Biol Chem. 1989 Feb 25;264(6):3301–3310. [PubMed] [Google Scholar]
- Côté J., Ruiz-Carrillo A. Primers for mitochondrial DNA replication generated by endonuclease G. Science. 1993 Aug 6;261(5122):765–769. doi: 10.1126/science.7688144. [DOI] [PubMed] [Google Scholar]
- Dake E., Hofmann T. J., McIntire S., Hudson A., Zassenhaus H. P. Purification and properties of the major nuclease from mitochondria of Saccharomyces cerevisiae. J Biol Chem. 1988 Jun 5;263(16):7691–7702. [PubMed] [Google Scholar]
- Foury F. Repair of mitochondrial DNA in Saccharomyces cerevisiae. Induction of cytoplasmic petite mutants in a nuclear mutant exhibiting thermosensitive mitochondrial deoxyribonuclease activity. J Biol Chem. 1982 Jan 25;257(2):781–787. [PubMed] [Google Scholar]
- Fraser M. J., Cohen H. Intracellular localization of Neurospora crassa endo-exonuclease and its putative precursor. J Bacteriol. 1983 Apr;154(1):460–470. doi: 10.1128/jb.154.1.460-470.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser M. J., Hatahet Z., Huang X. T. The actions of Neurospora endo-exonuclease on double strand DNAs. J Biol Chem. 1989 Aug 5;264(22):13093–13101. [PubMed] [Google Scholar]
- Fraser M. J., Koa H., Chow T. Y. Neurospora endo-exonuclease is immunochemically related to the recC gene product of Escherichia coli. J Bacteriol. 1990 Jan;172(1):507–510. doi: 10.1128/jb.172.1.507-510.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D., Weber K., Geisler N., Blobel G. Binding of two desmin derivatives to the plasma membrane and the nuclear envelope of avian erythrocytes: evidence for a conserved site-specificity in intermediate filament-membrane interactions. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6780–6784. doi: 10.1073/pnas.84.19.6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenson M., Low R. L., Loehr J. Levels of the mitochondrial endonuclease during rat cardiac development implicate a role for the enzyme in repair of oxidative damage in mitochondrial DNA. J Mol Cell Cardiol. 1994 Jan;26(1):31–40. doi: 10.1006/jmcc.1994.1005. [DOI] [PubMed] [Google Scholar]
- Harosh I., Mezzina M., Harris P. V., Boyd J. B. Purification and characterization of a mitochondrial endonuclease from Drosophila melanogaster embryos. Eur J Biochem. 1992 Dec 1;210(2):455–460. doi: 10.1111/j.1432-1033.1992.tb17442.x. [DOI] [PubMed] [Google Scholar]
- Houmiel K. L., Gerschenson M., Low R. L. Mitochondrial endonuclease activity in the rat varies markedly among tissues in relation to the rate of tissue metabolism. Biochim Biophys Acta. 1991 Aug 30;1079(2):197–202. doi: 10.1016/0167-4838(91)90125-j. [DOI] [PubMed] [Google Scholar]
- Jacquemin-Sablon H., Jacquemin-Sablon A., Paoletti C. Yeast mitochondrial deoxyribonuclease stimulated by ethidium bromide. 1. Purification and properties. Biochemistry. 1979 Jan 9;18(1):119–127. doi: 10.1021/bi00568a019. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linn S., Lehman I. R. An endonuclease from mitochondria of Neurospora crassa. J Biol Chem. 1966 Jun 10;241(11):2694–2699. [PubMed] [Google Scholar]
- Low R. L., Buzan J. M., Couper C. L. The preference of the mitochondrial endonuclease for a conserved sequence block in mitochondrial DNA is highly conserved during mammalian evolution. Nucleic Acids Res. 1988 Jul 25;16(14A):6427–6445. doi: 10.1093/nar/16.14.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low R. L., Cummings O. W., King T. C. The bovine mitochondrial endonuclease prefers a conserved sequence in the displacement loop region of mitochondrial DNA. J Biol Chem. 1987 Nov 25;262(33):16164–16170. [PubMed] [Google Scholar]
- Morosoli R., Lusena C. V. An endonuclease from yeast mitochondrial fractions. Eur J Biochem. 1980 Sep;110(2):431–437. doi: 10.1111/j.1432-1033.1980.tb04884.x. [DOI] [PubMed] [Google Scholar]
- Parks W. A., Couper C. L., Low R. L. Phosphatidylcholine and phosphatidylethanolamine enhance the activity of the mammalian mitochondrial endonuclease in vitro. J Biol Chem. 1990 Feb 25;265(6):3436–3439. [PubMed] [Google Scholar]
- Richter C., Park J. W., Ames B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond J. Purification and properties of an endonuclease from the mitochondrion of Saccharomyces cerevisiae. Eur J Biochem. 1981 Dec;120(3):541–546. doi: 10.1111/j.1432-1033.1981.tb05734.x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Renaud J. Endonuclease G: a (dG)n X (dC)n-specific DNase from higher eukaryotes. EMBO J. 1987 Feb;6(2):401–407. doi: 10.1002/j.1460-2075.1987.tb04769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tomkinson A. E., Linn S. Purification and properties of a single strand-specific endonuclease from mouse cell mitochondria. Nucleic Acids Res. 1986 Dec 22;14(24):9579–9593. doi: 10.1093/nar/14.24.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper J. N., Bennett J. L., Clayton D. A. A role for RNAase MRP in mitochondrial RNA processing. Cell. 1992 Jul 10;70(1):16–20. doi: 10.1016/0092-8674(92)90529-l. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent R. D., Hofmann T. J., Zassenhaus H. P. Sequence and expression of NUC1, the gene encoding the mitochondrial nuclease in Saccharomyces cerevisiae. Nucleic Acids Res. 1988 Apr 25;16(8):3297–3312. doi: 10.1093/nar/16.8.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walberg M. W., Clayton D. A. Sequence and properties of the human KB cell and mouse L cell D-loop regions of mitochondrial DNA. Nucleic Acids Res. 1981 Oct 24;9(20):5411–5421. doi: 10.1093/nar/9.20.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Zassenhaus H. P., Hofmann T. J., Uthayashanker R., Vincent R. D., Zona M. Construction of a yeast mutant lacking the mitochondrial nuclease. Nucleic Acids Res. 1988 Apr 25;16(8):3283–3296. doi: 10.1093/nar/16.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tigerstrom R. G. Purification and characteristics of a mitochondrial endonuclease from the yeast Saccharomyces cerevisiae. Biochemistry. 1982 Dec 7;21(25):6397–6403. doi: 10.1021/bi00268a012. [DOI] [PubMed] [Google Scholar]