Abstract
The aim of this work was to increase the solubility, stability and permeation of resveratrol by complexation with cyclodextrin-based nanosponges (NS). Nanosponges are recently developed hyper-cross-linked cyclodextrin polymers nanostructured to form three-dimensional networks; they are obtained by reacting cyclodextrin with a cross-linker such as carbonyldiimidazole. They have been used to increase the solubility and stability of poorly soluble actives. This study aimed at formulating complexes of resveratrol with β-cyclodextrin nanosponges in different weight ratios. DSC, FTIR and X-ray powder diffraction (XRPD) studies confirmed the interaction of resveratrol with NS. XRPD showed that the crystallinity of resveratrol decrease after encapsulation. The particle sizes of resveratrol-loaded NS are in between 400 to 500 nm with low polydispersity indices. Zeta potential is sufficiently high to obtain a stable colloidal nanosuspension. TEM measurement also revealed a particle size around 400 nm for NS complexes. The in vitro release and stability of resveratrol complex were increased compared with plain drug. Cytotoxic studies on HCPC-I cell showed that resveratrol formulations were more cytotoxic than plain resveratrol. The permeation study indicates that the resveratrol NS formulation showed good permeation in pigskin. The accumulation study in rabbit mucosa showed better accumulation of resveratrol NS formulation than plain drug. These results signify that resveratrol NS formulation can be used for buccal delivery and topical application.
Key words: complexation, cytotoxicity, nanosponges, resveratrol/cyclodextrin
INTRODUCTION
Cyclodextrin-based nanosponges (NS) are hyper-cross-linked cyclodextrin synthesized by the procedure mentioned elsewhere (1). Briefly, NS can be obtained by cross-linking different type of cyclodextrin (CD) with carbonyldiimidazole compound as a cross-linker. They are solid particles with spherical morphology that have been reported to have a very high solubilising power for poorly soluble molecules (2), and they are proposed to form inclusion and non-inclusion complexes with different drugs (3). The CD cross-linker ratio can be varied during their preparation to improve the drug loading and solubility.
Resveratrol is a polyphenolic phytoalexin that is found, free and conjugated, in high concentrations in grape juice, peanuts, mulberries and in other plant extracts (4,5). Phytoalexines are low molecular weight secondary metabolites made by plants as a defence response to microbial infections (6). Extracts containing resveratrol have been already used from decades in medicine for treatment of many diseases like inflammation, cardiovascular diseases, dermatitis, gonorrhea, fever and hyperlipidemia (7,8). The earlier studies showed that resveratrol is responsible for the cardiovascular benefits associated with moderate red wine consumption also modulates the lipid metabolism (9–11) inhibits platelet activation and aggregation (12) resveratrol has been shown to provide health-promoting activities such as cancer chemopreventive activity (13–16). In addition, it has been studied that resveratrol has antibacterial activity and antifungal that are major etiological agents in human skin infections (17).
However, due to hydrophobicity, dissolution is a rate-limiting step for in vivo absorption, which constitutes the serious problem for oral bioavailability (18), the extensive research has been carried out to overcome this problem (19,20).
The present work focused on, preparation, characterisation, cytotoxicity and permeation of a novel formulation of resveratrol with NS.
MATERIALS AND METHODS
Materials
Resveratrol and carbonyldiimidazole were purchased from Sigma Aldrich (Milan, Italy), β-CD was provided by Wacker Chemie (Germany). All other chemical and reagents were analytical grade. Milli Q water (Millpore) was used throughout the studies.
Method
Synthesis of β-CD Nanosponges
β-Cyclodextrin (MW 1,135 g/mol) nanosponges were prepared as reported (1). Briefly, 100 mL of anhydrous dimethylformamide (DMF) were placed in a round bottom flask and 17.42 g of anhydrous β-cyclodextrin (15.34 mmol) were added to achieve complete dissolution. Then 9.96 g of carbonyldiimidazole (61.42 mmol) were added and the solution allowed to react for 4 h at 100◦C. Once the condensation polymerization was completed, the transparent block of hyper-cross-linked cyclodextrin was roughly ground and an excess of deionized water added to remove DMF. Finally, residual by-products or unreacted reagents were completely removed by Soxhlet extraction with ethanol. The white powder thus obtained was dried overnight in an oven at 60°C and subsequently ground in a mortar. The fine powder obtained was dispersed in water. The colloidal part that remained suspended in water was recovered and lyophilized. The nanosponges recovered are sub-micron in dimension and with a spherical shape. The cyclodextrin:cross-linker molar ratio can vary (i.e. 1:2, 1:4, 1:8). Nanosponges can be classified according to the molar ratio with the cross-linker used in their preparation (i.e. nanosponges, 1:4) (2,17,21,22).
Preparation of Resveratrol-Loaded NS
Resveratrol NS 1:2 and 1:4 complexes were prepared at two different weight ratios of 1:5 and 1:10 (Drug, BNS w/w). Accurately, weighed quantities of NS 1:2 or 1:4 were suspended in 20 mL of Milli Q water using a magnetic stirrer, then the calculated amount of resveratrol was added, the mixture was sonicated for 10 min and was kept for 24 h understirring in the dark. The suspensions were centrifuged at 2,000 rpm for 10 min to separate the uncomplexed drug as a residue below the colloidal supernatant. The supernatant was lyophilized at −20°C temperature and operating pressure13.33 mbar. The dried powder was stored in a desiccator. The batch size was kept at 1.0 g for all batches.
Characterization of Resveratrol NS Complexes
Solubilisation Efficiency of NS
The resveratrol solubilisation efficiency of two types of NS viz. (1:2 and 1:4) was investigated to evaluate solubilisation enhancement capacity. An excess quantity of resveratrol was suspended with fixed quantity (15 mg) of NS in 20 mL Milli Q water. The volumetric flasks were placed on a mechanical shaker at ambient temperature. After equilibrium (24 h), the suspension was filtered through centrifugal filter device and filtrate analysed using a calibration curve for resveratrol concentration by UV spectrophotometer (Beckman Coulter DU 730) at 303 nm.
Loading Efficacy of Resveratrol
Weighed amount of loaded NS complexes were dissolved in methanol, sonicated for 5 min to break the complex, diluted suitably and then analysed by UV spectrophotometer at 303 nm (23).
Quantitative Determination of Resveratrol
The quantitative determination of resveratrol was carried out by high-performance liquid chromatography (HPLC) method (24) the chromatographic system consists of Perkin–Elmer HPLC system with LC-95 Detector and LC-250 pump was used. The chromatographic separation was performed using a Microsorb MV 100-5 C18 analytical column (4.6 × 250 mm) from Varian analytical Instruments. The mobile phase consisted of a mixture of 0.5% (v/v) acetic acid in methanol and water (52:48 v/v), filtered through a 0.45 μm nylon membrane and ultrasonically degassed prior to use. The mobile phase was delivered at a flow rate of 1 mL/min, the detection wavelength was 303 nm, and the attenuation was 0.001 and the injection volume in 100 μl. The HPLC method was validated for parameters linearity, limit of detection and quantitation. The method was found to be linear in the range of 0.5 to 2.5 μg/mL of resveratrol with regression coefficient of 0.999. Limit of detection and quantification were found out to be 0.04 and 0.220 μg/mL, respectively.
Particle Size, Polydispersity and Zeta Potential Determination
NS sizes and polydispersity index were measured by dynamic light scattering using a 90 plus particle sizer (Brookhaven Instruments Corporation), with MAS Option particle sizing software was used at the fixed angel of 90°. The samples were suitably diluted with water prior to measurements. Zeta potential measurements were also made using an additional electrode in same instruments. The mean hydrodynamic diameter (Dh) and polydispersity index (PI) of the particles were calculated in intensity using the cumulant analysis after averaging the three measurements.
Fourier Transform Infrared–Attenuated Transmitted Reflectance Spectroscopy
Resveratrol, NS, physical mixture and complex were subjected to Fourier transform infrared (FTIR) spectroscopic studies by using Perkin–Elmer system 2000 FTIR Spectrophotometer in the region of 4,000 to 650 cm−1.
Differential Scanning Calorimetry
Resveratrol, physical mixture and complex were subjected to differential scanning calorimetry (DSC) studies using Perkin–Elmer DSC instruments. Empty pan was used as a reference material and samples were scanned at the rate of 10°C/min in the range of 30°C to 450°C.
X-ray Powder Diffraction
Resveratrol, physical mixture and complex were subjected to X-ray powder diffraction (XRPD) studies using Huber Guinier camera G670 (simultaneous with Bragg-Brentano geometry (sequential collection between 2.5° and 100° at 2θ).
Transmission Electron Microscopy
The suspension of complex was observed using a Philips CM 10 transmission electron microscope to elucidate the particle shape and size. The particle size was measured by using the NIH image software.
Scanning Electron Microscopy
The surfaces of NS and complex were examined using a scanning electron microscope (SEM; Quanta W (FEI Company). The coated pellets were mounted onto stubs using double sided adhesive tape. The samples were sputter coated with platinum–palladium (80:20) in an argon atmosphere and examined at 15 kV accelerating voltage.
In Vitro Release Studies
The release of the resveratrol from the optimized formulation was studied using multi compartment (n = 6) rotating cell with dialysis membrane (cut-off 12,000 Da). The donor phase consisted of nanosuspensions 2 mL, (250 μg/mL) of resveratrol and resveratrol-loaded NS complex in distilled water. The receptor phase also contains the same medium. The receptor phase was withdrawn completely after fixed time intervals, suitably diluted with distilled water and was analysed by UV spectrophotometer at 303 nm (r2, 0.9993, CV < 2%) for resveratrol release.
Photodegradation Study
The photodegradation of resveratrol and resveratrol-loaded NS complex was performed under UV lamp (Philips 40 W TL K05). The samples were kept at distance of 10 cm from the lamp for 1 h stirring under dark, simultaneously the samples were quantitatively analysed by HPLC.
Cytotoxicity Studies Against HCPC-I
The HCPC-I is a continuous cell line derived from a chemically induced cancer 7, 12-dimethylben anthracene (via DMBA) in the buccal mucosa of the pocket of Syrian hamsters. Cells were cultured in 75 cm2 flasks in medium (Roswell Park Memorial Institute medium (RPMI) 1640), entire (with 10% w/v bovine serum) and with 100 U/mL penicillin G, 40 μm/mL of gentamycin sulphate and 2.5 μg of amphotericin B at 37°C and with 5% Co2. The cells were detached every 3–4 days with 0.25% w/v of trypsin. The in vitro cytotoxicity of NS with and without drug were evaluated by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay in 96-well plate, each well contained HCPC-I up to 5 × 102/100 μl in RPMI 1640 with 10% serum. To assess their toxicity culture medium was replaced with higher concentration after 1 day. After 24 and 48 h of incubation, the cells were added to 10 μL stock solution (5 mg/mL) of MTT in phosphate buffer. At least 3 h later the culture medium was replaced with a 100-μL dimethyl sulphoxide (DMSO) and plates were read at 560 nm with microplate reader model 450. The cell viability was expressed in average percentage of absorbance of treated cells compared with control.
Accumulation of Resveratrol in the Buccal Mucosa of Rabbit Ex Vivo Study
The freshly excised buccal tissue obtained from the rabbit was used within 2 h of removal. Most of underline tissue was removed from the mucosa with surgical scissors making sure that the basal membrane was still present (24). Then mucosa was washed with physiological solution after drying mucosa were taken in cell and resveratrol NS complex 0.2 mM in 0.2% w/v hydroxyethyl cellulose in water was put on the mucosa for 24 h. Similarly plain resveratrol sample was prepared in mixture of ethanol and water (50:50 v/v). After 24 h, the mucosa was washed with physiological solution and cut into small pieces. To extract the drug from the mucosa the pieces were kept in amber colour vial in methanol for 3 h with stirring, samples were analysed by HPLC method (25).
Permeation of Resveratrol Through the Pigskin Ex Vivo Study
The pigskin was used for the permeation study because the pig stratum corneum is the most similar to human stratum corneum in terms of lipid composition (26). The permeation studies of freshly excised pigskin were performed in triplicate (at least three times in order to achieve statistical significance) by means of vertical Franz diffusion cells with an effective diffusion area of 1.53 cm2. The donor compartment was filled with resveratrol NS complex 0.2 mM in 0.2% hydroxyethyl cellulose in water (2 mL) at room temperature, similarly plain resveratrol sample was prepared in mixture of ethanol and water (50:50 v/v). The samples were taken from the receptor compartment at predetermined intervals, replaced with same volume of fresh medium and subsequently assayed by an HPLC method (25).
RESULTS AND DISCUSSION
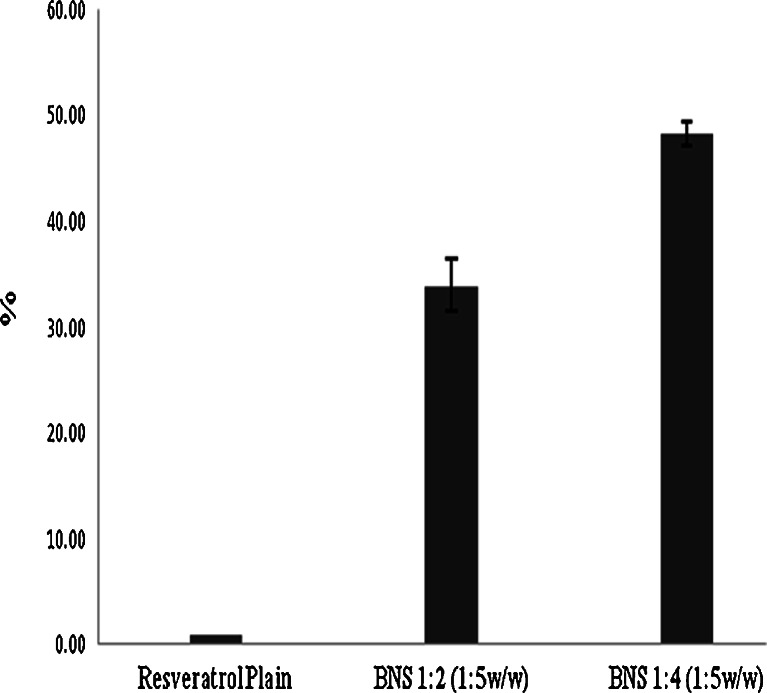
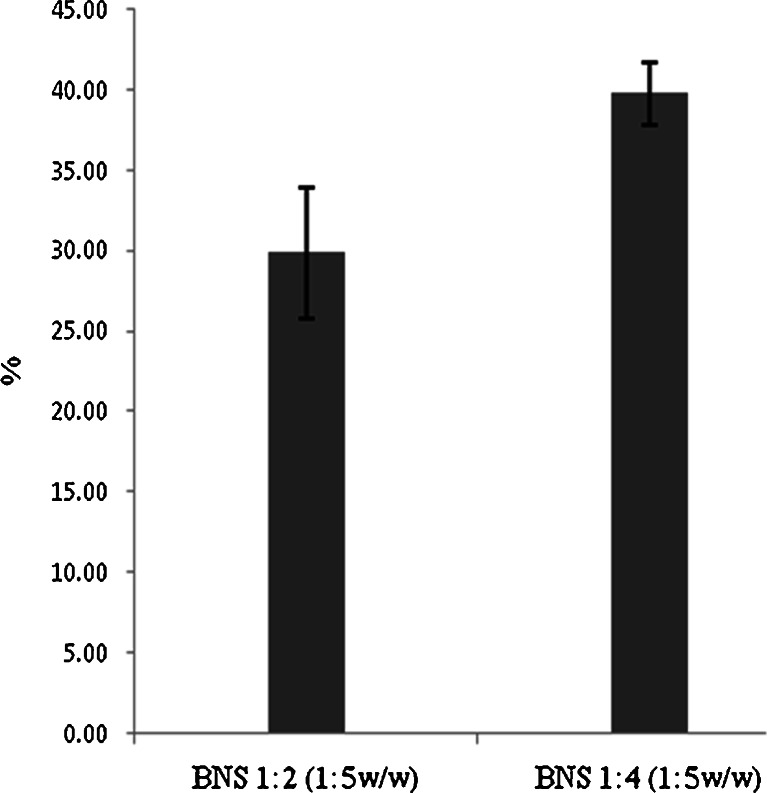
β-Cyclodextrin nanosponges NS with 2° of cross-link ratios, were synthesized (1,2) and standardized prior to use. In this study, we showed the resveratrol NS complexes, which had good encapsulation, solubility, stability and permeation. Figure 1 represents the percentage solubilisation of resveratrol by NS 1:2 and 1:4. The increase in solubility of resveratrol with NS 1:2 and 1:4 in 1:5 weight ratios is might be due to complexation of resveratrol with NS. The degree of cross-linking may be a factor affecting the extent at which the NS form inclusion complexation with the drug. The total solubility effect exerted by NS may be because of entrapment in the matrix as well as the formation of inclusion complex with drug. The solubilisation enhancement factor was 33 and 48 for resveratrol NS 1:2 and NS 1:4 complexes in 1:5 weight ratios (drug to NS by weight) respectively. Figure 2 indicates that loading efficacy of resveratrol by NS 1:2 and 1:4 in 1:5 weight ratios. NS 1:4 showed high loading compared with NS 1:2 may be because of high degree of cross-linking ratio.
Fig. 1.
Comparison of solubilisation of resveratrol by NS
Fig. 2.
Percentage loading of resveratrol on NS
The solubility of resveratrol increases as the NS concentration increases in the system up to 1:5 weight ratios (drug, NS). Above this concentration, there is no significant enhancement in solubility of resveratrol this might be because of saturation solubility of resveratrol in NS.
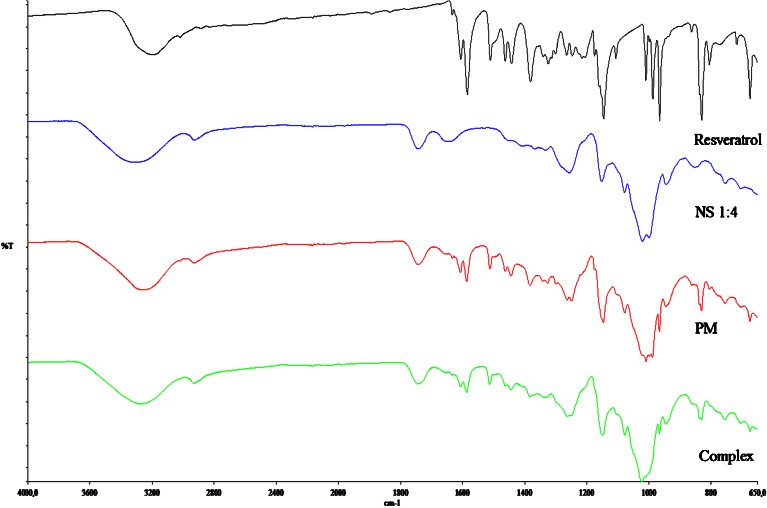
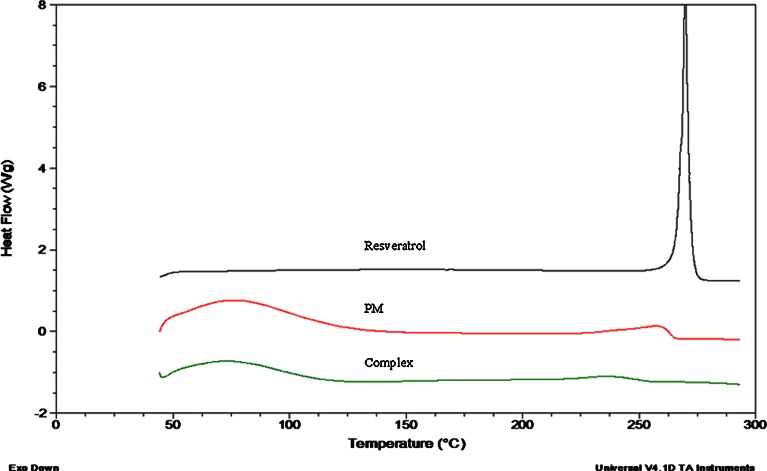
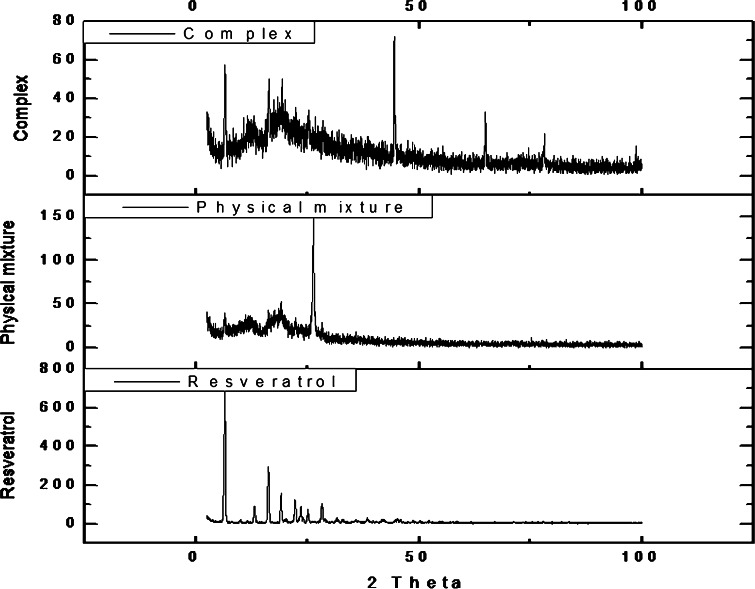
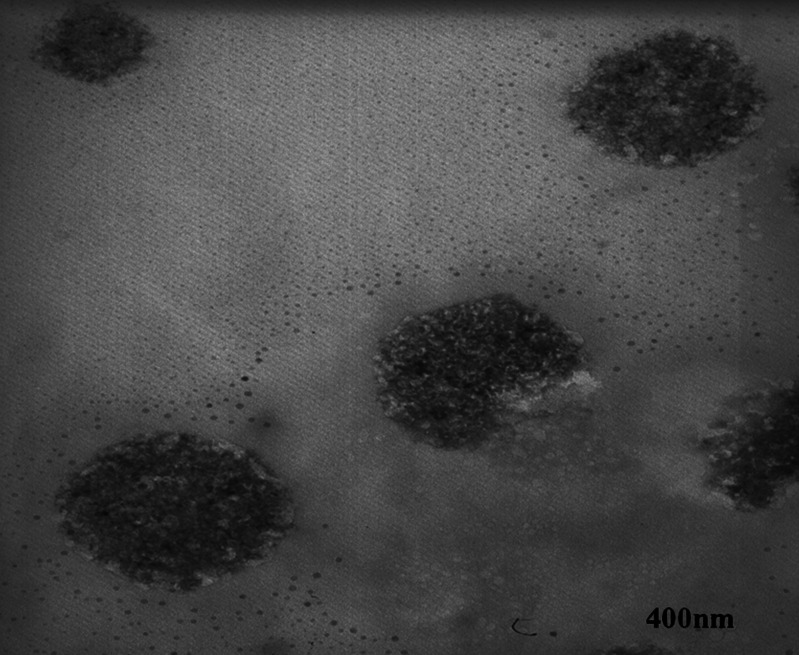
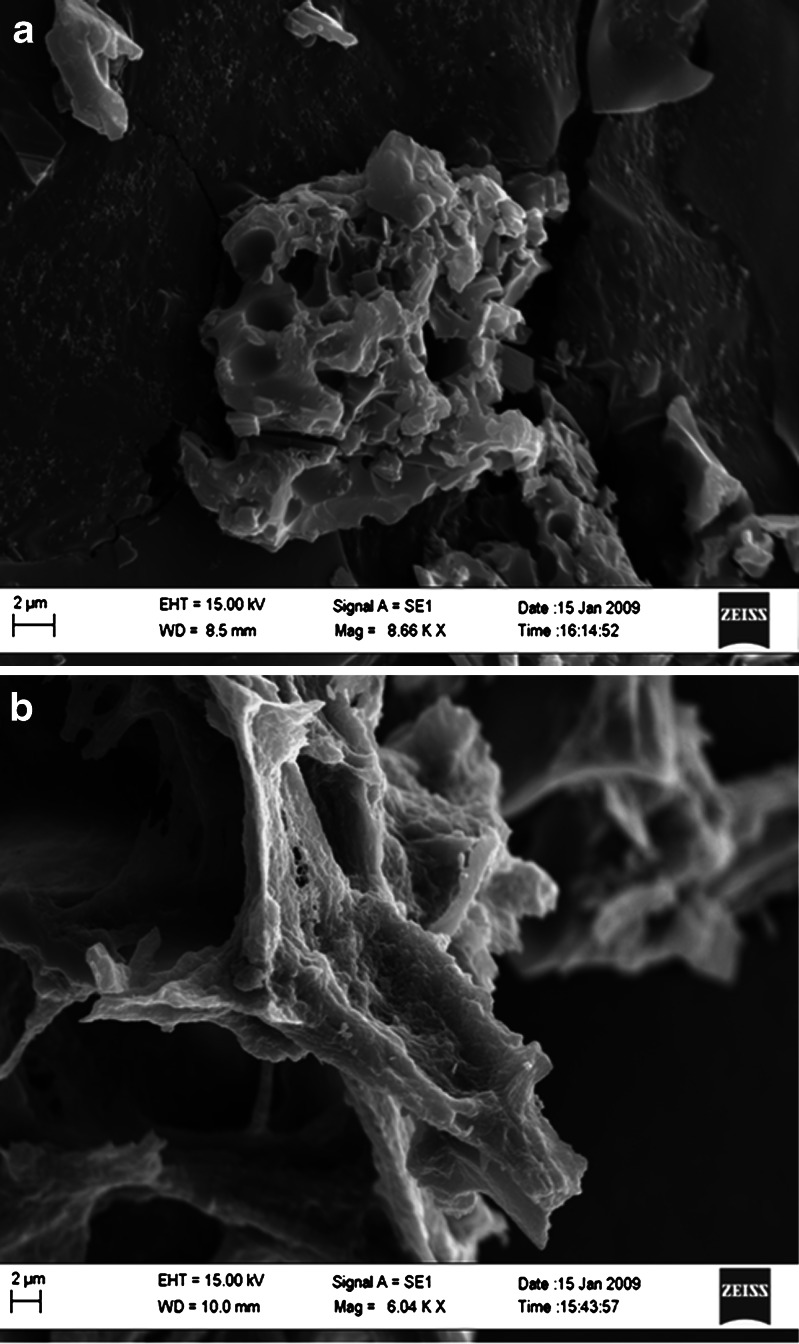
The particle size analysis of NS and resveratrol NS complex suspension revealed that the average particle size measured by laser light scattering method is around 400 to 500 nm. The particle size distribution is unimodal and having a narrow range as seen in the Table I. The Comparison of FTIR spectra of resveratrol and complex Fig. 3 showed that there is a major change in the fingerprint region i.e. 900 to 1,400 cm−1. There is intense peak due to O–H stretching typical to the carbohydrates in the spectrum of NS which is shifted to 3,273 cm−1 in the complex indicating there is interaction of OH groups of nanosponges with resveratrol. DSC thermograms of resveratrol, physical mixture and complex as showed in Fig. 4. Resveratrol showed exotherm at 267°C. The physical mixture showed this exotherm similar to the plain resveratrol sample but with very less intensity. However, in the complex this exotherm was suppressed indicating the partial protection due to the encapsulation of resveratrol with NS. The percentage crystallinity was drastically changed in NS complex indicating resveratrol was dispersed in NS loosing most of its crystallinity. This might be because of inclusion as well as non-inclusion phenomenon of NS with resveratrol. It can be seen from Fig. 5 that the intensity of resveratrol plain drug is considerably higher than that of physical mixture and complex. The comparison of complex and physical mixture showed that there was significant change in the intensities of powder, 2θ values. The lyophilization process gives rise to fluffy mass powder showing highly porous structure losing all its crystallinity which was confirmed by XRPD study. Transmission electron microscopy (TEM) studies showed that the regular spherical shape and size of NS that are unaffected even after drug encapsulation Fig. 6. TEM measurement also revealed a particle size around 400 nm for NS complex and DLS results were in agreement with TEM studies Table I. A cluster forming capacity when suspended in water and have a highly porous structure as seen in SEM studies Fig. 7a, b.
Table I.
Particle Size Analysis of Resveratrol NS Complex
| Sample | Mean hydrodynamic diameter ± SD (nm) | Polydispersity Index | Zeta potential (mV) |
|---|---|---|---|
| BNS 1:2 complex | 447 ± 45 | 0.23 ± 0.005 | −15.80 ± 2.34 |
| BNS 1:4 complex | 436 ± 38 | 0.23 ± 0.005 | −20.21 ± 2.21 |
Fig. 3.
FTIR spectrum of resveratrol, NS, physical mixture and complex
Fig. 4.
DSC thermograms of resveratrol, physical mixture and complex
Fig. 5.
XRPD pattern of resveratrol, physical mixture and complex
Fig. 6.
TEM of resveratrol-loaded NS complex (magnification, ×46,000)
Fig. 7.
a SEM of NS; b SEM of complex
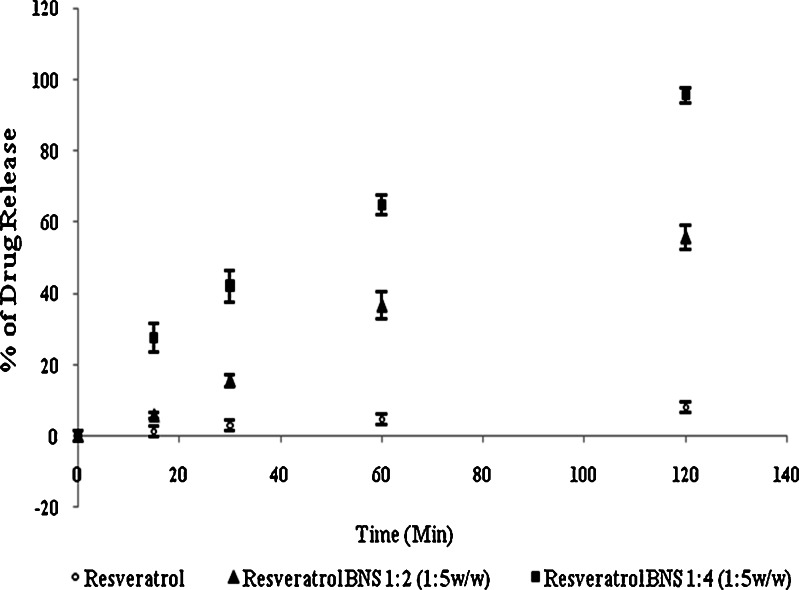
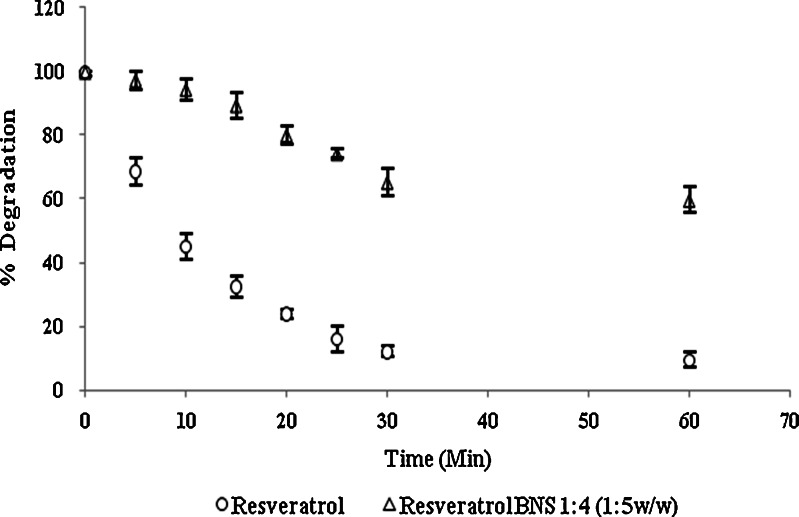
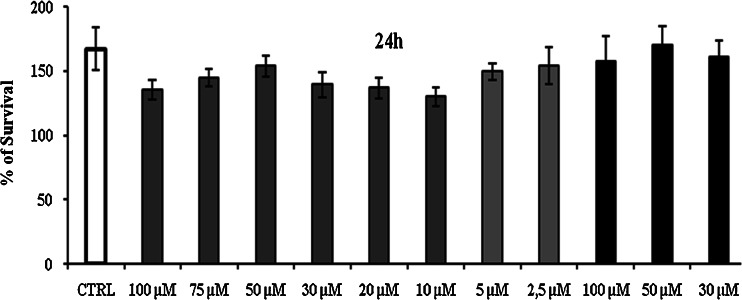
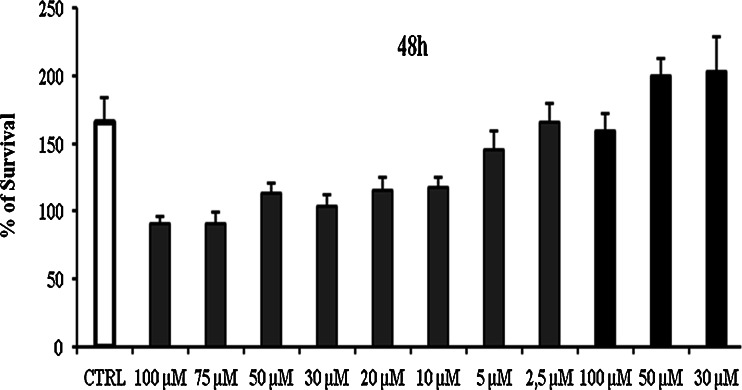
From in vitro release, study Fig. 8 it was found that the complex showed a significant improvement in the rate of release as compared with the pure drug. The first order rate constant for resveratrol plain drug, physical mixture and resveratrol complex are −0.96, −0.98 and −0.99, respectively. This may be due to the increased solubility and wettability caused by complex formation and processes. From the degradation study, it was clear that NS complex was more photostable than plain drug Fig. 9. This might be because of strong encapsulation of resveratrol with NS. The mechanism by which the resveratrol NS complex showed better inhibition of cell proliferation is yet not understood. The increased in solubility of resveratrol due to complexation compared with plain drug may be attributed for its better activity against HCPC-I cell cultures. Dose and time dependant effect of resveratrol and complex on proliferation of HCPC-I cancer cells after 24- and 48-h incubation is shown in Figs. 10 and 11. It was found that rapid loss of cell proliferation occurred with the increasing concentration of resveratrol this might be due to increase in the solubility of resveratrol because of complexsation with NS. It can be seen that inhibition of cell proliferation induced by the drug was dependant on both concentration and incubation time. The resveratrol NS complexes showed better inhibition of cell proliferation than its free drug counterpart did in all concentrations studied.
Fig. 8.
In vitro dissolution profiles of complexes
Fig. 9.
Photodegradation of resveratrol and complex
Fig. 10.
Cytotoxicity after 24 h; control, resveratrol BNS complex and BNS
Fig. 11.
Cytotoxicity after 48 h; control, resveratrol BNS complex and BNS
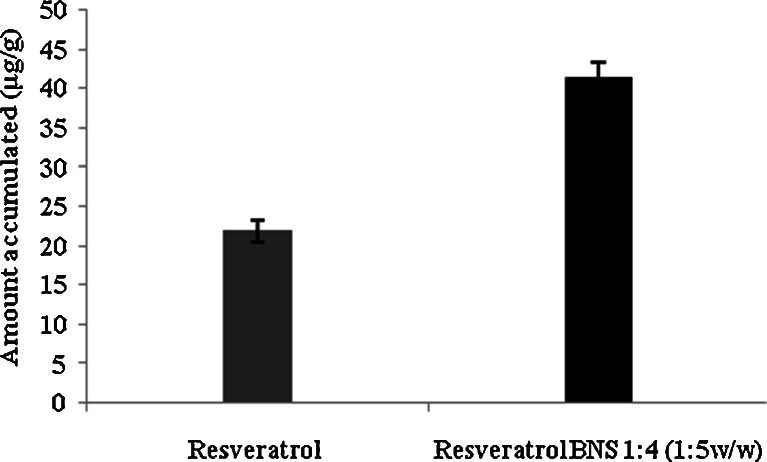
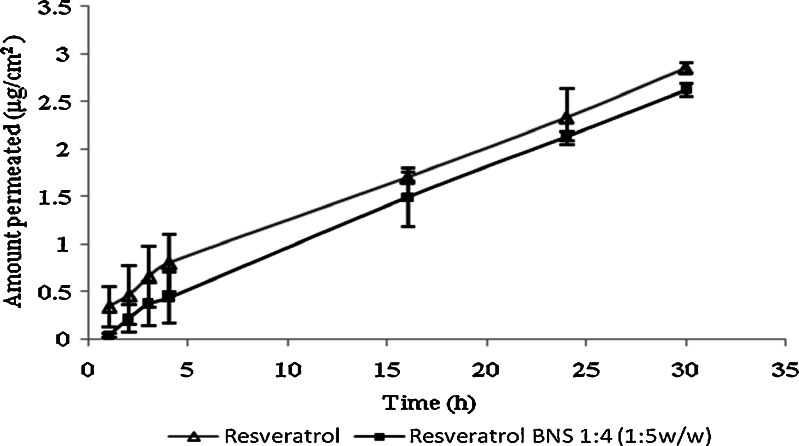
Figure 12 indicated that resveratrol NS complex showed almost twofold accumulation of resveratrol compared with plan drug, as complex samples were made in water and plain drug samples were made in ethanol water (50/50 v/v). Permeation studies results Fig. 13 showed that NS complex in water giving good permeation when compared with resveratrol plain in ethanol water mixture (50/50 v/v). The better permeation through pigskin might be because of increase in solubility of resveratrol due to inclusion as well as non-inclusion phenomenon of NS with resveratrol. The permeation and accumulation results showed that we can use NS resveratrol complex for buccal delivery and topical application.
Fig. 12.
Accumulation study of resveratrol and complex in rabbit mucosa
Fig. 13.
Permeation study of resveratrol and complex by using pig skin
CONCLUSIONS
In the light of these findings, it can be concluded that resveratrol-loaded NS are of appropriate particle size obtained by conventional inclusion complexation techniques. All characterisation results indicate that the drug is encapsulated within the matrix in the cyclodextrin chains. This nanosponge based formulation display significantly better permeation, stability and cytotoxicity against HCPC-I cells. This way it is possible to administer resveratrol NS complex as buccal delivery and topical application.
Acknowledgements
The Ministry of Education, University and Research (MIUR) Italian government and University grant commission (UGC) India supported this work.
References
- 1.Trotta F, Tumiatti W. Cross-linked polymers based on cyclodextrin for removing polluting agents. WO 03/085002; 2003.
- 2.Trotta F, Cavalli R. Characterization and applications of new hyper-cross-linked cyclodextrins. Compos Interface. 2009;16:39–48. doi: 10.1163/156855408X379388. [DOI] [Google Scholar]
- 3.Cavalli R, Trotta F, Tumiatti W. Cyclodextrin-based nanosponges for drug delivery. J Incl Phenom Macrocycl Chem. 2006;56:209–213. doi: 10.1007/s10847-006-9085-2. [DOI] [Google Scholar]
- 4.Lamuela-Raventos RM, Romero-Perez AI, Andrew L, Waterhouse M, de la Torre-Boronat C. Direct HPLC analysis of cis- and trans-resveratrol and piceid isomers in Spanish red Vitis vinifera wines. J Agric Food Chem. 1995;43:281–283. doi: 10.1021/jf00050a003. [DOI] [Google Scholar]
- 5.Romero-Pérez AI, Ibern-Gómez M, Lamuela-Raventós RM, de la Torre-Boronat C. Piceid the major resveratrol derivative in grape juices. J Agric Food Chem. 1999;26:1533–1536. doi: 10.1021/jf981024g. [DOI] [PubMed] [Google Scholar]
- 6.Dixon RA. Natural products and plant disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- 7.Bertacche V, Lorenzi N, Nava D, Pini E, Sinico C. Host–guest interaction study of resveratrol with natural and modified cyclodextrins. J Incl Phenom Macrocycl Chem. 2006;55:279–287. doi: 10.1007/s10847-006-9047-8. [DOI] [Google Scholar]
- 8.Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998;82:1111–1129. doi: 10.1161/01.res.82.11.1111. [DOI] [PubMed] [Google Scholar]
- 9.Renaud S, De Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-F. [DOI] [PubMed] [Google Scholar]
- 10.Chun JC, Wei Y, Yu-Cai F, Xin W, Ji-Lin L, Wei W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1–FoxO1 pathway. Biochem Biophys Res Commun. 2009;378:389–393. doi: 10.1016/j.bbrc.2008.11.110. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel E, Somoza V. Metabolism and bioavailability of trans-resveratrol. J Pharm Sci. 2005;49(5):472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 12.Pace A, Rounova CR, Hahn O. Wines and grape juices as modulators of platelet aggregation in healthy human subjects. Clin Chim Acta. 1996;246:163–182. doi: 10.1016/0009-8981(96)06236-5. [DOI] [PubMed] [Google Scholar]
- 13.Jang M, Cai L, Udeani G, Slowing KV, Thomas CF, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 14.Clement MV. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92:996–1002. [PubMed] [Google Scholar]
- 15.Bertelli A. Plasma and tissue resveratrol concentrations and pharmacological activity. Drugs Exp Clin Res. 1998;24:133–138. [PubMed] [Google Scholar]
- 16.Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the 'French paradox. Eur J Endocrinol. 1998;138:619–620. doi: 10.1530/eje.0.1380619. [DOI] [PubMed] [Google Scholar]
- 17.Chan MMY. Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogen of skin. Biochem Pharm. 2002;63:99–104. doi: 10.1016/S0006-2952(01)00886-3. [DOI] [PubMed] [Google Scholar]
- 18.Paola V, Stefano S, Gianni G, Cristiana G, Nicola C. Bioavailability of trans-resveratrol from red wine in humans. J Pharm Sci. 2005;49(5):495–504. doi: 10.1002/mnfr.200500002. [DOI] [PubMed] [Google Scholar]
- 19.Lu Z, Cheng Bo, Hu Yeli, Zhang Youhoung, Zou Guolin. Complexation of resveratrol with cyclodextrins: solubility and antioxidant activity. Food. Chemistry. 2009;113(1):17–20. doi: 10.1016/j.foodchem.2008.04.042. [DOI] [Google Scholar]
- 20.Lucas-Abellán C, Fortea I, López-Nicolás JM, Núñez-Delicado E. Cyclodextrins as resveratrol carrier system. Food Chem. 2007;104(1):39–44. doi: 10.1016/j.foodchem.2006.10.068. [DOI] [Google Scholar]
- 21.Vavia PR, Swaminathan S, Trotta F, Torne SJ. Formulation of β-cyclodextrin based nanosponges of itraconazole. J Incl Phenom Macrocycl Chem. 2007;57(1–4):89–94. [Google Scholar]
- 22.Swaminathan S, Pastero L, Serpe L, Troota F, Vavia P, Aquilano D, Trotta M, Zara G, Cavalli R. Cyclodextrin-based nanosponges encapsulating camptothecin: physicochemical characterization, stability and cytotoxicity. Eur J Pharm Biopharm. 2009;74:193–201. doi: 10.1016/j.ejpb.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Torne SJ, Ansari KA, Vavia PR, Trotta F, Cavalli R. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded nanosponges. Drug Deliv. 2010;17(6):419–425. doi: 10.3109/10717541003777233. [DOI] [PubMed] [Google Scholar]
- 24.Hoogstrate AJ, Senel S, Cullander C, Verhoef J, Junginger HE, Boddè HE. Effects of bile salts on transport rates and routes of FITC labelled compounds across porcine buccal epithelium in vitro. J Control Release. 1996;40:211–221. doi: 10.1016/0168-3659(95)00187-5. [DOI] [Google Scholar]
- 25.Hoogstraate AJ, Senel S, Cullander C, Verhoef J, Junginger HE, Hui H, Xijing C, Guangji W, Jiping W, Andrew KD. High-performanceliquid chromatography spectrometric analysis of trans-resveratrol in rat plasma. J of Chromatography B. 2006;832:177–180. doi: 10.1016/j.jchromb.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Cilurzo F, Minghetti Paola, Sinico C. Newborn pig skin as model membrane in vitro drug permeation studies. AAPS PharmSciTech. 2007;8(4):E1–E4. doi: 10.1208/pt0804094. [DOI] [PMC free article] [PubMed] [Google Scholar]