Abstract
Nanoparticles composed of naturally occurring biodegradable polymers have emerged as potential carriers of various therapeutic agents for controlled drug delivery through the oral route. Chitosan, a cationic polysaccharide, is one of such biodegradable polymers, which has been extensively exploited for the preparation of nanoparticles for oral controlled delivery of several therapeutic agents. In recent years, the area of focus has shifted from chitosan to chitosan derivatized polymers for the preparation of oral nanoparticles due to its vastly improved properties, such as better drug retention capability, improved permeation, enhanced mucoadhesion and sustained release of therapeutic agents. Chitosan derivatized polymers are primarily the quaternized chitosan derivatives, chitosan cyclodextrin complexes, thiolated chitosan, pegylated chitosan and chitosan combined with other peptides. The current review focuses on the recent advancements in the field of oral controlled release via chitosan nanoparticles and discusses about its in vitro and in vivo implications.
KEY WORDS: chitosan, chitosan derivatives, controlled release, oral, nanoparticles
INTRODUCTION
From the beginning of the pharmaceutical era, the oral route has always dominated over any other routes of drug delivery. This can be accredited to the numerous advantages of the oral route, such as ease of administration of drug, patient compliance, economical production methods, easy approvals from regulatory bodies and so on. Despite being the most superior route of administration, it is not always possible to deliver every therapeutic agent through the oral route (1,2). The major problem faced while delivering a therapeutic agent through the oral route is poor oral bioavailability due to incomplete and/or erratic absorption through the gastrointestinal tract (GIT), degradation of the drug or drug carriers due to varying pH of the stomach and enzymatic degradation of many proteins and peptide drugs (3,4). Hence, the importance of different drug delivery matrices is realized to improve the oral absorption and bioavailability by improving the stability of the therapeutic agent within the harsh condition of the GIT. Among the different drug delivery systems (DDS) available, the biodegradable polymeric nanoparticles (NPs) have shown immense potential for oral delivery of different types of therapeutic agents including drugs, vaccines, genes, proteins and peptides (1,5–8). Due to their nanosize and possibility of surface modification, these nanoparticulate systems possess several advantages over other forms of conventional and novel delivery systems like tablets, capsules, beads, liposomes, microparticles, microemulsions etc. (9–12). It is observed that therapeutic agents are much stable in the GIT when encapsulated within a nanoparticulate system. Moreover, the carrier itself can modulate the physicochemical properties of the encapsulated drug. It can control the release of the payload, can provide triggered release of the content, may enhance the cellular uptake and transmucosal transport and may even demonstrate targeting ability when attached to a suitable targeting moiety (1,13).
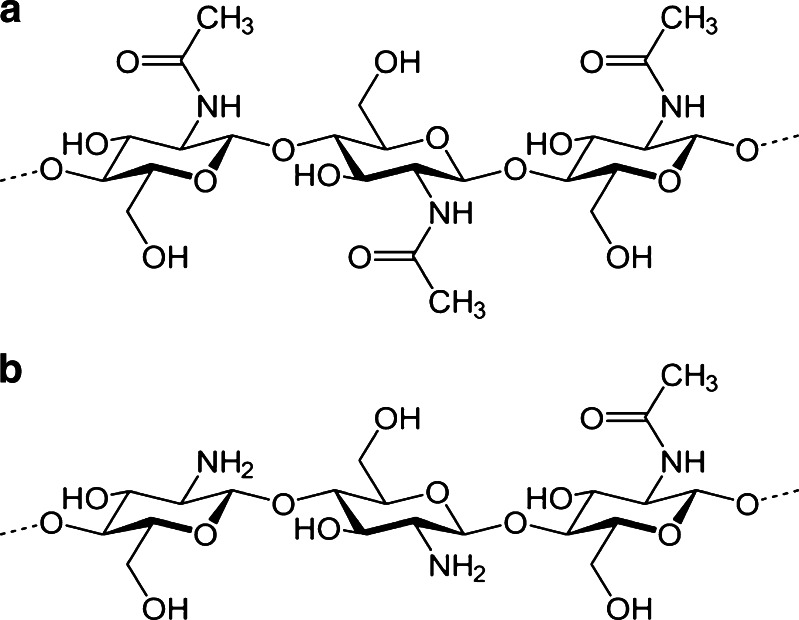
To date, most of the advance nanoparticulate drug carriers have been developed by utilising either synthetic or natural polymers or by their combination (14). Among the various natural polymers available, chitosan (CS) is perhaps one of the most widely used biopolymers for the preparation of NPs (8,15–19). This biodegradable polymer is synthesised by alkaline deacetylation of chitin (Fig. 1a) (20). CS (Fig. 1b) is made up of N-acetylglucosamine and glucosamine and is available in different molecular weights, viscosity and degree of deacetylation (21). Although CS has shown potential therapeutic activity as anti-microbial (22), wound healing (23), hypo-cholesterolemic (24) and anti-ulcer agent (20), its potential application still lies in formulating drug delivery systems (16,17,25,26). In the past decades, CS has been extensively used for delivery of small molecules (6,27), peptides (25), vaccines (28,29) and genes (30,31) via mucosal (29,32–34), nasal (25,35–39), colon (40), topical (41), oral (4,15) or parenteral route (20,25,42–47). However, its chemical versatility to form derivatives or cross-links (4,13,29), mucoadhesive property (4,48–51), permeation-enhancing capability (4,49,52), low toxicity (21,53,54) and ability to control the release of therapeutic agents (55,56) make it an ideal candidate for fabricating oral nanoparticulate DDS (17,57).
Fig. 1.
Chemical structure of chitin (a) and chitosan (b)
Although CS is one of the most commonly exploited polymer for the oral delivery of insulin, the focus has recently shifted from CS to CS derivatized biomaterial for the oral delivery of proteins and peptides (13,25,29). This is because the NPs should remain intact during their transit through the stomach for the effective delivery of proteins and peptides and should be able to protect their degradation by proteolytic enzymes (1,58–60). However, the high solubility of CS at low pH impedes the delivery of proteins to the intestine (57). To overcome this problem, CS derivatives have been developed in the long run, which not only protect the proteins from degradation in the GIT but also provide a sustained release delivery platform (57). Synthesis of CS derivatives would not have been possible without the versatile chemical nature of the compound. CS has free amino and hydroxyl groups which enable substitution or modification with different chemical entities to form CS derivatives with desired properties for oral drug delivery (4).
Thus, the objective of this review is to give an overview of the recent advancements in the field of CS-based nanoparticulate systems which have been utilised for the controlled oral delivery of different therapeutic agents. This review mainly focuses on different NPs prepared using CS or CS derivatives and discusses about the various prospects of the formulations and their in vitro and in vivo implications.
UNMODIFIED CHITOSAN
Several researchers have earlier utilised this polymer itself for preparing micro and NPs of different drugs, proteins and peptides (17,26). However, in 2002, Pan and co-workers first prepared NPs only with CS in an attempt to improve the oral bioavailability of insulin (61). They prepared insulin-loaded chitosan nanoparticles (CS-NPs) by ionotropic gelation method using tripolyphosphate (TPP) anions for cross-linking. These particles showed about 80% of insulin association and exhibited a pH-sensitive in vitro release property. Importantly, the NPs could extensively improve the intestinal absorption of insulin when compared to the aqueous solution of CS during in vivo evaluation in rats. Though the hypoglycaemic effect was prolonged for more than 15 h, bioavailability of the NPs was only 14.9% when compared to subcutaneous (s.c.) injection of insulin (61). Following this study, another group performed similar studies with insulin encapsulated CS-NPs and evaluated their pharmacological impact in rats (62). Since it was found from a previous study that pH of the formulation had a significant impact on the association efficiency and in vitro release of insulin from CS-NPs (63), the researchers altered the pH of the formulation to pH of 5.3 and 6.1 (62). However, the results obtained were similar to the previous study, and the formulation was unable to maintain the hypoglycaemic effect for more than 11 h.
Other than insulin, researchers have utilised this system for the delivery for ammonium glycyrrhizinate. Ammonium glycyrrhizinate is a salt of pharmacologically active glycyrrhetic acid, having potential anti-inflammatory, anti-tumorigenic and anti-hepatotoxic properties (64,65). However, the compound has poor oral absorption, which limits its clinical application and thus requires an effective delivery system (66). The study showed that ionic gelation of CS with TPP could efficiently load the drug into the cationic CS-NPs. The study revealed the importance of formulation parameters like CS molecular weight, CS concentration and ammonium glycyrrhizinate concentration (67) and investigated the effect of polyethylene glycol (PEG) coating on the CS-NPs characteristics. The intention of PEG coating was to stabilise the formulation in physiological fluids as well as to potentiate the controlled release of the drug. However, the results obtained indicated that the inclusion of PEG loosened the NP structure, thus increased its size. Furthermore, with an increase in PEG concentration, the encapsulation efficiency of ammonium glycyrrhizinate decreased considerably. The researchers proposed that such decrease in loading efficiency could be due to the entanglement of PEG chains with CS molecules which resulted in decreased availability of CS for interaction with drug (67).
In a study (68), dorzolamide hydrochloride (Dorzo) and pramipexole hydrochloride (Prami) have also been individually encapsulated in unmodified CS-NPs. While the CS-NP of Dorzo was for ocular delivery, CS-NP of Prami was targeted as oral sustained drug delivery system for the treatment of Parkinson’s disease. Here, loading efficiency of Prami did not correlate with the drug/CS ratio, and a decreasing trend was observed with an increase in drug proportion. Results from differential scanning calorimetry and Fourier transform infrared spectroscopy (FTIR) studies revealed that Prami’s inclusion into the NPs was in the form of molecular dispersion rather than drug crystals as per general conception. The NPs showed sustained release of Prami in simulated intestinal fluid (SIF). However, the NPs showed mucoadhesive properties only when the drug content was less, thus limiting its potential application (68).
In another study, sustained release ciprofloxacin hydrochloride-loaded CS-NPs have also been prepared, but its application was more for localised treatment of skin infections and corneal ulcers (69). Since the formulation does not fall directly under this topic, interested readers are advised to refer to the original article.
In line with the above study, aminoglycosidic antibiotics like streptomycin, gentamicin and tobramycin were individually encapsulated into CS-NPs (70). The antibiotics showed high drug incorporation, and their size was within the nanoscale. In acidic pH, these NPs showed in vitro controlled release of the drugs for about 6 h. Among the different antibiotics, streptomycin-loaded CS-NPs showed potential activity when administered orally in an in vivo mouse model of Mycobacterium tuberculosis (70). When administered at a dose of 100 mg/kg, the streptomycin-loaded CS-NPs showed similar activity equivalent to its subcutaneous injection. Overall, these aminoglycosidic CS-NPs showed potential to be developed as an oral dosage form, and the method may be applied for preparation other oral antibiotic-NPs.
Recently, CS-NPs of catechin have been developed by the ionotropic gelation method using TPP anions. The purpose of the study was to provide a controlled delivery system of this pharmacologically potential polyphenolic compound, for its nutraceuticals application. The NPs demonstrated high encapsulation efficiency of about 90% with good mucoadhesive property and controlled drug release of only 32% over a period of 24 h. This effect was attributed to better cross-linking of CS with TPP during NP formation. In addition, enhanced interaction due to hydrogen bonding was observed between the phenolic groups of catechin and unreacted amino groups of CS, as indicated by the FTIR data (71).
Though all these studies with unmodified CS used almost similar preparatory conditions and formulations parameters, the results indicate that the encapsulation efficiency and release properties of the NPs were more depended on the nature of the drug molecule itself rather than the inherent property of the CS-NP system. Thus, to effectively control the release of its content, it was required to develop some advance CS nanoparticulate systems which could precisely anticipate the drug release on oral administration, yet enhance the bioavailability of the drug. Hence at different stages, CS derivatives were utilised to prepare the NPs or CS was combined with other compounds to serve this purpose. Table I has summarised the main features of all these unmodified and modified CS-NP formulations.
Table I.
Main Features of Chitosan Nanoparticles Used for Oral Controlled Release of Therapeutic Agents
| Chitosan derivative | Therapeutic agent | Main features of the formulation or pharmacological effect | References |
|---|---|---|---|
| Unmodified chitosan | Insulin | Improved the in vivo intestinal absorption | (61) |
| Good insulin bioavailability | |||
| Insulin | Altered pH of the formulation | (62,63) | |
| Enhanced interaction with the intestinal epithelium | |||
| Ammonium glycyrrhizinate | Initial burst effect followed by continuous release of drug | (67) | |
| Pramipexole hydrochloride | Enhanced mucoadhesive properties; sustained release of drug in simulated intestinal fluid | (68) | |
| Ciprofloxacin hydrochloride | Controlled drug release | (69) | |
| Streptomycin, gentamicin, tobramycin | Controlled drug release | (70) | |
| Catechin | High encapsulation efficiency | (71) | |
| Controlled drug release | |||
| Trimethyl chitosan | Insulin | High insulin association efficiency | (72) |
| Diethylmethyl chitosan | Insulin | Better insulin loading | (73) |
| Tri-ethyl chitosan | Insulin | Enhanced colonic absorption of insulin | (74,75) |
| Dimethyl-ethyl chitosan | Insulin | Enhanced colonic absorption of insulin | (74,75) |
| N-Trimethyl chitosan | Insulin | High mucoadhesive property | (77–79) |
| Better penetration into duodenum and jejunum | |||
| Alginate–chitosan | Insulin | High association efficiency | (14,87) |
| Prolonged hypoglycaemic effect | |||
| Quaternized chitosan–alginate | Insulin | Enhanced absorption | (56) |
| Controlled drug release at the intestine | |||
| Chitosan and poly(γ-glutamic acid) | Insulin | Increased paracellular permeability | (88,93–95) |
| Effective reduction of blood glucose level | |||
| Chitosan and poly (aspartic acid) | Bovine serum albumin | pH-dependent release | (96) |
| Chitosan–heparin | Heparin | Effective against local H. pylori infection | (97) |
| N,O-carboxymethyl chitosan | Insulin | pH-dependent release | (100) |
| Chitosan and methyl methacrylate | Insulin | High encapsulation efficiency | (60) |
| Controlled drug release | |||
| Chitosan and poly (methyl methacrylate) | Insulin | pH-dependent release | (101) |
| Chitosan cyclodextrin | Triclosan, furosemide | Enhanced drug loading | (102) |
| Delayed release profile | |||
| Insulin, heparin | Good incorporation efficiency | (104) | |
| Glutathione | Enhanced absorption | (106) | |
| Thiolated chitosan | DNA | Augmented mucoadhesive property | (109) |
| Enhanced paracellular permeation | |||
| – | Enhanced bioadhesion | (52,110–114) | |
| Control release | |||
| Lauryl succinyl chitosan | Insulin | Improved mucoadhesive property | (117) |
| Enhanced paracellular permeation | |||
| Reduced blood sugar level | |||
| Chitosan and Arabic gum | Insulin | Lower encapsulation than CS-NPs | (118) |
MODIFIED CHITOSAN
Several therapeutic agents have been orally delivered using NPs prepared from different CS derivatives and CS complexes (Table I).
Trimethyl Chitosan
Jintapattanakit and co-workers utilised the CS derivative, trimethyl chitosan (TMC) and PEG-graft-TMC for the preparation of insulin NPs and polyelectrolyte complexes (PEC). Interestingly, it was observed that PEC with TMC and PEG-graft-TMC copolymer were more stable in SIF and could efficiently protect insulin from proteolytic enzymes when compared to the NPs (72). Despite this drawback, other researchers have continued their investigation on CS derivatives for NP preparation for oral delivery of therapeutic agents.
Quaternized Derivatives of Chitosan
Quaternized derivatives of CS (QCS) have broadened the application of CS for successful delivery for proteins and peptides at neutral or weakly alkaline pH of the small intestine. Recently, CS-NPs were prepared from newly synthesised diethylmethyl CS (DEMC) and TMC by ionotropic gelation method or by PEC (73). Similarly, another group reported the preparation of NPs from newly synthesised tri-ethyl chitosan (TEC) and dimethyl-ethyl chitosan (DMEC) (74,75). In both these studies, insulin NPs were delivered to the colon via the oral route. Insulin encapsulation in the positively charged CS derivatized NPs was about 70% (TMC, DEMC) to 90% (TEC, DMEC) when prepared by PEC method. This high encapsulation efficiency of insulin in NPs was thought to be due to electrostatic interactions between the negatively charged acidic groups of insulin with the positively charged amino groups of CS derivatives (76). The NPs made from TEC and DMEC showed sustained release of insulin for 5 h with minimum burst release effect at the initial hours; however, no significant difference in release profile was observed in phosphate-buffered saline (PBS) at different pH (6.8 and 7.4). The derivatives showed higher insulin release than CS-NPs itself as the quaternized derivatives were more soluble than the polymer itself at the neutral and alkaline pH (75). However, the ex vivo release was faster and was likely due to degradation of the NPs itself due to presence of enzymes and mucin in vivo (74). When Bayat and co-workers performed the in vivo studies in diabetic rats, the NPs composed of QCS demonstrated enhanced colonic absorption of insulin compared to free insulin or CS embedded insulin (74). Another current study with insulin-loaded TMC (35% quaternization degree) NPs (77) have shown higher penetration into duodenum and jejunum epithelial cells by endocytosis and specially to jejunum tissue (pH 6–6.5) due to its high mucoadhesive property (77–79). Though the above studies indicated that the NPs are efficiently absorbed via the paracellular pathway or by endocytosis due to smaller size (<200 nm), testing them in an appropriate in vivo study via the oral route is still required rather than direct delivery to the colon (74) or via the in vitro (77,80) or ex vivo studies (74,77) as done so far. Moreover, another similar study showed that these QCS (TMC, TEC, DEMC and DMEC) as free soluble polymers can improve the paracellular transport of insulin across the Caco-2 cell monolayer much more than the corresponding NPs (80). This was because the positive charge available on the surface of the NPs decreased, and hence, the particles were unable to open the tight junctions of the Caco-2 cell monolayer. In addition, TMC and DEMC in the free form exhibited higher antibacterial properties when compared to the nanoparticulate form (73). Thus, the question arises whether QCS itself is more suitable for oral delivery of proteins and peptides rather than QCS-NPs.
Alginate-Modified Chitosan
Alginate is a biocompatible, biodegradable and mucoadhesive polyanionic copolymer of (1–4)-linked b-d-mannuronic acid and a-l-guluronic acid widely used in the oral controlled delivery of drugs, proteins, peptides and cells (14,57,81). While pure CS is soluble in low pH and insoluble in higher pH, alginate shows the opposite trend. However, a stable rigid drug delivery matrix of the alginate–CS polyelectrolyte complex can be produced by the electrostatic interaction between the carboxyl groups of alginic acid and amino groups on CS, which can protect the drug from the harsh acidic environment of the stomach (51,82–86). Recently, this alginate–CS polyelectrolyte complex have also been utilised for the preparation of oral NPs (14,56,87). In a study, Sarmento and co-workers prepared insulin-loaded alginate–CS-NPs by ionotropic pre-gelation of dilute alginate solution with calcium chloride followed PEC formation with CS (87). Particle size, association efficiency of insulin into alginate NPs and loading capacity were influenced by various process and formulation variables such as time and speed of stirring, alginate guluronic acid content, CS molecular weight and initial alginate/CS mass ratio. Upon optimisation, high association efficiency (92%) and loading capacities (14.3%) of insulin were achieved, and the NPs displayed controlled release in gastric pH (50%) for up to 24 h while extensive insulin release was noticed in intestinal pH (75%) (87). The pharmacological effect of alginate–CS insulin NPs was evaluated in diabetic rats via oral administration (14). When insulin-loaded NPs at an insulin dose of 25 or 50 or 100 IU/kg were orally administered to the diabetic mice, it showed a significant reduction in plasma glucose level (8 to 14 h after administration) when compared to the empty NPs. A dose-dependent effect was observed; however, no significant difference was seen between the dose of 50 and 100 IU/kg probably due to saturation of the insulin absorption sites. More importantly, when empty NPs were administered in insulin solution (50 IU/kg), only a minor hypoglycaemic effect was observed between 2 and 8 h after administration. Thus, the results emphasized on nanoencapsulation of insulin into the alginate–CS-NPs, since this protected the protein from enzymatic degradation. Secondly, the results indicated prolonged hypoglycaemic effect up to 24 h when compared to previously formulated insulin encapsulated CS-NPs where the effect was only up to 12 h or less (62,88). Most importantly, insulin’s secondary structure analyses by Fourier transform infrared and Far-UV-CD (circular dichroism) spectra confirmed the structural integrity of insulin after encapsulation, thus by far preserving its bioactivity (89).
In another recent study by Li et al. (56), a partially quaternized derivative of CS, N-(2-hydroxyl) propyl-3-trimethyl ammonium CS, was used to prepare a quaternized CS–alginate NPs by ionotropic gelation method. This water-soluble CS derivative enhanced the absorption of the NPs via the paracellular transport pathway, and its pH-sensitive nature protected its content from acidic environment whereas allowed for controlled drug release at the intestine. The degree of substitution and average molecular weight of the quaternized CS affected the bovine serum albumin (BSA) loading and release when administered orally (56). Recently, a study by Zhang et al. (90) showed that insulin encapsulated in CS–alginate NPs was protected from degradation and release under simulated gastrointestinal conditions, on forming complex with cationic β-cyclodextrin polymer. This hemocompatible and water-soluble cationic β-cyclodextrin polymer was previously synthesised by the same group by one-step polycondensation of β-cyclodextrin (β-CD), epichlorohydrin and choline chloride (91). Their previous attempt of delivering insulin complexed with this cationic β-cyclodextrin via alginate/CS microspheres did not show desirable effect probably due to relatively large size of the microspheres (92). In this study, Zhang et al. prepared NPs of 150–350 nm in size. These NPs could effectively retain insulin within the CS–alginate NP core via complex formation with cationic β-cyclodextrin. Here the association between insulin with cationic β-cyclodextrin was both via electrostatic attraction and formation of inclusion complex between the negatively charged insulin groups with the positively charged side chains of β-cyclodextrin (90). However, due to the large molecular size of insulin, it could not be wholly included into the CD hydrophobic cavity. The NPs showed high association efficiency of up to 87% depending on the CS–alginate mass ratio and could also preserve the insulin structure within the NP. The NPs demonstrated effective protection and controlled release of insulin in simulated gastric fluid (SGF) and SIF, respectively, when compared with non-complexed and un-encapsulated insulin release. About 40% of total insulin was released in SIF after 6 h of oral delivery, whereas another 48% was released in the SGF, which does not account for the bioavailability (90). However, since the oral bioavailability of insulin in vivo is usually much lower than the results obtained in vitro, this system showed limited hope for clinical trials, unless further improvement is made.
Chitosan and Its Derivatives Combined with Different Peptides
Lin and co-workers prepared NPs composed of CS and poly(γ-glutamic acid) (γ-PGA) by a simple ionic gelation method for oral insulin delivery (88). γ-PGA is a naturally occurring anionic peptide which is biodegradable, non-toxic and relatively non-immunogenic. It was assumed that amide linkages of l-glutamic acids could conjugate with Zn ions of insulin. The NPs displayed a pH-dependent insulin release where only 20% of insulin was released at acidic pH (pH 2.5–6.6), whereas a sudden release occurred at pH 7.4. Noticeably, no significant conformation change of insulin was detected at pH 7.4 by circular dichroic spectra when compared to the standard insulin. The results showed that NPs bearing a positive surface charge were able to increase the paracellular permeability of Caco-2 cells by transiently opening the tight junctions. These in vitro results were corroborated by in vivo results which demonstrated an effective reduction of blood glucose level in diabetes induced rat model at a dose as low as 15 and 30 IU/kg. However, the hypoglycaemic effect lasted only up to 10 h, which opened some space for improvement in the formulation (88). In addition, the major drawback of the formulation was that the NPs became unstable and disintegrated in the alkaline pH of the gastrointestinal tract, limiting its potential for the oral delivery of insulin. Hence, to overcome the issue, the same group freeze-dried the NPs and delivered it orally through an enteric-coated capsule (93). Small angle X-ray scattering profiles of insulin showed that the structure of insulin remained same in spite of going through the process of freeze drying and it neither got fragmented nor aggregated upon release. This enteric-coated capsule was able to protect its content from the acidic environment of the stomach and release the NPs at the intestine providing a controlled and prolonged insulin release. However, the formulation was unable to improve the bioavailability of insulin, which was found to be nearly 20% (93). Hence, further modification of the NPs was required to get the desired result. For further development, a pH-responsive multi-ion-cross-linked NP was prepared from CS and γ-PGA with TPP and MgSO4 (94). Physical characterization with FTIR and X-ray diffraction indicated ionically cross-linked network structure which was stable for 10 weeks as suspension in deionised water. These multi-ion-cross-linked NPs were also stable over a wide pH range when compared to the NPs made only of CS or γ-PGA. Transepithelial electrical resistance (TEER), confocal microscopy images and transport experiments demonstrated effective transport and permeation of insulin via the paracellular pathway when compared to its counterparts (94). When administered orally in an in vivo diabetic rat model, it showed that the NPs efficiently adhered on to the mucosal surface. The NPs did not show any toxicity at an 18 times higher dose and demonstrated a hypoglycaemic action for about 10 h. However, the formulation was unable to improve the bioavailability of insulin which was found to be about 15.1% (95).
Comparable to the above study with γ-PGA, another newly generated NP was made from QCS and poly(aspartic acid) and was evaluated for its potential to deliver protein drugs orally (96). This formulation also showed variable potential based on formulation parameters like QCS/poly(aspartic acid) ratios, QCS molecular weight, poly(aspartic acid) concentration and BSA (model protein) concentration. The optimised formulation showed pH-dependent release of BSA from NPs. Though the formulation showed potential application, its therapeutic efficacy needs to be further evaluated before any definite conclusion could be made.
Chitosan–Heparin
In a recently published article, researchers have utilised negatively charged heparin, a well-known anticoagulant, to produce pH-responsive NPs by ionic gelation with positively charged CS (97). Heparin has been reported to promote gastric ulcer healing by mucosal regeneration, proliferation and angiogenesis (98). Hence, this nanoparticulate system was expected to protect the encapsulated drug from gastric acids, adhere to the gastric mucosa, penetrate through the mucosa and act on the Helicobacter pylori infection by releasing the drug due to difference in pH. The formulation showed that the positively charged NPs interacted with the negatively charged sites and opened the tight cellular junctions and was effective against the local H. pylori infection (97).
N,O-Carboxymethyl Chitosan
A pH-sensitive controlled release insulin-loaded NP was prepared from N,O-carboxymethyl CS (NOCC) (99). NOCC is a water-soluble CS derivative, where the amino and primary hydroxyl sites have been replaced by carboxymethyl substituent. Previously, a pH-sensitive hydrogel system has been prepared with this CS derivative for controlled protein drug delivery (100). Here the insulin-loaded NPs were prepared by ionic gelation of NOCC with TPP. Formulation variables had significant impact on the insulin loading and release profiles. It was observed that cumulative release of insulin from these NPs decreased with decreasing NOCC-to-TPP weight ratio; however, reverse trend was observed with decreasing initial concentration of insulin. The NPs protected insulin from acid attack as higher release of insulin was observed only at alkaline pH of the phosphate-buffered saline. Though the study demonstrated pH-dependent release of insulin, it cannot be correlated to the insulin bioavailability in vivo. Thus, results obtained so far are not conclusive enough to state the effective and enhanced delivery of insulin via oral absorption.
Chitosan and Methyl Methacrylate Derivatives
In 2006, Qian and co-workers prepared different types of functionalized graft copolymer NPs from CS with different types of methyl methacrylate. They utilised the monomer itself, as well as N-dimethylaminoethyl methacrylate hydrochloride and N-trimethylaminoethyl methacrylate chloride. Insulin was used as the model protein drug for encapsulation. The significance of the study lied in the fact that it did not utilise any organic solvents or high-energy sources for insulin loading; rather, free radical polymerisation was applied for the NP preparation. Up to 100% insulin loading could be achieved, and the NPs showed improved solubility in wide range of pH. The release study in PBS at pH 7.4 showed an initial burst release followed by controlled release for up to 4 days. However, release of insulin in the acidic and alkaline pH of stomach was not published. When these different NPs were orally fed to male Sprague–Dawley rats, it showed an enhanced absorption and improved bioavailability of insulin in comparison with the phosphate buffer solution of insulin. However, in comparison with the subcutaneous injection of insulin, the difference in plasma glucose level was not statistically significant (60).
In a recent study by the same group, a pH-sensitive NP has been prepared by combining CS with poly(methyl methacrylate) (PMMA). Here PMMA was used as a hydrophobic backbone to the carboxylated CS which consists of hydrophilic branches. This chemical structure enabled them to form instantaneous NPs through graft polymerization (101). Insulin was loaded into this NPs and the pharmacodynamic behaviour of the particles was studied in normal and diabetic rats. The NPs were of spherical shape and had insulin located within their core–shell structure. The NPs were aggregated in SGF and had slower release rate, whereas in SIF they were in a separated state and demonstrated faster insulin release. Though the NPs showed good biocompatibility and pH-specific insulin release, the pharmacological bioavailability of the orally administered insulin was only around 9.7%.
Chitosan–Cyclodextrin Complex
Alonso and co-workers first developed the CS cyclodextrin NPs by ionic cross-linking of CS with sodium TPP in the presence of hydroxypropyl-β-cyclodextrin (HPCD) (102). It is well-known that these oligosaccharides have a hydrophobic core but hydrophilic outer surface which enables them to form inclusion complexes with lipophilic drugs (103). HPCD was preferred since it had higher water solubility and better biocompatibility. Two-model hydrophobic drugs, namely triclosan and furosemide, were complexed with the cyclodextrin and subsequently encapsulated in the CS nanocarrier. The CD–drug complex enhanced the drug loading capacity and also demonstrated delayed release profile following an initial burst effect (102). This novel formulation opened the path to explore more CD-CS-NPs for oral drug delivery. The same group have then explored NPs consisting of CS and the anionic carboxymethyl-β-CD and studied the possibility of association with macromolecules. This anionic CD was expected to interact favourably with the cationic functional groups of CS and provide a more stable complex with proteins. The study demonstrated considerable incorporation efficiency with insulin and heparin as model drugs; however, the release of the drug was not dependent on the nanocarrier itself but rather on the macromolecule’s association with the matrix (104). This made the system more unpredictable, and hence, further modification was required to explore the scope of oral controlled drug release of these macromolecules via CD complexed CS-NPs.
In order to do so, Alonso and co-workers have also developed the CD-CS nanoparticulate system with sulphobutylether-β-CD (SBE-β-CD) which had a CD-rich inner core, with CS-rich outer layer capable of encapsulating both hydrophilic and hydrophobic drugs (105). They later investigated the oral delivery of the glutathione (GSH) peptide with this nanoparticulate system and compared it with NPs composed of only CS or with a CS/α-CD NP. X-ray photoelectron spectroscopy analysis showed stable complexation of the peptide with SBE-β-CD at the inner core of the NPs. But surprisingly this complexation was so stable that it did not show any GSH release in gastric stimulated conditions. Though SBE-β-CD composed CS-NPs were found to enhance the absorption of GSH from all segments of duodenum, its applicability in terms of oral delivery system would remain a concern due to the poor in vitro drug release (106).
Thiolated Chitosan
Thiolated CS were synthesised and utilised for oral controlled drug delivery due to its mucoadhesive nature and high stability of the carrier matrix which provided good controlled release of the drug (49,107,108). Here, the primary amino groups of CS were coupled with the thiol-bearing functional groups to form thiolated CS (108). This disulphide bond containing polymeric material demonstrated 6–100-fold augmented mucoadhesive property and enhanced paracellular permeation when compared to unmodified CS. Hence, this promising scaffold was utilised for NP preparation for the purpose of oral controlled delivery of drugs and macromolecules. Thiolated CS-NPs made from thioglycolic acid and CS were first prepared to deliver DNA to the cells via the oral route (109). The DNA thiolated CS-NPs showed significant improvement in transfection rate when compared to DNA encapsulated unmodified CS-NPs. Though the results were promising for accomplishing successful non-viral gene delivery, further investigation is required before clinical application.
Since the strong mucoadhesive property of thiolated CS is primarily due to disulphide bond formation between the thiolated polymer and cysteine-rich mucus layer, the addition of polyanionic excipients during ionotropic gelation of NPs could actually lead to a reduced mucoadhesive and permeation-enhancing properties of CS due to loss of its inherent positive charge. Recently Bravo-Osuna and co-workers have proposed several approaches to elaborate the application of thiolated CS by preserving its mucoadhesive property and permeation-enhancing ability. They have utilised the emulsion polymerization method to prepare thiolated CS-NPs coated with poly-isobutylcyanoacrylate (110–113). They evaluated the impact of different formulation factors like the effect of molecular weight and CS–thiobarbituric acid ratio for surface modification. Their results indicated improved penetration through the mucus layer due to enhanced bioadhesion when CS of high molecular weight was utilised for the preparation of NPs. This was because more thiol groups were present to form more covalent bonds with the cysteine-rich residues of the mucus glycoproteins (114). Their other studies also showed an enhanced calcium binding ability of this CS coated NPs, when compared to the poly-isobutylcyanoacrylate NPs. In addition, when the in vitro paracellular permeability of these NPs was measured by TEER studies, it demonstrated the importance of an optimal CS/CS–TBA ratio for an effective mucoadhesion and permeation (113). In another study, the same group modified the inner core of the NPs with methyl methacrylate in order to provide a more controlled release of the encapsulated content (112). Thus, these newly developed thiolated NP systems had the combined advantage of both thiolated polymers and colloidal particles and were proposed as a mucosal drug carrier system of biotechnological products.
Following the above concept, Moghaddam and co-workers developed a thiolated CS-NP by coating with polyhydroxyl ethyl methacrylate (pHEMA) (52). pHEMA has been a known carrier for hydrophilic drugs and has previously shown improved hydrophilicity and biocompatibility when co-delivered with CS (41). The NPs were prepared by emulsion polymerization method utilising different molecular weights of CS. Cerium (IV) ammonium nitrate was used as an initiator. This formulation also revealed the importance of formulation parameters on the permeability and mucoadhesive properties of the NPs (52). Since till now only in vitro and ex vivo studies have been performed with all these different types of thiolated CS-NPs systems, it cannot be concluded that they will serve as the optimal oral DDS for less bioavailable drugs and macromolecules.
Pegylated Chitosan
Another type of CS derivative which has found potential application in oral controlled release of therapeutic agents are the PEG derivatized CS-NPs. Prego et al. developed CS–PEG NPs with an objective to improve the biocompatibility of CS and to prolong its stability in biological fluids like intestinal fluids and so on (2). Different degrees of pegylation (0.5% and 1%) were used, and its impacts on in vitro and in vivo behaviour of the CS-NPs were tested. The NPs were prepared by solvent displacement technique. The CS–PEG NPs demonstrated substantial stability in gastrointestinal fluid and thus could ameliorate the in vivo intestinal absorption of salmon calcitonin in male Sprague–Dawley rats (2).
Lauryl Succinyl Chitosan
Earlier researches have shown that salts of fatty acids with medium carbon chain length such as caprylate (C 8), caprate (C 10) and laurate (C 12) are able to improve the paracellular permeability of hydrophilic drugs (115,116). Among them, sodium laurate has been reported to have the most promising permeation-enhancing ability (115). This concept was applied by Rekha and co-workers to synthesise a new derivative of CS, named as lauryl succinyl CS and prepared insulin-loaded NPs via cross-linking with sodium TPP (117). Their results indicated that the presence of both a hydrophobic (lauryl) and hydrophilic moiety (succinyl) in the NP matrix helped to prolong the controlled release of insulin. It also improved the mucoadhesive property and paracellular membrane permeation, which was reflected by reduced blood glucose levels in diabetic rats. However, the particles were unable to maintain the hypoglycaemic effect for too long (117).
Chitosan and Arabic Gum
In a recent study, researchers have utilised the biocompatible Arabic gum (acacia) to develop CS-NPs based on the basic principle of ionic gelation method (118). Acacia is a widely used pharmaceutical excipient with its primary application as a suspending and emulsifying agent (119). The presence of negative charge on the acacia molecule facilitates its interaction with this polycationic polymer CS. Release of insulin from these NPs was proposed to be dependent on a number of mechanisms such as diffusion, dissolution of CS in acidia media or due to swelling of acacia in pH > 6.5. However, the study showed that insulin loading in acacia-CS-NPs was less than CS-NPs prepared with TPP (118). Hence, to be considered for further evaluation, modifications to the current formulation should be made.
TOXICOLOGICAL ISSUES OF CHITOSAN NANOPARTICLES
CS is approved by Food and Drug Administration (USA) for wound dressing and is generally regarded as a non-toxic and biocompatible polymer (53,120,121). The LD50 of CS in mice after oral administration is 16 g/kg body weight, which is nearly equivalent to household sugar or salt (17,122). No side effects were reported in human up to 4.5 g/day oral administration of CS. However, when taken regularly for 12 weeks, it showed mild nausea and constipation in humans (53,122,123). Although CS alone is considered to be safe for oral administration, its properties may change completely upon chemical modification (122). Moreover, it is well-known that the pharmacokinetic properties of a drug or excipient change considerably when included in a nanoparticulate system (9,53,122). This is because the size, charge and surface modifications of the NPs often decide their fate in vivo (9,53,122). This in turn can alter the toxicity profile of the NP itself, as these properties influence the way the NPs interacts with different types of cells, thus modifying their cellular uptake, absorption through the GIT, tissue distribution and excretion (122). This is the reason that the generally recognized as safe (GRAS) status of CS does not apply for nanoparticulate formulations and primarily depends upon the conditions of intended use (122). In addition, it has been found that the pharmacokinetic properties and toxicity of CS and its derivatives are influenced by its molecular weight and degree of deacetylation (124,125). Thus, each and every derivative should be assessed individually both in the free form and nanoparticulate form. Recently, few studies have been performed to assess the toxicity of CS-NPs upon parenteral and oral administration (126,127). In addition, several studies have also identified the IC50 of CS derivatives in different cell lines (126–130). A recently published review by Kean and Thanou (122) has summarised all the current findings related to CS toxicity.
CURRENT PERSPECTIVE OF CHITOSAN NANOPARTICLES FOR CLINICAL USE
Although several oral CS-NP formulations have been developed in the past few years, as per our knowledge, there are no clinical trials under progress (131). It can be said that these orally administered CS-NPs are still in their early stage of pre-clinical evaluation and would require further investigation before being considered for evaluation in humans. So far, most of the results obtained are based on in vitro (49,52,110,132–135) and ex vivo (74,77,78) studies. Although some pre-clinical studies for CS-NPs (oral, parenteral etc.) have been performed in mice (35,42,46,47,134,136,137) and rats (61,74), it becomes very difficult to extrapolate these results to their in vivo performance in humans. In addition, very few studies have addressed the toxicity related issues which remains a major concern with these newly emerged nanoparticulate systems (46,47,136–140). Perhaps, more pre-clinical studies in the coming years may provide an important insight on the role of CS-NPs for the delivery of proteins, peptides and drugs and would initiate the need of clinical trials in humans.
CONCLUSIONS
In this review, we have discussed about different CS-based NPs for oral controlled delivery of therapeutic agents. These nanoparticulate systems have shown potential to be successful carriers for proteins, peptides, DNA and small drug molecules. They have shown superior stability, enhanced mucoadhesion and increased paracellular transportation in comparison to the other drug carrier systems or solutions of unmodified CS. Most importantly, these carriers could effectively protect the drug from enzymatic degradation and from the variable pH of the GIT. In addition, NPs prepared from CS derivatives could alter the rate of release of its encapsulated content and hence could be regarded as an effective oral control drug delivery system. Although very few studies have been performed on the in vivo evaluation of these formulations, the promising in vitro results do open up a scope for further investigation. Overall, it can be concluded that the chitosan-based nanoparticles have a promising future as an oral controlled drug delivery system.
Contributor Information
Anumita Chaudhury, Phone: +65-65163120, Email: g0700516@nus.edu.sg.
Surajit Das, Phone: +65-67963853, Email: surajit_das@ices.a-star.edu.sg.
REFERENCES
- 1.Des Rieux A, Fievez V, Garinot M, Schneider YJ, Preat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Prego C, Torres D, Fernandez-Megia E, Novoa-Carballal R, Quinoa E, Alonso MJ. Chitosan-PEG nanocapsules as new carriers for oral peptide delivery. Effect of chitosan pegylation degree. J Control Release. 2006;111(3):299–308. doi: 10.1016/j.jconrel.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Woodley JF. Enzymatic barriers for GI peptide and protein delivery. Crit Rev Ther Drug Carrier Syst. 1994;11(2–3):61–95. [PubMed] [Google Scholar]
- 4.Werle M, Takeuchi H, Bernkop-Schnurch A. Modified chitosans for oral drug delivery. J Pharm Sci. 2009;98(5):1643–1656. doi: 10.1002/jps.21550. [DOI] [PubMed] [Google Scholar]
- 5.Singh R, Singh S, Lillard JW., Jr Past, present, and future technologies for oral delivery of therapeutic proteins. J Pharm Sci. 2008;97(7):2497–2523. doi: 10.1002/jps.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Simerska P, Moyle PM, Olive C, Toth I. Oral vaccine delivery—new strategies and technologies. Curr Drug Deliv. 2009;6(4):347–358. doi: 10.2174/156720109789000537. [DOI] [PubMed] [Google Scholar]
- 8.Allemann E, Leroux J, Gurny R. Polymeric nano- and microparticles for the oral delivery of peptides and peptidomimetics. Adv Drug Deliv Rev. 1998;34(2–3):171–189. doi: 10.1016/S0169-409X(98)00039-8. [DOI] [PubMed] [Google Scholar]
- 9.Chiu GN, Wong MY, Ling LU, Shaikh IM, Tan KB, Chaudhury A, et al. Lipid-based nanoparticulate systems for the delivery of anti-cancer drug cocktails: implications on pharmacokinetics and drug toxicities. Curr Drug Metab. 2009;10(8):861–874. doi: 10.2174/138920009790274531. [DOI] [PubMed] [Google Scholar]
- 10.Muller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs—a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol. 2004;113(1–3):151–170. doi: 10.1016/j.jbiotec.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Kularatne SA, Low PS. Targeting of nanoparticles: folate receptor. Methods Mol Biol. 2010;624:249–265. doi: 10.1007/978-1-60761-609-2_17. [DOI] [PubMed] [Google Scholar]
- 12.Praetorius NP, Mandal TK. Engineered nanoparticles in cancer therapy. Recent Pat Drug Deliv Formul. 2007;1(1):37–51. doi: 10.2174/187221107779814104. [DOI] [PubMed] [Google Scholar]
- 13.Prabaharan M. Review paper: chitosan derivatives as promising materials for controlled drug delivery. J Biomater Appl. 2008;23(1):5–36. doi: 10.1177/0885328208091562. [DOI] [PubMed] [Google Scholar]
- 14.Sarmento B, Ribeiro A, Veiga F, Sampaio P, Neufeld R, Ferreira D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm Res. 2007;24(12):2198–2206. doi: 10.1007/s11095-007-9367-4. [DOI] [PubMed] [Google Scholar]
- 15.Bowman K, Leong KW. Chitosan nanoparticles for oral drug and gene delivery. Int J Nanomedicine. 2006;1(2):117–128. doi: 10.2147/nano.2006.1.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prego C, Torres D, Alonso MJ. The potential of chitosan for the oral administration of peptides. Expert Opin Drug Deliv. 2005;2(5):843–854. doi: 10.1517/17425247.2.5.843. [DOI] [PubMed] [Google Scholar]
- 17.Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release. 2004;100(1):5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Wong TW. Chitosan and its use in design of insulin delivery system. Recent Pat Drug Deliv Formul. 2009;3(1):8–25. doi: 10.2174/187221109787158346. [DOI] [PubMed] [Google Scholar]
- 19.Damge C, Reis CP, Maincent P. Nanoparticle strategies for the oral delivery of insulin. Expert Opin Drug Deliv. 2008;5(1):45–68. doi: 10.1517/17425247.5.1.45. [DOI] [PubMed] [Google Scholar]
- 20.Felt O, Buri P, Gurny R. Chitosan: a unique polysaccharide for drug delivery. Drug Dev Ind Pharm. 1998;24(11):979–993. doi: 10.3109/03639049809089942. [DOI] [PubMed] [Google Scholar]
- 21.Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15(9):1326–1331. doi: 10.1023/A:1011929016601. [DOI] [PubMed] [Google Scholar]
- 22.Rabea EI, Badawy ME, Steurbaut W, Rogge TM, Stevens CV, Smagghe G, et al. Fungicidal effect of chitosan derivatives containing an N-alkyl group on grey mould Botryti77s cinerea and rice leaf blast Pyricularia grisea. Commun Agric Appl Biol Sci. 2005;70(3):219–223. [PubMed] [Google Scholar]
- 23.Azad AK, Sermsintham N, Chandrkrachang S, Stevens WF. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J Biomed Mater Res B Appl Biomater. 2004;69(2):216–222. doi: 10.1002/jbm.b.30000. [DOI] [PubMed] [Google Scholar]
- 24.Sugano M, Watanabe S, Kishi A, Izume M, Ohtakara A. Hypocholesterolemic action of chitosans with different viscosity in rats. Lipids. 1988;23(3):187–191. doi: 10.1007/BF02535456. [DOI] [PubMed] [Google Scholar]
- 25.Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010;62(1):59–82. doi: 10.1016/j.addr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Masotti A, Ortaggi G. Chitosan micro- and nanospheres: fabrication and applications for drug and DNA delivery. Mini Rev Med Chem. 2009;9(4):463–469. doi: 10.2174/138955709787847976. [DOI] [PubMed] [Google Scholar]
- 27.Mourya VK, Inamdar NN. Trimethyl chitosan and its applications in drug delivery. J Mater Sci Mater Med. 2009;20(5):1057–1079. doi: 10.1007/s10856-008-3659-z. [DOI] [PubMed] [Google Scholar]
- 28.Sayin B, Somavarapu S, Li XW, Thanou M, Sesardic D, Alpar HO, et al. Mono-N-carboxymethyl chitosan (MCC) and N-trimethyl chitosan (TMC) nanoparticles for non-invasive vaccine delivery. Int J Pharm. 2008;363(1–2):139–148. doi: 10.1016/j.ijpharm.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 29.van der Lubben IM, Verhoef JC, Borchard G, Junginger HE. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur J Pharm Sci. 2001;14(3):201–207. doi: 10.1016/S0928-0987(01)00172-5. [DOI] [PubMed] [Google Scholar]
- 30.Borchard G. Chitosans for gene delivery. Adv Drug Deliv Rev. 2001;52(2):145–150. doi: 10.1016/S0169-409X(01)00198-3. [DOI] [PubMed] [Google Scholar]
- 31.Koping-Hoggard M, Tubulekas I, Guan H, Edwards K, Nilsson M, Varum KM, et al. Chitosan as a nonviral gene delivery system. Structure–property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivo. Gene Ther. 2001;8(14):1108–1121. doi: 10.1038/sj.gt.3301492. [DOI] [PubMed] [Google Scholar]
- 32.Bonferoni MC, Sandri G, Rossi S, Ferrari F, Caramella C. Chitosan and its salts for mucosal and transmucosal delivery. Expert Opin Drug Deliv. 2009;6(9):923–939. doi: 10.1517/17425240903114142. [DOI] [PubMed] [Google Scholar]
- 33.Li YH, Fan MW, Bian Z, Chen Z, Zhang Q, Yang HR. Chitosan–DNA microparticles as mucosal delivery system: synthesis, characterization and release in vitro. Chin Med J. 2005;118(11):936–941. [PubMed] [Google Scholar]
- 34.Carvalho EL, Grenha A, Remunan-Lopez C, Alonso MJ, Seijo B. Mucosal delivery of liposome–chitosan nanoparticle complexes. Methods Enzymol. 2009;465:289–312. doi: 10.1016/S0076-6879(09)65015-1. [DOI] [PubMed] [Google Scholar]
- 35.Borges O, Silva M, de Sousa A, Borchard G, Junginger HE, Cordeiro-da-Silva A. Alginate coated chitosan nanoparticles are an effective subcutaneous adjuvant for hepatitis B surface antigen. Int Immunopharmacol. 2008;8(13–14):1773–1780. doi: 10.1016/j.intimp.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Dai H, Jiang X, Tan GC, Chen Y, Torbenson M, Leong KW, et al. Chitosan–DNA nanoparticles delivered by intrabiliary infusion enhance liver-targeted gene delivery. Int J Nanomedicine. 2006;1(4):507–522. doi: 10.2147/nano.2006.1.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51(1–3):81–96. doi: 10.1016/S0169-409X(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 38.Kim BG, Kang IJ. Evaluation of the effects of biodegradable nanoparticles on a vaccine delivery system using AFM, SEM, and TEM. Ultramicroscopy. 2008;108(10):1168–1173. doi: 10.1016/j.ultramic.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 39.Krauland AH, Leitner VM, Grabovac V, Bernkop-Schnurch A. In vivo evaluation of a nasal insulin delivery system based on thiolated chitosan. J Pharm Sci. 2006;95(11):2463–2472. doi: 10.1002/jps.20700. [DOI] [PubMed] [Google Scholar]
- 40.Shimono N, Takatori T, Ueda M, Mori M, Higashi Y, Nakamura Y. Chitosan dispersed system for colon-specific drug delivery. Int J Pharm. 2002;245(1–2):45–54. doi: 10.1016/S0378-5173(02)00344-7. [DOI] [PubMed] [Google Scholar]
- 41.dos Santos KS, Coelho JF, Ferreira P, Pinto I, Lorenzetti SG, Ferreira EI, et al. Synthesis and characterization of membranes obtained by graft copolymerization of 2-hydroxyethyl methacrylate and acrylic acid onto chitosan. Int J Pharm. 2006;310(1–2):37–45. doi: 10.1016/j.ijpharm.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 42.Kim JH, Kim YS, Park K, Kang E, Lee S, Nam HY, et al. Self-assembled glycol chitosan nanoparticles for the sustained and prolonged delivery of antiangiogenic small peptide drugs in cancer therapy. Biomaterials. 2008;29(12):1920–1930. doi: 10.1016/j.biomaterials.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 43.Zhu B, Qie Y, Wang J, Zhang Y, Wang Q, Xu Y, et al. Chitosan microspheres enhance the immunogenicity of an Ag85B-based fusion protein containing multiple T-cell epitopes of Mycobacterium tuberculosis. Eur J Pharm Biopharm. 2007;66(3):318–326. doi: 10.1016/j.ejpb.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 44.Yu JM, Li YJ, Qiu LY, Jin Y. Polymeric nanoparticles of cholesterol-modified glycol chitosan for doxorubicin delivery: preparation and in-vitro and in-vivo characterization. J Pharm Pharmacol. 2009;61(6):713–719. doi: 10.1211/jpp.61.06.0003. [DOI] [PubMed] [Google Scholar]
- 45.Wilson B, Samanta MK, Santhi K, Kumar KP, Ramasamy M, Suresh B. Chitosan nanoparticles as a new delivery system for the anti-Alzheimer drug tacrine. Nanomedicine. 2010;6(1):144–152. doi: 10.1016/j.nano.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q, Zhang L, Hu W, Hu ZH, Bei YY, Xu JY, et al. Norcantharidin-associated galactosylated chitosan nanoparticles for hepatocyte-targeted delivery. Nanomedicine. 2010;6(2):371–381. doi: 10.1016/j.nano.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Kim JH, Kim YS, Park K, Lee S, Nam HY, Min KH, et al. Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J Control Release. 2008;127(1):41–49. doi: 10.1016/j.jconrel.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 48.Dhawan S, Singla AK, Sinha VR. Evaluation of mucoadhesive properties of chitosan microspheres prepared by different methods. AAPS PharmSciTech. 2004;5(4):e67. doi: 10.1208/pt050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernkop-Schnurch A, Guggi D, Pinter Y. Thiolated chitosans: development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery system. J Control Release. 2004;94(1):177–186. doi: 10.1016/j.jconrel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Ferrari F, Rossi S, Bonferoni MC, Caramella C, Karlsen J. Characterization of rheological and mucoadhesive properties of three grades of chitosan hydrochloride. Farmaco. 1997;52(6–7):493–497. [PubMed] [Google Scholar]
- 51.Wittaya-areekul S, Kruenate J, Prahsarn C. Preparation and in vitro evaluation of mucoadhesive properties of alginate/chitosan microparticles containing prednisolone. Int J Pharm. 2006;312(1–2):113–118. doi: 10.1016/j.ijpharm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Moghaddam FA, Atyabi F, Dinarvand R. Preparation and in vitro evaluation of mucoadhesion and permeation enhancement of thiolated chitosan–pHEMA core–shell nanoparticles. Nanomedicine. 2009;5(2):208–215. doi: 10.1016/j.nano.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 53.Baldrick P. The safety of chitosan as a pharmaceutical excipient. Regul Toxicol Pharmacol. 2010;56(3):290–299. doi: 10.1016/j.yrtph.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Singla AK, Chawla M. Chitosan: some pharmaceutical and biological aspects—an update. J Pharm Pharmacol. 2001;53(8):1047–1067. doi: 10.1211/0022357011776441. [DOI] [PubMed] [Google Scholar]
- 55.Gan Q, Wang T. Chitosan nanoparticle as protein delivery carrier—systematic examination of fabrication conditions for efficient loading and release. Colloids Surf B Biointerfaces. 2007;59(1):24–34. doi: 10.1016/j.colsurfb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Li T, Shi XW, Du YM, Tang YF. Quaternized chitosan/alginate nanoparticles for protein delivery. J Biomed Mater Res A. 2007;83(2):383–390. doi: 10.1002/jbm.a.31322. [DOI] [PubMed] [Google Scholar]
- 57.George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—a review. J Control Release. 2006;114(1):1–14. doi: 10.1016/j.jconrel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 58.Vila A, Sanchez A, Tobio M, Calvo P, Alonso MJ. Design of biodegradable particles for protein delivery. J Control Release. 2002;78(1–3):15–24. doi: 10.1016/S0168-3659(01)00486-2. [DOI] [PubMed] [Google Scholar]
- 59.Mukherjee B, Santra K, Pattnaik G, Ghosh S. Preparation, characterization and in-vitro evaluation of sustained release protein-loaded nanoparticles based on biodegradable polymers. Int J Nanomedicine. 2008;3(4):487–496. doi: 10.2147/IJN.S3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qian F, Cui F, Ding J, Tang C, Yin C. Chitosan graft copolymer nanoparticles for oral protein drug delivery: preparation and characterization. Biomacromolecules. 2006;7(10):2722–2727. doi: 10.1021/bm060065f. [DOI] [PubMed] [Google Scholar]
- 61.Pan Y, Li YJ, Zhao HY, Zheng JM, Xu H, Wei G, et al. Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int J Pharm. 2002;249(1–2):139–147. doi: 10.1016/S0378-5173(02)00486-6. [DOI] [PubMed] [Google Scholar]
- 62.Ma Z, Lim TM, Lim LY. Pharmacological activity of peroral chitosan–insulin nanoparticles in diabetic rats. Int J Pharm. 2005;293(1–2):271–280. doi: 10.1016/j.ijpharm.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 63.Ma Z, Yeoh HH, Lim LY. Formulation pH modulates the interaction of insulin with chitosan nanoparticles. J Pharm Sci. 2002;91(6):1396–1404. doi: 10.1002/jps.10149. [DOI] [PubMed] [Google Scholar]
- 64.Takeda S, Ishthara K, Wakui Y, Amagaya S, Maruno M, Akao T, et al. Bioavailability study of glycyrrhetic acid after oral administration of glycyrrhizin in rats; relevance to the intestinal bacterial hydrolysis. J Pharm Pharmacol. 1996;48(9):902–905. doi: 10.1111/j.2042-7158.1996.tb05998.x. [DOI] [PubMed] [Google Scholar]
- 65.Ichikawa T, Ishida S, Sakiya Y, Sawada Y, Hanano M. Biliary excretion and enterohepatic cycling of glycyrrhizin in rats. J Pharm Sci. 1986;75(7):672–675. doi: 10.1002/jps.2600750711. [DOI] [PubMed] [Google Scholar]
- 66.Ishida S, Sakiya Y, Ichikawa T, Awazu S. Pharmacokinetics of glycyrrhetic acid, a major metabolite of glycyrrhizin, in rats. Chem Pharm Bull. 1989;37(9):2509–2513. doi: 10.1248/cpb.37.2509. [DOI] [PubMed] [Google Scholar]
- 67.Wu Y, Yang W, Wang C, Hu J, Fu S. Chitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinate. I Int J Pharm. 2005;295(1–2):235–245. doi: 10.1016/j.ijpharm.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 68.Papadimitriou S, Bikiaris D, Avgoustakis K, Karavas E, Georgarakis M. Chitosan nanoparticles loaded with dorzolamide and pramipexole. Carbohydr Polym. 2008;73:44–54. doi: 10.1016/j.carbpol.2007.11.007. [DOI] [Google Scholar]
- 69.Jain D, Banerjee R. Comparison of ciprofloxacin hydrochloride-loaded protein, lipid, and chitosan nanoparticles for drug delivery. J Biomed Mater Res B Appl Biomater. 2008;86(1):105–112. doi: 10.1002/jbm.b.30994. [DOI] [PubMed] [Google Scholar]
- 70.Lu E, Franzblau S, Onyuksel H, Popescu C. Preparation of aminoglycoside-loaded chitosan nanoparticles using dextran sulphate as a counterion. J Microencapsul. 2009;26(4):346–354. doi: 10.1080/02652040802365182. [DOI] [PubMed] [Google Scholar]
- 71.Dudhani AR, Kosarajua SL. Bioadhesive chitosan nanoparticles: preparation and characterization. Carbohydr Polym. 2010;81:243–251. doi: 10.1016/j.carbpol.2010.02.026. [DOI] [Google Scholar]
- 72.Jintapattanakit A, Junyaprasert VB, Mao S, Sitterberg J, Bakowsky U, Kissel T. Peroral delivery of insulin using chitosan derivatives: a comparative study of polyelectrolyte nanocomplexes and nanoparticles. Int J Pharm. 2007;342(1–2):240–249. doi: 10.1016/j.ijpharm.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 73.Sadeghi AM, Dorkoosh FA, Avadi MR, Saadat P, Rafiee-Tehrani M, Junginger HE. Preparation, characterization and antibacterial activities of chitosan, N-trimethyl chitosan (TMC) and N-diethylmethyl chitosan (DEMC) nanoparticles loaded with insulin using both the ionotropic gelation and polyelectrolyte complexation methods. Int J Pharm. 2008;355(1–2):299–306. doi: 10.1016/j.ijpharm.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 74.Bayat A, Dorkoosh FA, Dehpour AR, Moezi L, Larijani B, Junginger HE, et al. Nanoparticles of quaternized chitosan derivatives as a carrier for colon delivery of insulin: ex vivo and in vivo studies. Int J Pharm. 2008;356(1–2):259–266. doi: 10.1016/j.ijpharm.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 75.Bayat A, Larijani B, Ahmadian S, Junginger HE, Rafiee-Tehrani M. Preparation and characterization of insulin nanoparticles using chitosan and its quaternized derivatives. Nanomedicine. 2008;4(2):115–120. doi: 10.1016/j.nano.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 76.Mao S, Bakowsky U, Jintapattanakit A, Kissel T. Self-assembled polyelectrolyte nanocomplexes between chitosan derivatives and insulin. J Pharm Sci. 2006;95(5):1035–1048. doi: 10.1002/jps.20520. [DOI] [PubMed] [Google Scholar]
- 77.Sandri G, Bonferoni MC, Rossi S, Ferrari F, Boselli C, Caramella C. Insulin-loaded nanoparticles based on N-trimethyl chitosan: in vitro (Caco-2 model) and ex vivo (excised rat jejunum, duodenum, and ileum) evaluation of penetration enhancement properties. AAPS PharmSciTech. 2010;11(1):362–371. doi: 10.1208/s12249-010-9390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandri G, Bonferoni MC, Rossi S, Ferrari F, Gibin S, Zambito Y, et al. Nanoparticles based on N-trimethylchitosan: evaluation of absorption properties using in vitro (Caco-2 cells) and ex vivo (excised rat jejunum) models. Eur J Pharm Biopharm. 2007;65(1):68–77. doi: 10.1016/j.ejpb.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 79.Snyman D, Hamman JH, Kotze AF. Evaluation of the mucoadhesive properties of N-trimethyl chitosan chloride. Drug Dev Ind Pharm. 2003;29(1):61–69. doi: 10.1081/DDC-120016684. [DOI] [PubMed] [Google Scholar]
- 80.Sadeghi AM, Dorkoosh FA, Avadi MR, Weinhold M, Bayat A, Delie F, et al. Permeation enhancer effect of chitosan and chitosan derivatives: comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur J Pharm Biopharm. 2008;70(1):270–278. doi: 10.1016/j.ejpb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 81.Wee S, Gombotz WR. Protein release from alginate matrices. Adv Drug Deliv Rev. 1998;31(3):267–285. doi: 10.1016/S0169-409X(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 82.Gotoh T, Matsushima K, Kikuchi K. Preparation of alginate–chitosan hybrid gel beads and adsorption of divalent metal ions. Chemosphere. 2004;55(1):135–140. doi: 10.1016/j.chemosphere.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 83.Sezer AD, Akbuga J. Release characteristics of chitosan treated alginate beads: II. Sustained release of a low molecular drug from chitosan treated alginate beads. J Microencapsul. 1999;16(6):687–696. doi: 10.1080/026520499288636. [DOI] [PubMed] [Google Scholar]
- 84.Sezer AD, Akbuga J. Release characteristics of chitosan treated alginate beads: I. Sustained release of a macromolecular drug from chitosan treated alginate beads. J Microencapsul. 1999;16(2):195–203. doi: 10.1080/026520499289176. [DOI] [PubMed] [Google Scholar]
- 85.Tapia C, Ormazabal V, Costa E, Yazdani-Pedram M. Study of dissolution behavior of matrices tablets based on alginate–gelatin mixtures as prolonged diltiazem hydrochloride release systems. Drug Dev Ind Pharm. 2007;33(6):585–593. doi: 10.1080/03639040601085359. [DOI] [PubMed] [Google Scholar]
- 86.Takka S, Gurel A. Evaluation of chitosan/alginate beads using experimental design: formulation and in vitro characterization. AAPS PharmSciTech. 2010;11(1):460–466. doi: 10.1208/s12249-010-9406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sarmento B, Ribeiro AJ, Veiga F, Ferreira DC, Neufeld RJ. Insulin-loaded nanoparticles are prepared by alginate ionotropic pre-gelation followed by chitosan polyelectrolyte complexation. J Nanosci Nanotechnol. 2007;7(8):2833–2841. doi: 10.1166/jnn.2007.609. [DOI] [PubMed] [Google Scholar]
- 88.Lin YH, Mi FL, Chen CT, Chang WC, Peng SF, Liang HF, et al. Preparation and characterization of nanoparticles shelled with chitosan for oral insulin delivery. Biomacromolecules. 2007;8(1):146–152. doi: 10.1021/bm0607776. [DOI] [PubMed] [Google Scholar]
- 89.Sarmento B, Ferreira DC, Jorgensen L, van de Weert M. Probing insulin’s secondary structure after entrapment into alginate/chitosan nanoparticles. Eur J Pharm Biopharm. 2007;65(1):10–17. doi: 10.1016/j.ejpb.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 90.Zhang N, Li J, Jiang W, Ren C, Li J, Xin J, et al. Effective protection and controlled release of insulin by cationic beta-cyclodextrin polymers from alginate/chitosan nanoparticles. Int J Pharm. 2010;393(1–2):212–218. doi: 10.1016/j.ijpharm.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 91.Li J, Xiao H, Li J, Zhong Y. Drug carrier systems based on water-soluble cationic beta-cyclodextrin polymers. Int J Pharm. 2004;278(2):329–342. doi: 10.1016/j.ijpharm.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 92.Huang L, Xin J, Guo Y, Li J. A novel insulin oral delivery system assisted by cationic B-cyclodextrin polymers. J Appl Polym Sci. 2010;115:1371–1379. doi: 10.1002/app.30775. [DOI] [Google Scholar]
- 93.Sonaje K, Chen YJ, Chen HL, Wey SP, Juang JH, Nguyen HN, et al. Enteric-coated capsules filled with freeze-dried chitosan/poly(gamma-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials. 2010;31(12):3384–3394. doi: 10.1016/j.biomaterials.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 94.Lin YH, Sonaje K, Lin KM, Juang JH, Mi FL, Yang HW, et al. Multi-ion-crosslinked nanoparticles with pH-responsive characteristics for oral delivery of protein drugs. J Control Release. 2008;132(2):141–149. doi: 10.1016/j.jconrel.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 95.Sonaje K, Lin YH, Juang JH, Wey SP, Chen CT, Sung HW. In vivo evaluation of safety and efficacy of self-assembled nanoparticles for oral insulin delivery. Biomaterials. 2009;30(12):2329–2339. doi: 10.1016/j.biomaterials.2008.12.066. [DOI] [PubMed] [Google Scholar]
- 96.Wang T, Xu Q, Wu Y, Zeng A, Li M, Gao H. Quaternized chitosan (QCS)/poly (aspartic acid) nanoparticles as a protein drug-delivery system. Carbohydr Res. 2009;344(7):908–914. doi: 10.1016/j.carres.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 97.Lin YH, Chang CH, Wu YS, Hsu YM, Chiou SF, Chen YJ. Development of pH-responsive chitosan/heparin nanoparticles for stomach-specific anti-Helicobacter pylori therapy. Biomaterials. 2009;30(19):3332–3342. doi: 10.1016/j.biomaterials.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 98.Li Y, Wang HY, Cho CH. Association of heparin with basic fibroblast growth factor, epidermal growth factor, and constitutive nitric oxide synthase on healing of gastric ulcer in rats. J Pharmacol Exp Ther. 1999;290(2):789–796. [PubMed] [Google Scholar]
- 99.Lin CC, Lin CW. Preparation of N, O-carboxymethyl chitosan nanoparticles as an insulin carrier. Drug Deliv. 2009;16(8):458–464. doi: 10.3109/10717540903353090. [DOI] [PubMed] [Google Scholar]
- 100.Chen SC, Wu YC, Mi FL, Lin YH, Yu LC, Sung HW. A novel pH-sensitive hydrogel composed of N, O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J Control Release. 2004;96(2):285–300. doi: 10.1016/j.jconrel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 101.Cui F, Qian F, Zhao Z, Yin L, Tang C, Yin C. Preparation, characterization, and oral delivery of insulin loaded carboxylated chitosan grafted poly(methyl methacrylate) nanoparticles. Biomacromolecules. 2009;10(5):1253–1258. doi: 10.1021/bm900035u. [DOI] [PubMed] [Google Scholar]
- 102.Maestrelli F, Garcia-Fuentes M, Mura P, Alonso MJ. A new drug nanocarrier consisting of chitosan and hydroxypropylcyclodextrin. Eur J Pharm Biopharm. 2006;63(2):79–86. doi: 10.1016/j.ejpb.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 103.Stella VJ, Rajewski RA. Cyclodextrins: their future in drug formulation and delivery. Pharm Res. 1997;14(5):556–567. doi: 10.1023/A:1012136608249. [DOI] [PubMed] [Google Scholar]
- 104.Krauland AH, Alonso MJ. Chitosan/cyclodextrin nanoparticles as macromolecular drug delivery system. Int J Pharm. 2007;340(1–2):134–142. doi: 10.1016/j.ijpharm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 105.Trapani A, Garcia-Fuentes M, Alonso MJ. Novel drug nanocarriers combining hydrophilic cyclodextrins and chitosan. Nanotechnology. 2008;19:185101. doi: 10.1088/0957-4484/19/18/185101. [DOI] [PubMed] [Google Scholar]
- 106.Trapani A, Lopedota A, Franco M, Cioffi N, Ieva E, Garcia-Fuentes M, et al. A comparative study of chitosan and chitosan/cyclodextrin nanoparticles as potential carriers for the oral delivery of small peptides. Eur J Pharm Biopharm. 2010;75(1):26–32. doi: 10.1016/j.ejpb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 107.Roldo M, Hornof M, Caliceti P, Bernkop-Schnurch A. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: synthesis and in vitro evaluation. Eur J Pharm Biopharm. 2004;57(1):115–121. doi: 10.1016/S0939-6411(03)00157-7. [DOI] [PubMed] [Google Scholar]
- 108.Bernkop-Schnurch A, Hornof M, Guggi D. Thiolated chitosans. Eur J Pharm Biopharm. 2004;57(1):9–17. doi: 10.1016/S0939-6411(03)00147-4. [DOI] [PubMed] [Google Scholar]
- 109.Martien R, Loretz B, Thaler M, Majzoob S, Bernkop-Schnurch A. Chitosan–thioglycolic acid conjugate: an alternative carrier for oral nonviral gene delivery? J Biomed Mater Res A. 2007;82(1):1–9. doi: 10.1002/jbm.a.31135. [DOI] [PubMed] [Google Scholar]
- 110.Bravo-Osuna I, Millotti G, Vauthier C, Ponchel G. In vitro evaluation of calcium binding capacity of chitosan and thiolated chitosan poly(isobutyl cyanoacrylate) core-shell nanoparticles. Int J Pharm. 2007;338(1–2):284–290. doi: 10.1016/j.ijpharm.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 111.Bravo-Osuna I, Schmitz T, Bernkop-Schnurch A, Vauthier C, Ponchel G. Elaboration and characterization of thiolated chitosan-coated acrylic nanoparticles. Int J Pharm. 2006;316(1–2):170–175. doi: 10.1016/j.ijpharm.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 112.Bravo-Osuna I, Ponchel G, Vauthier C. Tuning of shell and core characteristics of chitosan-decorated acrylic nanoparticles. Eur J Pharm Sci. 2007;30(2):143–154. doi: 10.1016/j.ejps.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 113.Bravo-Osuna I, Vauthier C, Chacun H, Ponchel G. Specific permeability modulation of intestinal paracellular pathway by chitosan–poly(isobutylcyanoacrylate) core–shell nanoparticles. Eur J Pharm Biopharm. 2008;69(2):436–444. doi: 10.1016/j.ejpb.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 114.Bravo-Osuna I, Vauthier C, Farabollini A, Palmieri GF, Ponchel G. Mucoadhesion mechanism of chitosan and thiolated chitosan–poly(isobutyl cyanoacrylate) core–shell nanoparticles. Biomaterials. 2007;28(13):2233–2243. doi: 10.1016/j.biomaterials.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 115.Lindmark T, Kimura Y, Artursson P. Absorption enhancement through intracellular regulation of tight junction permeability by medium chain fatty acids in Caco-2 cells. J Pharmacol Exp Ther. 1998;284(1):362–369. [PubMed] [Google Scholar]
- 116.Lindmark T, Nikkila T, Artursson P. Mechanisms of absorption enhancement by medium chain fatty acids in intestinal epithelial Caco-2 cell monolayers. Pharmacol Exp Ther. 1995;275(2):958–964. [PubMed] [Google Scholar]
- 117.Rekha MR, Sharma CP. Synthesis and evaluation of lauryl succinyl chitosan particles towards oral insulin delivery and absorption. J Control Release. 2009;135(2):144–151. doi: 10.1016/j.jconrel.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 118.Avadi MR, Sadeghi AM, Mohammadpour N, Abedin S, Atyabi F, Dinarvand R, et al. Preparation and characterization of insulin nanoparticles using chitosan and Arabic gum with ionic gelation method. Nanomedicine. 2010;6(1):58–63. doi: 10.1016/j.nano.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 119.Verbeken D, Dierckx S, Dewettinck K. Exudate gums: occurrence, production, and applications. Appl Microbiol Biotechnol. 2003;63(1):10–21. doi: 10.1007/s00253-003-1354-z. [DOI] [PubMed] [Google Scholar]
- 120.Skaugrud O, Hagen A, Borgersen B, Dornish M. Biomedical and pharmaceutical applications of alginate and chitosan. Biotechnol Genet Eng Rev. 1999;16:23–40. doi: 10.1080/02648725.1999.10647970. [DOI] [PubMed] [Google Scholar]
- 121.Zhang J, Xia W, Liu P, Cheng Q, Tahirou T, Gu W, et al. Chitosan modification and pharmaceutical/biomedical applications. Mar Drugs. 2010;8(7):1962–1987. doi: 10.3390/md8071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev. 2010;62(1):3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 123.Gades MD, Stern JS. Chitosan supplementation and fecal fat excretion in men. Obes Res. 2003;11(5):683–688. doi: 10.1038/oby.2003.97. [DOI] [PubMed] [Google Scholar]
- 124.Yang YM, Hu W, Wang XD, Gu XS. The controlling biodegradation of chitosan fibers by N-acetylation in vitro and in vivo. J Mater Sci Mater Med. 2007;18(11):2117–2121. doi: 10.1007/s10856-007-3013-x. [DOI] [PubMed] [Google Scholar]
- 125.Zhang H, Neau SH. In vitro degradation of chitosan by bacterial enzymes from rat cecal and colonic contents. Biomaterials. 2002;23(13):2761–2766. doi: 10.1016/S0142-9612(02)00011-X. [DOI] [PubMed] [Google Scholar]
- 126.CarrenoGomez B, Duncan R. Evaluation of the biological properties of soluble chitosan and chitosan microspheres. Int J Pharm. 1997;148(2):231–240. doi: 10.1016/S0378-5173(96)04847-8. [DOI] [Google Scholar]
- 127.Zhang C, Qu GW, Sun YJ, Yang T, Yao Z, Shen WB, et al. Biological evaluation of N-octyl-O-sulfate chitosan as a new nano-carrier of intravenous drugs. Eur J Pharm Sci. 2008;33(4–5):415–423. doi: 10.1016/j.ejps.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 128.Kean T, Roth S, Thanou M. Trimethylated chitosans as non-viral gene delivery vectors: cytotoxicity and transfection efficiency. J Control Release. 2005;103(3):643–653. doi: 10.1016/j.jconrel.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 129.Mao SR, Shuai XT, Unger F, Wittmar M, Xie XL, Kissel T. Synthesis, characterization and cytotoxicity of poly(ethylene glycol)-graft-trimethyl chitosan block copolymers. Biomaterials. 2005;26(32):6343–6356. doi: 10.1016/j.biomaterials.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 130.Opanasopit P, Aumklad P, Kowapradit J, Ngawhiranpat T, Apirakaramwong A, Rojanarata T. Effect of salt forms and molecular weight of chitosans on in vitro permeability enhancement in intestinal epithelial cells (Caco-2) Pharm Dev Technol. 2007;12(5):447–455. doi: 10.1080/10837450701555901. [DOI] [PubMed] [Google Scholar]
- 131.ClinicalTrial.gov. A service of the U.S. National Institutes of Health. http://clinicaltrials.gov/ct2/results?term=chitosan.
- 132.Le Garrec D, Gori S, Luo L, Lessard D, Smith DC, Yessine MA, et al. Poly(N-vinylpyrrolidone)-block-poly(D, L-lactide) as a new polymeric solubilizer for hydrophobic anticancer drugs: in vitro and in vivo evaluation. J Control Release. 2004;99(1):83–101. doi: 10.1016/j.jconrel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 133.Cui F, Shi K, Zhang L, Tao A, Kawashima Y. Biodegradable nanoparticles loaded with insulin-phospholipid complex for oral delivery: preparation, in vitro characterization and in vivo evaluation. J Control Release. 2006;114(2):242–250. doi: 10.1016/j.jconrel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 134.Chen F, Zhang ZR, Yuan F, Qin X, Wang M, Huang Y. In vitro and in vivo study of N-trimethyl chitosan nanoparticles for oral protein delivery. Int J Pharm. 2008;349(1–2):226–233. doi: 10.1016/j.ijpharm.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 135.Aral C, Akbuga J. Preparation and in vitro transfection efficiency of chitosan microspheres containing plasmid DNA:poly(L-lysine) complexes. J Pharm Pharm Sci. 2003;6(3):321–326. [PubMed] [Google Scholar]
- 136.Zheng F, Shi XW, Yang GF, Gong LL, Yuan HY, Cui YJ, et al. Chitosan nanoparticle as gene therapy vector via gastrointestinal mucosa administration: results of an in vitro and in vivo study. Life Sci. 2007;80(4):388–396. doi: 10.1016/j.lfs.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 137.Hwang HY, Kim IS, Kwon IC, Kim YH. Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles. J Control Release. 2008;128(1):23–31. doi: 10.1016/j.jconrel.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 138.Rajeshkumar S, Venkatesan C, Sarathi M, Sarathbabu V, Thomas J, Anver Basha K, et al. Oral delivery of DNA construct using chitosan nanoparticles to protect the shrimp from white spot syndrome virus (WSSV) Fish Shellfish Immunol. 2009;26(3):429–437. doi: 10.1016/j.fsi.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 139.Rajesh Kumar S, Ishaq Ahmed VP, Parameswaran V, Sudhakaran R, Sarath Babu V, Sahul Hameed AS. Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in Asian sea bass (Lates calcarifer) to protect from Vibrio (Listonella) anguillarum. Fish Shellfish Immunol. 2008;25(1-2):47–56. doi: 10.1016/j.fsi.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 140.Loh JW, Yeoh G, Saunders M, Lim LY. Uptake and cytotoxicity of chitosan nanoparticles in human liver cells. Toxicol Appl Pharmacol. 2010;249:148–157. doi: 10.1016/j.taap.2010.08.029. [DOI] [PubMed] [Google Scholar]