Abstract
Although several guidelines do exist for freeze-drying process development and scale-up, there are still a number of issues that require additional attention. The objective of this review article is to discuss some emerging process development and scale-up issue with emphasis on effect of load condition and freeze-drying in novel container systems such as syringes, Lyoguard trays, ampoules, and 96-well plates. Understanding the heat and mass transfer under different load conditions and for freeze-drying in these novel container systems will help in developing a robust freeze-drying process which is also easier to scale-up. Further research and development needs in these emerging areas have also been addressed.
KEY WORDS: freeze-drying, heat and mass transfer, novel container systems, partial load, process development, scale-up
INTRODUCTION
Freeze-drying is widely used to convert solutions of unstable materials into solid form by removing the solvent, usually water, to improve the long-term storage stability. Freeze-drying is often perceived as a very difficult process to design and scale-up from laboratory to manufacturing. The focus of this review chapter is to address some of the most important freeze-drying process development and scale-up issues that have gone largely ignored or at least have been given inadequate recognition and attention.
In freeze-drying, the aqueous solution is filled into the vials and placed onto the temperature-controlled shelves of the freeze-dryer. The shelf temperature is lowered to around −40°C, usually in several steps, which results in the transformation of most of the water into ice. During this freezing step, some of the drugs and excipients may also crystallize. However, in most cases the solute remains amorphous in the freeze concentrated state. Typically, the amount of unfrozen water in the amorphous phase is about 20% (1). Once the material has completely solidified, the vacuum pump is turned on to evacuate the product chamber. Usually the chamber pressure is maintained between 50 and 200 mTorr such that the chamber pressure is well below the vapor pressure of ice at the target product temperature, resulting in high sublimation rate. Next, the shelf temperature is raised to facilitate sublimation of ice. This step is referred to as primary drying, wherein water is removed by sublimation of ice. During primary drying, product temperature is maintained below the maximum allowable product temperature, which is the collapse temperature for an amorphous solute or the eutectic temperature for a crystalline solute. Drying above the maximum allowable temperature results in loss of cake structure which further results in rejection of the entire batch due to lack of pharmaceutical elegance. In the last step, referred to as secondary drying, most of the unfrozen water is removed by desorption at elevated shelf temperature with the chamber still under vacuum. The residual water content at the end of secondary drying is about 1% (2).
The freeze-drying process is a problem in coupled heat and mass transfer, and heat and mass transfer issues must be recognized to achieve process control and optimization. The freeze-drying process parameters (i.e., shelf temperature, ramp rate, and chamber pressure) are largely determined by a trial and error approach, which results in non-optimal processes with long processing times and scale-up problems. Every single step of the freeze-drying process presents a unique process development and scale-up challenge. The ice nucleation temperature during the freezing step determines the size and morphology of the ice crystals. The ice crystals are removed by sublimation during primary drying leaving behind a porous cake, which is frequently a “template” of the ice crystal structure. In a recent report, the specific surface area of the dried cake was shown to correlate with the degree of supercooling, which is the temperature difference between the equilibrium freezing point and the temperature at which ice first nucleates in the solution (3). The degree of supercooling depends on the presence of particulate matter in the solution (i.e., low degree of supercooling in a laboratory environment compared to class 100 in manufacturing) and impacts both primary and secondary drying. A higher degree of supercooling results in faster secondary drying and longer primary drying time, as a higher degree of supercooling results in smaller pore size (i.e., higher specific surface area) and hence higher product resistance. Thus, controlling the degree of supercooling presents process development challenges not only in the laboratory dryer due to intervial differences in drying rate (due to a non-uniform ice nucleation temperature), but it also presents a scale-up challenge due to differences in ice nucleation temperature between laboratory and manufacturing scale (4).
The ultimate objective of the freeze-drying process is to maintain product quality which is consistent not only within the batch but also from batch to batch. Consequently, the product temperature becomes a critical product quality attribute during freeze-drying. Consistent product quality is ensured when the product has the same thermal history within the batch, from batch to batch and also between lab scale, pilot scale, and production scale dryers. Since freeze-drying is an expensive process with a typically long processing time, a key objective during freeze-drying process development is to improve process economics by reducing the cycle time (i.e., increasing throughput). Since the primary drying step is the longest of all the three steps, optimization of the primary drying time is normally the focus in the industry.
The theory of heat and mass transfer during freeze-drying is well established and several process development and scale-up issues are well addressed in the literature. During primary drying, atypical radiation effects arise from the walls to the door of the dryer that run at a higher temperature than the shelf set point (5). As a result, the vials on the edge sublime faster and run at higher temperatures than do the rest of the vials. Also, differences in heat and mass transfer performance between freeze-dryers may occur due to inherent design differences (6). During primary drying, the shelf surface temperature is not necessarily uniform, and there could be hot and cold spots on the shelf surface that need to be identified. The mean vial heat transfer coefficient may be significantly different between different dryers depending on the emissivities of the surfaces (shelf, walls, and door). Also, depending on the dryer design, there are differences in maximum sublimation rate supported by the freeze-dryer and the minimum attainable chamber pressure (6,7).
While systematic guidelines have been developed to address many of these scale-up issues (6), there remain many issues that require additional attention. The aim of this review article is to address the new emerging freeze-drying process development and scale-up issues.
EFFECT OF LOAD ON FREEZE-DRYING PROCESS DESIGN
The dryer load condition is an important process variable during freeze-drying process development and scale-up. A full dryer load is defined as vials occupying essentially all available shelf surface area. There are several reasons to run a dryer under partial load conditions. Generally, insufficient active pharmaceutical ingredient (API) is available early in the manufacturing history, so to meet the immediate production needs freeze-drying is carried out under partial load conditions.
Under partial load conditions, one may never notice the potential for dryer overload (i.e., choked flow or condenser overload) during a high heat and mass flux freeze-drying process. Thus, under partial load, the process runs well, but under full load condition dryer overload occurs with loss of chamber pressure control. Partial load may also result in the shelf surface temperature running slightly higher than under full load condition. In addition, as the load on a given shelf decreases, a greater fraction of the vials become edge vials (i.e., vials experiencing higher heat transfer from the walls to the door of the dryer). As a result, the overall heat transfer to the batch increases (i.e., mean vial heat transfer coefficient increases, product temperature increases and drying time decreases). Lastly, there could be a load condition where the number of vials on the shelf is not sufficient to maintain the molar flux of water much higher than the molar flux of nitrogen, and the gas composition in the chamber changes from 100% water vapor to a significant level of nitrogen. The gas composition in the chamber is important since the heat transfer via gas conduction depends on the thermal conductivity of the gas. If the gas composition were 100% nitrogen, the overall vial heat transfer coefficient decreases (by roughly 30%) in most applications, product temperature decreases and hence drying time increases.
The effect of load condition on critical process parameters, such as product temperature and drying time, on a lab scale, pilot scale, and a clinical scale dryer has been recently investigated (8). In going from full load to partial load condition, drying time decreased and product temperature increased. The radiation effect was reported to be the dominant cause of these trends as the fraction of edge vials increased at lower load conditions. Thus, the freeze-drying cycle may need to be adjusted (i.e., shelf temperature or chamber pressure is changed) to achieve the same product temperature profile and drying times under different load conditions.
FREEZE-DRYING IN NOVEL CONTAINER SYSTEMS
Freeze-Drying in Syringes
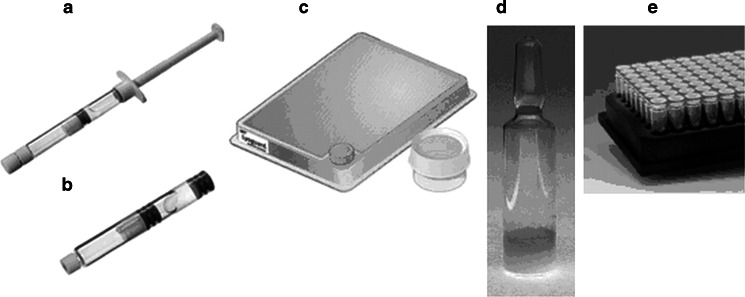
In the past few decades there has been great advancement in the field of freeze-drying in terms of process development and scale-up knowledge. The freeze-drying process which was once designed by “trial and error” methods may now be designed based on a sound understanding of the formulation characteristics and fundamentals of heat and mass transfer. Progress has also been made in the container closure systems used for freeze-drying. Traditionally, the product is freeze-dried in a glass vial. The freeze-dried product is reconstituted with a diluent and administered using a syringe. Current market needs demand alternate packaging for many emerging biopharmaceutical drug products. Thus, many freeze-dried products are now supplied in dual chamber syringes (Fig. 1a) or cartridges (Fig. 1b) (9). The freeze-dried product is in one chamber and the diluent is in the other chamber. Typically, the product is freeze-dried first and then a stopper is pushed into the syringe barrel to separate the two chambers and then the diluent is filled in the other chamber. Reconstitution is quickly and conveniently achieved at the push of the plunger. Prefilled syringes are much convenient from the end user perspective to run less risk of sterility compromise since they do not require several steps before administration. Also, the dosing is more accurate compared to vials and hence requires less overfill compared to vials (10,11).
Fig. 1.
Novel container systems for freeze-drying. a Dual chamber syringe, b dual chamber cartridge, c Gore™ Lyoguard® tray and container, d ampoule, and e VirTis 96-well freeze-drying system
Freeze-drying in these novel container systems poses a unique challenge to freeze-drying process development and scale-up. Glass vials are widely used and are well characterized in terms of heat and mass transfer during freeze-drying (12,13). However, there are few reports in the literature describing heat and mass transfer for freeze-drying in syringes. In a recent publication, it has been reported that there are no freezing difference between the vial and the syringe (14). Homogeneous ice crystal morphology was achieved because of uniform cooling. During primary drying, the sublimation rate decreased with increasing chamber pressure and, as expected, sublimation rate increased with increasing shelf temperature (15). The overall heat transfer coefficient increased with increasing gas temperature, and hence it was concluded that the major mode of heat transfer for syringes suspended above the shelf is via gas convection. Lastly, no significant differences were observed for drying in a syringe as compared to a vial in terms of sublimation rate.
However, these results are in sharp contradiction to another report (16). In this study, glass syringes were suspended through a plexiglass holder. A relatively lower degree of super cooling and hence lower product resistance were found for product in syringes. In agreement with an earlier report (15), a decrease in sublimation rate with increasing chamber pressure was observed, but the syringe heat transfer coefficient was found to be independent of pressure. The dominant mode of heat transfer was demonstrated to be via radiation. This difference with the earlier study is important since if gas convection were the dominant mode of heat transfer, one might expect a severe scale-up challenge since the gas flow dynamics in the drying chamber would likely vary with the size and geometry of the freeze-dryer. For radiation heat transfer, the scale-up issue is not as severe since the contribution from radiation heat transfer can be estimated from the geometric view factor to the emissivities of the surfaces. Also, in this second study, significant differences were observed for freezing drying in a syringe compared to a vial.
Additionally, an improved holder system, which was custom designed from an aluminum (Al) block, was also investigated for freeze-drying in syringes. The syringes were placed inside holes drilled in the Al block. Heat transfer from the shelf to the Al block as well as from the Al block to the syringes was via all three modes: gas conduction, contact conduction, and radiation. With the Al block, heat transfer was greatly improved relative to the plexiglass holder. However, under the same conditions of shelf temperature and chamber pressure, the product temperature was lower and the drying time was longer when compared to freeze-drying in vials.
Bulk Freeze-Drying
Since many biopharmaceutical API’s are not stable in solutions they are commonly freeze-dried. Usually, metal trays (stainless steel) are used for bulk freeze-drying.
Metal Trays
Heat transfer from the shelf to the trays is via all three modes: gas conduction, contact conduction, and radiation. Bulk freeze-drying is traditionally done in open stainless steel trays, which have several drawbacks. One issue is the contamination of the equipment due to product blowout, for high sublimation rate processes, resulting in loss of material and cleanup problems. Also, the trays become warped with repeated use, resulting in poor and variable heat transfer from the shelf to the tray (12,13). If thermocouples are used to determine the end point of primary drying they might indicate that primary drying is done in the local region around the sensor, but there could still be regions of ice in the tray resulting in product melt back if the cycle is progressed into secondary drying (17). In such cases, it is particularly important to have a technique that would monitor the entire batch based on gas composition in the drying chamber, such as Pirani vs. Capacitance Manometer differential pressure or dew point (18). In general, aluminum trays, because of their relatively higher thermal conductivity, are preferred over stainless steel trays. However, the drug cannot be exposed to aluminum and hence laminated pans, with stainless steel inside and aluminum outside, would be required.
LYOGUARD® Trays and Containers
The LYOGUARD® trays and containers (Fig. 1b) by W.L. Gore & Associates, Inc. represent a recent innovation for bulk freeze-drying which have several potential advantages over metal trays, including the fact that the product can be freeze-dried sterile (19). The tray is sealed at the top with a semipermeable membrane made out of GORE-TEX expanded polytetrafluoroethylene laminate developed specifically for freeze-drying sterile contents. The semipermeable membrane allows water vapor to flow easily with little resistance but at the same time prevents any product from flying off (up to 0.2 μm particles). The bottom of the tray is sealed with a thin, transparent, and flexible film made of polypropylene. For the trays, a port with screw cap is provided in the front to fill the product. The material of construction, with which the product comes in contact, is polypropylene.
Some preliminary characterization of the trays by the manufacturer demonstrated that the heat transfer in Lyoguard trays is more uniform and better than in stainless steel trays (19). The semipermeable membrane does impose a resistance to mass transfer; however, the membrane resistance is insignificant compared to the normal dry layer resistance for a product. Thus, the membrane resistance is similar to the stopper resistance in vial freeze-drying, which accounts for only ~10–15% of the total mass transfer resistance. However, there are no published literature focused on the quantitative characterization of heat and mass transfer for freeze-drying in Lyoguard trays. Such data would help in rational design of the freeze-drying process conditions and would ultimately help in scaling up the process.
A general concern with freeze-drying in trays is the inability to seal the container inside the freeze-dryer. For hygroscopic material, this deficiency could be a serious issue as the product could pick up moisture when exposed to room conditions before transferring into a fully sealed container system for long-term storage. With the Lyoguard trays, because of the semipermeable membrane, in principle, there should be little uptake of atmospheric water during the small period of sample handling (since convection is absent, the water molecules would have to diffuse through the semipermeable membrane). However, no data confirming this hypothesis are available in the literature. Finally, preformed foil pouches are available to seal the Lyoguard trays. Thus, the product can be stored and shipped in the Lyoguard trays sealed in foil pouches.
Freeze-Drying in Glass Ampoules
Glass ampoules (Fig. 1c) are commonly used to freeze-dry biological reference materials, which are of high purity and high value. These reference materials are intended for extremely long storage and hence high stability is an essential requirement. The World Health Organization has recommended using glass ampoules for the preparation of biological reference materials to minimize the risk of moisture or oxygen entry which may occur when rubber stoppers are used to seal the glass vials (20). The level of moisture and oxygen within a rubber sealed vial has been reported in the literature to increase due to equilibration of moisture in the rubber stopper with the product (20–26). The oxygen and moisture content during storage of formulated 5% human serum albumin in a glass ampoule was compared with that in a glass vials (26). The oxygen and moisture content do not change in the glass ampoule over a storage period of 1 year at ambient and higher temperatures; however, a significant increase (≥1%) was observed in glass vials. Nevertheless, at low temperature (below 0°C) no difference was observed in the oxygen or moisture content between a vial and an ampoule. Since the rate of transfer is slowed down at low temperature, it is possible that there may be differences even at lower temperatures with longer term storage stability studies. For a product stored in a vial, there is an equilibration of water between the product and the stoppers, which results in an increase in residual water of a dry product or a decrease in residual water for a wet product. The vials with treated stoppers1 were as effective as ampoules for retention of alkaline phophatase activity during processing and storage under a range of temperature and humidity conditions (27). The stoppers are usually treated (steam-sterilized followed by vacuum drying) to remove excess water. The equilibrium water content depends on the history of stopper treatment. For stoppers vacuum dried for 8 h following steam sterilization, there is only a small increase in residual water level during storage (24). Additionally, a critical factor is the rubber stopper composition, e.g., Flurotec® stoppers manufactured by Daikyo Seiko, Ltd. are made from fluoro resins which prevent moisture release from the stoppers. The ampoules, on the other hand, need to be flame-sealed outside the freeze-dryer where the initial moisture and oxygen level may increase, and of course a product of combustion (i.e., the flame) is water. However, special capillary closure device has been designed to act as a stopper for ampoules and was found compatible with a production freeze-dryer (28). This closure system has been reported to reduce the ingress of moisture and oxygen by a factor of 13 and 17 (relative to the open ampoule), respectively, over a period of 30 min. However, even with this special closure system, the initial level of oxygen was higher in an ampoule than in a vial (26). Before flame sealing, air (i.e., oxygen) enters into the ampoule. Thus, the process of flame sealing the ampoules outside the freeze-dryer is a potential issue, especially when dealing with biopharmaceutical products that are both oxygen and moisture sensitive. In short, the ampoules may be no better than stoppered vials, at least when low moisture release stoppers (i.e., Flurotec®) are used.
A comparison of heat and mass transfer between the vial and an ampoule would facilitate changing the container closure system from ampoule to vial or the other way around. Such comparison would help in determining the process modifications required to achieve same product temperature profile when switching to new container system. Since both the ampoule and the vial are made from the same material (i.e., borosilicate glass) with similar geometry and are placed on the shelf in a similar hexagonal packing array, one would expect that the magnitude of heat transfer coefficient and mass transfer resistance for the ampoules would be qualitatively similar to that of a vial. However, no such data exists in the literature.
Freeze-Drying in 96-Well Plates
VirTis has developed a 96-well freeze-drying system (Fig. 1e) which consists of glass or plastic vials placed in a specially designed aluminum block for uniform heat transfer, eliminating the atypical edge vial effect seen in standard 96-well plastic plates. The Lyocap 96-well capmat stoppers with slots are used to stopper the wells either under vacuum or inert gas.
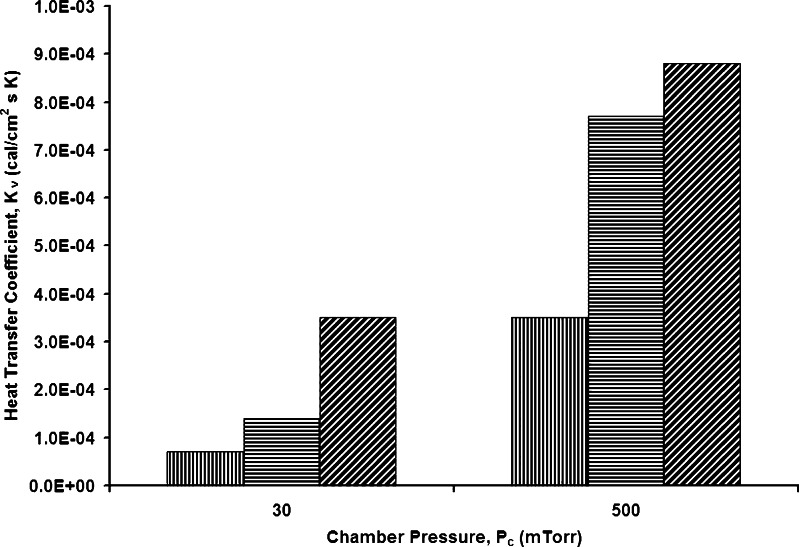
The heat transfer coefficient for freeze-drying in 96-well polymerase chain reaction (PCR) plates was characterized with and without a custom-made slightly oxidized aluminum block and also with the freeze-drying system designed by VirTis wherein the Al block is painted black (29). The results were compared against the traditional tubing vials. The heat transfer coefficient of the 2R tubing vials (Kvial) was greatest, and the heat transfer coefficient is least for PCR plates in the standard VirTis (black painted) Al block (Kvirtis). The heat transfer coefficient of the PCR plates in the custom designed Al block (KPCR) lie between the two (Fig. 2) (29). The heat transfer from the shelf to the wells, with and without Al block, is via gas conduction, contact conduction and radiation. However, without the Al block, there is little direct contact of the PCR plates with the shelf surface and hence contact conduction is small. Overall, the dominant mode of heat transfer for freeze-drying in PCR plates is via gas conduction (30). The heat transfer coefficient is greatly improved upon using Al block. Also, without the Al block, there is a large contribution from radiation heat transfer.
Fig. 2.
Comparison of heat transfer coefficient. VirTis freeze-drying system for PCR plates (vertical stripes), PCR plates with custom designed Al block (horizontal stripes), and 2R tubing vials (diagonal stripes). Graph plotted using data from (29)
An important consideration for freeze-drying in syringes and 96-well plates is the geometry of the ice sublimation interface. Since there is significant heat transfer from the walls of these container systems, a cone structure is usually formed during the sublimation test with pure ice when ~30% of the ice has sublimed; however, it must be acknowledged that a product dries differently than does pure ice, and the curvature of the ice–vapor interface is much less with a product. Thus, the container closure system is an important variable which needs due consideration in freeze-drying. An understanding of the heat and mass transfer in these novel container systems is essential to develop robust cycle conditions with minimal scale-up issues. Characterization of freezing differences and different modes of heat transfer during drying would help design cycles based on science rather than empiricism.
THEORETICAL MODELING
Freeze-drying is an expensive and time consuming process, and of the three steps the primary drying step during freeze-drying is normally the longest and hence is generally the focus in industry for process optimization. During primary drying, heat is transferred from the shelf to the product, and ice sublimes from the frozen solution leaving behind the dry layer. Thus, the coupled heat and mass transfer relationship governs the primary drying step of the freeze-drying process. The principles behind the freeze-drying process are now well understood and robust theoretical models which can accurately predict product temperature, residual water, glass transition temperature, and primary drying time have been developed. Several theoretical models are reported in the literature that describes the freeze-drying process (12, 31–37). There are differences between these models such as assumptions regarding the geometry of the ice sublimation interface, initial conditions, boundary conditions, and several other basic assumptions. Nevertheless, satisfactory agreement is observed, during primary drying, between theoretically calculated process parameters (such as drying time and product temperature) with those determined experimentally for various commonly used excipients. With theoretical modeling, one can predict position of the ice sublimation interface, moisture distribution, and the glass transition temperature in the dried layer, which is difficult to obtain experimentally. These theoretical models can be used to predict the effect of various process parameters (shelf temperature, ramp rate, chamber pressure, sublimation rate, and drying time) on critical product quality attributes (product temperature, residual water). Such an approach helps in creating the design space wherein the process will operate without any problems and at the same time meet the product specifications (38). Also, theoretical modeling minimizes the number of experiments required during process development and scale-up. However, experiments are required! A systematic characterization of the formulation and the container closure system is required to obtain the values of input parameters for these models. Further, it would always be prudent to validate the theoretical calculations by comparisons between theory and experiment for a subset of the configurations of interest.
SUMMARY/CONCLUSION
Differences do exist in heat and mass transfer depending on the load condition, dryer design, and the container closure system. These differences need to be taken into consideration during process development and scale-up to obtain the same product temperature history throughout the drying process independent of the dryer scale. Also, it is important to understand the limitations of the freeze-dryer (i.e., minimum achievable chamber pressure, maximum sublimation). The container closure system is another important process variable in freeze-drying. Apart from the characterization of interaction between the product and the container closure system, what is important for freeze-drying process development and scale-up is the proper selection and characterization of heat and mass transfer in these container closure systems, which could be significantly different from the traditionally used glass vial. The product may fail to meet the desired specifications if fixed cycle time from a lab scale dryer is used on a pilot or production scale dryer. Thus, the freeze-drying cycle needs to be adjusted by changes in shelf temperature or chamber pressure when the process is scaled-up from laboratory to pilot and ultimately to production scale. The process should be designed based on sound principles of heat and mass transfer rather than a trial and error approach. The basic physics is well understood, and this understanding should be effectively used in Quality by Design programs. From the end user perspective, prefilled syringes are preferred over vials, but it must be recognized that a process optimized for vials will generally not be optimized for syringes. Traditionally stainless steel trays have been used for bulk freeze-drying. However, Lyoguard trays and containers do offer the advantage of product containment and possibly also provide advantages in reproducibility of heat transfer, since stainless steel pans are subject to serious warping with continued use. Also, depending on the final application, freeze-drying may be carried out in other container closure systems such as ampoules and 96-well plates. For long-term storage of biological standards, vials with appropriate stoppers seem to be at least as effective as the ampoules. Lastly, the theoretical models for freeze-drying are useful tools that utilize the basic knowledge of heat and mass transfer in freeze-drying to provide an understanding of the effect of various process parameters on the critical product quality attributes.
Footnotes
Treated with methylated spirit and air dried.
REFERENCES
- 1.Hatley RHM, Mant A. Determination of the unfrozen water content of maximally freeze-concentrated carbohydrate solutions. Int J Biol Macromol. 1993;15(4):227–32. doi: 10.1016/0141-8130(93)90042-K. [DOI] [PubMed] [Google Scholar]
- 2.Pikal MJ. Freeze-drying of proteins: process, formulation, and stability. ACS Symp Ser. 1994;567:120–33. doi: 10.1021/bk-1994-0567.ch008. [DOI] [Google Scholar]
- 3.Rambhatla S, Ramot R, Bhugra C, Pikal MJ. Heat and mass transfer scale-up issues during freeze drying: II. Control and characterization of the degree of supercooling. AAPS PharmSciTech. 2004;5(4):e58. doi: 10.1208/pt050458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SM, Pikal MJ. Process analytical technologies (PAT) in freeze-drying of parenteral products. Pharm Dev Technol. 2009;14(6):567–87. doi: 10.3109/10837450903295116. [DOI] [PubMed] [Google Scholar]
- 5.Rambhatla S, Pikal MJ. Heat and mass transfer scale-up issues during freeze-drying, I: atypical radiation and the edge vial effect. AAPS PharmSciTech. 2003;4(2):111–20. doi: 10.1208/pt040214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rambhatla S, Tchessalov S, Pikal MJ. Heat and mass transfer scale-up issues during freeze-drying, III: control and characterization of dryer differences via operational qualification tests. AAPS PharmSciTech. 2006;7(2):E39. doi: 10.1208/pt070239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Searles J. Observation and implications of sonic water vapor flow during freeze-drying. Am Pharm Rev. 2004; 7(2):58, 60, 62, 64, 66–68, 75.
- 8.Patel SM, Jameel F, Pikal MJ. The effect of dryer load on freeze-drying process design. J Pharm Sci. 2010;99(10):4363–79. doi: 10.1002/jps.22132. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds G. The market need for reconstitution systems: manufacturers and consumers can benefit from these advanced systems. Pharm Process. 2006
- 10.Polin JB. The ins and outs of prefilled syringes. Pharm Med Packag News. 2003;11(5):40–3. [Google Scholar]
- 11.Swain E. Functional packages protect and deliver. Pharm Med Packag News. 2001
- 12.Pikal MJ. Use of laboratory data in freeze drying process design: heat and mass transfer coefficients and the computer simulation of freeze drying. PDA J Pharm Sci Technol. 1985;39(3):115–39. [PubMed] [Google Scholar]
- 13.Pikal MJ, Roy ML, Shah S. Mass and heat transfer in vial freeze-drying of pharmaceuticals: role of the vial. J Pharm Sci. 1984;73(9):1224–37. doi: 10.1002/jps.2600730910. [DOI] [PubMed] [Google Scholar]
- 14.Hottot A, Andrieu J, Vessot S, Shalaev E, Gatlin LA, Ricketts S. Experimental study and modeling of freeze-drying in syringe configuration. Part I: freezing step. Drying Technol. 2009;27(1):40–8. doi: 10.1080/07373930802565806. [DOI] [Google Scholar]
- 15.Hottot A, Andrieu J, Hoang V, Shalaev EY, Gatlin LA, Ricketts S. Experimental study and modeling of freeze-drying in syringe configuration. Part II: mass and heat transfer parameters and sublimation end-points. Drying Technol. 2009;27(1):49–58. doi: 10.1080/07373930802565814. [DOI] [Google Scholar]
- 16.Patel SM, Pikal MJ. Freeze-drying in novel container system: characterization of heat and mass transfer in glass syringes. J Pharm Sci. 2010;99(7):3188–204. doi: 10.1002/jps.22086. [DOI] [PubMed] [Google Scholar]
- 17.Roy ML, Pikal MJ. Process control in freeze drying: determination of the end point of sublimation drying by an electronic moisture sensor. PDA J Pharm Sci Technol. 1989;43(2):60–6. [PubMed] [Google Scholar]
- 18.Patel SM, Doen T, Pikal MJ. Determination of end point of primary drying for freeze-drying process control. AAPS PharmSciTech. 2010;11(1):73–84. doi: 10.1208/s12249-009-9362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gassier M, Rey L. Development of a new concept for bulk freeze-drying: LYOGUARD freeze-dry packaging. Drugs Pharm Sci. 2004;137:325–48. [Google Scholar]
- 20.WHO Guidelines for the preparation, characterization and estabishment of international and other standards and reference reagents for biological standardiation. WHO Tech Rep Ser. 1990;800:181–214. [Google Scholar]
- 21.de Grazio FL. Closure and container considerations in lyophilization. Drugs Pharm Sci. 2004;137:277–97. [Google Scholar]
- 22.Donovan PD, Corvari V, Burton MD, Rajagopalan N. Effect of stopper processing conditions on moisture content and ramifications for lyophilized products: comparison of “low” and “high” moisture uptake stoppers. PDA J Pharm Sci Technol. 2007;61(1):51–8. [PubMed] [Google Scholar]
- 23.Earle JP, Bennett PS, Larson KA, Shaw R. The effects of stopper drying on moisture levels of Haemophilus influenzae conjugate vaccine. Dev Biol Stand. 1992;74:203–10. [PubMed] [Google Scholar]
- 24.Pikal MJ, Shah S. Moisture transfer from stopper to product and resulting stability implications. Dev Biol Stand. 1992;74:165–79. [PubMed] [Google Scholar]
- 25.Templeton AC, Placek J, Xu H, Mahajan R, Hunke WA, Reed RA. Determination of the moisture content of bromobutyl rubber stoppers as a function of processing: implications for the stability of lyophilized products. PDA J Pharm Sci Technol. 2003;57(2):75–87. [PubMed] [Google Scholar]
- 26.Matejtschuk P, Rafiq S, Johnes S, Gaines Das R. A comparison of vials with ampoules for the storage of biological reference materials. Biologicals. 2005;33(2):63–70. doi: 10.1016/j.biologicals.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Ford AW, Dawson PJ. Effect of type of container, storage temperature and humidity on the biological activity of freeze-dried alkaline phosphatase. Biologicals. 1994;22(2):191–7. doi: 10.1006/biol.1994.1026. [DOI] [PubMed] [Google Scholar]
- 28.Phillips PK, Dawson PJ, Delderfield AJ. The use of DIN glass ampoules to freeze-dry biological materials with a low residual moisture and oxygen content. Biologicals. 1991;19(3):219–21. doi: 10.1016/1045-1056(91)90038-L. [DOI] [PubMed] [Google Scholar]
- 29.Graberg S, Hyla W, Gieseler H. Freeze Drying from small product containers to its implication on freeze-drying process design: evaluation of heat transfer coefficient of a new 96-well freeze drying system in comparison to 2R tubing vials and polypropylene 96-well PCR-plates. CPPR Freeze Drying of Pharmaceuticals and Biologicals. Breckenridge, Colorado; 2008
- 30.Graberg S, Gieseler H. Freeze drying in non-vial container systems: evaluation of heat transfer coefficients of PCR-plates and correlation to freeze-drying cycle design. CPPR Freeze-Drying of Pharmaceuticals and Biologicals. Garmisch-Partenkirchen, Germany; 2006
- 31.Hottot A, Peczalski R, Vessot S, Andrieu J. Freeze-drying of pharmaceutical proteins in vials: modeling of freezing and sublimation steps. Drying Technol. 2006;24(5):561–70. doi: 10.1080/07373930600626388. [DOI] [Google Scholar]
- 32.Millman MJ, Liapis AI, Marchello JM. An analysis of the lyophilization process using a sorption-sublimation model and various operational policies. AIChE J. 1985;31(10):1594–604. doi: 10.1002/aic.690311003. [DOI] [Google Scholar]
- 33.Pikal MJ, Cardon S, Bhugra C, et al. The nonsteady state modeling of freeze drying: in-process product temperature and moisture content mapping and pharmaceutical product quality applications. Pharm Dev Technol. 2005;10(1):17–32. doi: 10.1081/pdt-35869. [DOI] [PubMed] [Google Scholar]
- 34.Sadikoglu H, Liapis AI. Mathematical modeling of the primary and secondary drying stages of bulk solution freeze-drying in trays: parameter estimation and model discrimination by comparison of theoretical results with experimental data. Drying Technol. 1997;15(3 & 4):791–810. doi: 10.1080/07373939708917262. [DOI] [Google Scholar]
- 35.Sheehan P, Liapis AI. Modeling of the primary and secondary drying stages of the freeze drying of pharmaceutical products in vials: numerical results obtained from the solution of a dynamic to spatially multi-dimensional lyophilization model for different operational policies. Biotechnol Bioeng. 1998;60(6):712–28. doi: 10.1002/(SICI)1097-0290(19981220)60:6<712::AID-BIT8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Velardi SA, Barresi AA. Development of simplified models for the freeze-drying process and investigation of the optimal operating conditions. Chem Eng Res Des. 2008;86(1):9–22. doi: 10.1016/j.cherd.2007.10.007. [DOI] [Google Scholar]
- 37.Nakagawa K, Hottot A, Vessot S, Andrieu J. Modeling of freezing step during freeze-drying of drugs in vials. AIChE J. 2007;53(5):1362–72. doi: 10.1002/aic.11147. [DOI] [Google Scholar]
- 38.Patel SM, Chaudhuri S, Pikal MJ. Choked flow and importance of Mach 1 in freeze-drying process. Chem Eng Sci. 2010;65 (21):5716–27.