Abstract
Numerous presentations and articles on manual inspection of pharmaceutical drug products have been released, since the pioneering articles on inspection by Knapp and associates Knapp and Kushner (J Parenter Drug Assoc 34:14, 1980); Knapp and Kushner (Bull Parenter Drug Assoc 34:369, 1980); Knapp and Kushner (J Parenter Sci Technol 35:176, 1981); Knapp and Kushner (J Parenter Sci Technol 37:170, 1983). This original work by Knapp and associates provided the industry with a statistical means of evaluating inspection performance. This methodology enabled measurement of individual inspector performance, performance of the entire inspector pool and provided basic suggestions for the conduct of manual inspection. Since that time, numerous subject matter experts (SMEs) have presented additional valuable information for the conduct of manual inspection Borchert et al. (J Parenter Sci Technol 40:212, 1986); Knapp and Abramson (J Parenter Sci Technol 44:74, 1990); Shabushnig et al. (1994); Knapp (1999); Knapp (2005); Cherris (2005); Budd (2005); Barber and Thomas (2005); Knapp (2005); Melchore (2007); Leversee and Ronald (2007); Melchore (2009); Budd (2007); Borchert et al. (1986); Berdovich (2005); Berdovich (2007); Knapp (2007); Leversee and Shabushing (2009); Budd (2009). Despite this abundance of knowledge, neither government regulations nor the multiple compendia provide more than minimal guidance or agreement for the conduct of manual inspection. One has to search the literature for useful information that has been published by SMEs in the field of Inspection. The purpose of this article is to restate the sound principles proclaimed by SMEs with the hope that they serve as a useful guideline to bring greater consistency to the conduct of manual inspection.
KEY WORDS: false rejection (RAG), particulate, reject zone efficiency (RZE), RZE/RAG plane, visual acuity
INTRODUCTION
Sterile pharmaceutical and biological drug products are inspected prior to labeling to ensure that the drug product container meets predetermined specifications for container/closure integrity and that the drug product be “essentially free” of visible particulate matter. Control points for correct fill volume and correct components exist before the inspection area, but manual inspection serves as a secondary check for these defects. The primary objectives of inspection after filling and sealing is to ensure that the container/closure is free of defects that could lead to a breach in sterility and that the drug product inside the container is free of visible particulate matter.
Modern fully automated inspection systems use cameras and/or light emitting diode technology to effectively detect defects, but their use is not practical for all operations. Many companies cannot justify the capital expense and associated validation activities for products that are manufactured in small batch sizes or products that are infrequently manufactured. Another consideration is that existing facilities may not have the required space to accommodate the large footprint of a fully automated inspection system. Until there is a practical alternative to the large and expensive fully automated inspection systems, manual inspection will continue to be used by the industry. The Parenteral Drug Association (PDA) surveys conducted in 1996, 2003, and 2008 indicate that 33–46% of participating companies use manual inspection (1). Manual inspection will remain the method of choice for small batch sizes, infrequently manufactured products, stability samples, and operations with limited production budgets.
MANUAL INSPECTION PROCESS
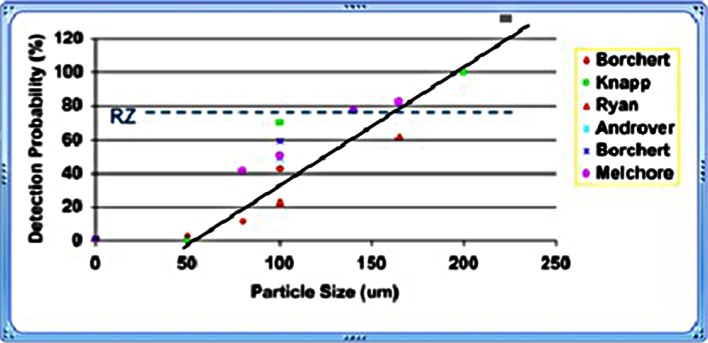
Manual inspection results have the reputation of great variability due to a number of factors that affect human visual inspection. The compilation of proven control measures, referenced in this article, provide a means to minimize human inspection variability. The data presented in Fig. 1 provides documented evidence that manual inspection variability can be minimized if inspection conditions are controlled (2). The Knapp methodology labels the probability of detecting rejects as reject zone efficiency (RZE) and the data in Fig. 1 indicates that six different studies had similar RZE values when manual inspection was controlled (2).
Fig. 1.
Human visual inspection performance
As with validation of other systems and processes, there is more than one way to validate. The practices documented in this article provide latitude for validation of the manual inspection process, while using established practices essential for the conduct of manual inspection.
The 2008 PDA preliminary survey indicates that approximately 69% of participating companies use a manual baseline to validate automated inspection systems (1). If a manual baseline is used to validate an automated inspection system, the manual data must be consistent for a meaningful validation of the automated inspection system.
INSPECTOR QUALIFICATION
Inspectors must pass an eye exam before training. Most, if not all, companies require an eye examination before inspector training and the latest PDA survey indicates that 79% of participating companies require 20/20 near focus visual acuity (1). It is the opinion of this investigator and other subject matter experts (SMEs) that the requirement for 20/20 near focus visual acuity (corrected) should be a mandatory requirement to minimize variability (3–9). One investigator had a more stringent requirement of 14/16 near focus visual acuity (10). Starting an inspection program with inspectors not having a minimum of 20/20 visual acuity (corrected) immediately adds variability to the inspection process.
The latest PDA inspection survey indicates that 68% of participating companies also test for color blindness (1). The incorporation of color blindness testing can easily be performed with visual acuity testing. This color blindness requirement is recommended for inspectors of commercial product. It must be an absolute requirement for those inspectors performing inspection of stability samples, where subtle color changes provide significant information on the stability of a product.
After meeting the visual acuity requirements, inspector candidates should have formal training on the importance of inspection and an overview of inspection methodology. Formal training is followed by extensive “hands-on training” with containers that are defect free and containers that have representative defects. At some point during the inspection of challenge containers, the instructor will review the candidate’s results and determine if or in what area(s) additional training is required. There is no substitute for personal interaction between the instructor and student at various time points along training. The instructor must ensure that the candidate can recognize and differentiate defect types and that their questions have been answered. In addition to visual acuity, the ideal inspector must be focused, have a good work ethic, have a commitment to quality, good manual dexterity, patience, and be capable of performing tedious work for relatively long time periods (5,11).
Approaches to training vary with each site. The goal is to ensure that the future inspector can recognize all types of defects and has the ability to differentiate between acceptable and reject containers that occur in production. How this is done is up to the discretion of the site management. The training program should include clearly defined acceptance criteria; a library of containers combined with digital pictures of representative container defects, and documented inspector records that are available for review. It should also include a formally documented standard operating procedure and a validation master plan when many inspection methods are used (7).
Personal experience indicates that focusing on one type of defect may be advantageous for training. For example, container/closure defects are easier to detect than particulate matter and training should start with this type of inspection. Once satisfied that the inspector candidate understands and can recognize container/closure defects, then instruction can move on to the more difficult to detect particulate matter.
When the instructor believes that the candidate is ready for qualification testing, the candidate is given a set of characterized containers having both container/closure defects and containers having particulate matter mixed together simulating actual production. For analyzing inspector performance, the data for the particulate and container/closure containers should be calculated separately. Placing data for a container/closure defect in with particulate data will lead to erroneous results and confusion. Simply stated, container/closure defects should not be confused with particulate detection. Keeping the data separate for each type of inspection is the only way to determine the inspector’s performance for each type of defect. It will simplify the evaluating performance for each type of inspection. Such data can be valuable to the instructor and reveal areas where further training is required for one type of defect.
Establishing a Manual Baseline
Establishing a manual baseline for inspector performance is a one-time activity used to qualify an inspector pool and set the in-house standard for qualification of inspectors (7,12,13). Inspection programs and training must take into consideration current good manufacturing practices, compendial requirements, and business concerns with excessive false rejection (RAG). Knapp’s methodology is based on developing the statistical rejection probability for each individual container in the challenge set. For qualification and establishing a manual baseline, containers need to be inspected multiple times to develop a probability of rejection. Containers that are rejected <30% of the time are classified as acceptable. Containers that are rejected >30–70% are classified as “Grey Zone” containers. Containers having a rejection probability greater than 70% are classified as rejects.
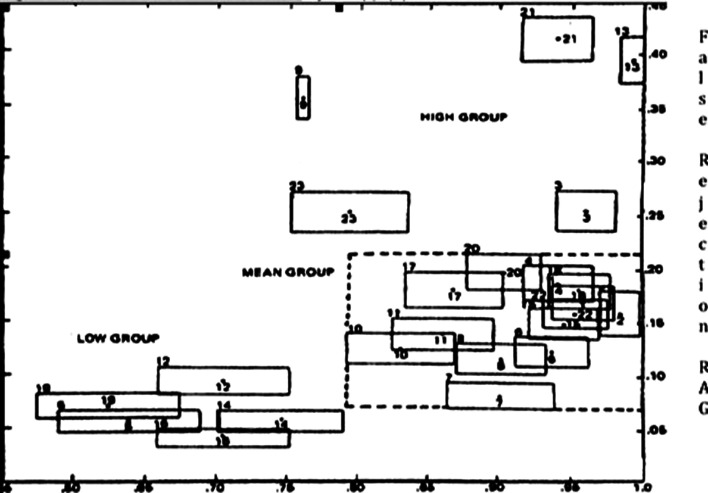
False rejection is a business concern that is due to rejection of “Acceptable” and “Grey Zone” containers (RAG), which are sacrificed to ensure that the must-reject containers having a rejection probability greater than 70% are rejected (14). Rejection of these containers provides a buffer zone that helps to ensure that the “Must Reject” containers are detected (14). The key to successful qualification is to detect and reject defective containers, while minimizing the rejection of good and gray zone containers (AGN). A thoroughly characterized challenge set will be inspected multiple times by numerous inspectors. The group data is used to calculate the rejection probability for each container in the set. A manual baseline is established from this data. This baseline reflects in-house capability and robustness of the inspection program. The data is used to evaluate group performance and individual inspector performance compared to the group. The RZE/RAG plane published by Knapp (13) is a pragmatic approach for comparing individual performance to group performance. The RZE is used to set the minimum accuracy for dejection of defective containers, while the RAG is used to set the maximum acceptable level of False Rejection of defect free containers.
Use of statistical tools, such as the Knapp RZE/RAG plane, enables the comparison of manual inspection performance to semi-automated or fully automated inspection systems (15). Other approaches may be devised, but they must be based on multiple inspections of the challenge set to develop the required statistics required for validation. The use of more than one challenge set can be used to facilitate the process, but strict controls must be in place to ensure that each challenge set is equivalent. Unless there is sufficient expertise and procedures in place to make identical challenge sets, it is better to use one challenge set for testing or purchase identical challenge sets from a qualified laboratory. Qualification of inspectors and validation of automated inspection systems depend upon a thoroughly characterized challenge set. Either purchasing an entire set of containers or a partial set of challenge containers by a qualified laboratory is recommended. The contents, size, and composition of each particulate should be verified and documented. Only qualified laboratories should be used to manufacture a customized challenge set for this critical testing. Challenge sets should include a vial inventory with the identified size and composition of the particulates, certificate of analysis and expiration date. All challenge containers should be examined upon receipt by a qualified inspector or other support personnel qualified to verify the container attributes before being used for testing (Fig. 2).
Fig. 2.
The RZE/RAG plane created by Knapp (13)
PERIODIC REQUALIFICATION
Manual inspection is a dynamic process that must be maintained in a controlled state. Understanding process capability and the variables that can be controlled must be monitored closely (16). Periodic requalification of inspectors by eye examination and testing with a well-characterized challenge set are essential elements of a manual inspection program. Periodic testing and an eye examination are required since human visual acuity and ability to detect contrast decrease with age. The latest survey indicates that 100% of participating companies use a standard challenge set for training (1). The same survey also indicates that only 79% of these same companies require 20/20 visual acuity (1).
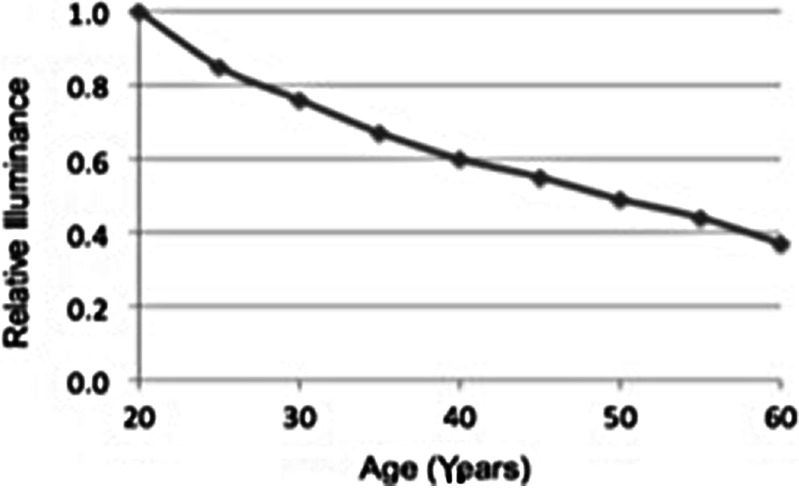
An eye examination requiring 20/20 visual acuity combined with performance testing, using a characterized challenge set, should be mandatory requirements for qualification and requalification of each inspector. The latest survey indicates that 100% of the companies perform testing with a characterized challenge container set (1). It also indicates that periodic requalification decreased from 92% in the 2003 survey to 79% in the 2008 survey (1). This negative trend could be due to variability in the survey or the possibility that there is an undesirable trend for requalification of inspectors. Requalification is an essential element of maintaining control of an inspection program and should be performed periodically. The latest survey indicates that 63% of participating companies requalify their inspectors annually (1). Visual acuity decreases with age, as well as, the ability to detect contrast as shown in Fig. 3. This data in Fig. 3 was taken from the 9th edition of the Illumination Engineering Society of North America Lighting Handbook (IESNA) (17).
Fig. 3.
Aging and relative illumination
Simply stated, the data in Fig. 3 indicate that sufficient light would be provided for an inspector, 65 years of age (17).
INSPECTION LIGHTING
The PDA surveys indicate a trend towards increased use of fluorescent lighting that is controlled by a high frequency ballast to eliminate flicker. According to the survey, 68% of participating companies use fluorescent lighting (1). The use of incandescent lighting has declined to 16% (1). Incandescent bulbs have a color shift into the yellow light spectrum and they deteriorate quickly as the filament is consumed during use.
The Japanese Pharmacopoeia specifies a light intensity of 100 fc for general inspection (18), which is significantly less than the Ph. Eur. requirement. Many investigators have used this light intensity range for qualification studies and maintenance of their production inspection lighting.
The median light intensity reported in the PDA survey ranged from 270 median foot candles with fluorescent lighting and 215 median foot candles with incandescent lighting at the point of inspection (1). The light intensities reported in the survey fit into the 200–375 fc range specified in the Ph. Eur. (19). The initial studies performed by Knapp were performed using 225 fc (11–14,20) and the six studies documented in Fig. 1 was conducted using a light intensity range of 200–300 fc (2). The United States Pharmacopoeia (USP) does not provide a recommended light intensity for inspection (21).
Recent studies by Knapp (9) and Budd (8) indicate that inspection with a light intensity of 550 fc will increase detection of particulates. Knapp’s data indicates that the use of 550 fc light intensity, combined with an 18% gray background, enabled 95% detection of a 150-μm particle 95% of the time (4). This is a significant improvement of the multiple studies in Fig. 1, where a 163 μ particle was detected ≈83% of the time, using 200–375 fc and a black/white background. This data supports an approximate 13% improvement in detection with the use of the 550 fc light intensity for prolonged inspection at near-focus distance (4,8,22). This light intensity studied by Knapp and Budd, since 2005, also coincides with the IESNA recommendation for performing an exacting task over a long period of time (17).
The data of Knapp and Budd indicates that increased light intensity improves detection of particles; however, supportive studies are required to ensure that the increased light intensity does not increase inspector eye fatigue or result in excessive false rejection. The industry should continue to strive towards manufacturing excellence, but we must also ensure that the inspection process does not exceed the capabilities of upstream manufacturing. Knapp’s data indicated an increase in false rejection when the light intensity was increased to 900 fc (3).
The industry has performed many studies using a light intensity of 225–350 fc. The usage of the Ph. Eur. recommended light intensity range of 250–375 fc should serve as the pragmatic guideline until more data is available to support using an increased light intensity (18). A light intensity of 200–375 foot candles was used in the studies plotted in Fig. 1. This data supports visual detection of a 163 μ particle ≈83% of the time when using a light intensity of 200–375 fc. Increasing light intensity to 550 fc may become the new standard for light intensity providing supportive studies indicate that the increased light intensity does not result in inspector eyestrain or cause excessive false rejection.
The Japanese Pharmacopoeia is the only compendium that recognizes the need to use an alternate light intensity for the inspection of different type containers. It specifies a light intensity of 800–1,000 fc (18) for the inspection of opaque plastic bottles. This recommendation is consistent with years of experience with Eisai automated inspection machines that have multiple light intensity settings required to optimize inspection of pigmented products, amber glass, and opaque containers. The lighting sources and position are adjustable on the Seidenader semi-automated inspection system. Some inspection booths provide control over light intensity and position, which is desirable for inspecting multiple product/container configurations.
Use of manual inspection booths and semi-automated inspection stations should have the provision to adjust light intensity and position for each type of inspection. Common sense dictates that an increased light intensity is required for opaque plastics, amber containers and pigmented products. The data supports the position that one-light intensity does not satisfy all inspection requirements and whatever intensity is selected it must be qualified. Each site should have the freedom to qualify and use and qualify the light intensity as required for a specific product/container combination.
CHALLENGE SETS
The criticality of a well-characterized challenge set must be understood. For initial training, testing and requalification of manual inspectors, a challenge set of containers must be manufactured with particulates that are characterized (e.g., fiber, glass, stainless steel, etc.) and measured for size. This challenge set can contain some containers with standard sized spheres, but must also contain particulates apt to be seen during manufacturing (7,22).
Standard spheres are useful for determining size threshold of manual inspectors, but are poor training substitutes in preparation for commercial production. Simply stated, an inspector will not see a standard sized round stainless steel sphere in production. Standards must be a benchmark for comparing inspection methods. Challenge containers must be well characterized, repeatable, and traceable (23). Standards must be stable, the nature of the particulate must be known, the ideal set will include accept zone, gray zone, and reject zone, the ideal set should contain a reject population large enough to obtain a statistical picture of the inspection process. The ideal set challenge set should contain particles that have similar properties as production rejects (24).
Standard sized spheres meeting National Institute of Standards and Technology sizes, made of various materials, have been used to plot calibration curves (3,4,25,26,28). Standard spheres are useful for plotting calibration curves and comparing groups, but they do not look like or behave like real particulate matter when spun.
The reject population of a sample set should be greater than actual manufacturing, but the percentage of reject containers should never exceed 20% of the population to avoid sensitization of inspectors (13). Creation of a quality container challenge set is time consuming and exacting. Containers must be absolutely free of visible particles or have one and only particulate per container (7,8). The rationale for one having only one defect per container is that it provides absolutely certainty the reason for container rejection. The practice of using multiple particles should be discouraged, since it is not possible to know which defect led to the container rejection. Also, having more than one defect adds a multiplying effect for detection.
The challenge set should be manufactured under a high efficiency particulate air filtered hood, using a vehicle such as water for injection with the optional use of a preservative and surfactant. Taking these precautions, in combination with stable components that have been meticulously prepared, will protect the investment in the challenge set. If in-house expertise or manpower is not available, a certified laboratory should be used to provide this vial set. A certified laboratory will provide a certificate of analysis, expiration date, and guarantee the contents of each container (24). This challenge set needs to be stored under controlled secured conditions and if properly maintained with provide a means for testing new inspectors and requalifying existing inspectors for a number of years (23).
INSPECTION ROOM ENVIRONMENT
A well-designed inspection room is free from distractions, extraneous light and is ergonomically designed for inspector comfort (2,4,8,26). Inspection rooms may consist of a number of independent inspection booths operated as separate units or they may be connected in series on a paced line. Both arrangements are acceptable providing the pacing time provides effective inspection and is qualified.
The three articles written by Budd (4,8,26) clearly discuss concepts on inspection environment. These articles demonstrate by illustration on how to hold the vial at the optimal angle for agitation and inspection. One illustration clearly demonstrates the angle of the eye to the object. Another illustration clarifies the distance between the pupil and the object being inspected. This information specifies the correct distance of close focus position of the eye and object being inspected (150–250 mm). These papers also discuss the art of mixing to put particles in motion, while not creating air bubbles.
INSPECTION BOOTH BACKDROP
The Ph. Eur. has a drawing of a traditional inspection station that has a backdrop consisting of one half black and one half white colors (18). Recent studies by Knapp indicate that a single 18% gray backdrop may be as effective as the black/white backdrop and has the advantage of reducing inspection dwell (3). Additional data comparing the black/white background versus the use of an 18% gray background needs to become available in order to make an informed choice. Each site should have the latitude to test and qualify the backdrop for on-site use.
MAGNIFICATION
According to the companies participating in the PDA surveys, the use of magnification has declined through the years. At present, approximately 26% of participating companies use magnification (1). As discussed by Budd (26), the curvature of a vial’s circular shape and the index of refraction of filling fluid create an imperfect lens due to distortion. In a similar way, the curved surface of a magnifying lens creates similar distortion, which makes the particle size vary depending where it is located. Containers, such as small syringes, are difficult to examine and the use of magnification may be advantageous in such circumstances as long as the optical distortion is understood and controlled (4). Once qualified, the use of magnification must be used consistently for that specific product. The line inspector is not free to make a decision on its use, once the process is qualified.
SEMI-AUTOMATED INSPECTION
A number of companies perform in-line container/closure inspection with the aid of semi-automated inspection systems. Semi-automated systems provide container handling for the inspector, but the inspection process is still manual inspection. A common use of semi-automated inspection occur in-line after filling or before labeling. In-line inspection using semi-automated stations provides adequate inspection time for container/closure defects, but it is less suitable for particulate inspection, due to the high speeds of filling or packaging lines which are in the range of 60–100 containers per minute. Inspection studies using an alternative process such as fully automated or semi-automated inspection systems must demonstrate that the replacement technology has a detection probability that is greater than or equal to manual baseline (15).
Inspectors have much less time to inspect when inspection is performed at speeds that greatly exceed the inspection time compared to inspection booths installed off-line (27). Common sense dictates that inspection with a semi-automated system installed in line running at 60–100 containers per minute is not as effective as an inspection booth where a 5-s inspection duration is used in front of each color background. If these systems are installed off-line and run at greatly reduced speeds, adequate particulate inspection is possible and performance must be greater than or equal to manual inspection baseline performed in an inspection booth. Semi-automated systems do offer various light configurations, polarized light and optional magnification. The lighting and container rotation on the semi-automated systems reduce inspection time, but inspection duration must be sufficient so that semi-automated detection performance is greater than or equal to manual inspection baseline.
If in-line use of semi-automated roller systems must be used, the use of two consecutive systems performing two consecutive inspections in series can be considered. The last disadvantage of semi-automated particulate inspection is that a group of four containers is constantly being passed in front of the inspector.
SUMMARY
The pharmaceutical and biotechnology industries perform 100% inspection of containers filled with drug product to ensure that the container is free of container/closure defects and that the drug product in the container is free of particulate matter. Either type of defect can have harmful effects on the patient if missed. Container/closure defects can potentially lead to a breach in sterility. Particulate matter in the drug product indicates potentially dangerous contamination introduced during the upstream process. Both types of defects are serious and USP chapter <1> injections, requires 100% inspection of sterile injectable products (21).
Manual inspection can be controlled and produce acceptable inspection security if the control measures addressed in this article are used. Effective manual inspection begins with an inspector having 20/20 visual acuity. Inspector candidates must then be trained using a combination of formal training, practice with characterized challenge container sets, and interaction with the trainer. Once a statistically sound baseline is established, the inspection program must be maintained by periodic eye examinations, inspector testing with characterized challenge containers, change control production, trending rejection rates, and a periodic review of the inspection process. Distractions such as extraneous light, other activities in the inspection area and noise must be minimized. The importance of a well-characterized challenge container set(s) cannot be over emphasized. Frequently, validation failures or excessive false rejection rates are due to a poorly constructed challenge set.
Quality manual inspection is essential in situations where the use of a fully automated inspection system is not practical. Hopefully, the top manufacturers of automated inspection systems will manufacture systems in the future that are suitable for small sized operations, small-scale batches and have a smaller footprint to fit into available space. A majority of companies that participated in the PDA survey reported that they use manual data to validate automated inspection systems. For this reason, manual inspection data must have minimal variation if it is to be used as a baseline for the validation of an automated inspection system.
The intent of this article is to incorporate the sound manual inspection practices established by numerous subject matter experts into one document that can be used as a guideline document for the conduct of manual inspection. Hopefully, this effort will lead to the creation of a document similar to PDA Technical Report Number 29, entitled “Points to Consider for Cleaning Validation” (27). Such a document would provide guidance; establish procedures that must be followed, while permitting varied approaches to validation. If used, these control measures will elevate manual inspection to a level previously not thought possible.
In the absence of comprehensive information in the Code of Federal Regulations or Compendia, a guidance document for the conduct of manual inspection is severely needed. This guidance document contains the essential requirements for conducting manual inspection, while providing latitude to qualify different product/container combinations. The collation of the knowledge of the numerous subject matter experts referenced in this article stresses what the thinking we have in common and focus less on our differences. This guidance document should present the basic concepts agreed to by most SMEs, while providing latitude for site validation. It can serve as a temporary guideline for the conduct of manual inspection until a more substantive document such as a technical report is written by the PDA.
REFERENCES
- 1.Leversee RL, Shabushnig JG. “A survey of industry practice for the visual inspection of injectable products, (PDA Preliminary Report)” 2008.
- 2.Shabushnig JG, Melchore JA, Geiger M, Chrai S, Gerger ME. “A proposed working standard for the validation of particulate inspection in sterile solutions,” podium presentation at PDA annual meeting; Philadelphia, PA 1994.
- 3.Knapp J. “The bridge between visible particle data and the accuracy and repeatability requirements of PAT, one safe step at a time,” Paper presented at: PDA Visual Inspection Forum, Bethesda, MD 2005.
- 4.Budd G. “Optimizing the correlation of automated inspection data obtained from electronic sensors to (NIST Traceable) referee level particle standards,” paper presented at: PDA visual inspection forum, Bethesda, MD 2005.
- 5.Barber, Thomas A. “Training and qualification of inspectors for manual visual inspection,” Paper presented at: PDA Visual Inspection Forum, Bethesda MD. 2005.
- 6.Melchore JA. “Lessons learned regarding particulate inspection,” Presentation at PDA metro chapter, 2007, Somerset NJ (2007).
- 7.Melchore JA. “Establishing a statistically sound manual inspection baseline,” Paper presented at: PDA Visual Inspection Forum, Bethesda, MD 2009.
- 8.Budd G. “Accurate NIST traceable contaminating particle detection and measurement technology, Presented at The PDA Visual Inspection Forum, Bethesda MD 2007.
- 9.Knapp JZ. “Overview of accurate NIST traceably sized dimensions of visible particulate contamination data in sealed containers of injectable products: an essential validation and product acceptance tool,” Paper presented at: PDA Visible Inspection Forum, Bethesda MD. 2007.
- 10.Leversee, Ronald. “Training and performance monitoring of the injectable inspector,” Paper presented at: PDA Visual Inspection Forum, Bethesda, MD. 2007.
- 11.Knapp JZ, Kushner HK. A validation procedure for the particulate inspection of parenteral products, “generalized methodology for evaluation of parenteral inspection procedures. J Parenter Drug Assoc. 1980;34:14. [PubMed] [Google Scholar]
- 12.Knapp JZ, Zeiss JG, Thompson BJ, Crane JS, Dunn P. Inventory and measurement of particulates in sealed sterile containers. J Parenter Sci Technol. 1983;37:170. [PubMed] [Google Scholar]
- 13.Knapp JZ. The scientific basis visible particle detection. PDA J Pharma Technol. 1999;53:291. [PubMed] [Google Scholar]
- 14.Knapp JZ, Abramson LR. Automated particulate inspection systems: strategies and implications, particulate inspection of parenteral products: an assessment. J Parenter Sci Technol. 1990;44:74. [PubMed] [Google Scholar]
- 15.Knapp JZ. The reject rate of a USP validated 100% inspection for visible particles versus the attribute sampling inspection for improved quality at lower cost, PAT compatibility one safe step at a time,” Paper presented at: PDA Visual Inspection Forum, Bethesda MD. 2005.
- 16.Cherris RT. “Visible particulate inspection,” paper presented at: PDA visual inspection forum, Bethesda, MD 2005.
- 17.The Illuminating Engineering Society of North America (IESNA) Lighting Handbook, 9th edition.
- 18.Japanese Pharmacopeia, “Foreign insoluble matter test for injection volume," 15, 6.06, 2007.
- 19.European Pharmacopoeia, “Particulate contamination; visible particulates,” Volume 6, Appendix XIII B (Ph. Eur. Method 2.9.20 2008
- 20.Knapp JZ, Kushner HK, Abramson LR. Particulate inspection of parenteral products: an assessment. J Parenter Sci Technol. 1981;35:176. [PubMed] [Google Scholar]
- 21.United States Pharmacopoeia-National Formulary (USP-NF), “Chapter <1>injections- foreign and particulate matter,” USP33-NF28 reissue, 2010.
- 22.Berdovich D. “Use visual inspection standards,” PDA Visible Inspection Forum, Bethesda MD. 2007.
- 23.Leversee RL, Shabushnig JG. “The use of standard particles in the qualification, validation and routine control operation of visual inspection operations,” Paper presented at: PDA Visible Inspection Forum, Bethesda MD. 2009.
- 24.Borchert SJ, Maxwell RJ, Davidson RL, Aldrich DS. “Standard particulate sets for visual inspection systems: their preparation, evaluation and applications,” J. Parenter. Sci. & Technol. 1986. [PubMed]
- 25.Berdovich D. “Visual inspection standards for human inspector qualification” Presented at: PDA Visual Inspection Forum, Bethesda, MD. 2005.
- 26.Budd GW. “Referee level NIST traceable particle standards in sealed containers used for the creation of a visible calibration curve,” Paper presented at: PDA Visible Inspection Forum, Bethesda MD. 2009.
- 27.PDA Pharmaceutical Validation Task Force, Technical Report No. 29, “Points to Consider for Cleaning Validation 1998. [PubMed]
- 28.Borchert, Steven J, Abe A, Aldrich S, Fox LE, Freeman JE, White RW. Particulate matter in parenteral products: a review. J Parenter Sci Technol. 1986;40:212. [PubMed] [Google Scholar]