Abstract
The purpose of the current study was to mask the taste of cetirizine HCl and to incorporate the granules produced in oral disintegrating tablets (ODT). The bitter, active substance was coated by fluidized bed coating using Eudragit® RL30-D at levels between 15% and 40% w/w. The ODTs were developed by varying the ratio of superdisintegrants such as sodium croscarmellose, crospovidone grades and low substituted hydroxypropyl cellulose (L-HPC). A direct compression process was used to compress the ODTs under various compaction forces to optimize tablet robustness. The properties of the compressed tablets including porosity, hardness, friability and dissolution profiles were further investigated. The in vitro and in vivo evaluation of the tablet disintegration times showed almost identical rapid disintegration below 10 s at the optimal levels of each superdisintegrant. Finally, the taste and sensory evaluation in human volunteers demonstrated excellence in masking the bitter active and tablet palatability.
Keywords: Fluidized bed coating, oral disintegrating tablets, superdisintegrants, taste masking
INTRODUCTION
The development of oral disintegrating tablets (ODTs) has attracted increased interest among researchers and pharmaceutical industries over the last decade. The ODTs are designed to disintegrate or dissolve rapidly on contact with saliva, without the need for water, which makes them advantageous to conventional tablet forms. ODTs, as novel dosage forms present several characteristics to distinguish them from more traditional dosage forms. A major advantage of the ODT formulations is that they combine the properties of both liquid and conventional tablet formulations (1,2). They provide the convenience of a tablet formulation yet are easy to swallow similar to a liquid formulation. ODTs are ingested simply by placing them on the tongue, thus eliminating the need to chew the tablet, swallow an intact tablet, or take the tablet with water. Furthermore, administration of ODTs is beneficial to paediatric and geriatric patients or people who find swallowing difficult and for the treatment of patients where compliance is difficult.
However, the taste masking of bitter active substances is a critical hurdle to overcome in the development of ODTs. Several active substances leave an unpleasant taste in the oral cavity immediately after tablet disintegration.
Therefore, taste masking is of critical importance for the formulation of an acceptable ODT. Oral administration of bitter active substances through ODT formulations should provide an improved degree of palatability, increased patient compliance and a concomitantly beneficial therapeutic effect. Current methods of taste masking in fast dissolving/disintegrating tablets in some cases include sweeteners and flavours. Nevertheless, these additives are not a sufficient means for taste masking. Fortunately, recent developments in technology have presented viable dosage alternatives to taste mask bitter drugs. Several approaches have been reported which implement complexation (3,4), freeze-drying (5), microencapsulation (6,7), fluidized bed coating (8,9), high shear mixing (10) and supercritical fluids (11,12) for taste-masking purposes.
Despite the fact that fluidized bed coating represents one of the oldest pharmaceutical techniques, it is still regularly used in the manufacturing of pharmaceutical dosage forms (8). Fluidized bed coating is mainly used to develop modified release (13), pulsatile release (14) or to increase drug bioavailability (15). There are only a few studies reported where fluidized bed coating was implemented for taste-masking purposes (9,16,17). For example, ibuprofen (9) was successfully taste masked by using a combination of hydroxypropylmethylcellulose and ethylcellulose. In another case, indeloxazine hydrochloride (16) was coated with a mixture comprising of hydrogenated oil and surfactants in a fluidized bed using a side-spray method.
Cetirizine HCl (CTZ) is a second-generation histamine H1 receptor antagonist, with a rapid onset, a long duration of activity and is used in the treatment of allergies, hay fever, angioedema and uticaria (18). It is available over the counter as, Zyrtec, in the form of immediate release and chewable, immediate release tablets. Recently, it was reported that Eurand Pharmaceuticals, Inc. has developed a fast disintegrating cetirizine HCl tablet using a combination of the microcaps® and advaTab® technologies where the bitter API is microencapsulated through coacervation to produce taste-masked microparticles (19).
The aim of this twofold study was to mask the taste of a model active substance through fluidized bed coating and to incorporate the granules produced in robust ODT formulations. A methacrylic pH-sensitive copolymer was used as masking agent. To evaluate the taste a sensory test was implemented using ten healthy volunteers, which revealed significant taste masking. For the development of ODTs, the effect of the amount of superdisintegrant(s) on ODT hardness, friability and disintegration times was assessed in order to identify the optimum formulation. The use of superdisintegrants is a well-known approach to formulate ODTs (2). The physicochemical nature of the disintegrant determines the disintegration mechanism and consequently affects the disintegration times.
MATERIALS AND METHODS
Materials
Cetirizine HCl (CTZ) was purchased from Ultratech India Limited (New Mumbai, India). Eudragits E100 and Eudragit RL-30D poly(methacrylate) polymers were kindly donated from Evonik Pharma Polymers (Darmstadt, Germany). Crospovidone (Polyplasdone XL10), croscarmellose, (Vivasol, JRS Pharma, Rosenberg, Germany), Pearlitol C160 (Roquette, France), microcrystalline cellulose (Avicel 102, FMC), sodium stearyl fumarate (PRUV, JRS Pharma, Rosenberg, Germany) and sodium phosphate dehydrate (Emcompress, JRS Pharma, Rosenberg, Germany) were also donated and used as tablet excipients. Low substituted hydroxypropylcellulose (L-HPC) was kindly donated from Shin-Etsu (UK). PVP K25 was purchased from Sigma (UK). The high-performance liquid chromatography (HPLC) solvents acetonitrile and methanol were analytical grade and purchased from Fisher Chemicals (UK). All materials were used as received.
Methods
Granulation and Fluidized Bed Coating
Taste masking of cetirizine HCl carried out in two steps. Initially, cetirizine HCl (1 kg) was granulated with Emcompress (50:50 w/w) with 5% (w/w) PVP K25. In brief, CTZ and Emcompress were mixed in a tumble mixer for 15 min at 35 rpm and then granulated with the binder solution for 8 min in a Diosna Pharma Mixer P/5 (600 rpm). The granules produced were passed through a 0.3-mm diameter sieve. The drying process was carried out in an airflow oven for 12 h at 40°C. The dried granules were passed again through a 0.3-mm diameter sieve to break any agglomerates. The granules that did not pass the sieve where mixed again with the rest of the sample.
The latter step included taste masking of the CTZ granules with Eudragit RL-30D. The coating process was conducted in a Glatt fluid bed GPCG 1.1 coater. The spraying suspension was applied with talc (12%) as glidant and triethyl citrate as plasticizer (5%). Talc and triethyl citrate were added in demineralised water and homogenized for 15 min with an Ultra Turrax K50 homogenizer. The suspension was poured slowly into the Eudragit RL-30D suspension while stirring gently until the coating process was complete. A Schlick 930D/S3 spray gun was used for the coating process at 1.8 bars atomizing pressure, 7.6 g/min spray rate and 380-min coating time. The inlet temperature varied between 42°C and 47°C whilst the exhaust temperature varied between 35°C and 31°C.
Particle Size Distribution Measurements
The particle size distribution of the produced cetirizine HCl (CTZ HCl) granules in each step of the production process was measured by dry sieving. The method involved stacking of the sieves on the top of each other and then placing the test powder (100 g) on the top sieve. The nest of sieves was subjected to a standardized period of agitation (20 min) and then the weight of the material retained on each sieve was accurately determined to give the weight percentage of powder in each sieve size range.
Tablet Preparation
Development batches were prepared using batch sizes of 50 g. All materials were passed through a mesh sieve with an aperture of 500 μm before use. The batches were blended with sodium stearyl fumarate (0.5%) in a Turbula TF2 mixer (Basel, Switzerland) for 5 min. Blends were directly compressed on a Flexitab trilayer tablet press (Oystar Manesty, Germany) using 10-mm normal flat punches. Dwell time was set at 30 ms and the compaction force varied from 4 to 12 kN.
In Vitro Drug Disintegration
The tablet disintegration test was carried out using an Erweka disintegration tester according to BP monograph (2007). The same tests were repeated by using a TA-XDPlus texture analyzer (Stable Micro Systems) similarly to a recent study (20). The apparatus was calibrated with a 5 kg load cell and fitted with a flat-bottomed cylindrical stainless steel probe (P/25, 12 mm in diameter and 25 mm in height). In summary, the completely dry tablet is placed on the perforated grid and it is not in contact with the disintegration medium. The probe descends until a trigger force is detected where it gets in contact with the tablet placed on the grid and pushes the whole system downwards. The tablet then touches the medium and starts disintegrating. At this point, the TA apparatus is set to maintain a predetermined nominal force (50 g) for a given period of time (60 s). For all the disintegration studies artificial saliva (pH 5.8) was used as a solvent medium in order to simulate oral disintegration.
In Vivo Drug Disintegration and Bitterness/Roughness Evaluation
In vivo disintegration and taste-masking evaluation was performed on ten healthy human volunteers (21,22) from whom informed consent was first obtained (approved by the Ethics Committee of the University of Greenwich). The healthy volunteers of either sex (age, 18–25 years) were selected, trained and then one tablet was held in the mouth after rinsing with water and the time required for complete disintegration of the tablet was recorded. The time from when the tablet was placed on the tongue till it disintegrated without leaving any lumps was taken as end point. The disintegrated material was held in the mouth for another 60 s, and then spat out. The mouth was rinsed with water without swallowing the disintegrated material and, finally, the perceived roughness levels were recorded on a numerical scale ranging from 0 to 3 where 0, 1, 2 and 3 indicate none, slight, moderate and high roughness, respectively.
The equivalent of 10 mg of pure cetirizine HCl was held in the mouth for 10 s and then spat out, and 1 ODT (containing equal amounts of cetirizine) was held in the mouth until complete disintegration (three tablets per trained volunteer). Bitterness was recorded immediately and at several intervals for 15 min according to the bitterness intensity scale from 0 to 3 where 0, 0.5, 1, 2 and 3 indicate none, threshold, slight, moderate and strong bitterness.
Flow Properties and Compressibility
Compressibility index I (Carr’s index) values of the different formulations were determined by measuring the tapped bulk and poured bulk density of the powders after subjecting to 100 taps in a graduated measuring cylinder using the following equation (23):
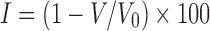 |
1 |
Evaluation of Tablets
The prepared tablets were evaluated for the uniformity of thickness, hardness (Erweka TBH 28, Frankfurt, Germany), friability (Erweka friabilator, model A3R, Frankfurt, Germany), and disintegration time (Erweka, model ZT4, Heusenstamm, Germany) according to USP22 tests. The diametral compression test devised by Fell and Newton (24) was used to determine the tensile strength T, using the formula:
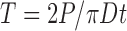 |
2 |
Where P (kP) is the applied stress, D (cm) is the diameter of the tablet, and t (cm) is the tablet thickness. Three tablets from each batch were subjected to tensile strength determination.
The solid fraction (SF) and porosity (e) were calculated based on the true density (ρtrue), tablet volume (v), and tablet weight (Wt) as below [25]:
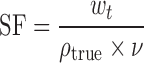 |
3 |
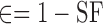 |
4 |
Scanning Electron Microscopy
The surface morphology of the pure CTZ crystals and coated CTZ granules was also examined. The samples were attached to aluminium stubs with double side adhesive carbon tape then gold coated with a sputter coater and examined using a scanning electron microscope (Jeol 5200, scanning electron microscopy (SEM)).
Powder X-ray Diffraction
Samples of pure or granulated drugs were evaluated using a Kristalloflex 810 Siemens diffractometer. The samples were radiated using Ni filtered CuKa radiation operated at 40 kV and 30 mA. The granule samples were scanned from 5° to 45o (2θ) at scanning speed of 0.5 θ/min.
In vitro Drug Release Studies
In vitro drug release studies were carried out in 750 ml of 0.1-M hydrochloric acid for 2 h using a Varian 705 DS dissolution paddle apparatus (Varian Inc. North Carolina, USA) at 100 rpm and 37 ± 0.5°C. After a 2-h operation, 250 ml of 0.20-M solution of trisodium phosphate dodecahydrate were added into the vessel (buffer stage, pH 6.8) that had been equilibrated to 37°C. Samples were withdrawn for HPLC assay at predetermined time intervals. All dissolution studies were performed in triplicate. The above method (BP, Appendix XII D) is recommended for delayed release formulations. The reason for the selection of such method is based on the nature of the coating polymer RL-30D which is water permeable but not gastro-soluble and high coating percentages could result drug release beyond the first 2 h.
HPLC Analysis
The amounts of released CTZ were determined by HPLC. An Agilent Technologies system equipped with a wavelength detector at 264 nm and a Spherisorb S5ODSI column was used for the CTZ HPLC assay. The mobile phase consisted of acetonitrile/water/orthophopshoric acid (75:24.8:0.2 v/v). The flow rate was 1.5 ml/min and the retention time of CTZ was 3.0 min.
RESULTS AND DISCUSSION
Cetirizine HCl Granulation and Fluidized Bed Coating
In the current study, an attempt was made to develop robust cetirizine HCl taste-masked ODTs. Cetirizine is an extremely bitter substance and cannot be formulated into ODTs without standard taste-masking approaches. Another major obstacle to overcome with CTZ is its chemical incompatibility with a number of commonly used excipients, which can lead to unstable products. Thus the masking of CTZ required careful selection of the masking agent.
Initially, CTZ was granulated to obtain fine granules prior to the fluidized bed coating process. The granulation process was repeated three times for reproducibility purposes and the particle size of the CTZ granules obtained varied from 50 to 500 μm as depicted in Fig. 1. For the taste-masking process Eudragit® RL-30D was selected as the masking agent. The development of a taste-masked ODT formulation usually requires the selection of a gastro-soluble, modifying agent as the coating material. The selection process ensures negligible drug release in the oral cavity and simultaneously rapid drug release when the API reaches the stomach environment. EUDRAGIT® RL-30D is a copolymer of ethyl acrylate, methyl methacrylate with a low content of methacrylic acid ester with quaternary ammonium groups. The ammonium groups are present as salts and make the polymers permeable and pH independent. Although RL-30D is not gastro-soluble, we anticipated rapid CTZ release due to the high permeability of the polymer. We have initially incorporated EUDRAGIT® EPO polymer as the masking agent, which is soluble in gastric fluids up to pH 5.0. Further drug/polymer compatibility studies (data not shown) revealed undesired interactions that led to drug decomposition due to CTZs chemical incompatibility as mentioned before.
Fig. 1.
Particles size distribution of coated and uncoated cetirizine HCl granules
The yield of the produced granules with particle size <100 μm was too low (~6%) and hence for the fluidized bed coating we used granules with a particle size distribution of 100–500 μm. The fluidized bed coating process was straightforward and the RL-30D coating suspension was applied within normal parameter ranges to obtain homogenously coated granules. The production yield was up to 90.1% compared with the theoretical yield. The excellent coating process, as depicted in Fig. 1, produced coated granules with approximately 87% of the particle size distribution lying between 100 and 500 μm. The granules/excipients particle size distribution plays an important role in the development of ODTs in terms of their disintegration time.
The results were also supported by SEM images of primary CTZ particles and coated granules at the end of the coating process. Figure 2 depicts the appearance of pure CTZ crystals (2A) with an acicular shape and the Eudragit coated granules (2B–C).
Fig. 2.
SEM images of a pure CTZ crystals and b Eudragit RL30D coated granules
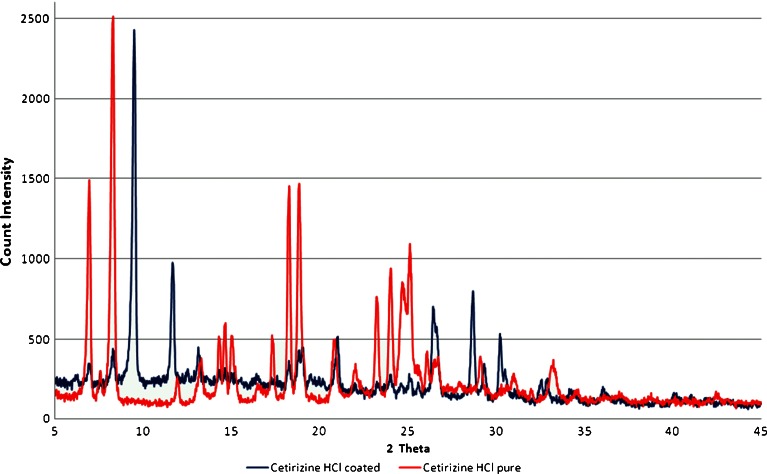
XRD was implemented to investigate whether CTZ retains its crystalline state in the coated granules produced. Pure forms of the drugs and the drug/RL-30D coated forms were scanned as shown in Fig. 3. The diffractogram of pure CTZ shows numerous distinct lines of high intensity indicating that they are in a highly crystalline state. The diffraction peaks for CTZ appeared at 2θ values of 6.9°/8.1°/11.9°/13.2°/14.2°/14.5°/14.9°/17.3°/18.2°/18.8°/20.7°/23.2°/23.9°/24.6°/25.0° respectively while for the coated crystals at 6.7°/8.0°/13.1°/14.1°/14.4°/14.7°/18.1°/18.6°/20.8°/23.8°. The comparison of the diffraction peaks in the two diffractograms clearly indicates that CTZ retains a crystalline structure. Several sharp and intense peaks can be identified clearly in the coated CTZ diffractogram at the same 2θ values as in pure CTZ. However, the disappearance of CTZ peaks located at 11.9°, 17.3° and 23.2° indicate the formation of a new solid phase with lower degree of crystallinity for CTZ. Finally, for the coated granules the peaks appeared at 9.5°/28.6° belong to talc and the one at 11.65° belongs to calcium hydrogen phosphate (Emcompress).
Fig. 3.
X-ray diffraction patterns of pure cetirizine HCl and coated with Eudragit RL 30D, respectively
Powder and ODTs Characterization
The coated CTZ granules were blended with five different superdisintegrants at concentrations varying from 2% to 20% (w/w) as shown in Table I prior to tablet compression. Each powder blend was characterized in terms of compressibility by Carr’s index determination. All batches presented very good compressibility except the croscarmellose sodium batches with 10% and 20% (w/w) that proved to be non-compressible blends. The batch flowability and compressibility properties were attributed to the presence of microcrystalline cellulose, MCC, (Avicel 102), which is an excellent filler/flow-aid for direct compression (average particle size, 90 μm). In addition, the combination of MCC with mannitol (Pearlitol C160) can further improve tablet compressibility. However, it is worth noting that compressibility decreased when the amount of superdisintegrants were increased in all formulations (Table II). In addition, the presence of MCC (disintegrant nature) and mannitol (soluble diluents) in many cases has been reported to facilitate the tablet disintegration. In the studies described below this was not the case as formulations prepared without the presence of superdisintegrants showed high disintegration times (data not shown). As a result the improved disintegration times should not attributed to the existence of the above two excipients.
Table I.
Composition of ODT Formulations
| CTZ/Excipients | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | F13 | F14 | F15 | F16 | F17 | F18 | F19 | F20 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | ||||||||||||||||||||
| CTZa | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Avicel 102 | 50.0 | 48.5 | 46.0 | 41.0 | 50.0 | 48.5 | 46.0 | 41.0 | 50.0 | 48.5 | 46.0 | 41.0 | 50.0 | 48.5 | 46.0 | 41.0 | 50.0 | 48.5 | 46.0 | 41.0 |
| XL10 | 2.0 | 5.0 | 10.0 | 20.0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Vivasol | – | – | – | – | 2.0 | 5.0 | 10.0 | 20.0 | ||||||||||||
| XL | – | – | – | – | – | – | – | – | 2.0 | 5.0 | 10.0 | 20.0 | – | – | – | – | – | – | – | – |
| SF | – | – | – | – | – | – | – | – | – | – | – | – | 2.0 | 5.0 | 10.0 | 20.0 | – | – | – | – |
| L-HPC | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2.0 | 5.0 | 10.0 | 20.0 |
| Mannitol | 27.5 | 26.0 | 23.5 | 18.5 | 27.5 | 26.0 | 23.5 | 18.5 | 27.5 | 26.0 | 23.5 | 18.5 | 27.5 | 26.0 | 23.5 | 18.5 | 27.5 | 26.0 | 23.5 | 18.5 |
| Pruv | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
a CTZ coated granules
Table II.
Physical Properties of Powder Blends and Compressed Tablets (n = 3)
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | F13 | F14 | F15 | F16 | F17 | F18 | F19 | F20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | ||||||||||||||||||||
| Bulk density (g/cm3) | 32.00 | 32.20 | 32.00 | 33.00 | 32.50 | 32.00 | 32.00 | 31.50 | 30.00 | 32.00 | 32.00 | 32.00 | 32.00 | 32.00 | 34.00 | 44.00 | 30.60 | 32.00 | 32.00 | 32.00 |
| Tapped density (g/cm3) | 29.70 | 30.10 | 29.50 | 29.90 | 29.80 | 29.70 | 25.00 | 24.00 | 28.00 | 29.10 | 29.30 | 29.20 | 29.80 | 28.80 | 30.00 | 37.80 | 28.00 | 27.30 | 27.70 | 26.00 |
| aDensity (g/cm3) | 1.80 | 1.50 | 1.48 | 1.45 | 1.79 | 1.49 | 1.46 | 1.44 | 1.81 | 1.52 | 1.49 | 1.47 | 1.81 | 1.52 | 1.49 | 1.47 | 1.56 | 1.53 | 1.56 | 1.48 |
| Carr’s Index (%) | 7.18 | 6.52 | 7.81 | 9.39 | 8.30 | 7.19 | 21.88 | 23.80 | 6.66 | 9.06 | 8.44 | 8.75 | 6.88 | 10.00 | 11.76 | 14.09 | 8.50 | 14.69 | 13.44 | 18.75 |
| Tensile strength (kP/cm2) | 14.11 | 16.09 | 12.92 | 8.81 | 13.13 | 12.45 | – | – | 13.45 | 16.39 | 12.39 | 12.30 | 15.78 | 12.59 | 13.68 | 12.54 | 15.15 | 14.32 | 12.39 | 9.51 |
| Porosity | 0.32 | 0.11 | 0.21 | 0.17 | 0.34 | 0.35 | – | – | 0.36 | 0.07 | 0.26 | 0.20 | 0.36 | 0.4 | 0.28 | 0.03 | 0.24 | 0.13 | 0.16 | 0.12 |
The rationale of using various superdisintegrants was to evaluate the influence of their concentration on the disintegration time under different compression forces and also on the ODTs performance. Generally, the concentration of a disintegrating agent influences the relationship between the applied compression force and the disintegration time (26). For this purpose we used three different grades of crosslinked N-Vinylpyrrolidone (XL, XL10 and CL-SF), sodium croscarmellose (Vivasol®) and L-HPC. The selected superdisintegrants differ in their particle size distribution, particle shape, porosity, compressibility, flowability and disintegration mechanism. It is worth mentioning that L-HPC and CL-SF act as disintegrants by absorbing water and the subsequent swelling leads to separation of the tablet particles. On the contrary, XL10, XL and croscarmellose facilitate wicking via capillary action due to their high porosity. As a result water is rapidly absorbed and disrupts the interparticular matrix bonds causing the tablet to fall apart (27).
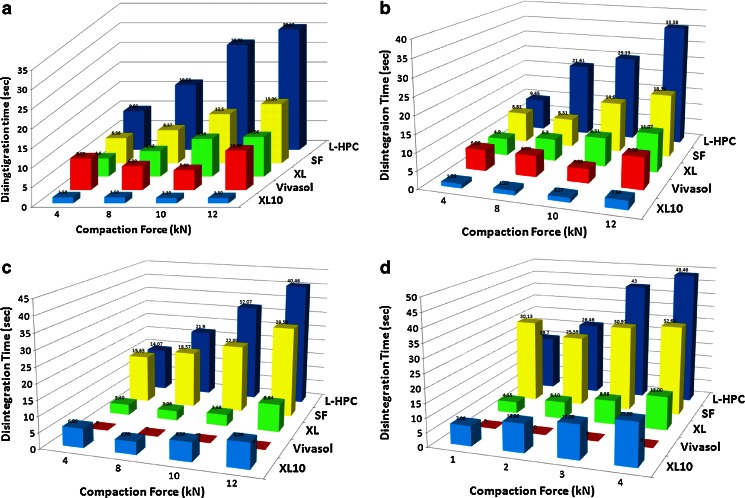
The superdisintegrant concentration profiles versus the compaction force in Fig. 4a–d revealed interesting results regarding the disintegration time performance of each superdisintegrant. At first, it can be seen that XL10 outperformed all superdisintegrants at low levels (2–5% w/w) while XL outperformed at high levels (10% w/w), respectively. The disintegration performance can be arranged in descending order for low levels as follows: XL10 > XL > Vivasol > CL-SF > L-HPC while for high levels the order is XL > XL10 > Vivasol > CL-SF > L-HPC. For XL10 the optimum concentration level was between 2% and 5% w/w without however significant disintegration time differences. At 2% the increase of compaction force led to faster disintegration times for XL10 but above this level increase of the compaction force led to increased disintegration time. On the other hand, optimum level of XL was at 10% (w/w) showing substantial reduction on the disintegration times (Fig. 4c). When the compaction force was increased the disintegration times were also increased. It also appears that XL10 performs better than XL in terms of disintegration times at various compaction forces. This can be attributed to the smaller particle size of XL10 (30–50 μm) compared with XL (100–130 μm) facilitating faster water absorbance.
Fig. 4.
Schematic diagram of ODTs disintegration times at various compaction forces with a 2%, b 5%, c 10% and d 20% superdisintegrants
Vivasol showed good disintegration times at low levels (2–5% w/w). However, it was impossible to compress tablets at higher levels (10–20% w/w) due to sticking problems on the tablet punches. Interestingly, Vivasol showed better disintegration times at compaction force of 10 kN. Finally, CL-SF and L-HPC showed the slowest disintegration times with increasing superdisintegrant levels and compaction forces respectively.
The differences between the disintegration times among the five superdisintegrants are attributed to the different disintegration mechanisms. The Polyplasdone grades (XL10, XL) and Vivasol have both swelling and wicking properties. All the disintegration studies carried out at pH 5.6 where these crosslinked polymers have similar swelling capacity (92%, 90% and 85%, respectively). However, for XL10 and XL, swelling is not the primary mechanism of disintegration. Due to their porous particle size morphology XL10 and XL can rapidly absorb water (wicking) to generate rapid volume expansion and increased hydrostatic pressure which cause tablet disintegration. In contrast, Vivasol has a fibrous non-porous particle structure which swells at slower rates and thus presents slower disintegration times. The Kollidon CL-SF grade has a fibrous particle shape (10–30 μm) and non-porous surface with swelling as the main disintegration mechanism. Similarly the micronized L-HPC swells with the slowest rate and therefore presents increased disintegration times.
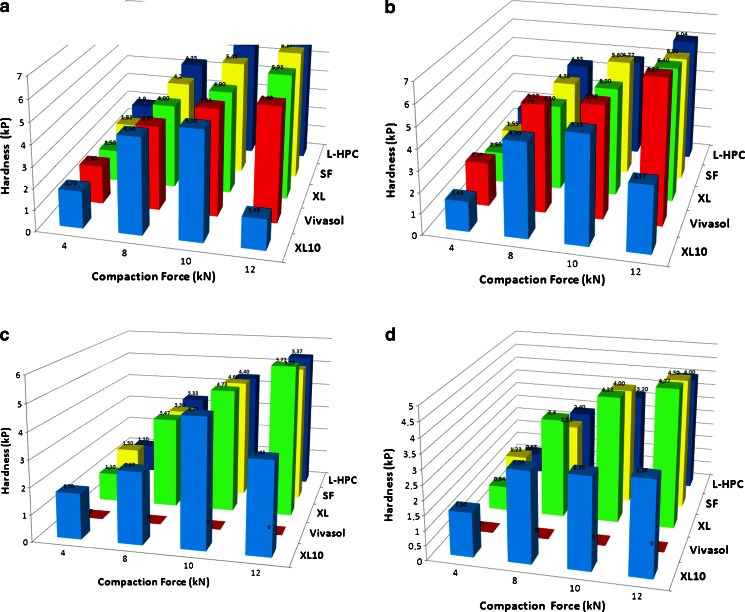
Figure 5a–d shows the relationship between hardness and disintegration times of the superdisintegrants at various concentrations and compaction forces. Disintegration time increased with increasing crushing tolerance. As it can be seen for all superdisintegrants the hardness increased with concentrations up to 5% (w/w). When high disintegrant concentrations (>10% w/w) were used, tablets became softer and the hardness decreased as a result of poor compressibility. Another interesting observation is that hardness also increased with the applied compaction force for all disintegrants except XL10. In this case, XL10 tablet hardness exhibited a gradual increase from 4 to 10 kN and then was considerably decreased at 20 kN compaction force. However, the XL10 disintegration times constantly increased up to and including 20 kN.
Fig. 5.
Schematic diagram of ODTs hardness at various compaction forces with a 2%, b 5%, c 10% and d 20% superdisintegrants
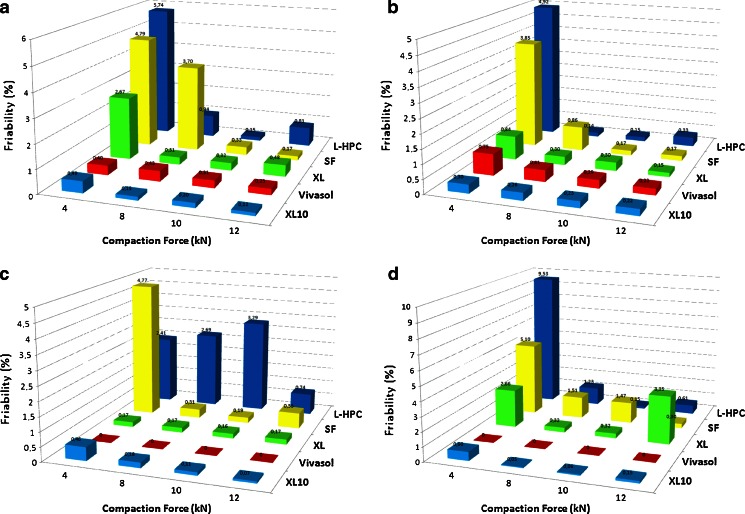
The ODTs friability was also investigated as a function of the superdisintegrant level and the applied compaction force (Fig. 6a-d). The results obtained from these studies indicate dissimilar behaviour for each disintegrant. For instance, XL10 (2–20% w/w) and Vivasol (2–5% w/w) displayed no impact of disintegrant level at different compaction forces. On the other hand, XL showed increased friability at low compaction forces (4 kN) and negligible impact at compaction forces >8 kN. For CL-SF low compression forces (4–8 kN) and high disintegrant levels (20% w/w) had significant negative effect on the tablet friability. In the case of L-HPC, low compaction forces (4 kN) and 10% (w/w) disintegrant level showed also a negative effect on tablet friability.
Fig. 6.
Schematic diagram of ODTs friability at various compaction forces with a 2%, b 5%, c 10% and d 20% superdisintegrants
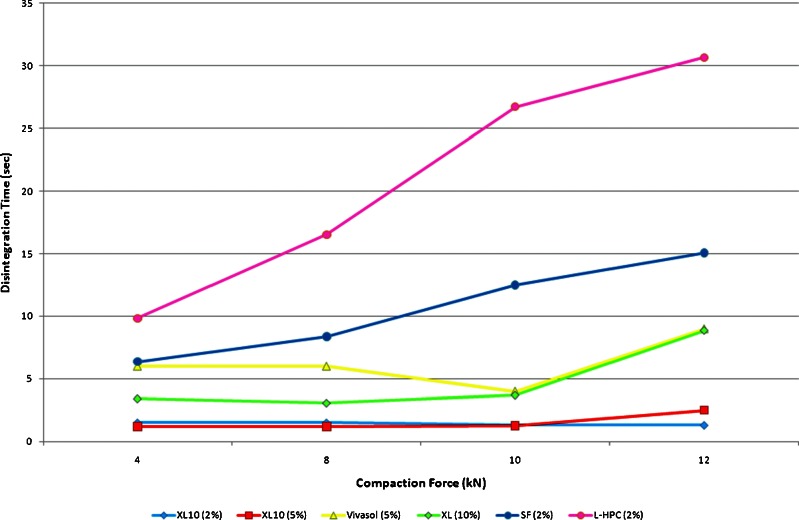
It was possible to identify the optimum superdisintegrant use level, as a function of compaction forces, which provided the faster disintegration time, from the above studies. In Fig. 7, we compared the disintegration time of each superdisintegant at its optimum level. For XL10 we included 2% and 5% levels as there are minor differences in the disintegration times. It is obvious that polyplasdones (XL10 and XL) outperform the other disintegrants followed by sodium croscarmellose (Vivasol). By taking into account the friability results from Fig. 6a–d of these three disintegrants, we could confirm the robustness of the developed ODTs. In most ODT formulations the disintegration times were less than 10 s.
Fig. 7.
Schematic diagram of ODTs disintegration times at various compaction forces with supedisintegrants at their optimal levels
In Vivo Disintegration Time and Taste-Masking Evaluation
The ODTs disintegration times were evaluated using the compendial test, the texture analyzer approach and oral disintegration. The actual average disintegration times in Table III have not revealed significant differences between the three methodologies. However, the texture analyzer times are almost identical to the oral disintegration. The reason is that the texture analyzer method (artificial saliva, 37°C) better simulated the oral disintegration of ODTs providing a convenient means for accurate and reproducible determination of the disintegration times.
Table III.
Comparison of Disintegration Times of ODTs at Various Compaction Forces (n = 3)
| Formulations (kN) | Disintegration time (s) | ||
|---|---|---|---|
| USP apparatus | Texture analyzer | In vivo disintegration | |
| F10 (4) | 4.90 | 4.27 | 4.35 |
| F10 (8) | 6.30 | 6.01 | 6.00 |
| F10 (10) | 8.31 | 7.81 | 7.96 |
| F10 (12) | 11.07 | 11.81 | 11.77 |
| F11 (4) | 3.40 | 3.04 | 3.10 |
| F11 (8) | 3.06 | 3.36 | 3.30 |
| F11 (10) | 3.68 | 4.05 | 3.95 |
| F11 (12) | 8.84 | 8.36 | 8.25 |
| F13 (4) | 6.46 | 6.81 | 6.78 |
| F13 (8) | 8.37 | 8.35 | 8.36 |
| F13 (10) | 12.50 | 11.93 | 11.90 |
| F13 (12) | 15.06 | 15.56 | 15.51 |
The taste-masking of the developed ODTs was of critical importance for this study. The application of a physical barrier between the bitter CTZ HCl granules and the taste buds proved an efficient masking platform. In Table IV the perceived taste intensity studies in human volunteers showed considerable masking of CTZ HCl bitter taste. The degree of bitterness was zero after 10 min for most of the formulations. In addition, the use of XL10, XL and CL-SF at 10% (w/w) gave a smooth mouth feel. When L-HPC and Vivasol were incorporated in the ODT formulations the roughness appeared to be at the threshold value (0.5) after 1 min. These observations were attributed to the superdisintegrants’ nature. The overall taste and sensory tablet evaluation proved excellent palatability.
Table IV.
Taste Evaluation of ODTs on Healthy Human Volunteers (n = 10)
| Formulationsa | Degree of bitterness (DB)/roughness (DR) after time | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 s | 1 min | 2 min | 5 min | 7 min | 10 min | |||||||
| DB | DR | DB | DR | DB | DR | DB | DR | DB | DR | DB | DR | |
| CTZ HCl | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 1 |
| F2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5+ | 0 | 0.5+ | 0 |
| F3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F5 | 0 | 0 | 0 | 0.5+ | 0 | 0.5+ | 0 | 0.5+ | 0.5+ | 0 | 0.5+ | 0 |
| F6 | 0 | 0 | 0 | 0.5+ | 0 | 0.5+ | 0 | 0.5+ | 0.5+ | 0 | 0.5+ | 0 |
| F10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5+ | 0 | 0.5+ | 0 |
| F11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5+ | 0 | 0.5+ | 0 |
| F15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 | 0 |
| F17 | 0 | 0 | 0 | 0.5+ | 0 | 0.5+ | 0 | 0.5+ | 0.5+ | 0 | 0.5+ | 0 |
| F18 | 0 | 0 | 0 | 0.5+ | 0 | 0.5+ | 0 | 0.5+ | 0.5+ | 0 | 0.5+ | 0 |
aSuperdisintegrants’ amounts for the evaluated formulations varied from 2% to 10%
In Vitro Dissolution
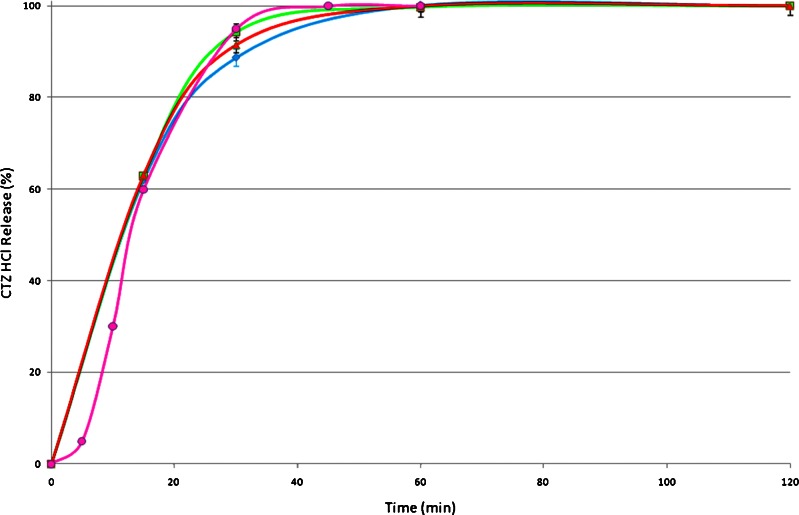
The main challenge of this study was to achieve sufficient CTZ HCl taste-masking and to provide simultaneous rapid release. The Eudragit RL-30D polymer grade is highly water permeable but the increased coating amounts (30%) could delay the release of the active substance. In Fig. 8, it can be seen that this condition was not realized as almost 100% of CTZ HCl was released in the first 30 min. It can also be seen that three different tablet formulations with different compaction forces were studied showing similar release patterns. By applying a Kruskal–Wallis non-parametric test (p > 0.05) no significant difference was observed. The comparison of the commercial IR tablets showed similar release profiles with those of the selected ODTs. In the commercial IR tablets CTZ was also released within the first 30 min.
Fig. 8.
Release profiles of CTZ HCl coated with Eudragit RL30D formulations F10 (12 kN, triangles), F11 (10 kN, squares) and F13 (10 kN, diamonds) from ODTs and commercial IR tablet (circles)
CONCLUSIONS
In this study we demonstrated the complete taste-masking of CTZ HCl coated with a highly water permeable methacrylate polymer and the rapid disintegration of the developed ODT tablets. For the purpose of the study, various superdisintegrants were evaluated in terms of disintegration time, hardness, friability and optimum levels. The in vivo taste-masking evaluation showed that fluidized bed coating can be efficiently used to mask the taste of bitter active substances without compromising tablet palatability. Finally, the release patterns of the produced ODTs were similar to the commercial IR CTZ tablets.
REFERENCES
- 1.Habibh W, Khankarik R, Hontz J. Fast-dissolve drug delivery system. Crit Rev Ther Drug Carrier Syst. 2000;17:61–72. [PubMed] [Google Scholar]
- 2.Douroumis D. Practical approaches of taste masking technologies in oral solid forms. Expert Opin Drug Deliv. 2007;4:417–426. doi: 10.1517/17425247.4.4.417. [DOI] [PubMed] [Google Scholar]
- 3.Mahesh A, Shastri N, Sadanandam M. Development of taste masked fast disintegrating films of levocetirizine dihydrochloride for oral use. Curr Drug Deliv. 2010;7:21–27. doi: 10.2174/156720110790396454. [DOI] [PubMed] [Google Scholar]
- 4.Dinge A, Nagarsenker M. Formulation and evaluation of fast dissolving films for delivery of triclosan to the oral cavity. AAPS PharmSciTech. 2008;9:349–356. doi: 10.1208/s12249-008-9047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kearney P, Wong SK. Method for making freeze dried drug dosage forms. US Patent. 1997;5(631):023. [Google Scholar]
- 6.Al-Omran MF, Al-Suwayeh SA, El-Helw AM, Saleh SI. Taste masking of diclofenac sodium using microencapsulation. J Microencapsul. 2002;19:45–52. doi: 10.1080/02652040110055612. [DOI] [PubMed] [Google Scholar]
- 7.Shah PP, Mashru RC, Rane YM, Thakkar A. Design and optimization of mefloquine hydrochloride microparticles for bitter taste masking. AAPS PharmSciTech. 2008;9:377–389. doi: 10.1208/s12249-008-9052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behzadi SS, Toegel S, Viernstein H. Innovations in coating technology. Recent Pat Drug Deliv Formul. 2008;2:209–230. doi: 10.2174/187221108786241633. [DOI] [PubMed] [Google Scholar]
- 9.Hamashita T, Matsuzaki M, Ono T, Ono M, Tsunenari Y, Aketo T, Watano S. Granulation of core particles suitable for film coating by agitation fluidized bed II. A proposal of a rapid dissolution test for evaluation of bitter taste of ibuprofen. Chem Pharm Bull (Tokyo) 2008;56:883–887. doi: 10.1248/cpb.56.883. [DOI] [PubMed] [Google Scholar]
- 10.Albertini B, Cavallari C, Passerini N, Voinovich D, González-Rodríguez ML, Magarotto L, Rodriguez L. Characterization and taste-masking evaluation of acetaminophen granules: comparison between different preparation methods in a high-shear mixer. Eur J Pharm Sci. 2004;21:295–303. doi: 10.1016/j.ejps.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Benoit JP, Rolland H, Thies C, Vande Velde V. Method of coating particles and coated spherical particles. US Patent. 2000;6(087):003. [Google Scholar]
- 12.Mazen H, York P. Particle formation methods and their products. US Patent. 2006;7(115):280. [Google Scholar]
- 13.He W, Fan LF, Du Q, Xiang B, Li CL, Bai M, Chang YZ, Cao DY. Design and in vitro/in vivo evaluation of multi-layer film coated pellets for omeprazole. Chem Pharm Bull. 2009;57:122–128. doi: 10.1248/cpb.57.122. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Liu S, Dai Q. Design and evaluation of pH-independent pulsatile release pellets containing isosorbide-5-mononitrate. Chem Pharm Bull. 2009;57:55–60. doi: 10.1248/cpb.57.55. [DOI] [PubMed] [Google Scholar]
- 15.Ji C, Xu H, Wu W. In vitro evaluation and pharmacokinetics in dogs of guar gum and Eudragit FS30D-coated colon-targeted pellets of indomethacin. J Drug Target. 2007;15:123–131. doi: 10.1080/10611860601143727. [DOI] [PubMed] [Google Scholar]
- 16.Sugao H, Yamazaki S, Shiozawa H, Yano K. Taste masking of bitter drug powder without loss of bioavailability by heat treatment of wax-coated microparticles. J Pharm Sci. 1998;87:96–100. doi: 10.1021/js970104g. [DOI] [PubMed] [Google Scholar]
- 17.Curran MP, Scott LJ, Perry CM. Cetirizine: a review of its use in allergic disorders. Drugs. 2004;64:523–561. doi: 10.2165/00003495-200464050-00008. [DOI] [PubMed] [Google Scholar]
- 18.Corbo M, Desai J, Patell M, Warrick R. Taste masking coating composition. US. 2002;6(663):893. [Google Scholar]
- 19.Harmon T. Beyond the first greneration of orally disintegrationg tablets. www.eurand.com/pdf/EURX_Article_Sep_2006.pdf (2006).
- 20.Abdelbary G, Eouani C, Prinderre P, Joachim J, Reynier JP, Piccerelle P. Determination of the in vitro disintegration profile of rapidly disintegrating tablets and correlation with oral disintegration. Int J Pharm. 2005;292:29–41. doi: 10.1016/j.ijpharm.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Khan S, Kataria P, Nakhat P, Pramod Yeole P. Taste masking of ondansetron hydrochloride by polymer carrier system and formulation of rapid-disintegrating tablets. AAPS PharmSciTech. 2007;8:E1–7. doi: 10.1208/pt0802046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goel H, Vora N, Rana V. A novel approach to optimize and formulate fast disintegrating tablets for nausea and vomiting. AAPS PharmSciTech. 2008;9:774–781. doi: 10.1208/s12249-008-9113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peppas NA, Buri PA. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Control Rel. 1985;2:257–275. doi: 10.1016/0168-3659(85)90050-1. [DOI] [Google Scholar]
- 24.Fell JT, Newton JM. Determination of tablet strength by diametral—compression test. J Pharm Sci. 1970;59:688–691. doi: 10.1002/jps.2600590523. [DOI] [PubMed] [Google Scholar]
- 25.Tye CK, Sun CC, Amidon GE. Evaluation of the effects of tableting speed on the relationships between compaction pressure, tablet tensile strength, and tablet solid fraction. J Pharm Sci. 2005;94:465–467. doi: 10.1002/jps.20262. [DOI] [PubMed] [Google Scholar]
- 26.Parrott EL. In: “Pharmaceutical dosage forms: tablets vol. 2,”. 2. Lieberman HA, Lachman L, Schwartz JB, editors. New York: Marcel Dekker; 1990. pp. 201–243. [Google Scholar]
- 27.Bi YX, Sunada H, Yonezawa Y, Danjo K. Evaluation of rapidly disintegrating tablets prepared by a direct compression method. Drug Dev Ind Pharm. 1999;25:571–581. doi: 10.1081/DDC-100102211. [DOI] [PubMed] [Google Scholar]