Abstract
The aim was to design a pH-sensitive pulsatile drug delivery system that allows for an on–off pulsed release of a drug using polyacrylic acid (PAA) blended with ethyl cellulose (EC) in different ratios. PAA, a polyelectrolyte polymer, exhibits a highly coiled conformation at low pH but a highly extended structure at high pH. Fumaric acid, which is an internal acidifying agent, was incorporated into the hydroxypropyl methylcellulose-based core tablets to create an acidic microenvironmental pH (pHM). The concentration of fumaric acid inside the core tablet and the ratio of PAA/EC in the coating layer were very crucial in modulating drug release behaviors. When the fumaric acid was retained in the core tablet, it gave a more acidic pHM, so that the PAA was kept in a highly coiled state in the coated film, which hindered drug release (“off” release pattern). Interestingly, the release profiles of the drug and fumaric acid from coated tablets showed the on–off pulsed pattern upon dissolution. Imaging analyses using scanning electron microscopy, near-infrared imaging, confocal laser scanning microscopy, and Fourier transform infrared spectroscopy confirmed this on–off release behavior of the drug and fumaric acid from coated tablets.
KEY WORDS: fumaric acid, microenvironmental pH, on–off drug release, pH-sensitive pulsatile drug delivery system, polyacrylic acid
INTRODUCTION
Pulsatile drug delivery systems have gained much interest lately due to their high applicability in delivering drugs to specific sites and at specific times, thus providing targeted drug therapies and increased patient compliance (1–3). The drug release of these systems is a complete and rapid release that is achieved in a pulsed manner after a predetermined lag time. These systems are very beneficial for drugs that are affected by circadian rhythms or drugs where nighttime dosing is required for treatment (such as treatments for asthma). In recent decades, chronotherapy, which is another term for controlled drug delivery systems, has been investigated to allow the pulsed release of the drug and the synchronization of drug release with circadian rhythms of the body to achieve maximum health benefits and minimum harmful effects (4). For example, moderate to severe asthma, particularly where severe nocturnal symptoms can occur during the night, may not be adequately controlled even with high doses (over 1,000 μg per day) of inhaled steroids (5). In addition, conventional β-agonist aerosol medications possess a rather short duration of action. Thus, an advanced drug delivery system that synchronizes asthma treatment with circadian biochemical changes and has a 24-h duration of activity would be effective.
Bambuterol (BAM) was selected as a model drug. BAM belongs to a group of long-acting β2-agonists and enables asthma treatment by once-daily dosing via the oral route. BAM is a prodrug that produces terbutaline as its active metabolite in a slow and controlled manner which exhibits a high affinity for lung tissue and a high pre-systemic metabolic stability (6–8). Because asthma worsens or asthma attacks frequently occur during nighttime in most patients, evening dosing using a pulsed release of BAM can be effective for nocturnal asthma treatment. Currently, numerous immediate released BAM tablets are commercially available. So far, there has been no pulsed delivery system that synchronizes the circadian release of BAM in a controlled manner after a lag time.
Polymeric coating techniques have been widely used in pharmaceuticals for many purposes including most applications for controlled release of drugs in the preparation of various dosage forms (9,10). EC is one of the well-known polymeric film-coating materials frequently used to control drug release from solid dosage forms (11–13). Importantly, EC is a water-insoluble and pH-independent polymer throughout the gastrointestinal tract and is generally recognized as a polymer that is nontoxic, nonallergenic, nonirritant, and safe (14). Because of the low permeability and slow drug release of EC films, a hydrophilic polymer such as hydroxypropyl methylcellulose (HPMC) is commonly added to the EC films. Unfortunately, these two polymers may be incompatible and produce inhomogeneous films. The HPMC levels commonly used in EC-based controlled-release coatings often fall into the flocculation region (15–17).
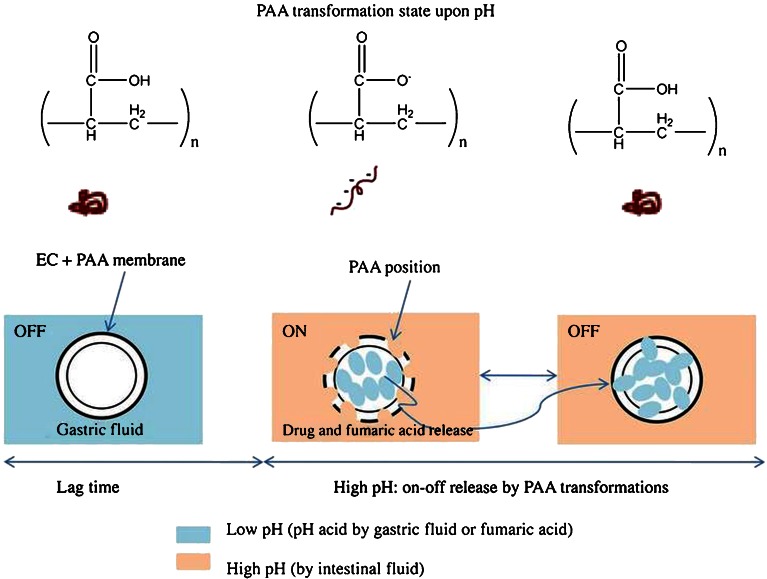
In this study, we designed a novel pH-sensitive oral delivery system using nonenteric polymeric coatings of a core drug-loaded tablet; these coatings consisted of a binary blend of ethyl cellulose (EC) and poly(acrylic acid) (PAA) and provided “on–off” pulsed release of BAM. PAA is a pH-responsive weak polyelectrolyte polymer that exhibits a highly coiled conformation at low pH but a highly extended state at high pH (18,19). In other words, PAA is uncharged at low pH but becomes highly anionic as the pH is increased. Furthermore, the PAA was combined with EC to efficiently control the release of BAM from the coated film. This binary blend of coated film was expected to render the drug sensitive to pH conditions for the on–off release system, i.e., a system with an “off” or “on” release when the PAA was tightly coiled or highly extended, respectively, upon the change of the pH environment. Furthermore, an internal acidic agent such as fumaric acid can be added to core tablets to create an acidic microenvironmental pH (pHM), which controls the on–off pulsed release of the drug even at the higher pH of the gastrointestinal tract. Although the pulsed release can be commonly obtained by enteric coating or other methods, we originally designed the on/off release of drug using pH-sensitive PAA with an aid of fumaric acid as a pH modifier in the dosage form. Moreover, it should be noticed that neither PAA nor EC is an enteric coating material. Scheme 1 illustrates a novel pH-sensitive delivery system for the on–off pulsed release of the drug upon pH changes, ensuring effective overnight drug release for the treatment of asthmatic disease.
Scheme 1.
Structural illustration of pH-sensitive matrix tablets coated with ethyl cellulose (EC) and poly(acrylic acid) (PAA) at different pH conditions
In addition to designing such a drug delivery system, we characterized the release rate of the drug in the gastric fluid (pH 1.2) and the intestinal fluid (pH 6.8). The release rate of fumaric acid was also evaluated to link microenvironmental pH changes of coated films with the control of the on–off release of BAM. The surface morphology of the coated films at different intervals of the dissolution test was characterized by scanning electron microscopy (SEM). Near-infrared imaging (NIR imaging) and confocal laser scanning microscopy (CLSM) were also utilized to investigate the on–off pulsed behaviors of the tablets’ coated films upon dissolution. The molecular interaction of the coated films was also investigated using Fourier transform infrared (FTIR) spectroscopy.
MATERIALS AND METHODS
Materials
BAM powder was received from Korea United Pharm. Inc. (Seoul, Korea). EC (45 cps), PAA, triacetin and fumaric acid were obtained from Sigma (St. Louis, MO, USA). HPMC (4,000 cps) was supplied by Richwood Inc. (Seoul, Korea). Lactose was obtained from Meggle (Wasserburg, Germany). Magnesium stearate (Mg stearate) was purchased from Katayama Chemical Co. (Osaka, Japan). The solvents used were high-performance liquid chromatography (HPLC) grade. All other chemicals were of analytical grade and used without further purification.
Solubility Study
The solubility of BAM was determined in simulated gastric fluid (pH 1.2), simulated intestinal fluid (pH 6.8), and distilled water by adding excess amounts of BAM to snap-cap Eppendorf tubes (Hamburg, Federal Republic of Germany) containing 1 mL of media, following the methods previously reported elsewhere (20,21). The resulting mixtures were sufficiently vortexed and then placed in a constant temperature in an incubator at 37°C for 2 days. Aliquots were centrifuged at 15,000 rpm for 10 min. The supernatant layer was carefully taken out and then diluted with a solution with components as the mobile phase in the HPLC analysis based on the preliminary solubility test. The concentration of BAM was then quantified by HPLC from a standard calibration curve.
Preparation of Core Tablets
Core HPMC tablets (160 mg) were prepared by the direct compressing method. All of drugs and excipients were dried under vacuum at room temperature before use. BAM, HPMC, fumaric acid, and lactose were thoroughly mixed for 10 min. The uniform mixture was then mixed with Mg stearate (1% w/w) for 3 min. Finally, the resultant powders were passed through a 40-mesh sieve (425 μm). Thereafter, the homogeneous mixtures were directly compressed into tablets using a rotary tablet machine (Korea Machine, Anyang, Korea). The diameter and hardness of core HPMC tablets were 5 mm and 50.0 ± 5 N, respectively. The theoretical drug content per core tablet was 20 mg. The detailed formulation compositions of core tablets are shown in Table I.
Table I.
Formulation Compositions of Core Tablets (Unit, Milligram; Tablet Weight, 160 mg)
| Codes | F1 | F2 | F3 |
|---|---|---|---|
| BAM | 20 | 20 | 20 |
| HPMC 4,000 | 60 | 40 | 20 |
| Fumaric acid | 8 | 8 | 8 |
| Lactose | 70.4 | 90.4 | 110.4 |
| Mg stearate | 1.6 | 1.6 | 1.6 |
HPMC hydroxypropyl methylcellulose, Mg magnesium
Preparation of Coating Solutions
PAA and triacetin were initially dissolved in ethanol; EC was added to this mixture; and the solution was stirred completely until homogeneous mixture was obtained. Triacetin was selected as a plasticizer at 5% or 10% (w/w compared with the total weight of polymers in the formulations). The coating solutions were stored overnight at room temperature until use. For the evaluation of tablet-coated films using CLSM, fluorescein (1% w/w compared with the polymer weight) was added into the coating solution. The detailed formulation compositions of coating solutions are shown in Table II.
Table II.
Formulation Compositions in 1,000 mL of Ethanol for the Preparation of Coating Solutions (Grams)
| Codes | C1 | C2 | C3 | C4 |
|---|---|---|---|---|
| EC | 12.62 | 11.36 | 10.33 | 10.33 |
| PAA | 6.31 | 7.57 | 8.61 | 8.61 |
| Triacetina | 1.89 (10% w/w) | 1.89 (10% w/w) | 1.89 (10% w/w) | 0.95 (5% w/w) |
| Ratio of EC/PAA | 3: 1.5 | 3:2 | 3:2.5 | 3:2.5 |
EC ethyl cellulose, PAA polyacrylic acid
aThe parenthesis indicates the percentage based on the total weight of EC and PAA
Tablet Coating Process
The coating process was carried out using a pan coater (Sejong, Korea) under the following conditions with the pan charge: inlet temperature (60°C), outlet temperature (37°C), tablet bed temperature (40°C), spray rate (6 mL/min), air pressure (2 kgf/cm2), and pan speed (10 rpm). The tablets were sampled at regular intervals and weighed to determine the coating levels based on the weight gains of tablets. When the required coating weight gain was achieved, spraying of the solution was stopped, and the coated tablets were then dried in the coating drum for another 10 min before the samples were taken out. Based on primarily screening of weight gains of tablet coating in the range from 3% to 7%, tablets with coating levels at 4% (w/w) weight gains were optimally obtained and stored in plastic bags at room temperature until use.
In Vitro Dissolution of Drug and Fumaric Acid
Dissolution studies of coated tablet were conducted using the USP I apparatus (100 rpm, 37°C, and a 900-ml dissolution medium) with a dissolution tester (DCM-D12, DCM Korea, Siheung, Korea). The immediate release BAM tablet (Bambec™ from AstraZeneca Korea) commercially available was also tested for comparison. Tablets were exposed to the simulated gastric fluid (pH 1.2) for the first 2 h and then switched to simulated intestinal fluid (pH 6.8). Samples were withdrawn at predetermined intervals and replaced with an equivalent amount of fresh medium to maintain a constant dissolution volume. The samples were filtered through a 0.45-μm membrane filter. The simulated gastric fluid and intestinal fluid were prepared according to the method reported previously (22). The concentrations of drug and fumaric acid were determined by HPLC as described below.
HPLC Analysis for the Determination of Drug and Acid Fumaric Concentrations
Based on the British Pharmacopoeia 2001 (23) and a research by Tran et al. (24), BAM and fumaric acid concentrations were analyzed by the HPLC system (Water, USA) consisting of the pump (Waters™ 600 Controller), the UV–VIS spectrophotometer detector at the wavelength of 214 nm (Waters™ Tunable Absorbance Detector), the autosampler (Waters™ 717 plus Autosampler), the degasser (Waters™ In-line Degasser) with the reverse phase column (Phenomenex® Luna C18, 150 × 4.6 mm, 5 μm diameter, 100 A), and the Borwin 1.20 software. The mobile phase consisted of the mixture of acetonitrile, methanol and 0.05 M potassium phosphate buffer adjusted to pH 3 by acid phosphoric at the ratio of 10/30/60 (% v/v). The flow rate was 1.3 mL/min, and the injection volume was 20 μL. The retention time was 5 and 3 min for BAM and fumaric acid, respectively. The entire solution was filtered using a 0.45-μM membrane filter (Millipore™, Millipore Corporation, Bedford) and degassed before running the HPLC analysis. Each result was obtained by triplicate measurement.
NIR Imaging of Coated Films
After some predetermined intervals (145, 155, 180, 230, and 235 min) of the dissolution test, tablets were removed from the dissolution vessels. After removing the excessive water from the samples using tissue paper, the tablets were dried at room temperature in the dark overnight. The coated films of the dried samples were then observed using a MatrixNIR™ chemical imaging microscope and Isys™ 3.1.1 chemical imaging software (Spectral Dimensions) to analyze the spatial distribution of PAA in the coatings.
CLSM of Coated Films
To visualize the transformation of the coated films upon on–off drug release, fluorescein was used as a marker and incorporated into the coating solution as mentioned above, and then CLSM (FLUOVIEW-FV300, Olympus, Japan) was utilized for taking images. Coated tablets containing fluorescein were exposed to the dissolution test in the dark and then collected from the dissolution vessel at different times corresponding to predetermined intervals given in NIR imaging. The excessive water of the samples was gently removed using tissue paper. The wetted tablet was then dried and placed onto the glass slide to observe the CLSM images of coated films at 488 nm using an argon ion laser.
Surface Morphology of Coated Films
Surface views of the tablet coatings before and during the dissolution test at different times corresponding to predetermined intervals given in NIR imaging were observed with a SEM. The dried tablets were coated with gold under an argon atmosphere using a Jeol JFC-1,100 sputter coater (Jeol, Japan) for about 2 min. Micrographs were taken with a Cambridge Stereo Scan 200 (London, UK) at an accelerating voltage of 15 kV.
FTIR Spectroscopy
A FTIR spectrophotometer (Excalibur Series UMA-500, Bio-Rad, USA) was used to investigate the molecular interaction of the coated films at different times corresponding to predetermined intervals taken in NIR imaging. The wavelength was scanned from 500 to 4,000 cm−1 with a resolution of 2 cm−1. KBr pellets were prepared by gently mixing 1 mg of the sample with 200 mg of KBr.
RESULTS AND DISCUSSION
Release Profiles of Drug and Fumaric Acid
The solubility of BAM was studied to determine its pH dependence, if any. The solubility of BAM in distilled water, simulated gastric fluid (pH 1.2), and simulated intestinal fluid (pH 6.8) were as follows: 183.59 ± 3.79 mg/mL, 173.35 ± 0.85 mg/mL, and 170.79 ± 0.47 mg/mL, respectively. This result indicates that BAM is a highly soluble drug, and this solubility is pH independent. Various cellulose derivatives such as HPMC and EC are commonly used in controlled-release drug delivery systems such as matrix tablets or coated tablets (25–27). HPMC is the most commonly used hydrophilic polymer among cellulose derivatives for oral sustained-release tablets (28,29). However, the design of core matrix tablets based on HPMC alone did not show a pulsatile drug release with a lag time of drug release rate, but, rather, it showed zero-order release (30–32). On the other hand, EC is the rate-controlling polymer well-known in the controlled-release dosage forms, but unluckily, the use of EC alone in a coating formulation usually leads to a very slow drug release (14) which is not suitable to deliver drugs in a pulsed manner for patients with nocturnal asthma.
In this study, we designed a new pH-sensitive polymeric system by coating of the core tablet using a binary blend of EC and PAA for on–off pulsed drug release to modulate an efficient retardation after a lag time. Furthermore, fumaric acid (acidic agent) added to the formulation of core tablets can readily create an acidic microenvironment to the coated films because of the intrinsic structural transformation of PAA from a highly coiled state to an extended form depending on the pH conditions. An acidic agent is widely used in the formulations to enhance poor water solubility of a weakly basic and pH-dependent drug (33,34). However, this method is useless because BAM is not a weakly basic drug with poor water solubility. Instead, fumaric acid is used to modulate the microenvironment of coated films and can temporarily turn off the drug release by the PAA transformation under low pH conditions. Among acidic agents, fumaric acid has a high acidic strength with pKa1 3.03 and pKa2 4.54 (25°C) and relatively low solubility in lower pH ranges. Thus, fumaric acid can provide a sufficiently low pH in the matrix tablet for a long period of time even at low proportion. Such an effect of fumaric acid for a well-maintained pH has been shown in many reports (24,35–38). Moreover, HPMC as a hydrophilic polymer has been shown by many previous studies to produce a sufficient matrix system containing acidic agents, especially for fumaric acid, for the controlled release of drugs (39–43). Two reasons to choose HPMC as a polymer of the core matrix tablet are as follows: a) its capability of maintaining fumaric acid inside the dosage for as long as possible, and b) the nonionic property that does not induce the pH effect on solubility and swelling behavior (40). For all of these reasons, the HPMC core matrix tablets containing fumaric acid coated with the coating solution of EC and PAA were introduced in the study. In addition, the coating levels of tablet were screened in the range from 3% to 7% and the coating level at 4% weight gain was optimally obtained with the desired pulsatile drug release profiles for the lag time as well as the complete drug release after the end of the dissolution test. On the other hand, drug release profiles from the tablets with a coating level at 3% weight gain could not acquire the lag time, whereas drug release from the tablets with coating level above 4% weight gain were too slow and were not completed after 8 h.
Figure 1 shows the photo images of the coated product, including intact coated tablet and cut coated tablet which clearly exposes the coating layer clearly separated from the inner core. Figure 2 shows the dissolution profiles of drugs from core tablets in simulated intestinal fluid (pH 6.8) for 6 h (Fig. 2a) as well as the coated tablets tested in simulated gastric fluid (pH 1.2) for 2 h and continued in simulated intestinal fluid (pH 6.8) for 6 h (Fig. 2b). The commercial product was also compared. Drug release rates from the core HPMC matrix tablets were retarded and completed in a near-zero-order manner irrespective of the ratio between drug and HPMC, whereas the drug release rates from the tablets coated with PAA and EC showed a lag time followed by a pulsed drug release. Conversely, the drug release from the commercial tablet was completed within 10 min at pH 1.2, indicating the immediate release of drug from this product. The core F2 tablet was chosen for further coating process due to its appropriate drug release compared with F1 and F3 core tablet. The extent of drug release rate from coated tablets after the lag time depended on the coating levels, showing an increased order of drug release after 8 h as follows: C1 (10.54%), C2 (30.07%), C3 (78.63%), and C4 (100%). As the amount of PAA relative to EC in the coating formulation with the same amount of plasticizer increased, the drug release rates highly increased (C1, C2, and C3). Obviously, the increased amount of PAA simultaneously reduced the ratio of EC in the coating formulation by decreasing the hydrophobic nature of this polymer, a key factor that obstructs tablets from imbibing water (44,45). Furthermore, the higher amount of plasticizer in the formulation at the same EC/PAA ratio contributed to the slower drug release (C3 compared with C4).
Fig. 1.
Photo images of the intact and cross section of the coated tablet
Fig. 2.
Release profiles of the drug from core tablets in simulated intestinal fluid (pH 6.8) for 6 h (a) and from coated tablets with various coating formulations in simulated gastric fluid (pH 1.2) for the first 2 h and in simulated intestinal fluid (pH 6.8) for 6 h (b)
An increased PAA amount could induce a higher grade of its structural transformation from the coiled to the extended forms upon the change of pH environment from low pH to intestinal fluid (pH 6.8), and hence, leading to a greatly increased drug release. In contrast, an increase of the plasticizer amount facilitated a higher penetration of the pH 1.2 fluid into the coated tablets due to the hydrophilic property of triacetin, leading to the maintenance of the highly coiled state of PAA for a longer period of time. Thus, the delayed time for an extended transformation of PAA in the coated films could decrease the drug release when switched to intestinal fluid. Based on the release profiles of the drug, the C4 formulation showing a 135-min lag time followed by a complete drug release could deliver the drug in a pulsatile manner and was chosen for the investigation of the pharmaceutical mechanism of coated films using instrumental analyses.
The in-depth impact of the PAA on the on–off release patterns of the drug and the fumaric acid from the coated tablet (C4) was further characterized from 120 to 240 min in the intestinal fluid, and the on–off release patterns during the dissolution periods could be clearly observed. Aliquots were withdrawn from the dissolution vessel every 5 min in this period. The equation to determine whether the sample taken out from the dissolution medium at the time corresponding to the “on” or “off” pattern was as follows:
 |
in which C1 and C2 are the cumulative percentage of drug released at a specific time point, t1 and t2 (t2 = t1 + 5 min), respectively. D (%) is the difference in the cumulative drug release between the two consecutive sampling times. The times when D (%) was at least 17% implied that the drug release was “on”. The “on” pattern of drug release was observed at time points such as 135, 145, 150, 160, 165, 180, 190, 200, 210, 220, 225, and 235 min; the “off” drug release occurred during the remaining time intervals.
The release profile of the fumaric acid was also well matched with the on–off drug release mechanism during the dissolution test. The release rate of fumaric acid was higher at some intervals but decreased a little or was almost unchanged and then greatly increased, which corresponded to the on–off drug release behaviors as described above. As the release of fumaric acid occurred, the release of the drug was induced and vice versa. In other words, fumaric acid was not released in the gastric media due to the highly coiled structure of PAA so that the release of the drug did not occur. When the intestinal fluid was penetrated into the coated tablet, the drug release started occurring in advance through the extended PAA structure and then the release of fumaric acid occurred due to the difference in solubility. This is why the release profile of fumaric acid was lower than that of the drug (Fig. 3). However, the release of the drug was stopped as the fumaric acid diffused to the coated films and induced the PAA to transform into a coiled structure. This is the reason that the on–off drug release is matched with the on–off release of the fumaric acid. Hence, fumaric acid was not detected in the gastric fluid and its appearance was further delayed for about 15 min in the intestinal fluid, producing a lag time of drug release.
Fig. 3.
Release profiles of the drug and fumaric acid from coated tablets (C4), showing the “on/off” pulsed release pattern
The amount of fumaric acid determined was low or constant at some interval times as there was no release of the acidic agent from the coated tablets. The fumaric acid remaining in the coated films could readily facilitate greater acidic pHM of the dosage form, which transformed the PAA into a highly coiled state that hindered drug release and hence, the release pattern was “off”. On the other hand, the higher amount of fumaric acid released together with the release of the drug in the intestinal media implied that the PAA on the coated films was transformed into a highly extended structure. The imbibing of the intestinal fluid could result in increased pHM of the dosage form to restart the release of the drug and the fumaric acid, which corresponded to the “on” pattern. As a result, the on–off release of the fumaric acid could modulate the structural transformation of PAA for the on–off drug release patterns because of the alteration of pHM in the coated films as the dosage form was placed in the intestinal medium. The pHM modulation between core (pH = 3 by fumaric acid) and outer intestinal media (pH = 6.8) could induce the structural transformation of PAA in a coated film for the pulsed on–off release. A complete drug release after a lag time ensured that this pH-sensitive delivery system was suitable for the asthmatic patient during night sleep against nocturnal asthma. The detailed structural transformations of PAA and the molecular interactions investigated using instrumental analyses were described below.
Mechanistic Investigation of Coated Films upon the On–Off Pulsed Drug Release Using Instrumental Analyses
Figure 4 shows the surface morphology of the coated films of the tablets at the different release times using SEM. The surface morphology of the coated films removed from the dissolution test at different interval times was compared with the intact coated tablet before introduction into the dissolution media. The coated tablets were taken out from the vessels at different release times, 145, 155, 180, 230, and 235 min, based on the dissolution results of the drug and fumaric acid. The surface morphology of the coated films before the test was homogenous and smooth. However, the coated films of the tablet taken out at 145, 180, and 235 min showed the numerous microcrystals on the surface whereas much fewer crystals appeared on the surface of the samples taken out at 155 and 230 min. This indicated that the appearance or disappearance of the microcrystals on the surface of the coated tablets resulted from the on–off release behaviors of the drug and fumaric acid on the coated films because of the changes in the pHM.
Fig. 4.
Surface morphology of coated films of the tablets (C4) at different release times using SEM
To observe the imaging analyses of the coated films that followed the on–off release behaviors of the drug and acidic agent, CLSM images and spatial PAA distributions of the coated films of the tablets using NIR spectroscopy at different release times are also visualized in Figs. 5 and 6, respectively. Both CLSM and NIR images were clearly matched with the on–off release behaviors of the drug and fumaric acid as mentioned previously (also see Fig. 3). For CLSM, fluorescein was selected as a marker because of its pH-sensitive fluorescence property (pKa ∼ 6.4), which is gradually reduced below pH 6 (46). This property of fluorescein allows for the observation of the structural transformation of the coated films, indicating the on–off pulsed release mechanism.
Fig. 5.
CLSM images of coated films of the tablets (C4) at different release times
Fig. 6.
Spatial distributions of PAA in coated films of the tablets (C4) using NIR spectroscopy at different release times
The CLSM images matched the dissolution behaviors of the drug and fumaric acid well; the fluorescence intensity of the samples at 155 and 230 min was reduced on some positions of the coated films, corresponding to the “off” release pattern. The very acidic pHM value created inside the coated films could transform the PAA to a highly coiled state to hinder drug release. In contrast, the fluorescence intensity of the samples at 145, 180, and 235 min was homogenous without any reduced fluorescence positions due to the higher pH of the intestinal fluid changing the coiled PAA to an extended state for “on” release pattern.
Similarly, the NIR imaging also displayed results that matched the on–off pulsed release of the drug. The NIR chemical imaging is practical and preferentially used nowadays based on the unique spectroscopic signature of a component, providing a visual distribution of the component within a sample (47,48). The structural transformation behaviors of the PAA from the coiled to an extended state were clearly visualized through NIR imaging. Figure 6 reveals the correlations between the PAA structures and the drug release kinetics. The higher NIR absorbance of the PAA was fixed all throughout the samples; the color red in the NIR images represents the higher concentration of the PAA in coated films. The intensity of the color red of the samples decreased at 145, 180, and 235 min but increased at 155 and 230 min, clearly indicating the transformation of PAA into a highly coiled or a highly extended state for the on–off pulsed release of the drug with the aid of the fumaric acid release.
FTIR spectroscopy often supplies information about the molecular interactions among functional groups, reflecting the structural behaviors of the current samples (49). In this study, the FTIR spectra of the coated films were used to figure out the structural transformation of PAA upon pH changes at different release times (Fig. 7). Samples used in this analysis included film coatings of tablets taken at 145, 155, 180, and 230 min compared to pure PAA and EC as references. Two distinct areas of the adsorption bands of the carboxylic acid functional groups of PAA were considered: one was the asymmetric stretching band of COO− in the range of 1,565–1,542 cm−1 and the other was the C = O stretching of the COOH in the range of 1,710–1,700 cm−1. Specifically, the display of the COO− band and C = O stretching at about 1,560 cm−1 and 1,710 cm−1, respectively, were analyzed. When the PAA was exposed at an acidic pH, the COOH acid peak appeared with high intensity but the COO− peak was of low intensity. In contrast, when the PAA was exposed at a higher pH towards a basic pH, the peak intensity of the COOH band decreased but the peak intensity of the COO- band increased as the acidic COOH groups become ionized (50). Based on this, there was evidence that the on–off pulsed drug release via the coated films occurred. The FTIR spectra in this study showed that the intensity of the COOH peak in coated films during the dissolution test was higher in the cases of samples at 155 and 230 min, corresponding to the lower intensity of COO− band of these samples. On the other hand, the COOH peak intensity was lower and the COO− band intensity was much higher for the samples at 145 and 180 min. Therefore, these results revealed that the coated films containing PAA were in a contact with an acidic pHM at 155 and 230 min, and that the PAA was transformed into a highly coiled states for the “off” drug release mechanism. Conversely, the “on” pulsed release mechanism was indicated through the FTIR spectra of samples taken at 145 and 180 min when the PAA encountered a basic pHM and adopted its extended form.
Fig. 7.
FTIR spectra of coated films of the tablets at different release times
CONCLUSIONS
Taking advantage of the structural transformation of PAA induced by pH changes allowed for the development of a pH-sensitive pulsatile drug delivery system of BAM to treat nocturnal asthma in a circadian rhythm. The incorporation of PAA into the EC-based film coating revealed complete drug release following on–off release behaviors after a lag time. The on–off pulsed drug release behaviors corresponded to those of fumaric acid. While the fumaric acid was retained in coated films to modulate the high acid pHM, drug release was hindered because of the structural transformation of PAA into a highly coiled state. These results were matched with SEM, CLSM, and NIR images. Furthermore, the FTIR analysis also indicated the changes of PAA characteristic peaks because of the structural transformation in the PAA leading to the on–off drug release. The current pH-sensitive pulsatile system is innovative and can be used to deliver drugs such as BAM, in coordination with circadian rhythms after a predetermined lag time, to treat asthmatic patients during nighttime sleep.
ACKNOWLEDGMENTS
This work was supported by the 2009 Small Business Administration, BK21 program and a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea. We would like to thank the Central Research Laboratory (Kangwon National University) for the use of the FTIR and SEM and the Research Institute of Pharmaceutical Sciences, Kangwon National University, for the use of their HPLC systems. Finally, we acknowledge KBSI (Chuncheon, Korea) for the CLSM and KAIST (Daejeon, Korea) for the NIR imaging.
REFERENCES
- 1.Stubbe BG, Smedt SCD, Demeester J. “Programmed polymeric devices” for pulsed drug delivery. Pharm Res. 2004;21:1732–1740. doi: 10.1023/B:PHAM.0000045223.45400.01. [DOI] [PubMed] [Google Scholar]
- 2.Arora S, Ali J, Ahuja A, Baboota S, Qureshi J. Pulsatile drug delivery systems: an approach for controlled drug delivery. Indian J Pharm Sci. 2006;68:295–300. doi: 10.4103/0250-474X.26655. [DOI] [Google Scholar]
- 3.Anal AK. Time-controlled pulsatile delivery systems for bioactive compounds. Recent Pat Drug Deliv Formul. 2007;1:73–79. doi: 10.2174/187221107779814096. [DOI] [PubMed] [Google Scholar]
- 4.Roy P, Shahiwala A. Multiparticulate formulation approach to pulsatile drug delivery: current perspectives. J Contr Rel. 2009;134:74–80. doi: 10.1016/j.jconrel.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Wallaert B, Brun P, Ostinelli J, Murciano D, Champel F, Blaive B, et al. The French bambuterol study group. A comparison of two long acting β-agonists, oral bambuterol and inhaled salmeterol, in the treatment of moderate to severe asthmatic patients with nocturnal symptoms. Respir Med. 1999;93:33–38. doi: 10.1016/S0954-6111(99)90074-4. [DOI] [PubMed] [Google Scholar]
- 6.D’alonzo GE, Smolensky MH, Feldman S, Gnosspelius Y, Karlsson K. Bambuterol in the treatment of asthma: a placebo-controlled comparison of once-daily morning vs. evening administration. Chest. 1995;107:406–412. doi: 10.1378/chest.107.2.406. [DOI] [PubMed] [Google Scholar]
- 7.Nyberg L, Rosenborg J, Weibull E, Jönsson S, Kennedy BM, Nilsson M. Pharmacokinetics of bambuterol in healthy subjects. Br J Clin Phamacol. 1998;45:471–478. doi: 10.1046/j.1365-2125.1998.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenborg J, Larsson P, Nyberg L. Pharmacokinetics of bambuterol during oral administration of plain tablets and solution to healthy adults. Br J Clin Phamacol. 2000;49:199–206. doi: 10.1046/j.1365-2125.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee B-J, Min G-H. Oral controlled release of melatonin using polymer-reinforced and coated alginate beads. Int J Pharm. 1996;144:37–46. doi: 10.1016/S0378-5173(96)04723-0. [DOI] [Google Scholar]
- 10.Cao Q-R, Choi H-G, Kim D-C, Lee B-J. Release behavior and photo-image of nifedipine tablet coated with high viscosity grade hydroxypropylmethylcellulose: effect of coating conditions. Int J Pharm. 2004;274:107–117. doi: 10.1016/j.ijpharm.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Ozturk AG, Ozturk SS, Palsson BO, Wheatley TA, Dressman JB. Mechanism of release from pellets coated with an ethylcellulose-based film. J Contr Rel. 1990;14:203–213. doi: 10.1016/0168-3659(90)90160-U. [DOI] [Google Scholar]
- 12.Borgquist P, Zackrisson G, Nilsson B, Axelsson A. Simulation and parametric study of a film-coated controlled-release pharmaceutical. J Contr Rel. 2002;80:229–245. doi: 10.1016/S0168-3659(02)00033-0. [DOI] [PubMed] [Google Scholar]
- 13.Muschert S, Siepmann F, Leclercq B, Carlin B, Siepmann J. Prediction of drug release from ethylcellulose coated pellets. J Contr Rel. 2009;135:71–79. doi: 10.1016/j.jconrel.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Siepmann F, Hoffmann A, Leclercq B, Carlin B, Siepmann J. How to adjust desired drug release patterns from ethylcellulose-coated dosage forms. J Contr Rel. 2007;119:182–189. doi: 10.1016/j.jconrel.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Sakellariou P, Rowe RC, White EFT. Polymer/polymer interaction in blends of ethyl cellulose with both cellulose derivatives and polyethylene glycol 6000. Int J Pharm. 1986;34:93–103. doi: 10.1016/0378-5173(86)90014-1. [DOI] [Google Scholar]
- 16.Sakellariou P, Rowe RC. The morphology of blends of ethylcellulose with hydroxypropyl methylcellulose as used in film coating. Int J Pharm. 1995;125:289–296. doi: 10.1016/0378-5173(95)00147-B. [DOI] [Google Scholar]
- 17.Wong D, Bodmeier R. Flocculation of an aqueous colloidal ethyl cellulose dispersion (Aquacoat) with a water-soluble polymer, hydroxypropyl methylcellulose. Eur J Pharm Biopharm. 1996;42:12–15. [Google Scholar]
- 18.Suh J, Paik H-J, Hwang BK. Ionization of poly(ethylenimine) and poly(allylamine) at various pH’s. Bioorg Chem. 1994;22:318–327. doi: 10.1006/bioo.1994.1025. [DOI] [Google Scholar]
- 19.Grunlan JC, Liu L, Regev O. Weak polyelectrolyte control of carbon nanotube dispersion in water. J Collo Int Sci. 2008;317:346–349. doi: 10.1016/j.jcis.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 20.Tran PHL, Tran HTT, Lee B-J. Modulation of microenvironmental pH and crystallinity of ionizable telmisartan using alkalizers in solid dispersions for controlled release. J Contr Rel. 2008;129:59–65. doi: 10.1016/j.jconrel.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Tran TT-D, Tran PH-L, Lee B-J. Dissolution-modulating mechanism of alkalizers and polymers in a nanoemulsifying solid dispersion containing ionizable and poorly water-soluble drug. Eur J Pharm Biopharm. 2009;72:83–90. doi: 10.1016/j.ejpb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Piao Z-Z, Lee M-K, Lee B-J. Colonic release and reduced intestinal tissue damage of coated tablets containing naproxen inclusion complex. Int J Pharm. 2008;350:205–211. doi: 10.1016/j.ijpharm.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 23.British Pharmacopoeia Commission . British Pharmacopoeia 2001 (BP 2001) London: Stationery Office; 2001. [Google Scholar]
- 24.Tran TT-D, Tran PH-L, Choi H-G, Han H-K, Lee B-J. The roles of acidifiers in solid dispersions and physical mixtures. Int J Pharm. 2010;384:60–66. doi: 10.1016/j.ijpharm.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 25.Siepmann J, Kranz H, Bodmeier R, Peppas NA. HPMC-matrices for controlled drug delivery: a new model combining diffusion, swelling, and dissolution mechanisms and predicting the release kinetics. Pharm Res. 1999;16:1748–1756. doi: 10.1023/A:1018914301328. [DOI] [PubMed] [Google Scholar]
- 26.Miranda A, Millán M, Caraballo I. Study of the critical points of HPMC hydrophilic matrices for controlled drug delivery. Int J Pharm. 2006;311:75–81. doi: 10.1016/j.ijpharm.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Nair A, Gupta R, Vasanti S. In vitro controlled release of alfuzosin hydrochloride using HPMC-based matrix tablets and its comparison with marketed product. Pharm Dev Tech. 2007;12:621–625. doi: 10.1080/10837450701563277. [DOI] [PubMed] [Google Scholar]
- 28.Lee B-J, Ryu S-G, Cui J-H. Formulation and release characteristics of hydroxypropylmethylcellulose matrix tablet containing melatonin. Drug Dev Ind Pharm. 1999;25:493–501. doi: 10.1081/DDC-100102199. [DOI] [PubMed] [Google Scholar]
- 29.Lee B-J, Ryu S-G, Cui J-H. Controlled release of dual drug-loaded hydroxypropyl methylcellulose matrix tablet using drug-containing polymeric coatings. Int J Pharm. 1999;188:71–80. doi: 10.1016/S0378-5173(99)00204-5. [DOI] [PubMed] [Google Scholar]
- 30.Baveja SK, Rao KVR, Devi KP. Zero-order release hydrophilic matrix tablets of β-adrenergic blockers. Int J Pharm. 1987;39:39–45. doi: 10.1016/0378-5173(87)90196-7. [DOI] [Google Scholar]
- 31.Devi KP, Rao KVR, Baveja S, Fathi M, Roth M. Zero-order release formulation of oxprenolol hydrochloride with swelling and erosion control. Pharm Res. 1989;6:313–317. doi: 10.1023/A:1015998424548. [DOI] [PubMed] [Google Scholar]
- 32.Danckwerts MP. Development of a zero-order release oral compressed tablet with potential for commercial tabletting production. Int J Pharm. 1994;112:37–45. doi: 10.1016/0378-5173(94)90259-3. [DOI] [Google Scholar]
- 33.Thoma K, Zimmer T. Retardation of weakly basic drugs with diffusion tablets. Int J Pharm. 1990;58:197–202. doi: 10.1016/0378-5173(90)90195-A. [DOI] [Google Scholar]
- 34.Gabr KE. Effect of organic acids on the release patterns of weakly basic drugs from inert sustained release matrix tablets. Eur J Pharm Biopharm. 1992;38:199–202. [Google Scholar]
- 35.Streubel A, Siepmann J, Dashevsky A, Bodmeier R. pH-independent release of a weakly basic drug from water-insoluble and -soluble matrix tablets. J Contr Rel. 2000;67:101–110. doi: 10.1016/S0168-3659(00)00200-5. [DOI] [PubMed] [Google Scholar]
- 36.Kranz H, Brun VL, Wagner T. Development of a multi particulate extended release formulation for ZK 811 752, a weakly basic drug. Int J Pharm. 2005;299:84–91. doi: 10.1016/j.ijpharm.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Kranz H, Guthmannb C, Wagner T, Lipp R, Reinhard J. Development of a single unit extended release formulation for ZK 811 752, a weakly basic drug. Eur J Pharm Sci. 2005;26:47–53. doi: 10.1016/j.ejps.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 38.Siepe S, Lueckel B, Krammer A, Ries A, Gurny R. Strategies for the design of hydrophilic matrix tablets with controlled microenvironmental pH. Int J Pharm. 2006;316:14–20. doi: 10.1016/j.ijpharm.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 39.Espinoza R, Hong E, Villafuerte L. Influence of admixed citric acid on the release profile of pelanserin hydrochloride from HPMC matrix tablets. Int J Pharm. 2000;201:165–173. doi: 10.1016/S0378-5173(00)00406-3. [DOI] [PubMed] [Google Scholar]
- 40.Varma MVS, Kaushal AM, Garg S. Influence of micro-environmental pH on the gel layer behavior and release of a basic drug from various hydrophilic matrices. J Contr Rel. 2005;103:499–510. doi: 10.1016/j.jconrel.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 41.Tatavarti AS, Hoag SW. Microenvironmental pH modulation based release enhancement of a weakly basic drug from hydrophilic matrices. J Pharm Sci. 2006;95:1459–1468. doi: 10.1002/jps.20612. [DOI] [PubMed] [Google Scholar]
- 42.Siepe S, Herrmann W, Borchert HH, Lueckel B, Kramer A, Ries A, et al. Microenvironmental pH and microviscosity inside pH-controlled matrix tablets: an EPR imaging study. J Contr Rel. 2006;112:72–78. doi: 10.1016/j.jconrel.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Siepe S, Lueckel B, Kramer A, Ries A, Gurny R. Assessment of tailor-made HPMC-based matrix minitablets comprising a weakly basic drug compound. Drug Dev Ind Pharm. 2008;34:46–52. doi: 10.1080/03639040701484106. [DOI] [PubMed] [Google Scholar]
- 44.Tiwari SB, Murthy TK, Pai MR, Mehta PR, Chowdary PB. Controlled release formulation of tramadol hydrochloride using hydro-philic and hydrophobic matrix system. AAPS Pharm Sci Tech. 2003;4:1–6. doi: 10.1208/pt040331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piao Z-Z, Lee K-H, Kim D-J, Lee B-J. Comparison of release-controlling efficiency of polymeric coating materials using matrix-type casted films and diffusion-controlled coated tablet. AAPS Pharm Sci Tech. 2010;11(2):630–636. doi: 10.1208/s12249-010-9377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammer M, Schweitzer D, Richter S, Königsdörffer E. Sodium fluorescein as a retinal pH indicator? Physiol Meas. 2005;26:N9–N12. doi: 10.1088/0967-3334/26/4/N01. [DOI] [PubMed] [Google Scholar]
- 47.Reich G. Near-infrared spectroscopy and imaging: basic principles and pharmaceutical applications. Adv Drug Deliv Rev. 2005;57:1109–1143. doi: 10.1016/j.addr.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 48.Awa K, Okumura T, Shinzawa H, Otsukac M, Ozaki Y. Self-modeling curve resolution (SMCR) analysis of near-infrared (NIR) imaging data of pharmaceutical tablets. Anal Chim Acta. 2008;619:81–86. doi: 10.1016/j.aca.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 49.Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60. doi: 10.1016/S0939-6411(00)00076-X. [DOI] [PubMed] [Google Scholar]
- 50.Choi J, Rubner MF. Influence of the degree of ionization on weak polyelectrolyte multilayer assembly. Macromolecules. 2005;38:116–124. doi: 10.1021/ma048596o. [DOI] [Google Scholar]