Abstract
The objectives of this study were to develop and evaluate a novel self-emulsifying floating drug delivery system (SEFDDS) that resulted in improved solubility, dissolution, and controlled release of the poorly water-soluble tetrahydrocurcumin (THC). The formulations of liquid self-emulsifying drug delivery system (SEDDS; mixtures of Labrasol, Cremophor EL, Capryol 90, Labrafac PG) were optimized by solubility assay and pseudo-ternary phase diagram analysis. The liquid SEDDS was mixed with adsorbent (silicon dioxide), glyceryl behenate, pregelatinized starch, sodium starch glycolate, and microcrystalline cellulose and transformed into pellets by the extrusion/spheronization technique. The resulting pellets with 22% liquid SEDDS had a uniform size and good self-emulsification property. The microemulsions in aqueous media of different self-emulsifying floating pellet formulations were in a particle size range of 25.9–32.5 nm. Use of different weight proportions of glyceryl behenate and sodium starch glycolate in pellet formulations had different effects on the floating abilities and in vitro drug release. The optimum formulation (F2) had a floating efficiency of 93% at 6 h and provided a controlled release of THC over an 8-h period. The release rate and extent of release of THC liquid SEDDS (80% within 2 h) and self-emulsifying floating pellet formulation (80% within 8 h) were significantly higher than that of unformulated THC (only 30% within 8 h). The pellet formulation was stable under intermediate and accelerated storage conditions for up to 6 months. Controlled release from this novel SEFDDS can be a useful alternative for the strategic development of oral solid lipid-based formulations.
Key words: controlled release, floating drug delivery systems, SEDDS, self-emulsifying systems, tetrahydrocurcumin
INTRODUCTION
Oral delivery of lipophilic drugs presents a major challenge due to the low aqueous solubility of such compounds. A self-emulsifying drug delivery system (SEDDS) can be used for the design of formulations in order to improve the oral absorption of lipophilic drugs. SEDDS are isotropic mixtures of oils and surfactants that form fine oil-in-water emulsions upon mild agitation in aqueous media such as GI fluids (1). SEDDS present the solubilized drugs in an oil-in-water emulsion of small droplet size (nanometers), and this therefore ultimately results in increased drug absorption (2,3). SEDDS are usually supplied in liquid form encapsulated in gelatin capsules. This type of system does have some disadvantages. The dosage form might present incompatibility problems with the capsule shell and are usually more expensive to produce. Recently, several researchers have successfully developed solid-SEDDS by incorporating SEDDS into pharmaceutical excipients to produce different solid dosage forms, such as pellets and tablets. The advantages of pellet formulations containing SEDDS are that they can disperse freely in the gastrointestinal tract, the dose can be readily modulated, and the pellets can be easily packed in hard gelatin capsules. Tuleu et al. (4) studied a self-emulsifying pellets of progesterone administered orally to dogs (2 mg/kg) using an extrusion/spheronization (E/S) technique. The SEDDS pellets resulted in a significant increased absorption of progesterone compared with the aqueous suspension formulation. Serratoni et al. (5) prepared self-emulsifying controlled release pellets by mixing two water-insoluble drugs into SEDDS and then coating the pellets with water-insoluble polymers. The study demonstrated that the designed solid self-emulsifying systems increased drug solubility, and the coating polymers provided a controlled release of the drug dissolved in the self-emulsifying system.
Floating drug delivery systems have been previously developed by several researchers. These have extended drug residence time in the stomach, leading to increased drug bioavailability (6–8). However, the development of a self-emulsifying floating drug delivery system (SEFDDS) has not been previously described. SEFDDS is a novel solid lipid-based formulation that has been developed in the present study. The use of SEDDS provides better absorption of hydrophobic drugs. The floating system prolongs the gastric residence time of the drug, since the pellets remain buoyant on the surface of the stomach liquid contents, without affecting the gastric emptying rate. Sustained-release agents used in this new SEFDDS system offer an effective controlled release in the stomach. Consequently, the drugs dissolved in SEDDS can act locally and are released continuously from the floating system.
Curcumin (Fig. 1a), a polyphenolic compound extracted from the rhizomes of turmeric (Curcuma longa Linn.), has a wide biological and pharmacological profile. It has also been reported to possess anti-oxidative, anti-inflammatory, anti-carcinogenic, and gastroprotective effects (9–14). Tetrahydrocurcumin (THC), one of the major metabolites of curcumin in vivo (15), was reported to exhibit the same physiological and pharmacological properties of curcumin (16). THC has been widely used in pharmaceutical and cosmetic preparations. THC (Fig. 1b), in a white to off-white powder form, has a molecular weight of 372.41 Da and a melting point of 85–100°C. THC is insoluble in water and soluble in alcohol, acetone, and glacial acetic acid. However, the pharmacological effect of THC is limited due to its low aqueous solubility. In addition, a relative short gastric emptying time can result in an incomplete release of THC from the dosage form at the site of absorption and lead to a diminished efficacy of the administered dose.
Fig. 1.
Chemical structures of curcumin (a) and tetrahydrocurcumin (b)
In this study, the solubility of THC was determined in various vehicles (oils, surfactants, co-surfactants, and co-solvents). Pseudo-ternary phase diagrams were constructed to identify efficient self-emulsification regions. Optimum ratios of excipient concentrations were selected to develop THC-SEDDS and THC-SEFDDS pellet formulations. THC was first dissolved in SEDDS, and this oily solution was then incorporated into a powder to produce pellets using the E/S technique. The effect of incorporating different weight proportions of a low-density waxy material, glyceryl behenate, which has previously been utilized as a retardant material for a sustained-release dosage form (17–20), was investigated. Furthermore, the effect of sodium starch glycolate (a disintegrant) levels on drug release was also determined.
The main objective was to develop a well-designed SEDDS formulation and then incorporate this formulation into a solid dosage form, namely pellets. The physicochemical characteristics of this novel formulation, including its floating ability and drug release, were also evaluated.
MATERIALS AND METHODS
Materials
THC (white to off-white powder, 99.52% purity, lot no. C61260) was from Sabinsa Corporation (Piscataway, NJ, USA). Capryol 90™ (propylene glycol monocaprylate), Labrafac PG™ (propylene glycol caprylate/caprate), Labrasol™ (caprylocaproyl macrogol-8 glycerides), and Compritol 888 ATO™ (glyceryl behenate) were from Gattefosse (Saint-Priest, France). Oleic acid, propylene glycol, and PEG 400 (polyethylene glycol) were from PC Drug Center Co., Ltd. (Bangkok, Thailand). Soybean oil and corn oil were from Thai Vegetable Oil Public Company Limited (Bangkok, Thailand). Ethyl oleate was from Sigma Aldrich (Buchs, Switzerland). Cremophor RH40™ (polyoxyethylene castor oil derivatives) and Cremophor EL™ (polyoxyethylene castor oil derivatives) were from BASF (Ludwigshafen, Germany). Peceol™ (glyceryl monooleate), Labrafac CC™ (caprylic/capric triglycerides), Lauroglycol FCC™ (propylene glycol laurate), Lauroglycol 90™ (propylene glycol monolaurate), Labrafil M2125 CS™ (linoleoyl macrogol-6-glycerides), and Plurol oleique™ (polyglyceryl-6 dioleate) were from Gattefosse (Saint-Priest, France). Sylysia 350™ (silicon dioxide) was from Fugi Sylysia Chemical Ltd. (Aichi, Japan). Starch 1500™ (pregelatinized starch) was from Colorcon (Indianapolis, IN, USA). Explosol™ (sodium starch glycolate) was from Blanver (Itapevi Unit, Brazil). Flocel 101™ (microcrystalline cellulose) was from Gujarat Microwax Private Limited (Mehsana, India). Hard gelatin capsules (size 00) were from Capsugel (Bangkok, Thailand). Acetonitrile and methanol (HPLC-grade) were from RCI Labscan (Bangkok, Thailand). All other chemicals were of analytical grade.
High-Performance Liquid Chromatography (HPLC) Analysis of THC
The quantitative determination of THC was performed using an Agilent HPLC photodiode array detector (HP 1100, Agilent, USA) with a VertiSep™ UPS C18 5-μm column (4.6 × 250 mm) and a guard VertiSep™ UPS C18 5-μm column (4.6 × 10 mm) (Ligand Scientific, Bangkok, Thailand). A linear gradient system was used for the chromatography of THC, with a mixture of 2% aqueous acetic acid (solvent A) and acetonitrile (solvent B) as the mobile phases. The gradient program was as follows: starting composition, A/B, 65:35% v/v; 0–4 min, then the percent v/v of B was increased from 35% to 65%; 4–7 min, then the percent v/v of B was increased from 65% to 70%; 7–12 min, and finally, the percent v/v of B was decreased from 70% to 35%. Before each injection, the HPLC column was stabilized for 30 min with the initial mobile phase composition of A/B, 65:35 (percent v/v). Detection was by UV spectroscopy at a wavelength of 280 nm, and the flow rate was 1 mL/min. The retention time of THC was about 9 min. Twenty microliters of the samples was injected directly onto the HPLC column for three separate times (n = 3). A methanolic stock solution of THC was prepared at a concentration of 0.1 mg/mL. The THC stock solution was diluted with methanol to provide working solutions that ranged from 0.1 to 20 μg/mL, and 20 μL of each was injected onto the HPLC column via the auto-injector three separate times (n = 3). The mean peak areas for each concentration were calculated, and standard calibration curves were constructed by plotting concentrations against peak areas. A good linearity was achieved with a correlation coefficient of 0.9999 over the concentration range of 0.1–20 μg/mL. The reproducibility of the method was demonstrated by repeated injections of THC standards. Five daily injections over a 5-day period gave intraday relative standard deviations that ranged from 0.39% to 1.22%, whereas the interday relative standard deviations ranged from 2.84% to 3.88%, respectively. The limit of detection value for THC was 20 ng/mL, and the limit of quantitation value was 50 ng/mL, respectively. The accuracy of the method was verified with recovery values of 98–102%.
Solubility Studies of THC in Different Vehicles
The solubility of THC in various vehicles, including oils (ethyl oleate, oleic acid, soybean oil, corn oil, Peceol, Labrafac CC, Labrafac PG, and Capryol 90), surfactants (Labrasol, Cremophor EL, and Cremophor RH40), co-surfactants (Lauroglycol 90, Lauroglycol FCC, Labrafil M2125 CS, and Plurol oleique), co-solvents (propylene glycol and PEG 400) was determined by the shake-flask method. An excess amount of THC (approximately 250 mg) was added to each cap vial containing 1 mL of the solution vehicles. After sealing, the mixture was vortexed using a mixer (Vortex-Genie 2, Scientific Industries, Inc., USA) at a maximum speed for 10 min in order to facilitate proper mixing of THC with the solution vehicles. Mixtures were then shaken in a water bath shaker (Heto Lab, Scientific Promotion, Thailand) maintained at room temperature until equilibrium (48 h) to facilitate solubilization. The mixtures were then centrifuged at 6,000 rpm for 10 min to remove the excess insoluble THC (Hettich Zentrifugen D-78532 Tuttlingen Model 16R, Germany). The supernatants were collected and diluted with methanol for quantification of THC by the validated HPLC method. The concentration of THC was calculated from the standard curve [peak area = 24.342 × conc. + 0.0698 (r2 = 0.9999)].
Construction of Pseudo-ternary Phase Diagrams
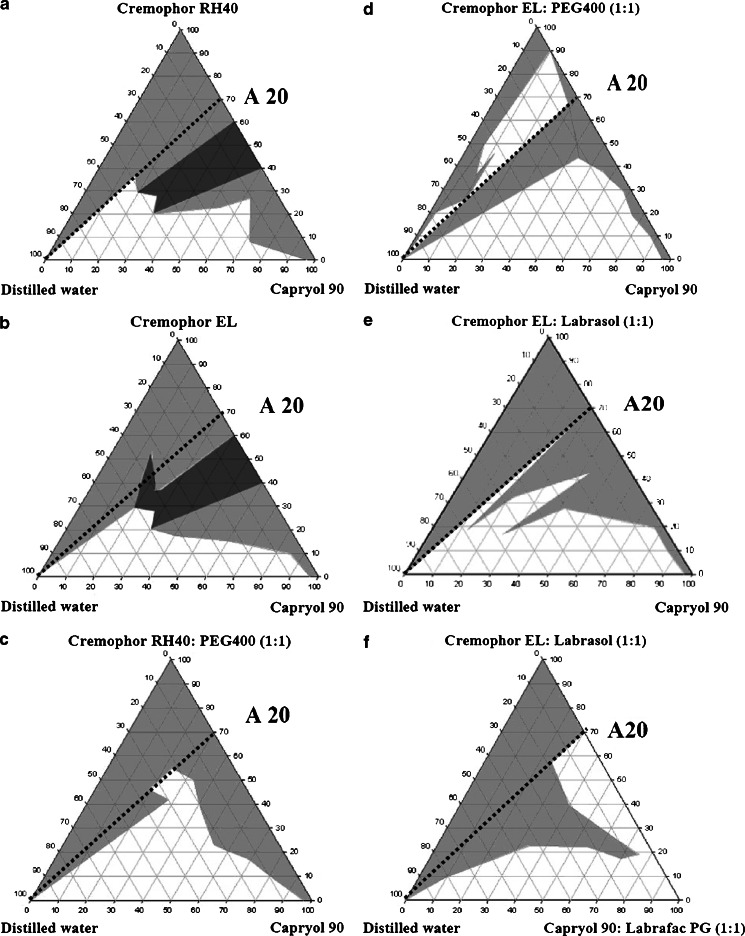
Pseudo-ternary phase diagrams (Fig. 2) were constructed using the water titration method. Mixtures (systems A–E) of the oil phase containing Capryol 90 with the surfactant phase, including Cremophor RH40 (system A); Cremophor EL (system B), a combination of surfactant and co-surfactant; Cremophor RH40/PEG 400, 1:1 weight ratio (system C); Cremophor EL/PEG 400, 1:1 weight ratio (system D); and a combination of two surfactants Cremophor EL/Labrasol, 1:1 weight ratio (system E) were prepared at certain weight ratios of 10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9, and 0:10. Similarly, mixtures (system F) of the oil phase containing a combination of two oils (Capryol 90/Labrafac PG, 1:1 weight ratio), with a combination of surfactant phase (Cremophor EL/Labrasol, 1:1 weight ratio), were also evaluated at the same weight ratios. The mixtures of the oil phase and surfactant phase of 11 different weight ratios were accurately weighed into 11 glass tubes. The mixtures in each tube were mixed homogeneously using a vortex mixer at room temperature until the oily liquid mixtures were obtained. Water was then added drop by drop using a dropper into each oily mixture. During the titration, samples were stirred vigorously for a sufficient length of time for homogenization and visually monitored against a dark background by illuminating the samples with white light. The concentration of water at which turbidity-to-transparency and transparency-to-turbidity transition occurred was derived from the weight measurements. These values were then used to determine the boundaries of the microemulsion regions that corresponded to the selected optimum ratios of combination vehicles for developing THC-SEDDS and THC-SEFDDS pellet formulations.
Fig. 2.
Pseudo-ternary phase diagrams composed of various oils and surfactants. The surfactant phase was as follows: Cremophor RH40 (a), Cremophor EL (b), Cremophor RH40/PEG 400 (1:1) (c), Cremophor EL/PEG 400 (1:1) (d), Cremophor EL/Labrasol (1:1) (e, f). The oil phase was as follows: Capryol 90 (a–e) and Labrafac PG/Capryol 90 (1:1) (f). The gray area represents the range of existence of a microemulsion, the white area represents the coarse emulsion ranges, and the gray–black area represents the gel-like phases. Broken line represents the aqueous dilution line A20 from the non-aqueous vehicle to the water axis (on this dilution line, the weight ratio of surfactant/oil phase is constant at 7:3)
Formulation and Preparation of THC-SEDDS
Six formulations of SEDDS (S1–S6, Table I) were prepared containing a fixed proportion of THC (8.93% w/w) dissolved in a mixture of solvent vehicles (91.07% w/w). The solvent vehicles were single or mixed surfactants, or mixtures of a surfactant and PEG 400 (70%), mixed with a Capryol 90, or a mixture of Capryol 90 and Labrafac PG (30%). A typical formulation (e.g., F6) contained 2 g of THC, 7.84 g of Cremophor EL, 7.84 g of Labrasol, 3.36 g of Capryol 90, and 3.36 g of Labrafac PG. These components were accurately weighed and mixed using a magnetic stirrer until a solution (THC-SEDDS) was obtained. All of the liquid formulations were left for 24 h at room temperature. Hard gelatin capsules (size 00) were manually filled with 122 mg ± 3 mg of S6, resulting in each capsule containing 10 mg THC. After filling, two-piece capsules were sealed with gelatin solution to enclose the liquid fill and to protect the leakage of the oily liquid from the capsule shells during the storage. The THC-SEDDS capsules were stored in air-tight glass containers and protected from light at room temperature until required for analysis.
Table I.
Composition of 10 mg THC-SEDDS Formulations S1–S6 in 112 mg of a Mixture of Surfactants (70% w/w) and an Oil Phase (30% w/w)
| Compositions | Formulations (mg) | |||||
|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | |
| THC | 10 | 10 | 10 | 10 | 10 | 10 |
| Surfactant phase | ||||||
| Cremophor RH40 | 78.4 | 39.2 | ||||
| Cremophor EL | 78.4 | 39.2 | 39.2 | 39.2 | ||
| Labrasol | 39.2 | 39.2 | ||||
| PEG 400 | ||||||
| Oil phase | 39.2 | 39.2 | ||||
| Capryol 90 | 33.6 | 33.6 | 33.6 | 33.6 | 33.6 | 16.8 |
| Labrafac PG | 16.8 | |||||
| Particle size (nm) | 212.6 ± 11.7 | 132.0 ± 2.5 | 261.0 ± 21.2 | 105.2 ± 1.1 | 91.2 ± 0.9 | 28.2 ± 0.3 |
THC-SEDDS tetrahydrocurcumin-self-emulsifying drug delivery system
Formulation of THC-SEFDDS Pellets
THC-SEDDS pellets were prepared by the E/S technique. The optimized liquid vehicle used for formulating the THC-SEFDDS pellets was the same as that judged to be the best vehicle for preparing THC-SEDDS (S6). Eight formulations of SEDDS pellets were prepared containing a fixed proportion of THC (1.98% w/w) dissolved in a mixture of oily liquid (22.18% w/w) and mixed with solid pharmaceutical excipients (75.84% w/w).
Four different formulations (F1–F4, Table II) of THC-SEFDDS pellets were prepared by the E/S technique, using constant weight proportions of all the ingredients except for glyceryl behenate (which was increased from 50% to 65% w/w) and microcrystalline cellulose (which was reduced from 22.39% to 7.39% w/w) in the four formulations. Four further formulations (F5–F8, Table III) were prepared where the weight proportion of sodium starch glycolate was increased from 0% to 3.2% w/w, with a corresponding decreased proportion (from 18.19% to 14.99% w/w) of microcrystalline cellulose. Formulations F2 and F6 have identical compositions.
Table II.
Composition of THC-SEFDDS Pellet Formulations with Different Weight Proportions of Glyceryl Behenate
| Ingredients | Formulations (% w/w) | |||
|---|---|---|---|---|
| F1 | F2a | F3 | F4 | |
| THC | 1.98 | 1.98 | 1.98 | 1.98 |
| Cremophor EL | 7.76 | 7.76 | 7.76 | 7.76 |
| Labrasol | 7.76 | 7.76 | 7.76 | 7.76 |
| Capryol 90 | 3.33 | 3.33 | 3.33 | 3.33 |
| Labrafac PG | 3.33 | 3.33 | 3.33 | 3.33 |
| Glyceryl behenate | 50 | 55 | 60 | 65 |
| Silicon dioxide | 0.15 | 0.15 | 0.15 | 0.15 |
| Microcrystalline cellulose | 22.39 | 17.39 | 12.39 | 7.39 |
| Pregelatinized starch | 2.5 | 2.5 | 2.5 | 2.5 |
| Sodium starch glycolate | 0.8 | 0.8 | 0.8 | 0.8 |
THC-SEFDDS tetrahydrocurcumin-self-emulsifying floating drug delivery system
aThe composition of formulation F2 is identical to that of formulation F6 (Table II)
Table III.
Composition of THC-SEFDDS Pellet Formulations with Different Weight Proportions of Sodium Starch Glycolate
| Ingredients | Formulations (% w/w) | |||
|---|---|---|---|---|
| F5 | F6a | F7 | F8 | |
| THC | 1.98 | 1.98 | 1.98 | 1.98 |
| Cremophor EL | 7.76 | 7.76 | 7.76 | 7.76 |
| Labrasol | 7.76 | 7.76 | 7.76 | 7.76 |
| Capryol 90 | 3.33 | 3.33 | 3.33 | 3.33 |
| Labrafac PG | 3.33 | 3.33 | 3.33 | 3.33 |
| Glyceryl behenate | 55 | 55 | 55 | 55 |
| Silicon dioxide | 0.15 | 0.15 | 0.15 | 0.15 |
| Microcrystalline cellulose | 18.19 | 17.39 | 16.59 | 14.99 |
| Pregelatinized starch | 2.5 | 2.5 | 2.5 | 2.5 |
| Sodium starch glycolate | 0 | 0.8 | 1.6 | 3.2 |
THC tetrahydrocurcumin, THC-SEFDDS tetrahydrocurcumin-self-emulsifying floating drug delivery system
aThe composition of formulation F6 is identical to that of formulation F2 (Table I)
A typical preparation (e.g., F2/F6) of pellets (202 g) contained THC (4 g), Cremophor EL (15.68 g), Labrasol (15.68 g), Capryol 90 (6.72 g), and Labrafac PG (6.72 g). These components were mixed using a magnetic stirrer until a solution (THC-SEDDS) was obtained. Glyceryl behenate (111.1 g), silicon dioxide (0.303 g), pregelatinized starch (5.05 g), sodium starch glycolate (1.616 g), and microcrystalline cellulose (35.131 g) were homogeneously mixed and prewetted with distilled water (5 mL). After that, wetting was completed by the gradual addition of the liquid THC-SEDDS leading to a damp mass. The wet mass was then extruded through an extruder developed in our laboratory, using a 2-mm pore size screen. The extrudates were then spheronized on a frictional plate with a cross-hatch geometry in a spheronizer (Yeo Heng, Bangkok, Thailand) at a rotation speed of 500 rpm for 1 min. The moist pellets were dried to constant weight (16 h) in an oven (Memmert, Germany) at 45 ± 2°C. Dried pellets (505 mg) contained 10 mg of THC (1.98% w/w). Pellets were manually filled with 505 mg±15 mg (n = 250) in size 00 hard gelatin capsules and the capsules stored in air-tight glass containers and protected from light at room temperature until required for analysis.
Physical Characterization of SEDDS and SEFDDS Pellets
Morphological Characterization of THC-SEDDS
The morphology of the optimum THC-SEDDS formulation (S6) was examined by transmission electron microscopy (TEM; JEOL Ltd., Tokyo, Japan). The liquid THC-SEDDS (0.12 g equivalent to THC 10 mg) was diluted with distilled water at a ratio of 1:25 and mixed by gentle shaking. A drop of sample obtained after dilution was placed on copper grids. Any excess was drawn off with filter paper. The samples were stained in 5% uranyl acetate for 10 min. The excess fluid was then removed, and the grid surface was air-dried at room temperature. TEM micrographs of THC microemulsions were photographed.
Emulsion Droplet Size Analysis
The droplet size of the emulsions formed with each of the formulations was determined by photon correlation spectroscopy (Zeta potential analyzer, Model ZetaPALS, Brookhaven, USA) using dynamic light scattering. THC-SEFDDS pellets (F1–F8; 0.50 g equivalent to THC 10 mg) were mixed with distilled water (200 mL) and subjected to mild agitation using a magnetic stirrer at a speed of 75 rpm for 8 h at room temperature and resulted in the formation of microemulsions. For comparison, the liquid THC-SEDDS formulation (S6; 0.12 g equivalent to THC 10 mg) was mixed with distilled water (200 mL) and emulsified as above for 5 min. The reconstituted microemulsions were loaded into cuvettes and their size measured after dilution to produce the required count rate (100–500 kcps) to allow for accurate measurements. The sample viscosity (0.890 cP) and the water refractive index (1.330) were factored into the particle size measurement using the instrument software. Light scattering was monitored at a 90° angle and at a temperature of 25°C. Distilled water filtered through a 0.45-μm filter was used as the dilution medium. Three replicate analyses were carried out for each formulation and the data presented as mean±SD.
Size and Friability of the THC-SEFDDS Pellets
THC-SEFDDS pellets from each of the formulations were randomly sampled. The diameter of each pellet (n = 100) was measured by a Vernier caliper (Kanon, Japan), and the mean diameter was calculated for each formulation. Pellet friability was conducted on 5 g of pellets combined with 5 g of glass beads (2 mm diameter) using an Erweka-type friabilator (KSL Engineering, Thailand). The drum was rotated at 25 rpm for 4 min. Loss of pellet weight with respect to the initial value was then calculated as the percentage friability (21).
Scanning Electron Microscope Observation of THC-SEFDDS Pellets
The scanning electron microscope (SEM) images of eight different formulations were identical. A representative of formulation F2 was selected to illustrate a typical SEM image. One gram of the THC-SEFDDS pellets was randomly sampled from pellets filled in the hard gelatin capsules. A few pellets of the formulations were mounted on the stub. This specimen was then sputter-coated with gold particles and observed with a LV-SEM 5800 (JEOL, Japan) at an accelerating voltage of 10 kV. SEM micrographs of the surfaces of THC-SEFDDS pellets were photographed, and SEM analysis of the internal pellet structure was also determined after splitting the samples.
Evaluation of Floating Properties
The floating properties of THC-SEFDDS pellets were evaluated using the USP30 dissolution apparatus 2 (Hanson Research Corporation, USA). Pellets (n = 100) were randomly sampled from each of the eight different formulations and filled in hard gelatin capsules. Each capsule was then individually placed in the 900 mL of simulated gastric fluid (SGF, pH 1.2) without pepsin at 37 ± 0.5°C. Gentle agitation was provided by a paddle rotating at 75 rpm. The number of floating pellets was estimated by photographing the liquid surface and the bottom of the liquid in the vessel and counting the number of floating pellets in the picture (22). The floating time was determined after 30, 60, 120, 180, 240, 300, 360, 420, and 480 min. The percentage of floating pellets at different time intervals was recorded.
Effect of Formulation Variables on THC Release In Vitro
Release profiles of the capsules filled with the SEFDDS pellets containing THC 10 mg were determined using the USP30 rotating paddle apparatus. One capsule of each of the different pellet formulations was placed in 450 mL of SGF (pH 1.2) without pepsin at 37 ± 0.5°C and a rotating speed of 75 rpm. The dissolution from THC-SEFDDS pellets was compared with the THC-SEDDS formulation (122 mg of the liquid SEDDS containing 10 mg THC per capsule) and with THC (10 mg of THC powder in a capsule). Samples (5 mL) were withdrawn and replaced with 5 mL of the medium after 5, 10, 15, 20, 25, 30, 45, 60, 90, 120, 180, 240, 300, 360, 420, and 480 min. The samples were filtered using a 0.45-μm filter and stored in glass vials at room temperature until analysis. Three replicate release studies were performed for each formulation, and the data is reported as mean±SD. A plot of the percent cumulative release of THC against time (0–480 min) was constructed to illustrate the release profile.
Stability Studies
Studies of the chemical and physical stability of the SEFDDS pellets (F2) was carried out according to the ICH guidelines (2003). The SEFDDS pellets containing 10 mg THC were filled in hard gelatin capsules size 00 (n = 100) and stored in air-tight glass containers protected from light. Samples were maintained in a stability chamber (Patron AH-80, Taiwan) under intermediate storage conditions [30 ± 2°C, 65 ± 5% relative humidity (RH)], and evaluated under accelerated storage conditions (45 ± 2°C, 75 ± 5% RH) with humidity and temperature control. The samples were evaluated at 0, 3, and 6 months for appearance, particle size, and drug content.
RESULTS AND DISCUSSION
Solubility Studies
An oil and surfactant, suitable for developing a SEDDS formulation, should have a high solubilization capacity for the drug and maintain the drug in a solubilized form in the oily phase. This will ensure that the drug is suitably dispersed from the formulation. The solubility of THC in various solution vehicles is presented in Table IV. THC had the highest solubility (207.93 ± 3.32 mg/mL) in PEG 400, and this was therefore chosen as a co-solvent. The high solubility of THC in PEG 400 is probably due to its ability to form a hydrogen bond with the polyethylene oxide (PEO) groups. Similarly, hydrophilic surfactants with a high hydrophilic-lipophilic balance (HLB = 13–15) such as Cremophor RH40, Cremophor EL, and Labrasol composed of PEO groups showed high solubilization capacities for THC. Capryol 90 and Labrafac PG that both afforded a high solubility of THC (40.78 ± 0.91 and 13.85 ± 0.20 mg/mL, respectively) were selected as the oil phases
Table IV.
The Solubility of THC in Various Solvent Vehicles
| Vehicles | Compositions | Solubility of THC (mg/ml), mean±SD (n = 3) |
|---|---|---|
| Oils | ||
| Ethyl oleate | Oleic acid ethyl ester | 5.66 ± 0.197 |
| Oleic acid | Long-chain fatty acid | 3.63 ± 0.05 |
| Soyabean oil | Long-chain fatty acid | 4.55 ± 0.06 |
| Corn oil | Long-chain fatty acid | 4.19 ± 0.33 |
| Peceol | Glyceryl monooleate | 10.98 ± 0.20 |
| Labrafac CC | Caprylic/capric triglycerides | 11.54 ± 0.39 |
| Labrafac PG | Propylene glycol caprylate/caprate | 13.85 ± 0.20 |
| Capryol 90 | Propylene glycol monocaprylate | 40.78 ± 0.91 |
| Co-surfactants | ||
| Labrafil M 2125 CS | Linoleoyl macrogol-6-glycerides | 16.63 ± 0.38 |
| Lauroglycol FCC | Propylene glycol laurate | 17.04 ± 0.56 |
| Lauroglycol 90 | Propylene glycol monolaurate | 20.86 ± 0.45 |
| Plurol oleique | Polyglyceryl-6 dioleate | 25.05 ± 1.64 |
| Surfactants | ||
| Labrasol | Caprylocaproyl macrogol-8 glycerides | 177.08 ± 1.94 |
| Cremophor EL | Polyoxyethylene castor oil derivatives | 159.61 ± 2.19 |
| Cremophor RH40 | Polyoxyethylene castor oil derivatives | 189.95 ± 4.42 |
| Co-solvents | ||
| Propylene glycol | 18.48 ± 0.29 | |
| PEG 400 | Polyethylene glycol 400 | 207.93 ± 3.32 |
THC tetrahydrocurcumin
Pseudo-ternary Phase Diagrams
Pseudo-ternary phase diagrams (Fig. 2) were constructed to identify the microemulsion regions and to optimize the concentrations of the selected solution vehicles (Cremophor RH40, Cremophor EL, Labrasol, PEG 400, Capryol 90, and Labrafac PG). For development of a SEDDS formulation, the optimum ratios of excipient concentrations were established by means of phase diagram studies that identified the area of the monophasic region. It is important to determine this area in order to ensure successful aqueous dilution without “breaking” the microemulsions (23,24). The phase diagrams for six different oil-surfactant–water systems contained different areas of clear microemulsions and coarse emulsions. The surfactant-to-oil ratios (7:3 correspond to a system that contained 70% w/w of the surfactant phase and 30% w/w of the oil phase) that can be fully diluted with water along the dilution line A20 without phase separation were therefore chosen to develop the SEDDS formulations.
Development of the Liquid THC-SEDDS
The composition of different THC-SEDDS formulations and the effect of the SEDDS formulations on the distribution of droplet size are summarized in Table I. Formulations (S1–S6) that were clear liquids rapidly formed fine oil-in-water emulsions with a transparent appearance when introduced into distilled water. All of the formulations showed no signs of phase separation and drug precipitation, even after 6 h. The mean particle size of the formulation S6 containing a 70% mixture of Cremophor EL and Labrasol (1:1) as surfactant and a 30% mixture of Labrafac PG and Capryol 90 (1:1) as oil was smaller than the other formulations. TEM micrographs (S6; Fig. 3) revealed that the droplet size was almost spherical in shape, with smooth surfaces, and the THC was mainly dispersed in the hydrophobic core consisting of oil and surfactants. S6 was therefore selected as the optimum formulation and used for preparing the SEFDDS pellet formulations.
Fig. 3.
TEM micrographs of THC-SEDDS (S6) (×100,000). Bar = 50 nm
The Formulation and Physical Characterization of the SEFDDS Pellets
To prepare pellets with the 22% oily liquid mixture using the E/S technique, the liquid SEDDS needs to be adsorbed to solid carriers such as pharmaceutical excipients to form a powder. Pellets then could be successfully prepared. SEDDS pellets based on microcrystalline cellulose as a spheronization aid appeared to have poor physical properties such as the formation of agglomerates, low hardness, and poor flowability (25). The adsorbents such as silicon dioxide (25,26) and binders such as pregelatinized starch, when incorporated into the SEDDS pellet system, provided good-quality pellets. The incorporation of glyceryl behenate or sodium starch glycolate had no influence on the formation of pellets.
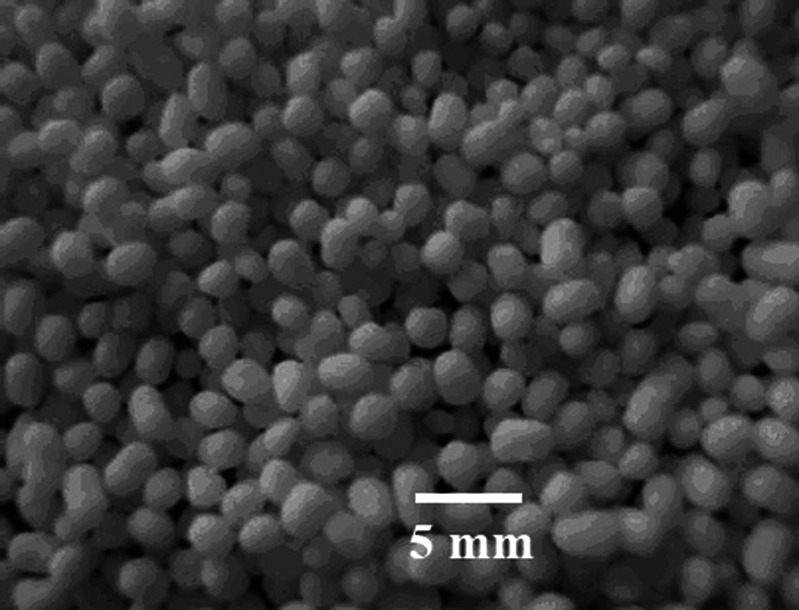
Pellets for all the different formulations (F1–F8, Tables II and III) were almost all of a spherical shape (Fig. 4). All formulations (F1–F8) produced pellets with a particle size in the range of 2.3–2.8 mm, and the percent friability of the pellets were in the range of 0.07–0.23%. The solid THC-SEFDDS of different formulations (F1–F8) preserved the self-emulsification performance of the liquid SEDDS, and the powder used for the pellets had no remarkable effect on the droplet size of the reconstituted microemulsions. The THC-SEFDDS pellets of eight different formulations (F1–F8) produced microemulsions with a droplet size in the range of 25.9–32.5 nm with a narrow distribution size, which was similar to the droplet size of the emulsion from the liquid SEDDS formulation (S6; 28.2 ± 0.3 nm).
Fig. 4.
Representative image of a THC-SEFDDS pellet of F2 formulation
SEM Imaging of the Pellets Before/After Drug Release
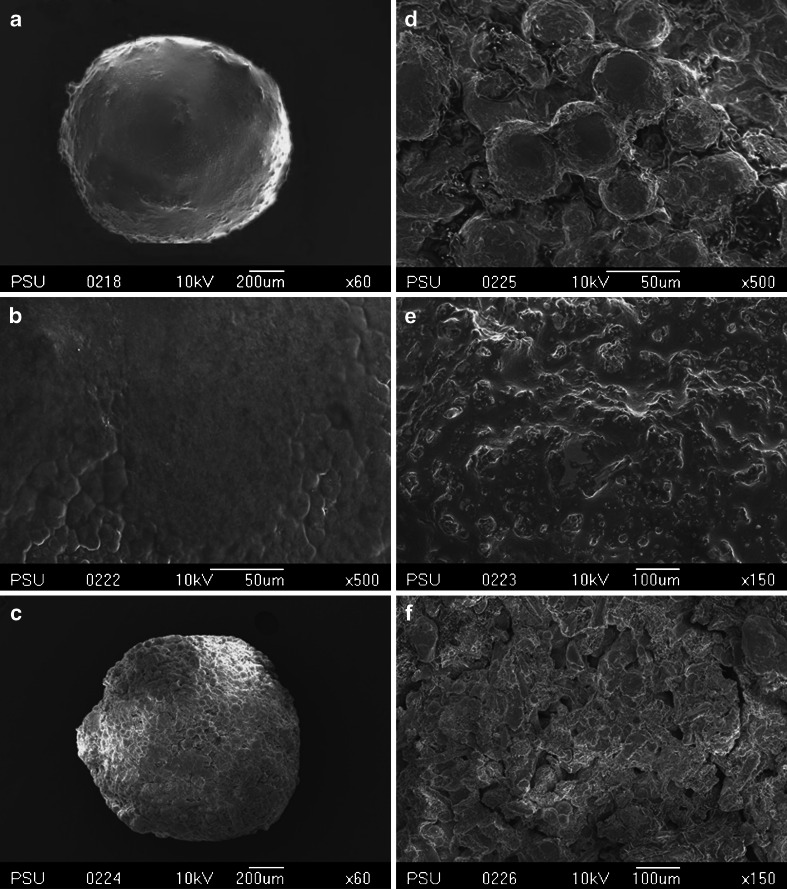
The imaging technique of the SEM can offer useful information about the morphology and porosity of the surface of the pellets. Therefore, SEM can be used to image the microstructures before and after the release of THC. Representative SEM images of the surface and cross-section of the matrix pellets (F2) after 8 h of THC release revealed that the pellets had a spherical shape (Fig. 5a). The THC-SEDDS appears to be dispersed on the surface of the pellets and is also entrapped in the matrices (5B and 5E). Fig. 5d shows that the surface of the pellets is full of pinholes, which probably allows the ingress of the aqueous phase into the matrix and/or allows for diffusion of the entrapped liquid THC-SEDDS out of the surface and core of the matrix. In either case, the liquid THC-SEDDS forms an oil-in-water microemulsion that is released through these mini-channels, thus making the THC ready for absorption as an oily droplet solution. The cross-section of the THC-SEFDDS after drug release also has a large number of narrow crevices on its cut surface, which is probably a reflection of the multiplicity of its porosity (Fig. 5f). Features such as the internal mini-tunnels with multiple voids have been described as a “microbore-network structure” (27).
Fig. 5.
Representative SEM micrographs of the surfaces and cross-sections of the THC self-emulsifying floating pellet formulation (F2). a (×60) and b (×500) surface of pellets before THC release; c (×60) and d (×500) surface of pellets after THC release; e (×150) cross-section of pellets before THC release; f (×150) cross-section of pellets after THC release
Effects of Formulation Variables on Floating Properties
The floating system should ideally allow for floating in a few minutes after contact with a gastric fluid to prevent the dosage forms from transiting into the small intestine (28). The influence of the waxy material and the effect of the disintegrant on floating ability were examined in this study. All the formulations of THC-SEFDDS pellets floated immediately upon contact with the release medium. This feature might be due to the hydrophobicity and low density (0.46 g/mL) of glyceryl behenate, which is a waxy material.
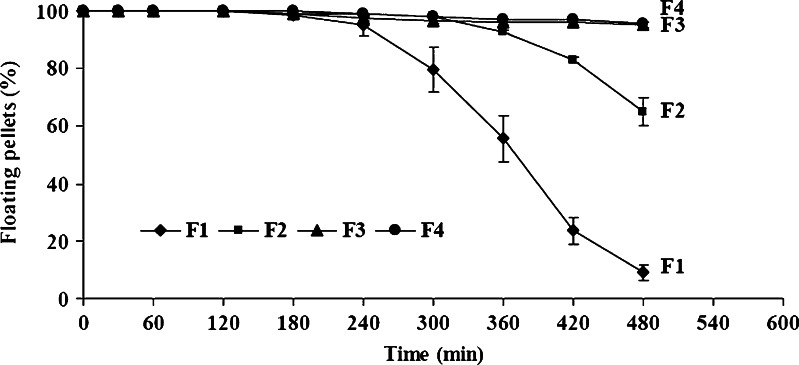
Figure 6 indicates the relationship between the percentages of pellets remaining afloat and time, with formulations that contained increasing proportions of the wax. Up to 3 h, all the four formulations behaved identically in that greater than 95% of the pellets remained afloat. After this time, only formulations that contained higher levels of glyceryl behenate (F3 and F4, containing 60 and 65% w/w, respectively) retained good floating ability, with greater than 95% of pellets still afloat up to 8 h. With formulations F1 and F2 (containing 50% and 55% w/w, respectively, of glyceryl behenate) floating ability gradually declined between 3 and 8 h, until only 10% of the pellets of formulation F1 and about 65% of the pellets of formulation F2 remained afloat at 8 h. It is clear that, as the levels of glyceryl behenate are increased, the lower water permeability of this waxy inert matrix helps maintain a higher floating efficiency for formulations F3 and F4.
Fig. 6.
Floating ability of THC-SEFDDS pellet formulations with different proportions of glyceryl behenate. Values represent a mean±SD of three replicates. Note: formulation F2 has the same composition as formulation F6 (Fig. 7)
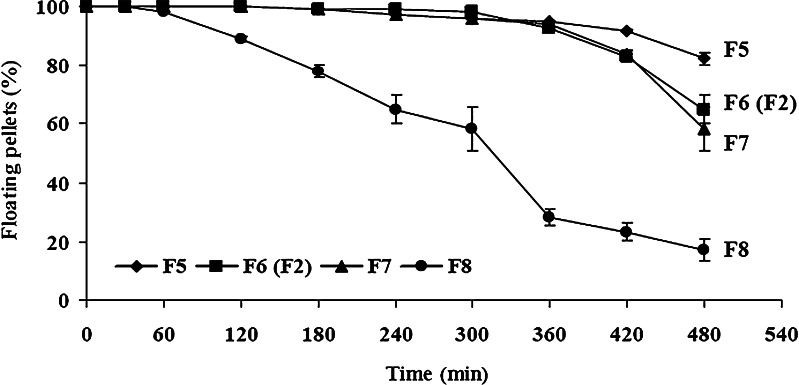
Figure 7 depicts the effect of increasing sodium starch glycolate levels, while maintaining glyceryl behenate at a fixed 55% w/w level. Increasing the amount of sodium starch glycolate from 0% to 3.2% w/w led to a decrease in floating efficiency. When this disintegrant was not incorporated (F5), 83% of the pellets remained afloat until 8 h. In contrast, incorporation of 3.2% w/w of sodium starch glycolate (F8) resulted in a large-scale disintegration of the floating pellets, with floating efficiency decreasing rapidly from about 1 h (95%) until only about 15% of the pellets remaining afloat at 8 h. The higher concentration of sodium starch glycolate clearly resulted in rapid uptake of water, followed by rapid and enormous swelling which resulted in easy pellet disintegration.
Fig. 7.
Floating ability of THC-SEFDDS pellet formulations with different proportions of sodium starch glycolate. Values represent a mean±SD of three replicates. Note: formulation F6 has the same composition as formulation F2 (Fig. 6)
The Effect of Formulation Variables on THC Release In Vitro
The release behavior of THC in the self-emulsifying system within the pellets from the matrix floating pellets was investigated. The liquid THC-SEDDS was apparently released with ease from the pellets to form a fine oil-in-water THC microemulsion which imparted a transparency to the clear aqueous medium.
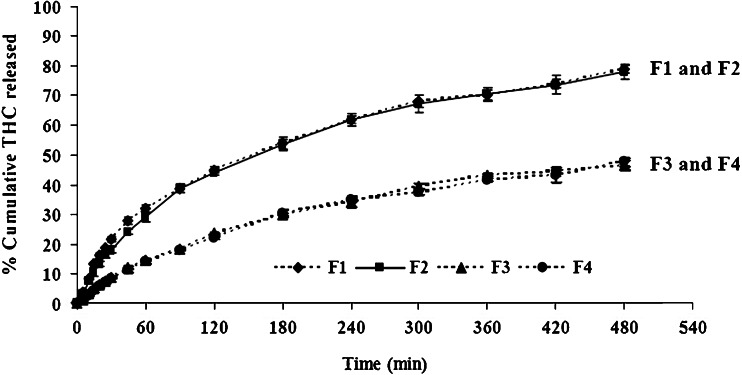
Figure 8 shows the release profiles for THC from the matrices with different proportions of glyceryl behenate (formulations F1–F4). The pellets prepared with 50% and 55% w/w of glyceryl behenate (F1 and F2) released nearly 80% of THC, while pellets containing 60–65% w/w of the wax material (F3 and F4) released only 45–48% of THC within 8 h. Therefore, increasing the amount of glyceryl behenate in the matrices resulted in a decrease in the release rate. The faster release of THC from the floating pellets with a lower amount of glyceryl behenate is probably explained by the higher penetration of the dissolution medium into the matrices.
Fig. 8.
Release profiles of THC from THC-SEFDDS with different proportions of glyceryl behenate in simulated gastric fluid (SGF, pH 1.2) without pepsin. Data represents the mean±SD (n = 3). Note: formulation F2 has the same composition as formulation F6 (Fig. 9)
Matrix floating pellets containing different proportions of sodium starch glycolate were prepared (formulations F5–F8) to examine the effect of this disintegrant on the release rate of THC. The combined use of sodium starch glycolate increases the THC release rate from the wax-based matrices significantly, see Fig. 9. Sodium starch glycolate in the range of 0.8–1.6% w/w (F6 and F7) resulted in a better controlled drug release and gradually increased the THC release to nearly 80% within 8 h. However, when the sodium starch glycolate level was increased to 3.2% w/w (F8), the release rate increases considerably, and therefore some of the controlled release ability is lost. Sodium starch glycolate that is homogeneously dispersed into the matrix provides water absorption and good swelling properties. The greater amount of sodium starch glycolate apparently creates a higher porosity and produces more channels in the matrices. This facilitates a more rapid penetration of the aqueous medium into the matrix. The drug embedded inside the matrix as an oily solution could therefore be released as an o/w microemulsion through the porous waxy networks.
Fig. 9.
Release profiles of THC from THC-SEFDDS with different proportions of sodium starch glycolate in simulated gastric fluid (SGF, pH 1.2) without pepsin. Data represents the mean±SD (n = 3). Note: formulation F6 has the same composition as formulation F2 (Fig. 8)
To explain the release kinetics of THC in the self-emulsifying system from the wax-based-matrix pellets, the mean data for the fraction of THC released between 5% and 80% was subjected to linear regression analyses using conventional mathematical models (29). The data for drug release, where different proportions of glyceryl behenate (F1–F4) and sodium starch glycolate (F5–F8) were used, was fitted to Higuchi's square root of time model and gave R2 close to 1 for all the pellet formulations. The release of THC from the lipidic matrices appeared to be a diffusion-controlled mechanism. THC was mainly released by diffusion through the channels formed in the matrix. The aqueous medium would continuously penetrate into these channels, leading to a gradual increase in formation of the o/w emulsion-solubilized drug in the surface and deeper sites of the waxy matrix (Table V).
Table V.
Coefficients of Determination from Linear Regression Analyses Using Various Mathematical Models
| Ingredient levels (% w/w) | Zero-order Q t = K 0 t + Q 0 | First-order Log Q t = k1t + log Q 0 | Higuchi Q t = K H t 1/2 |
|---|---|---|---|
| Glyceryl behenate | |||
| 50 (F1) | 0.9039 | 0.6323 | 0.9864 |
| 55 (F2a) | 0.9061 | 0.6391 | 0.9872 |
| 60 (F3) | 0.9408 | 0.6717 | 0.9958 |
| 65 (F4) | 0.9455 | 0.7090 | 0.9960 |
| Sodium starch glycolate | |||
| 0.0 (F5) | 0.8561 | 0.5456 | 0.9642 |
| 0.8 (F6a) | 0.9061 | 0.6391 | 0.9872 |
| 1.6 (F7) | 0.9100 | 0.6531 | 0.9878 |
| 3.2 (F8) | 0.7314 | 0.5130 | 0.8834 |
aFormulations F2 and F6 are identical
In Vitro Release of THC from the Optimum Formulation (F2) Compared with Release from THC-SEDDS and Unformulated THC
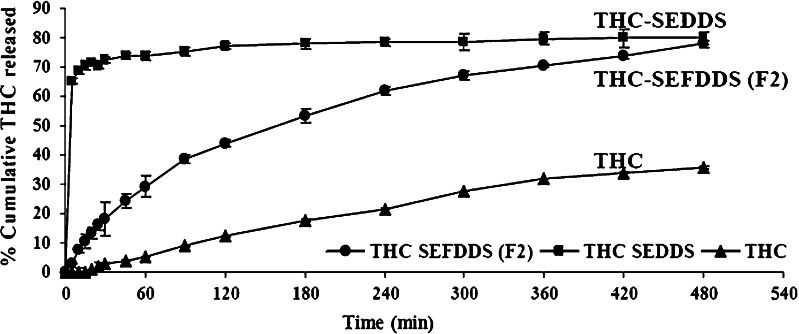
Base on the data from various earlier experiments, formulation F2 was judged to be the ideal formulation. This formulation utilized 55% w/w of glyceryl behenate and 0.8% w/w of sodium starch glycolate; the floating efficiency of F2 at 6 h was greater than 93%, and it provided a controlled release of 80% of the THC over an 8-h period. A comparative release study of unformulated THC, THC-SEDDS, and THC-SEFDDS (formulation F2) was carried out to ascertain the superiority of F2 over the THC and THC-SEDDS formulation. THC alone, as expected for a compound with low aqueous solubility, dissolved only slowly and only 30% of the drug was in solution at 8 h. THC release from the F2 floating pellets was nearly complete within 8 h. Liquid THC-SEDDS, as expected, release the THC almost instantly, since it rapidly forms an o/w microemulsion. Therefore, only the new developed THC-SEFDDS affords a controlled release of THC of about 80% of THC over an 8-h period (Fig. 10).
Fig. 10.
Release profiles of THC from THC-SEFDDS (F2) compared with release from THC-SEDDS and dissolution of unformulated THC in simulated gastric fluid (SGF, pH 1.2) without pepsin. Data represents the mean±SD (n = 3)
Stability Studies
There were insignificant variations found for the SEFDDS pellet formulation (F2) after being stored for various times under the influence of environmental factors such as temperature and humidity. There were no significant changes in the appearance, particle size, and the drug content up to 6 months under both intermediate storage condition (30 ± 2°C, 65 ± 5 %RH) and accelerated storage condition (45 ± 2°C, 75 ± 5 %RH; Table VI).
Table VI.
Stability Data of Capsules Filled with THC-SEFDDS (F2) Pellets
| Sampling time | Appearance | Particle size (nm)a | PDI | % Drug content |
|---|---|---|---|---|
| 30°C/65%RH | ||||
| 0 month | White spherical shape | 26.5 ± 0.1 | 0.081 ± 0.006 | 103.61 ± 2.49 |
| 3 month | White spherical shape | 26.9 ± 0.1 | 0.089 ± 0.011 | 102.81 ± 2.09 |
| 6 month | White spherical shape | 27.7 ± 0.2 | 0.073 ± 0.009 | 101.21 ± 1.27 |
| 45°C/75%RH | ||||
| 0 month | White spherical shape | 26.5 ± 0.1 | 0.081 ± 0.006 | 103.61 ± 2.49 |
| 3 month | White spherical shape | 32.3 ± 0.2 | 0.057 ± 0.018 | 101.43 ± 2.52 |
| 6 month | White spherical shape | 32.0 ± 0.2 | 0.082 ± 0.029 | 101.69 ± 2.02 |
THC-SEFDDS tetrahydrocurcumin-self-emulsifying floating drug delivery system, PDI polydispersity index, RH relative humidity
aData reported are mean±SD (n = 3)
CONCLUSIONS
In this study, a novel SEFDDS was successfully developed. The THC floating pellet formulations contained 70% mixtures of two surfactants: Cremophor EL and Labrasol (1:1), and 30% mixtures of oil/Labrafac PG and Capryol 90 (1:1) with silicon dioxide, glyceryl behenate, pregelatinized starch, sodium starch glycolate, and microcrystalline cellulose. The SEFDDS pellets readily released the lipid phase to form a fine oil-in-water emulsion, with a particle size in the range of 26.4–32.5 nm. Glyceryl behenate was used to impart a floating ability to the pellets, and this waxy material also provided a controlled release matrix from which THC was released gradually over an 8-h period in a simulated gastric fluid. Sodium starch glycolate increased the release rate without unduly affecting the controlled release matrix. In vitro release studies revealed that the THC-SEFDDS pellet formulation (F2) almost completely released the THC within 8 h. The release of about 80% of THC from THC-SEFDDS as an o/w microemulsion, compared with only 30% by an aqueous solution from the unformulated THC, indicates that the absorption from the THC-SEFDDS formulation should be considerably greater. The capsules filled with THC-SEFDDS were found to be stable over a period of 6 months under intermediate and accelerated storage conditions. These self-emulsifying floating pellets may provide a useful solid lipid-based dosage form for oral delivery of THC and other hydrophobic compounds.
Acknowledgments
Financial support was received from the Thailand Research Fund under the Royal Golden Jubilee Ph.D. program (PHD/0150/2549) and the Royal Thai Government through Prince of Songkla University. We also wish to thank Professor L.A. Damani and Dr. Brian Hobson for editing our manuscript.
References
- 1.Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm Res. 1995;12:1561–1572. doi: 10.1023/A:1016268311867. [DOI] [PubMed] [Google Scholar]
- 2.Charman SA, Charman WN, Rogge MC, Wilson TD, Dutko FJ, Pouton CW. Self-emulsifying drug delivery systems: formulation and biopharmaceutic evaluation of an investigational lipophilic compound. Pharm Res. 1992;9:87–93. doi: 10.1023/A:1018987928936. [DOI] [PubMed] [Google Scholar]
- 3.Shah NH, Carvajai MT, Patel CI, Infeld MH, Malick AW. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int J Pharm. 1994;106:15–23. doi: 10.1016/0378-5173(94)90271-2. [DOI] [Google Scholar]
- 4.Tuleu C, Newton M, Rose J, Euler D, Saklatvala R, Clarke A, Booth S. Comparative bioavailability study in dogs of a self-emulsifiying formulation of progesterone presented in a pellet and a liquid form compared with an aqueous suspension of progesterone. J Pharm Sci. 2004;93:1495–1502. doi: 10.1002/jps.20068. [DOI] [PubMed] [Google Scholar]
- 5.Serrtoni M, Newton M, Booth S, Clarke A. Controlled drug release from pellets containing water-insoluble drugs dissolved in a self-emulsifying system. Eur J Pharm Biopharm. 2007;65:94–98. doi: 10.1016/j.ejpb.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Desai S, Bolton S. A floating controlled-release drug delivery system: in vitro–in vivo evaluation. Pharm Res. 1993;10(9):1321–1325. doi: 10.1023/A:1018921830385. [DOI] [PubMed] [Google Scholar]
- 7.Streubel A, Siepmann J, Bodmeier R. Floating matrix tablets based on low density: effects of formulation and processing parameters on drug release. Eur J Pharm Sci. 2003;18:37–45. doi: 10.1016/S0928-0987(02)00223-3. [DOI] [PubMed] [Google Scholar]
- 8.Elkheshen SA, Yassin AEB, Alsuwayeh S, Alkhaled FA. In vitro and in vivo evaluation of floating controlled release dosage forms of verapamil hydrochloride. Pharm Ind. 2004;66(11):1364–1372. [Google Scholar]
- 9.Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumor and antioxidant activity of natural curcuminnoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-J. [DOI] [PubMed] [Google Scholar]
- 10.Gescher AJ, Sharma RA, Steward WP. Cancer chemoprevention by dietary constituents: a tale of failure and promise. Lancet Oncol. 2001;2:371–379. doi: 10.1016/S1470-2045(00)00392-2. [DOI] [PubMed] [Google Scholar]
- 11.Ireson CR, Jones DJL, Orr S, Coughtrie MWH, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomark Prev. 2002;11:105–111. [PubMed] [Google Scholar]
- 12.Lukita AW, Ito Y, Baker GL, McCuskey RS. Effect of curcuminoids as anti-inflammatory agents on the hepatic microvascular response to endotoxin. Shock. 2002;17:399–403. doi: 10.1097/00024382-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Chattopadhyay I, Bandyopadhyay U, Biswas K, Maity P, Banerjee RK. Indomethacin inactivates gastric peroxidase to induce reactive-oxygen-mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radic Biol Med. 2006;40:1397–1408. doi: 10.1016/j.freeradbiomed.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JJ, Mukhtar H. Curcumin for chemopreventive of colon cancer. Cancer Lett. 2007;255:170–181. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–494. [PubMed] [Google Scholar]
- 16.Sugiyama Y, Kawakishi S, Osawa T. Involvement of the ß-diketone moiety in the antioxidant mechanism of tetrahydrocurcumin. Biochem Phamacol. 1996;52:519–525. doi: 10.1016/0006-2952(96)00302-4. [DOI] [PubMed] [Google Scholar]
- 17.Sutanata W, Craig DQ, Newton JM. An evaluation of the mechanisms of drug release from glyceride bases. J Pharm Pharmacol. 1995;47:182–187. doi: 10.1111/j.2042-7158.1995.tb05775.x. [DOI] [PubMed] [Google Scholar]
- 18.Barthelemy P, Laforet JP, Farah N, Joachim J. Compritol 888 ATO: an innovative hot-melt coating agent for prolonged-release drug formations. Eur J Pharm Biopharm. 1999;47:87–90. doi: 10.1016/S0939-6411(98)00088-5. [DOI] [PubMed] [Google Scholar]
- 19.Faham A, Prinderre P, Piccerelle P, Farah N, Joachim J. Hot melt coating technology: influence of Compritol 888 Ato and granule size on chloroquine release. Pharmazie. 2000;55:444–448. [PubMed] [Google Scholar]
- 20.Mirghani A, Idkaidek NM, Salem MS, Najib NM. Formulation and release behavior of diclofenac sodium in Compritol 888 matrix beads encapsulated in alginate. Drug Dev Ind Pharm. 2000;26:791–795. doi: 10.1081/DDC-100101301. [DOI] [PubMed] [Google Scholar]
- 21.Abdalla A, Maeder K. Preparation and characterization of a self-emulsifying pellet formulation. Eur J Pharm Biopharm. 2007;66:220–226. doi: 10.1016/j.ejpb.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Hamdani J, Goole J, Moes AJ, Amighi K. In vitro and in vivo evaluation of floating riboflavin pellets developed using the melt pelletization process. Int J Pharm. 2006;323:86–92. doi: 10.1016/j.ijpharm.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 23.Narang AS, Delmarre D, Gaoc D. Stable drug encapsulation in micelles and microemulsions. Int J Pharm. 2007;345:9–25. doi: 10.1016/j.ijpharm.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 24.Spernath A, Aserin A, Garti N. Fully dilutable microemulsions embedded with phospholipids and stabilized by short-chain organic acids and polyols. J Colloid Interface Sci. 2006;299:900–909. doi: 10.1016/j.jcis.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Sun J, Wang Y, Liu X, Liu Y, Fu Q, Meng P, He Z. Solid selfemulsifying nitrendipine pellets: preparation and in vitro/in vivo evaluation. Int J Pharm. 2010;383:1–6. doi: 10.1016/j.ijpharm.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Podczeck F. A novel aid for the preparation of pellets by extrusion/spheronization. Pharm Tech Eur. 2008;20:26–31. [Google Scholar]
- 27.Li FQ, Hu JH, Deng JX, Su H, Xu S, Liu JY. In vitro controlled release of sodium ferulate from Compritol 888 ATO-based matrix tablets. Int J Pharm. 2006;324:152–157. doi: 10.1016/j.ijpharm.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Iannuccelli V, Coppi G, Bernabei MT, Cameroni R. Air compartment multiple-unit system for prolonged gastric residence. Part I. Formulation study. Int J Pharm. 1998;174:47–54. doi: 10.1016/S0378-5173(98)00229-4. [DOI] [Google Scholar]
- 29.Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–133. doi: 10.1016/S0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]