Abstract
Pulmonary delivery of therapeutic peptides and proteins has many advantages including high relative bioavailability, rapid systemic absorption and onset of action and a non-invasive mode of administration which improves patient compliance. In this study, we investigated the effect of spray-drying (SD) and spray freeze-drying processes on the stability and aerosol performance of parathyroid hormone (PTH) (1-34) microparticles. In this study, the stabilisation effect of trehalose (a non-reducing sugar) and Brij 97 (a non-ionic surfactant) on spray-dried PTH particles was assessed using analytical techniques including circular dichroism (CD), fluorescence spectroscopy, modulated differential scanning calorimetry and an in vitro bioactivity assay. Physical characterisation also included electron microscopy, tap density measurement and laser light diffraction. The aerosol aerodynamic performance of the formulations was assessed using the Andersen cascade impactor. Based on these studies, a formulation for spray freeze-drying was selected and the effects of the two particle engineering techniques on the biophysical stability and aerosol performance of the resulting powders was determined. CD, fluorescence spectroscopy and bioactivity data suggest that trehalose when used alone as a stabilising excipient produces a superior stabilising effect than when used in combination with a non-ionic surfactant. This highlights the utility of CD and fluorescence spectroscopy studies for the prediction of protein bioactivity post-processing. Therefore, a method and formulation suitable for the preparation of PTH as a dry powder was developed based on spray-drying PTH with trehalose as a stabiliser with the bioactivity of SD PTH containing trehalose being equivalent to that of unprocessed PTH.
Key words: parathyroid hormone, pulmonary delivery, spray drying, spray freeze-drying, stability
INTRODUCTION
Bone disorders such as osteoporosis are a common occurrence in the aging population. An estimated 150 million people are known to suffer from osteoporosis worldwide (1). Barret-Connor predicted that the number of hip fractures in the USA will exceed 650,000 and are likely to cost US $240 billion by 2045 (2). Parathyroid hormone (PTH), used either alone or in combination with other existing therapies has been recommended as the ideal treatment for osteoporosis (1). PTH is presently being marketed as Forteo® by Eli Lilly for administration via the subcutaneous (SC) route (3). Although, clinical trials have shown that injected PTH (1-34) increases both bone mineral density and bone mineral content in comparison to placebo, concerns still exist regarding its safety (3,4). The reported adverse effects experienced with the parenteral administration of PTH (1-34) and the inconvenience of chronic parenteral administration to the patient suggest that there is a need for the exploitation of alternative routes of administration.
The large absorptive surface area, thin alveolar epithelium, avoidance of first pass-hepatic metabolism, elevated blood flow and relatively low metabolic activity of the lungs are some of the advantages in delivering peptides by inhalation (5). Since many proteins do not have adequate long-term storage stability in a solution form, dry powder formulations are preferred (6). This is because molecular mobility of a protein is greatly reduced in the solid state resulting in improved biochemical stability (7). Dry powders are also favoured from a bioavailability standpoint. Transport studies conducted in our laboratory using human bronchial epithelial cell monolayers (Calu-3) showed that the level of PTH (1-34) transport was threefold higher with the dry powder formulation than with the solution formulation. This finding was in line with the study by Kobayashi et al. who found significant increase in the pulmonary bioavailability of salmon calcitonin when it was administered as a dry powder formulation to rats compared to the liquid formulation (8). Two studies have previously used PTH in a dry powder formulation for inhalation. One by Mannkind pharmaceuticals used the Technosphere technology that involved adsorbing PTH (1-34) onto proprietary, diketopiperazine-based excipients. Using this formulation, a relative bioavailability of 48% was reported in a clinical trial with ten healthy human volunteers (9). The other study by Codrons et al. used spray drying to produce inhalable dry powder formulations of PTH (1-34) containing a lipid, dipalmitoyl phosphatidyl choline as the main excipient (10). This study reported an absolute PTH (1-34) bioavailability of 34% after intra-tracheal administration of the formulation in rats.
The delivery of proteins and peptides through the lungs presents unique challenges including efficient delivery of expensive biomolecules to the lower respiratory tract, avoiding rapid clearance and degradation in the lungs and finally facilitating peptide transport across the airway epithelium. For optimal absorption of peptide particles via the lungs, an aerodynamic diameter of 1–5 μm is needed to reduce the loss of the drug either through inertial impaction of large particles (>5 μm) in the oro-pharyngeal region of the airways during inhalation or loss of small particles (<1 μm) by exhalation (11). Particle engineering can be used to prepare these inhalable particles. However, peptides are vulnerable to some of the stresses involved in many particle engineering processes including spray-drying and spray freeze-drying processes due to the lability of their higher order structures (12,13). Excipients, e.g. sugars and non-ionic surfactants are normally used to protect proteins during particle engineering (14,15).
The main focus of studies on inhaled PTH (1-34) has been on the bioavailability of the molecule in the lungs but no studies to-date have assessed the effect of the formulation manufacturing process on the conformational stability and biological activity of PTH (1-34) (9,10). In this study, we investigated the effect of spray drying and spray freeze-drying on the stability and aerosol performance of PTH (1-34) microparticles prepared for inhalation. Adsorption of proteins at the air–liquid interface or exposure to high temperatures can have an adverse impact on protein stability. Non-reducing sugars such as trehalose and sucrose are normally used to stabilise proteins against denaturation by acting as replacements for water molecules by forming hydrogen bonds with the protein molecule. This prevents/reduces protein unfolding and preserves its conformational stability (16). Furthermore, non-ionic surfactants such as Brij products and polysorbates help to exclude proteins from the air–liquid interface created during spray drying and spray freeze-drying and so help to stabilise proteins during these processes (6).
In this study, the stabilisation effect of trehalose (a non-reducing sugar) and Brij 97 (a non-ionic surfactant) on engineered peptide particles was assessed using analytical techniques such as circular dichroism, fluorescence spectroscopy, modulated differential scanning calorimetry (MDSC), and in vitro bioactivity assay. Physical characterisation was also performed using SEM, tap density measurement and laser light diffraction. The aerodynamic performance of the formulations was assessed using the Andersen cascade impactor.
MATERIALS AND METHODS
Materials
Recombinant human parathyroid hormone (rhPTH 1-34) acetate (4,117.8 Da) was supplied by Polypeptide Laboratories, California, USA. α-Trehalose dihydrate (>99.5%) was supplied by Fluka Biochemika, USA. Brij 97 (polyethylene glycol monooleyl ether), acetonitrile CHROMASOLV®, HPLC grade, sodium chloride (>99%) were all supplied by Sigma-Aldrich, Chemie GmbH, Buchs, Switzerland. Trifluoroacetic acid, Laboratory reagent grade was supplied by Fischer Scientific, Loughborough, UK.
Methods
Particle Manufacture
Spray Drying
Five milligrammes per milliltre of PTH (1-34) solutions were prepared by dissolving the appropriate amount of the peptide powder in water. Where appropriate, 25 mg/ml α-trehalose dihydrate and/or 0.29 mg/ml Brij 97 were added as stabilisers. The concentration of the Brij 97 used was its critical micelle concentration in water (0.29 mg/ml). Control formulations containing only 25 mg/ml of α-trehalose dihydrate were also prepared (see Table I for the various formulations spray dried). All the formulations were corrected to pH 7 using 0.1 M NaOH solution. Spray drying was performed using the B290 mini-spray dryer (Bϋchi, Switzerland). A nozzle size of 0.7 mm was used. The atomising air-flow rate was set 600 nL/h while the feed rate was 5 ml/min. The aspirator was set at 90% of its maximum capacity and the inlet temperature was 97°C. These parameters led to an outlet temperature of 47 ± 2°C. The powder yields ranged between 26–76%.
Table I.
Formulations of PTH (1-34)
| Formulation | PTH (mg/ml) | Trehalose (mg/ml) | Brij 95 (mg/ml) | Ratio by weight |
|---|---|---|---|---|
| 1a | 5 | 25 | – | 1:5 |
| 2 | 5 | – | – | – |
| 3 | 5 | 25 | 0.29 | 1:5:0.058 |
| 4 | – | 25 | – | – |
aThis formulation was both spray dried and spray freeze-dried while the rest were only spray dried
Spray Freeze-drying
For the spray freeze-drying process, solutions containing 5 mg/ml PTH (1-34) and 25 mg/ml of α-trehalose dihydrate that was atomised using a 0.7 mm co-axial nozzle (Bϋchi, Switzerland) into a container filled with liquid nitrogen at a feed rate of 5 ml/min. The atomising air-flow rate was set at 600 nL/h. The slurry formed was left for approximately 5 min on a bench to allow the nitrogen to evaporate before loading into a freeze dryer (Labconco Freezone 6, Missouri). The primary drying process was performed at −55°C and lasted for 30 h at 0.40 mbar before the secondary drying processed was initiated at approximately 25°C. This process lasted for 18 h at 0.13 mbar. The powder yield was approximately 98%.
Physical Characterisation
Scanning Electron Microscopy
The morphologies of the manufactured and unprocessed peptide particles were observed under Tescan Mira XMU Variable Pressure Field Emission Scanning Electron Microscope (Tescan USA Inc., USA). Powders were mounted onto aluminium stubs using double sided adhesive tape and were then made electrically conductive by coating in a vacuum with a thin layer of gold. The coated specimen was then examined under the microscope operated at an acceleration voltage of 5 kV.
Particle Size Analysis
The size distribution of manufactured powders was determined by laser light scattering with a Malvern MasterSizer Sirocco 2000 (Malvern Instrument Ltd., Worcestershire, UK). Approximately 25 mg of each powder was weighed into the sample holder. This bulk powder was then dispersed using an air pressure of 3 bar, feed rate of 100% for the bulk powder and an obscuration limit of <10%. Measurements were performed in triplicate.
Tap Density Measurements
The powder density (ρ) was determined by tap density according to the method used by Codrons et al. (2004). Briefly, specific amounts of the powder were weighed into a 5-ml measuring cylinder. The cylinder was then tapped 1,000 times to allow the density to plateau. The final volume was recorded and the tapped density determined as the ratio of the weight and the volume in grammes per millilitre. Measurements were performed in triplicate.
Circular Dichroism
Circular dichroism (CD) measurements were performed with Aviv CD Spectrophotometer 410 (Aviv Biomedical, Inc. New Jersey) using 80 μg/ml of reconstituted solution of PTH (1-34) in water. CD spectra were obtained in the far UV region (260–190 nm) using a quartz cell of 0.1 cm path length in order to probe the stability of the secondary structures of the engineered microparticles of PTH (1-34). A resolution of 0.1 nm and a scanning speed of 6 nm/min with a 2-s response time were applied. The CD signals were converted to molar ellipticity after an accumulation of five scans for each sample.
Intrinsic Fluorescence Spectroscopy
Intrinsic fluorescence spectra were obtained using Perkin-Elmer LB55 Luminescence Spectrometer. Intrinsic fluorescence was monitored between 300 and 420 nm, with an excitation wavelength of 280 nm. The excitation and emission slits were set to 10 mm. One microgramme per millilitre solution of PTH (1-34) was used to generate an accumulation of five scans for each sample.
In Vitro Bioactivity Assay for PTH (1-34)
Stimulation of cyclic AMP (cAMP) synthesis was used to determine the in vitro bioactivity of PTH (1-34) (17). MC3T3-E1 (subclone 4) cells were grown in minimum essential medium alpha medium (GIBCO) with 10% fetal bovine serum and 100 units/ml of penicillin and streptomycin. The cells were passaged every 4–5 days. They were plated at 50,000 cells/cm2 in 12-well plates and induced to differentiate with the addition of ascorbic acid (50 μg/ml) and β-glycerophosphate (10 mM). The cells were used for the bioactivity assay after 5–7 days, when maximal expression of the receptor for PTH (1-34) is reported to occur (18). The confluent cells in each well were treated for 5 min with 500 μl of 0.5 mM of phosphodiesterase inhibitor, isobutylmethylxanthine (IBMX; Sigma) in Kreb’s ringer buffer (KRB). To perform the assay, five different samples from each formulation were weighed out and dissolved in KRB to achieve a PTH (1-34) concentration of 100 ng/ml. Following treatment with IBMX, the cells were washed with KRB and subsequently treated with PTH (1-34) containing samples as prepared above. Samples equivalent to 45 ng of PTH (1-34) were added to each well. After incubating for 20 min at 37°C, cells were lysed using a lysis buffer (Catalog No. 250006, Cell Biolabs, Inc., CA, USA) and incubated at 4°C for 20 min. The cells were then scraped off the surface using a cell scraper. The cell suspension was then transferred to a centrifuge tube and stored at −20°C until analysed for cAMP. cAMP was measured using an enzyme immunoassay kit (Biomedical Technologies Inc., Stoughton, MA) following the manufacturer’s instructions. The frozen samples in the centrifuge tubes were thawed and centrifuged at 10,000 rpm for 10 min. The supernatants were used for cAMP estimation.
Differential Scanning Calorimetry
Solid-state thermal stability of PTH (1-34) particles was determined by MDSC using a Q100 MDSC (TA Instruments, Leatherhead, UK). Approximately 10 mg of PTH (1-34) powder was weighed into an aluminium pan, which was hermetically sealed. An empty hermetically sealed pan was loaded into the reference compartment while sample-containing pan was placed in the sample compartment. Equilibration was performed at −60°C. Sample was heated at a rate of 2°C/min between −60 and 250°C.
Andersen Cascade Impactor Testing (ACI)
The in vitro deposition patterns of the peptide formulations were assessed using the eight-stage ACI (Copley Scientific, Nottingham, UK). The flow rate through the ACI was set to 28.3 l/min using a mass flow metre (Copley Scientific, Nottingham, UK). For each formulation, powder containing an equivalent of 1 mg of peptide was weighed into HPMC capsules before loading the capsules into a Spinhaler® (Fisons Pharmaceuticals, UK) dry powder device. The content of the capsule was then sampled by the ACI before collection of the plates on each stage and washing with deionized water. The content of peptide on each plate was quantified by UV absorption at 280 nm.
Results and Discussion
Morphology of Peptide Microparticles
Figure 1 shows the morphologies of unprocessed and engineered PTH (1-34) microparticles. The spray-dried PTH/trehalose particles appear corrugated unlike the spray freeze-dried peptide particles which appear porous. In Fig. 1a, the unprocessed PTH (1-34) particles appear plate-like without any definite shape. However, following the spray drying process, the spray-dried neat PTH (1-34) (Fig. 1c) appear spherical with corrugated surfaces. Particles with such a corrugated morphology have been reported to display a superior flow property in comparison to particles with smooth surfaces (18). This is due to the fact that particles with corrugated surfaces have less inter-particulate contact area which leads to reduced Van der Waal forces. The improved flowability of the corrugated particles ultimately leads to higher fine particle fraction (FPF). The addition of trehalose to the peptide formulations at the ratio of 1:5 (peptide/trehalose) did not produce any discernible changes in the morphology of the microparticles (Fig. 1b, c) as compared with the corresponding neat peptides.
Fig. 1.
Scanning electron micrographs of PTH (1-34) microparticles. a Unprocessed PTH, b spray-dried PTH/trehalose 1:5, c spray-dried neat PTH, d spray-dried PTH/trehalose/Brij 97 (1:5:0.058), e spray-dried neat trehalose and f spray freeze-dried PTH/trehalose (1:5)
An interesting observation was made when Brij 97 was added to the PTH/trehalose formulation (1:5:0.058, PTH/trehalose:Brij 97) (Fig. 1d). While the morphology of the microparticles produced did not change, the particles appear more cohesive, forming agglomerates when compared to the Brij-free spray-dried PTH/trehalose microparticles. The presence of agglomerates may be attributed to the surface enrichment of the particles by the surfactant. The solute concentration of the formulations seemed to have an effect on the morphology and particle size of the spray freeze-dried peptide particles. The formulations containing trehalose (PTH/trehalose, 1:5) produced spherical but porous particles as seen in Fig. 1f.
Particle Size Analysis and Tapped Density Measurements
Table II shows the laser diffractometry-measured particle size distribution of the PTH (1-34) particles produced by both spray drying and spray freeze-drying in comparison to the unprocessed PTH (1-34) particles. While the unprocessed PTH (1-34) particles had a geometric diameter of approximately 27 μm, the spray-dried particles including the neat PTH, PTH/trehalose and PTH/trehalose/Brij had particle sizes between 3 and 4 μm. This suggests that the concentration of the solute did not have any discernible effect on the particle size spray-dried PTH (1-34) particles since all other parameters were kept constant. However, the particle size of the spray freeze-dried PTH/trehalose composite was approximately 13 μm. Particles manufactured by spray freeze-drying have been reported to have bigger particle sizes in comparison to spray-dried particles (19,20). Nevertheless, the porous nature of such particles makes them ideal for inhalation because they tend to have lower densities as seen in Table II, when compared to their spray-dried counterparts. Furthermore, their relatively bigger size helps to avoid engulfment by alveolar macrophages (11,21).
Table II.
Summary of the Particle Size Distribution and Tapped Densities of PTH (n = 3)
| Formulation | Particle size (MMDb, μm)a | Tapped density (g/ml)a |
|---|---|---|
| Unprocessed PTH | 27.3 ± 2.5 | 0.122 ± 0.001 |
| SD neat PTH | 3.9 ± 0.2 | 0.203 ± 0.001 |
| SD PTH/tre | 3.6 ± 0.1 | 0.165 ± 0.002 |
| SD PTH/tre/Brij | 3.2 ± 0.1 | 0.190 ± 0.001 |
| SFD PTH/tre | 12.6 ± 1.5 | 0.007 ± 0.001 |
aMean ± SD
bMass median diameter from laser diffractometry
Circular Dichroism
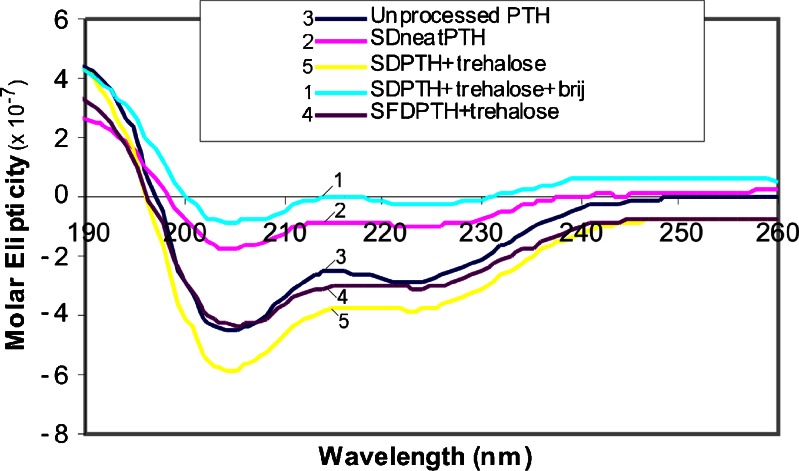
CD is a widely used technique in the determination of both the secondary and the tertiary structures of proteins/peptides (22,23). Information concerning the secondary structures of proteins is normally obtained in the far UV region (190–260 nm) while the near UV region (250–320 nm) gives information about the tertiary structure. The far UV region of the CD spectrum has been explored in this study in order to determine the effect of stabiliser (trehalose and Brij 97) on the secondary structure of PTH (1-34) following particle engineering. Reports from previous CD studies revealed that PTH (1-34) has an α-helical secondary structure (22,23). The result obtained from unprocessed PTH (1-34) is consistent with the published CD data for PTH (1-34) in the far UV spectrum. Figure 2 revealed that the unprocessed PTH (1-34) had an α-helical secondary structure. However, following spray drying, the neat PTH (1-34) spectra showed some perturbation in the secondary structure. The downward loop around 200 to 210 nm was not as intense as in the unprocessed PTH (1-34) which suggests a conversion to β-sheet (22). The same occurrence was observed in the CD spectra for the PTH:trehalose:Brij 97 composite. However, when trehalose alone was used as a stabiliser in the spray-dried and spray freeze-dried PTH (1-34) composites, the secondary structure was restored without any sign of perturbation (Fig. 2).
Fig. 2.
CD spectra (far UV region) of reconstituted PTH microparticles in double-distilled deionized water. From top to bottom: spray-dried PTH/trehalose/Brij 97, spray-dried neat PTH, unprocessed PTH, spray freeze-dried PTH and spray-dried PTH/trehalose
Intrinsic Fluorescent Intensity
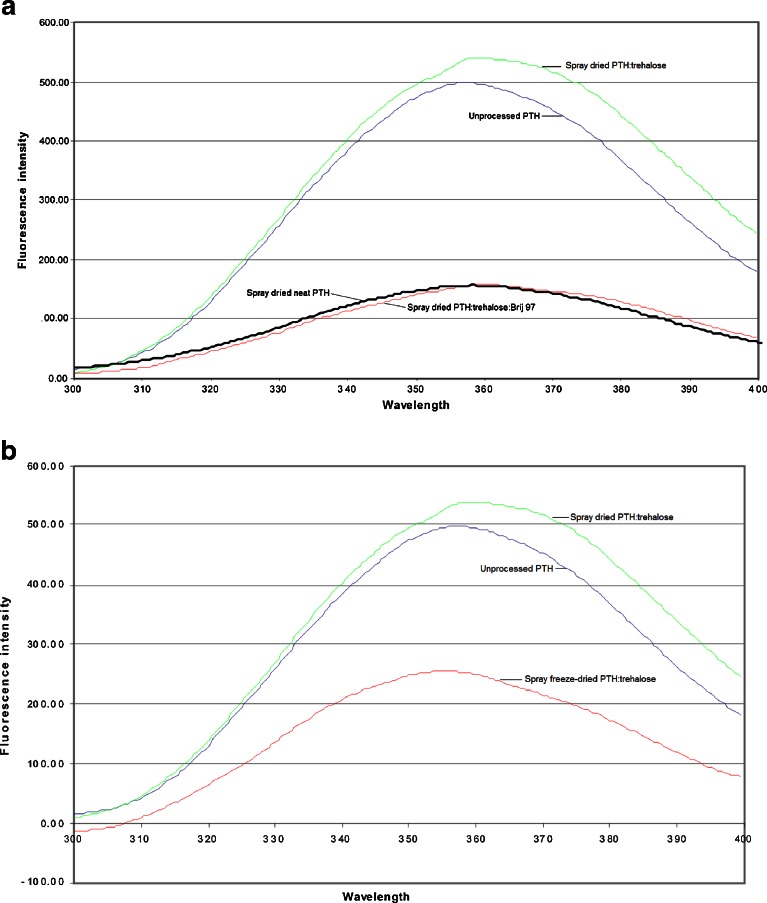
The stability of the tertiary structure of spray-dried and spray freeze-dried PTH (1-34) was determined using intrinsic fluorescence spectroscopy (24). Fluorescence measurement is a high-throughput method used in protein formulation and characterisation studies (25). Proteins that contain the hydrophobic amino acids tryptophan, tyrosine or phenylalanine are used in intrinsic fluorescence assays. Fluorescence intensity provides information on the local conformation and environment of these fluorophores. Being hydrophobic, tryptophan, tyrosine and phenylalanine produce a highly intensive fluorescence emission between 300–400 nm when the protein is in a folded state (25). However, fluorescence quenching occurs when unfolding (denaturation) occurs due to the hydrophobic amino acids being exposed to water (hydrophilic). A decrease in fluorescence intensity is normally observed when perturbations occur in the secondary structure of proteins. Figure 3a shows the fluorescence spectra of the different spray-dried PTH (1-34) formulations studied in comparison to the unprocessed PTH (1-34). The spectra of these PTH (1-34) formulations produced a similar lambda max (approximately 358 nm). However, while the unprocessed PTH (1-34) and the spray-dried PTH/trehalose formulations had maximum fluorescence intensities of 498 and 539 units, respectively, the spray-dried neat PTH (1-34) and PTH/trehalose/Brij formulations had intensities of 156 and 157 units, respectively. Figure 3b represents the fluorescence spectrum of the spray freeze-dried PTH/trehalose formulation in comparison to the corresponding spray-dried formulation and the unprocessed PTH (1-34). The maximum intensity of the spray freeze-dried PTH/trehalose formulation was 257 units in comparison to the 539 observed for its spray-dried counterpart.
Fig. 3.
a Fluorescence spectra comparing the tertiary structures of (from top to bottom) spray-dried PTH/trehalose, unprocessed PTH and spray-dried neat PTH and spray-dried PTH/trehalose/Brij 97. b Fluorescence spectra comparing the tertiary structures of (from top to bottom) spray-dried PTH/trehalose, unprocessed PTH and spray freeze-dried PTH/trehalose
In Vitro Bioactivity Assay of PTH
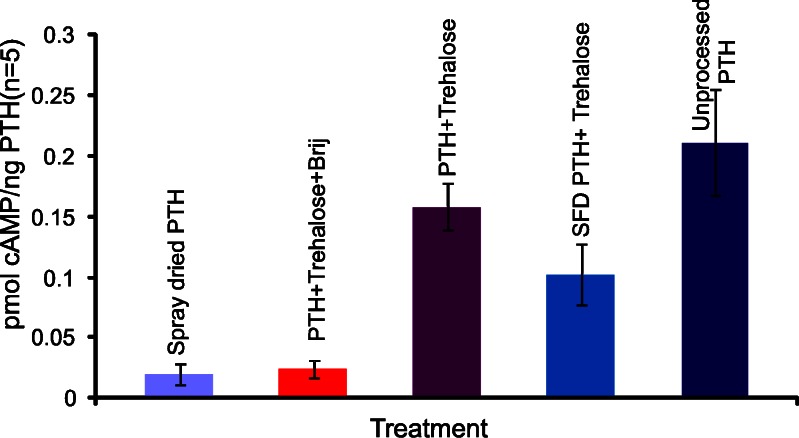
The in vitro activity of the engineered PTH (1-34) particles was determined using a previously published method (17). This is based on the in vitro stimulation of cAMP synthesis. The results are presented in Fig. 4. The in vitro bioactivity data show a correlation with the fluorescence and CD results. The spray-dried neat PTH (1-34) and spray-dried PTH/trehalose/Brij 97 formulations produced the lowest but similar bioactivity, stimulating the synthesis of approximately 0.016 ± 0.004 and 0.024 ± 0.008 pmol cAMP/ng of PTH (1-34) respectively. However, the spray-dried PTH/trehalose formulation produced a bioactivity comparable to that of the unprocessed PTH, 0.162 ± 0.037 pmol cAMP/ng of PTH for the former and 0.217 ± 0.052 pmol cAMP/ng of PTH for the latter. All values are denoted as mean ± SD. t Test statistical analysis showed that there was no significant difference (p > 0.05) in the bioactivity of the two formulations. The spray freeze-dried PTH/trehalose produced an inferior bioactivity (0.099 pmol cAMP/ng of PTH (1-34)) in comparison to its spray-dried counterpart.
Fig. 4.
The in vitro stimulation of cAMP synthesis by the different PTH formulations (mean ± SD; n = 5)
The spray freeze-drying process has been reported to subject proteins to different stresses such as the air–liquid interface and the freeze concentration induced aggregation (12,26). This could explain why the bioactivity of the spray freeze-dried PTH/trehalose formulation was lower than that of the corresponding spray-dried formulation. Nevertheless, spray drying also subjects proteins to the air–liquid interface which has been reported to be the main stress mechanism responsible for the denaturation/aggregation of proteins during the process (11). Data presented in Fig. 4 above suggest that proteins possibly react to stresses in different ways and that the effects of different stresses on the stability of proteins cannot be generalised.
Differential Scanning Calorimetry
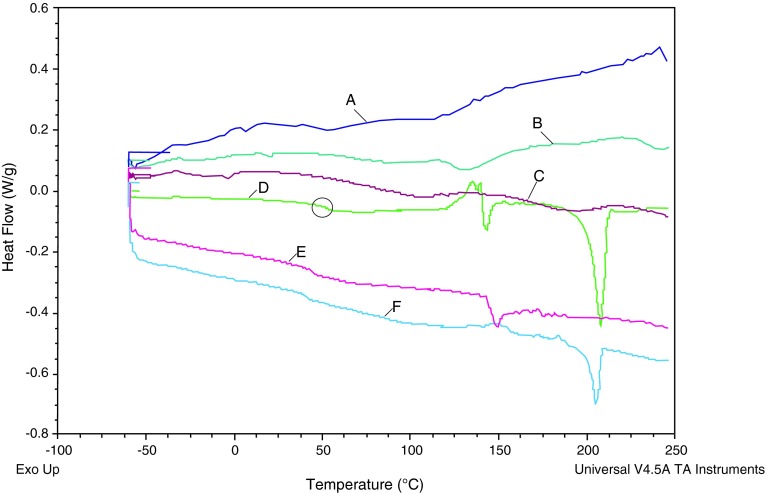
Figure 5 presents the MDSC thermal profiles of the different PTH (1-34) composites. Differential scanning calorimetry (DSC) has been used in this study to help with better understanding of the mechanism through which trehalose stabilizes spray-dried PTH (1-34) and also to understand whether any sort of interaction occurred between the Brij 97 and trehalose in the presence of PTH (1-34). The thermal profile of the spray-dried pure trehalose (Fig. 5d) shows a glass transition (Tg) at approximately 50°C. This transition was also observed in the spray-dried PTH/trehalose and spray-dried PTH/trehalose/Brij MDSC profiles suggesting that the presence of PTH (1-34) does not affect the Tg of trehalose. Nevertheless, the recrystallisation of the spray-dried trehalose to the α-polymorph was observed at approximately 145°C and its subsequent melting at approximately 150°C is represented by the endothermic peak (Fig. 5d). The endothermic peak at approximately 201°C represents the melting of the β-polymorph of trehalose (Fig. 5d) [26]. The thermal profile of the composite containing PTH/trehalose (Fig. 5f) shows the presence of the β-polymorph of trehalose only with only a “minor transition” around the α-polymorph. This suggests that the interaction of the PTH (1-34) with trehalose only allows for transition to the β-polymorph without any evidence of α-polymorph. An interesting profile was observed when Brij 97 was added to the PTH/trehalose composite (i.e. PTH/trehalose/Brij). Only the α-polymorph of trehalose was observed without any β-polymorphic transition (Fig. 5e). This suggests that Brij 97 actually interacts with the trehalose in the presence of PTH (1-34) and this probably explains why the combination of Brij and trehalose does not have the same stabilising effect as when only trehalose was used as the stabilising excipient.
Fig. 5.
MDSC thermal profiles of a spray freeze-dried PTH/trehalose, b spray-dried neat PTH, c unprocessed PTH, d spray-dried neat trehalose, e spray-dried PTH/trehalose/Brij and f spray-dried PTH/trehalose
Aerosol Performance Test Using ACI
Table III shows a summary of aerodynamic particle size distribution metrics using spinhaler® DPI device. The FPF (<4.7 μm aerodynamic diameter) of spray-dried PTH (1-34) formulation of mean 55 ± 15% was not significantly different from that produced by the spray freeze-dried PTH (1-34) formulation with a mean of 49 ± 13%. These FPFs correlate with the respective mass median aerodynamic diameters (MMAD). The MMAD of the spray-dried PTH/trehalose formulation was 2.4 ± 0.8 μm in comparison to that of the spray freeze-dried formulation which was 2.7 ± 0.5 μm. This is quite interesting as it is normally believed that large, porous particles always have superior aerodynamic properties in comparison to their solid particulate counterpart (19,21). It has been previously reported that the aerosol performance of dry powder formulations is not only dependent on the particle size and particle density but also on the morphology of the particles (27,28). Particles with corrugated surfaces may produce superior aerosol performance than particles with smooth surfaces because of the improved flow brought about by the reduced inter-particulate van der Waal forces. Particles with corrugated surfaces normally have reduced inter-particulate contact area which leads to reduced van der Waal forces (27,28). This leads to improved flow properties especially in dry powder formulations without a carrier. Furthermore, the spray-dried PTH/trehalose produced a higher FPF (55%) in comparison to the corresponding Brij 97 containing formulation which produced an FPF of 30% (Table III). This is in agreement with the SEM data which showed that the Brij 97 containing particles were agglomerated and this possibly explains the substantially lower FPF value.
Table III.
Summary of Aerodynamic Particle Size Distribution Metrics for PTH Using the Andersen Cascade Impactor (n = 3, Mean ± SD)
| Formulation | FPFa (%) | MMADb (μm) | GSDc | EDd (%) |
|---|---|---|---|---|
| Unprocessed PTH | 34 ± 4 | 3.9 ± 0.4 | 3.3 ± 0.4 | 95 ± 2 |
| SD neat PTH | 84 ± 10 | 2.0 ± 0.9 | 2.7 ± 0.2 | 84 ± 9 |
| SD PTH/tre | 55 ± 15 | 2.4 ± 0.8 | 3.2 ± 0.5 | 55 ± 15 |
| SFD PTH/trehalose | 49 ± 13 | 2.7 ± 0.5 | 3.8 ± 1.5 | 88 ± 6 |
| SD PTH/tre/Brij 97 | 30 ± 3 | 3.6 ± 0.4 | 3.5 ± 0.2 | 90 ± 3 |
aFine particle fraction
bMass median aerodynamic diameter from cascade impaction
cGeometric standard deviation (assuming particle size distribution is uni-modal and log normal)
dEmitted dose
CONCLUSIONS
The ACI and SEM data suggests that the morphology of particles in a DPI formulation has almost the same effect (if not greater) as the particle size and density of the particles. Spray-dried PTH powders produced equal or superior aerosol performance compared to the SFD PTH powder for inhalation despite the large, porous nature of the SFD PTH powder and this could be attributed to the surface roughness of the PTH particles produced by spray-drying. Furthermore, CD, fluorescence spectroscopy and bioactivity data suggest that trehalose when used alone as a stabilising excipient produced a superior stabilising effect than when used in combination with a non-ionic surfactant. DSC data suggest that an unfavourable interaction exists between the trehalose and Brij 97, which could be responsible for the detrimental effect observed when Brij was included in the formulation.
Acknowledgements
This research was supported by Science Foundation Ireland under grants SFI RFPENG0020 & SFI07/SRC/B1154.
References
- 1.Patton J. Pulmonary delivery of drugs for bone disorders. Adv Drug Deliv Rev. 2000;42(3):239–248. doi: 10.1016/S0169-409X(00)00064-8. [DOI] [PubMed] [Google Scholar]
- 2.Barrett-Connor E. The economic and human costs of osteoporotic fracture. Am J Med. 1995;98(2A):3S–8S. doi: 10.1016/S0002-9343(05)80037-3. [DOI] [PubMed] [Google Scholar]
- 3.Quattrocchi E, Kourlas H. Teriparatide: a review. Clin Ther. 2004;26(6):841–854. doi: 10.1016/S0149-2918(04)90128-2. [DOI] [PubMed] [Google Scholar]
- 4.Tashjian JRA, Chabner B. Commentary on clinical safety of recombinant human parathyroid hormone 1-34 in the treatment of osteoporosis in men and postmenopausal women. J Bone Miner Res. 2002;17:1151–1161. doi: 10.1359/jbmr.2002.17.7.1151. [DOI] [PubMed] [Google Scholar]
- 5.Shoyele S, Slowey A. Prospects of formulating proteins/peptides as aerosols for pulmonary drug delivery. Int J Pharm. 2006;314(1):1–8. doi: 10.1016/j.ijpharm.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Akers M, Vasudevan V, Stickelmeyer M. Formulation development of protein dosage forms. In: Nail SL, Akers MJ, editors. Development and manufacture of protein pharmaceuticals. New York: Kluwer; 2002. p. 47. [DOI] [PubMed] [Google Scholar]
- 7.Manning M, Patel K, Borchardt R. Stability of protein pharmaceuticals. Pharm Res. 1989;6(11):903–918. doi: 10.1023/A:1015929109894. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi S, Kondo S, Juni K. Pulmonary delivery of salmon calcitonin dry powders containing absorption enhancers in rats. Pharm Res. 1996;13(1):80–83. doi: 10.1023/A:1016081301369. [DOI] [PubMed] [Google Scholar]
- 9.Pfutzner A, Flacke F, Pohl R, Linkie D, Engelbach M, Woods R, et al. Pilot study with Technosphere/PTH (1-34)-a new approach for effective pulmonary delivery of parathyroid hormone (1-34) Horm Metab Res. 2003;35(5):319–323. doi: 10.1055/s-2003-41309. [DOI] [PubMed] [Google Scholar]
- 10.Codrons V, Vanderbist F, Verbeeck R, Arras M, Lison D, Préat V, et al. Systemic delivery of parathyroid hormone (1-34) using inhalation dry powders in rats. J Pharm Sci. 2003;92(5):938–950. doi: 10.1002/jps.10346. [DOI] [PubMed] [Google Scholar]
- 11.Shoyele S, Cawthorne S. Particle engineering techniques for inhaled biopharmaceuticals. Adv Drug Deliv Rev. 2006;58(9–10):1009–1029. doi: 10.1016/j.addr.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Costantino H, Firouzabadian L, Wu C, Carrasquillo K, Griebenow K, Zale S, et al. Protein spray freeze drying. 2. Effect of formulation variables on particle size and stability. J Pharm Sci. 2002;91(2):388–395. doi: 10.1002/jps.10059. [DOI] [PubMed] [Google Scholar]
- 13.Engstrom J, Simpson D, Lai E, Williams R, III, Johnston K. Morphology of protein particles produced by spray freezing of concentrated solutions. Eur J Pharm Biopharm. 2007;65(2):149–162. doi: 10.1016/j.ejpb.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Hillgren A, Aldén M. A comparison between the protection of LDH during freeze-thawing by PEG 6000 and Brij 35 at low concentrations. Int J Pharm. 2002;244(1–2):137–149. doi: 10.1016/S0378-5173(02)00322-8. [DOI] [PubMed] [Google Scholar]
- 15.Jovanovic N, Bouchard A, Hofland G, Witkamp G, Crommelin D, Jiskoot W. Distinct effects of sucrose and trehalose on protein stability during supercritical fluid drying and freeze-drying. Eur J Pharm Sci. 2006;27(4):336–345. doi: 10.1016/j.ejps.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Ameri M, Maa Y. Spray drying of biopharmaceuticals: stability and process considerations. Drying Technol. 2006;24(6):763–768. doi: 10.1080/03602550600685275. [DOI] [Google Scholar]
- 17.Jeon J, Puleo D. Formulations for intermittent release of parathyroid hormone (1-34) and local enhancement of osteoblast activities. Pharm Dev Technol. 2008;13(6):505–512. doi: 10.1080/10837450802282488. [DOI] [PubMed] [Google Scholar]
- 18.Johnson K. Preparation of peptide and protein powders for inhalation. Adv Drug Deliv Rev. 1997;26(1):3–15. doi: 10.1016/S0169-409X(97)00506-1. [DOI] [PubMed] [Google Scholar]
- 19.Maa Y, Nguyen P, Sweeney T, Shire S, Hsu C. Protein inhalation powders: spray drying vs spray freeze drying. Pharm Res. 1999;16(2):249–254. doi: 10.1023/A:1018828425184. [DOI] [PubMed] [Google Scholar]
- 20.Yu Z, Johnston K, Williams R., III Spray freezing into liquid versus spray-freeze drying: Influence of atomization on protein aggregation and biological activity. Eur J Pharm Sci. 2006;27(1):9–18. doi: 10.1016/j.ejps.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Edwards D, Hanes J, Caponetti G, Hrkach J, Ben-Jebria A, Eskew M, et al. Large porous particles for pulmonary drug delivery. Science. 1997;276(5320):1868. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- 22.Manavalan P, Johnson W. Protein secondary structure from circular dichroism spectra. J Biosci. 1985;8(1):141–149. doi: 10.1007/BF02703972. [DOI] [PubMed] [Google Scholar]
- 23.Kanaori K, Takai M, Nosaka A. Comparative study of chicken and human parathyroid hormone-(1-34)-peptides in solution with SDS. Eur J Biochem. 2004;249(3):878–885. doi: 10.1111/j.1432-1033.1997.00878.x. [DOI] [PubMed] [Google Scholar]
- 24.Demeule B, Lawrence M, Drake A, Gurny R, Arvinte T. Characterization of protein aggregation: the case of a therapeutic immunoglobulin. Biochimica et Biophysica Acta (BBA)-Proteins & Proteomics. 2007;1774(1):146–153. doi: 10.1016/j.bbapap.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Capelle M, Gurny R, Arvinte T. High throughput screening of protein formulation stability: practical considerations. Eur J Pharm Biopharm. 2007;65(2):131–148. doi: 10.1016/j.ejpb.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Costantino H, Firouzabadian L, Hogeland K, Wu C, Beganski C, Carrasquillo K, et al. Protein spray-freeze drying. Effect of atomization conditions on particle size and stability. Pharm res. 2000;17(11):1374–1382. doi: 10.1023/A:1007570030368. [DOI] [PubMed] [Google Scholar]
- 27.Lechuga-Ballesteros D. S, L. M., Vehring, R., Kuo M-C (eds). Use of a novel excipient to enable the preparation of stable and dispersible dry powder aerosol formulation by spray drying. In: 30th Annual Meeting and Symposium of the Controlled Release Society, Glasgow, Scotland; 2003
- 28.Chan H. Dry powder aerosol drug delivery—opportunities for colloid and surface scientists. Colloids Surf A. 2006;284:50–55. doi: 10.1016/j.colsurfa.2005.10.091. [DOI] [Google Scholar]