Abstract
An area of increasing concern and scientific scrutiny is the potential contamination of drug products by leachables entering the product during manufacturing and storage. These contaminants may either have a direct safety impact on the patients or act indirectly through the alteration of the physicochemical properties of the product. In the case of biotherapeutics, trace amounts of metal contaminants can arise from various sources, but mainly from contact with stainless steel (ss). The effect of the various factors, buffer species, solution fill volume per unit contact surface area, metal chelators, and pH, on metal leachables from contact with ss over time were investigated individually. Three major metal leachables, iron, chromium, and nickel, were monitored by inductively coupled plasma–mass spectrometry because they are the major components of 316L ss. Iron was primarily used to evaluate the effect of each factor since it is the most abundant. It was observed that each studied factor exhibited its own effect on metal leachables from contact with ss. The effect of buffer species and pH exhibited temperature dependence over the studied temperature range. The metal leachables decreased with the increased fill volume (mL) per unit contact ss surface area (cm2) but a plateau was achieved at approximately 3 mL/cm2. Metal chelators produced the strongest effect in facilitating metal leaching. In order to minimize the metal leachables and optimize biological product stability, each formulation factor must be evaluated for its impact, to balance its risk and benefit in achieving the target drug product shelf life.
Key words: biologics formulation factors, metal chelator, metal leachables, protein drug product/drug substance manufacturing, protein formulation stability
INTRODUCTION
Metal ions occur widely in association with proteins both in vivo and in vitro via direct covalent binding or electrostatic interactions. Alkali and alkaline earth metals bind proteins primarily through electrostatic interactions while the transition metals, such as Fe(II/III), Co(II), Cu(II), Al(III), and Mn(II), bind covalently to proteins (1) because the protein ligands donate an electron pair to form coordinate covalent bond with transition metals. The most common metal-binding amino acids are cysteine, histidine, aspartate, glutamate, and more rarely, glutamine, serine, methionine, threonine, and tyrosine because they contain electron donor elements, such as sulfur, nitrogen, or oxygen. The coordination numbers vary from one to eight among different metals. The solution pH and ionization state of the amino acid residue play an important role in determining the coordination number.
Metal ions mediate in vivo biological functions of about one third of the known proteins via directly binding to either protein catalytic sites or structural sites (1). Metal ions in proteins serve a variety of functions, including stabilizing protein structure and assisting protein function by inducing conformational changes. On the other hand, excessive metal ions introduced into biological systems have been implicated in the aggregation of proteins responsible for the pathophysiology of several neurodegenerative diseases, such as Alzheimer and Parkinson disease (2).
Although metal ions play a critical role in modulating physiological functions through binding to proteins in vivo, they can have a profound negative impact on biotherapeutic products when present as inadvertent contaminants. Trace levels of metal ions can induce biotherapeutic product degradation which potentially jeopardizes the quality of the products by altering physicochemical properties as well as reducing stability and shelf life. Metal ions migrating from rubber stoppers into a therapeutic protein liquid formulation have been reported to induce N-terminal degradation (3). Barium leached from glass vials reacted with sulfate in the formulation to form visible particles (3). Salts of tungsten oxide were shown to migrate from prefilled syringes into the drug product, where they triggered protein oxidation followed by aggregation to produce visible particles (4).
Contamination by leachables raises concerns about the safety and efficacy of the product due to the potential to cause acute toxicity or long-term health issues through chronic exposure. In the USA, the requirement for the proper evaluation of extractables and leachables (E&Ls) is governed by the FD&C Act as codified in 21 Code of Federal Regulations parts 210 and 211. Current Good Manufacturing Practice in Manufacturing, Processing, Packing or Holding of Drugs: General and Current Good Manufacturing Practice for Finished Pharmaceuticals, Subpart D-211.65 which states that “equipment shall be constructed so that surfaces that contact components, in-process materials, drug products shall not be reactive, additive, or adsorptive so as to alter the safety, identity, strength, quality, or purity of the drug product beyond the official or other established requirements.”
Specific guidance has been issued for monitoring and control of leachables as part of requirement for the documentation of primary packaging of drug product (5–9). Orally inhaled and nasal drug products as well as injection (solution and powder) products are of the highest degree of concern. The likelihood of interaction between packaging and dosage form is considered high for solutions or suspensions and medium for powders. Biological products are predominately formulated as liquid or freeze-dried products delivered by injection, which fall into the category of highest concern.
In biopharmaceutical drug product manufacturing and storage processes, the contact materials can be classified into three main categories: plastics/elastomers, glass, and stainless steel. Plastics/elastomers are by far the biggest source of extractables and leachables. Extensive research has been devoted to leachables/extractables from plastic materials (10–19). The strategy and guidance for selecting, evaluating, and qualifying appropriate container closures as well as strategies for safety evaluation have been reported (20–23). Other leachables/extractables from glass have also been studied (21, 22). However, very limited research has been carried out on metal leachables/extractables. Metals can leach out from drug substance and/or product contacting equipment in the manufacturing, packaging, shipping, and storage process, especially from stainless steel surfaces. Some plastic packages, glass vials, and/or rubber stoppers can also be the sources of metal leachates (24). Stainless steel is a typical contact material used for biologics product manufacturing, shipping, and storage. The stainless steel commonly used in biopharmaceutical applications is of the grade 316L and is an alloy containing mainly iron, nickel, and chromium with minor amounts of manganese and molybdenum. The grade 316L is a low-carbon formulation of austenitic stainless steel 316, and this modified chemistry is designed to reduce the propensity of the alloy to sensitize. Sensitization is the precipitation of chromium carbides (actually mixed iron/chromium carbides) along grain boundaries of stainless steel. This precipitation depletes the volume of metal near the grain boundaries of chromium in solution, thereby diminishing its corrosion resistance. The precipitation rate can be reduced by keeping carbon below 0.03%. The objective of this work is to elucidate the effect of various biologics formulation factors (e.g., buffer species, fill volume per unit stainless steel contact surface area, metal chelators, and pH) on the quantity of metal leachables from 316L stainless steel over time at different storage conditions.
MATERIALS AND METHODS
Materials
As shown in Fig. 1, the rectangular shape 316L stainless steel defined as coupons were utilized for this study. Prior to use, the coupons were chemically passivated by first immersing them in the solution of 1:4 (vol/vol) of CitruSurf solution (50%; Stellar Solution, McHenry, IL; http://www.citrisurf.com) to milliQ water for 30 min at 50–70°C and then were autoclaved after thorough rinsing with water. Two different sizes of stainless steel coupons were utilized, defined as type A and B. Type A had a surface area of 3.52 cm2 with the dimensions of 1.55 cm (length) × 0.7 cm (width) × 0.3 cm (thickness) while type B had a surface area of 6.0 cm2 with the dimensions of 2.5 cm (length) × 1.0 cm (width) × 0.15 cm (thickness).
Fig. 1.
Appearance of stainless steel coupons used
The 30-mL type I glass vials with 20 mm neck were purchased from Abbott Laboratories (Chicago, IL). The 10-mL type I glass vials with 20 mm neck and serum stoppers were obtained from West Pharmaceutical Services (Lionville, PA). USP/EP grade l-histidine and l-histidine monohydrochloride, analytical grade acetate acid, monobasic sodium phosphate, dibasic sodium phosphate, citric acid (anhydrous), and sodium citrate dihydrate, acetate acid and sodium acetate trihydrate, succinic acid, FeCl3, disodium edetate (Na2EDTA), and diethylenetriaminepentaacetic acid (DTPA) were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO). Polyvinylidene fluoride, 0.22 μm, filters were obtained from Millipore Inc. (Billerica, MA).
Methods
Inductively Coupled Plasma—Mass Spectrometry
Metal ion concentrations in the testing solutions were quantitated by an inductively coupled plasma–mass spectrometry (ICP-MS) method which utilized an Agilent 7500 CX ICP-MS system equipped with an autosampler. The instrument was qualified and calibrated with certified standard multi-element solution (BDH 10 ppm-Aristar) and maintained regularly. The accuracy of the method was evaluated by spiking recovery and inter-lab comparison with external GLP lab in Galbraith Laboratories, Inc. (Knoxville, TN). All vessels, pipettes, and other laboratory equipment that came in direct contact with solutions used in the analysis were made of plastic. Samples were analyzed in the “hydrogen mode” and 10× diluted certified standard multi-element solution (BDH 10 ppm-Aristar) was used as the internal standard. An external calibration curve for each element measured was established before analyzing the samples. A linear response curve between 1 and 500 ppb was established for iron, and 1–100 ppb for chromium and nickel. The limit of quantitation of iron, chromium and nickel was 1 ppb. A 50-ppb iron standard was analyzed every six samples to ensure the system performance. Samples were injected directly without dilution unless the metal ion concentration was outside the linear range as specified above. The sample volume required was 3.0 mL, and 1.5 mL aliquots were injected.
RP-HPLC Analyses for Chelator Concentration
The concentrations of both Na2EDTA and DTPA in the formulations were determined using an Agilent 1100 system coupled to a diode array detector. The system was calibrated and maintained regularly. The method accuracy was evaluated by spiking recovery. The separation was achieved isocratically on an Agilent Eclipse XDB-C18 column (5 μm, 4.6 × 150 mm) at a flow rate of 1 mL/min at ambient temperature. The mobile phase consisted of 30 mM sodium acetate, 2 mM tetrabutylammonium hydroxide, and 5% (v/v) methanol in water (pH 3.15). Excessive FeCl3 (5 mM final concentration) was added to both samples and standards. External calibration was established with varying levels of Na2EDTA and DPTA simultaneously. Of the sample, 20 μL was injected onto the column and the separation was monitored by UV detection at 254 nm.
EXPERIMENTS
To assess the effect of the five buffers on metal leachates from passivated 316L stainless steel at storage temperatures of −40°C to 25°C, the relationship between the testing solution volume (mL) and the coupon surface area (cm2) was controlled at 5 mL/cm2. All buffer solutions were prepared at 20 mM and pH 5.5 and sterile filtered with 0.22 μm polyvinylidene fluoride filters. Aliquots of 17.6 mL of the filtered buffer solutions were added into 30 mL type I glass vials with or without type A stainless steel coupons and sealed with serum stoppers and flip-off aluminum seals. The vials were stored in the stability chamber at either −40°C, −20°C, 2–8°C, or 25°C/60%RH separately for over 34 weeks. The vials without type A stainless steel coupons served as negative controls. The testing solutions and storage conditions are summarized in Table I. The selected buffer types, concentration, pH, and storage conditions were chosen because these are commonly used conditions in biotherapeutic drug substance and/or product development.
Table I.
Experimental Conditions to Assess the Effect of Buffer Species and Storage Conditions on Metal Leachates from 316 L Stainless Steel Coupons with a Surface Area of 3.52 cm2 in 30 mL Type I Glass Vials
| Storage condition (°C)/test solutions | −40°C | −20°C | 2–8°C | 25°C |
|---|---|---|---|---|
| 20 mM histidine–acetate at pH 5.5 | 1 vial w/ss | 1 vial w/ss | 1 vial w/ss | 1 vial w/ss |
| 1 vial w/o ss | 1 vial w/o ss | 1 vial w/o ss | 1 vial w/o ss | |
| 20 mM histidine–HCl at pH 5.5 | 1 vial w/ss | 1 vial w/ss | 1 vial w/ss | 1 vial w/ss |
| 1 vial w/o ss | 1 vial w/o ss | 1 vial w/o ss | 1 vial w/o ss | |
| 20 mM phosphate at pH 5.5 | 1 vial w/ss | 1 vial w/ss | 1 vial w/ss | 1 vial w/ss |
| 1 vial w/o ss | 1 vial w/o ss | 1 vial w/o ss | 1 vial w/o ss | |
| 20 mM citrate at pH 5.5 | 1 vial w/ss | 1 vial w/ss | 1 vial w/ss | 1 vial w/ss |
| 1 vial w/o ss | 1 vial w/o ss | 1 vial w/o ss | 1 vial w/o ss | |
| 20 mM succinate at pH 5.5 | 1 vial w/ss | 1 vial w/ss | 1 vial w/ss | 1 vial w/ss |
| 1 vial w/o ss | 1 vial w/o ss | 1 vial w/o ss | 1 vial w/o ss | |
| 20 mM acetate at pH 5.5 | 1 vial w/ss | 1 vial w/ss | 1 vial w/ss | 1 vial w/ss |
| 1 vial w/o ss | 1 vial w/o ss | 1 vial w/o ss | 1 vial w/o ss |
The test solution fill volume is 17.6 mL
ss stands for stainless steel coupon
To evaluate the impact of contact surface area on metal leachates, commonly used 20 mM histidine–HCl buffer at pH 5.5 was selected as a testing solution. The testing solution volume (mL) per unit stainless steel contact surface area (cm2) were selected to be between 1 and 5 mL/cm2, with 1 mL/cm2 as the worst case scenario for a stainless steel minitank used as a model for stability studies. The testing solution was prepared and processed as previously discussed. Aliquots of the testing solution of 17.6 mL for 5 mL/cm2, 14.08 mL for 4 mL/cm2, 10.56 mL for 3 mL/cm2, 7.04 mL for 2 mL/cm2, and 3.25 mL for 1 mL/cm2 were individually added to the vials with or without type A stainless steel coupons and stored at 25°C/60% RH in a stability chamber over the time period of 26 weeks. The vials without stainless steel coupons served as negative controls.
Many parenteral biologics drug products include metal chelators to improve product stability. To assess the effect of chelators on metal leachates, DTPA and Na2EDTA were tested as chelators at three concentrations of 0.0, 0.134, and 0.268 mM in 20 mM histidine–acetate buffer at pH 6. At this pH, DTPA (H5A), polyamino polycarboxylic acid, exists in equilibrium between H2A3− and H3A2−; however, in presence of metal ions, DTPA forms the metal complexes of MA3−/MA2− at equal molar ratios. For simplicity, the term DTPA and Na2EDTA are used in the text, regardless of their ionization forms. Here, the concentration of 0.134 mM is equivalent to 0.05 mg/mL Na2EDTA and 0.053 mg/mL DTPA. Similarly, the concentration of 0.268 mM is equivalent to 0.1 mg/mL Na2EDTA and 0.106 mg/mL DTPA. These two concentrations were selected because they are typical concentrations applied in parenteral formulations. The samples without chelators served as controls. The stainless steel coupons utilized in this experiment were of type B due to unavailability of the previously used type A coupons. In order for the coupons to be fully immersed in the solution, 10 mL type I glass vials were used as the sample containers. The fill volume of the testing solutions (mL) per unit stainless steel contact surface area (cm2) was controlled at 1 mL/cm2. The testing vials were filled with 6.0 mL of solution in glass vials with or without stainless steel coupons and were then stored in a 25°C/60% RH stability chamber for 34 weeks. The vials without stainless steel coupons served as negative controls.
It is well known that pH plays a major role in protein stability. To evaluate the effect of pH on metal leachates, 20 mM histidine–acetate solutions, of pH values ranging from 5.0 to 9.0, were tested. The stainless steel coupons, glass vials and fill volumes were the same as those utilized for the chelator evaluation in the previous experiment. The vials filled with testing solutions (6.0 mL) with or without stainless steel coupons (type B) were stored in a 25°C/60 RH stability chamber over the time period of 34 weeks. The vials without stainless steel coupons served as negative controls.
RESULTS
The amount of metal ions leaching into biologics products is subject to multiple variables, such as formulation components, pH, contact materials, drug substance/drug product contact surface area with stainless steel, surface area processing procedure, contact duration and temperature, etc. In this paper, commonly utilized buffers, the relationship of the solution volume and contact surface area, chelators, and pH were studied individually to explore their impact, if any, on metal leachates from 316L stainless steel coupons. 316L (US: S31603) is an alloy, mainly composed of iron (62–72%), Cr (16–18.5%), and Ni (10–14%) (25). Thus, in this study, all these three metals in the testing solutions were monitored by ICP-MS to evaluate the concentrations of metal ions potentially introduced into the products. Each measured metal ion concentration in the testing solution was corrected by subtracting the average amount detected in negative control solutions not containing stainless steel coupons. This allowed compensation for any metals possibly introduced from the glass and raw materials (26). Each sample was tested in triplicate and the relative standard deviation (RSD) for the triplicate measure was within 0.5%, thus the average was utilized here.
Effect of Buffers
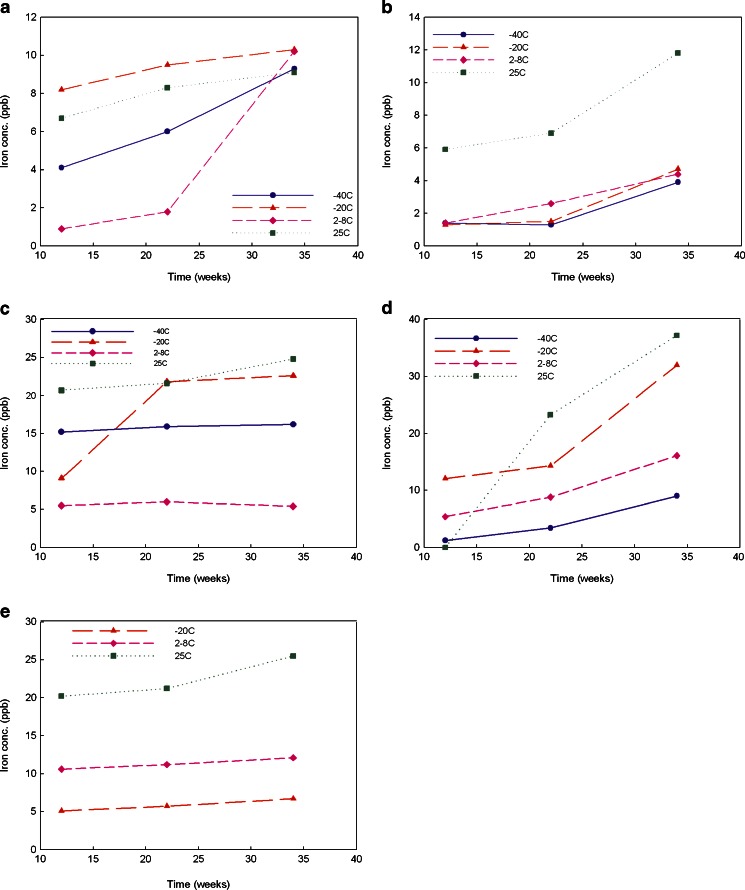
Buffers are added to a formulation to adjust the pH to optimize protein solubility and stability. Phosphate, citrate, acetate, histidine, and succinate are the most commonly used buffers in parenteral products. In this study, in order to compare the effect of the buffer species on metal leachates from stainless steel with time and storage conditions, the buffer concentration and pH were fixed at 20 mM and 5.5, respectively. Iron, nickel, and chromium were monitored in the media in contact or no contact with stainless steel coupons over 34 weeks. The sample storage temperatures were −40°C, −20°C, 2–8°C, and 25°C at 60%RH, the commonly accepted DS/DP storage or stressed conditions. At each time point, an individual vial was pulled for metal ion determination. The vials containing no stainless steel coupons served as negative controls. Due to glass vial breakage, the metal leachables data for 20 mM succinate buffer at the time points of 26 and 34 weeks stored at −40°C are not available. The concentrations of iron, nickel, and chromium leached from stainless steel coupons were very low. Nickel was not measurable quantitatively since its concentrations were below its limit of quantitation. Chromium was only determined quantitatively in the samples stored at 25°C at 60%RH. Among these three metal ions, iron was the most abundant and its concentrations were quantitatively determined by ICP-MS in all samples as shown in Fig. 2. Each buffer exhibited a different pattern on iron leachates. To further explore the differences in the impact of each buffer, the iron leachable data in each tested buffer were analyzed individually with the aid of John’s Macintosh Project (JMP; SAS Institute Inc., Cary, NC), a statistical data analysis software.
Fig. 2.
Iron leachate average concentrations for five different buffers (20 mM) at pH 5.5 stored at −40°C, −20°C, 2–8°C, and 25°C over 34 weeks (triplicate measurement with RSD of 0.5%). a for histidine-acetate, b for histidine–HCl, c for phosphate, d for citrate, and e for succinate buffer
Effect of Contact Surface Area
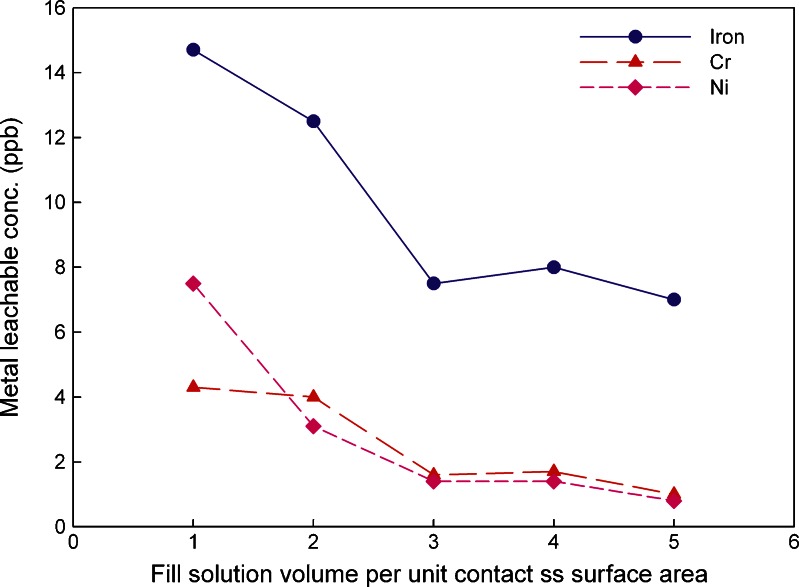
During drug substance manufacturing and the storage process, drug substances are in contact with stainless steel at different fill volume per unit contact surface area. In this study, the worst scenario has been chosen as 1 mL/cm2. Most practical situations will be significantly better than this, i.e., larger volume per unit surface area. The metal leachable data for iron, nickel, and chromium after 26 weeks at controlled room temperature are shown in Fig. 3.
Fig. 3.
Metal leachates average concentrations in 20 mM histidine–HCl buffer solution at pH 5.5 at different solution fill volumes per unit contact ss surface area (triplicate measurement with RSD of 0.5%)
Effect of Metal Chelators
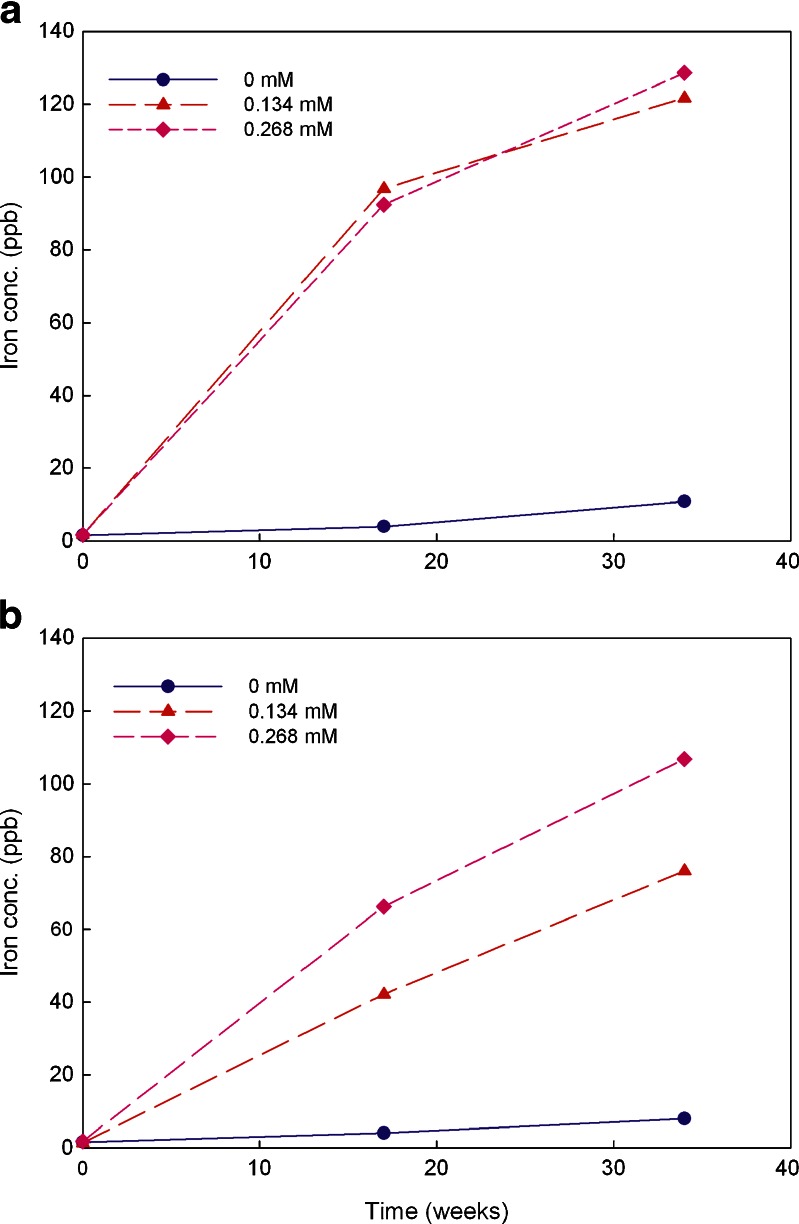
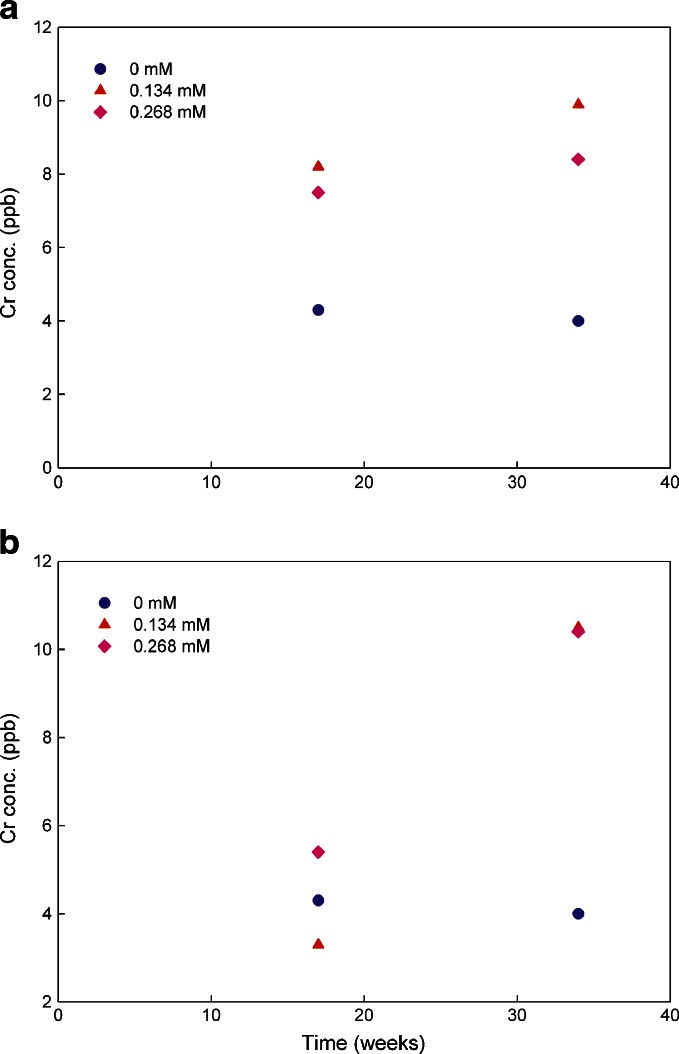
The metal chelator Na2EDTA has been commonly used in parenteral formulations. DTPA has also been used in several approved parenteral products. The efficiency of Na2EDTA and DTPA at inhibiting iron-induced degradation of an antibody was reported to be equivalent (27). Thus, in this study, the effect of the two chelators on metal leachates at two concentrations was explored. The testing solutions containing no chelators served as controls. The iron concentration data are plotted in Fig. 4 and that for chromium in Fig. 5. No data analysis was performed on nickel since the observed levels were either below or at the limit of quantitation.
Fig. 4.
Iron leachate average concentrations in 20 mM histidine–acetate buffer solutions (pH 6.0) containing Na2EDTA and DTPA at the concentrations of 0, 0.134, and 0.268 mM over 34 weeks stored at 25°C (triplicate measurement with RSD of 0.5%). a for Na2EDTA and b for DTPA
Fig. 5.
Chromium leachate average concentrations in 20 mM histidine–acetate buffer solutions (pH 6.0) containing Na2EDTA and DTPA at the concentrations of 0, 0.134, and 0.268 mM over 34 weeks stored at 25°C (triplicate measurement with RSD of 0.5%). a for Na2EDTA and b for DTPA
Effect of pH
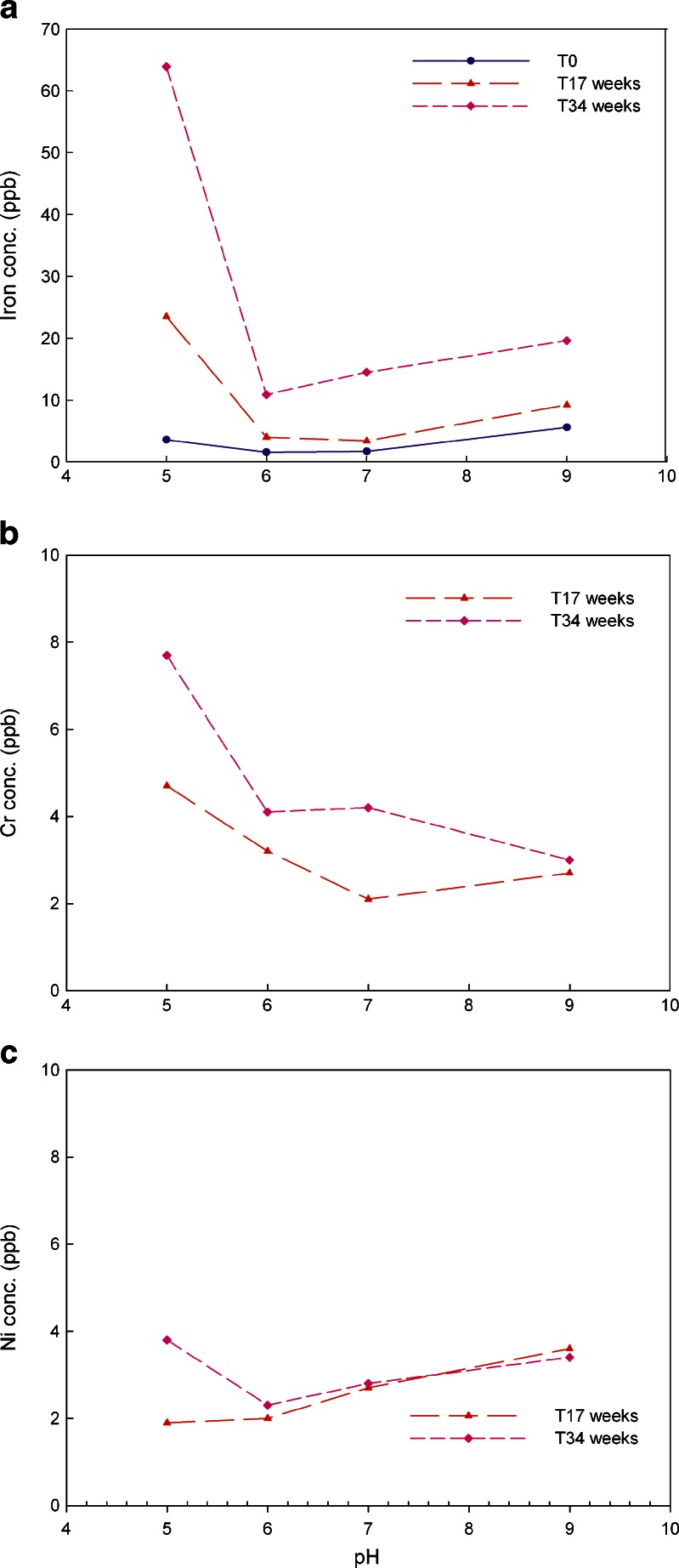
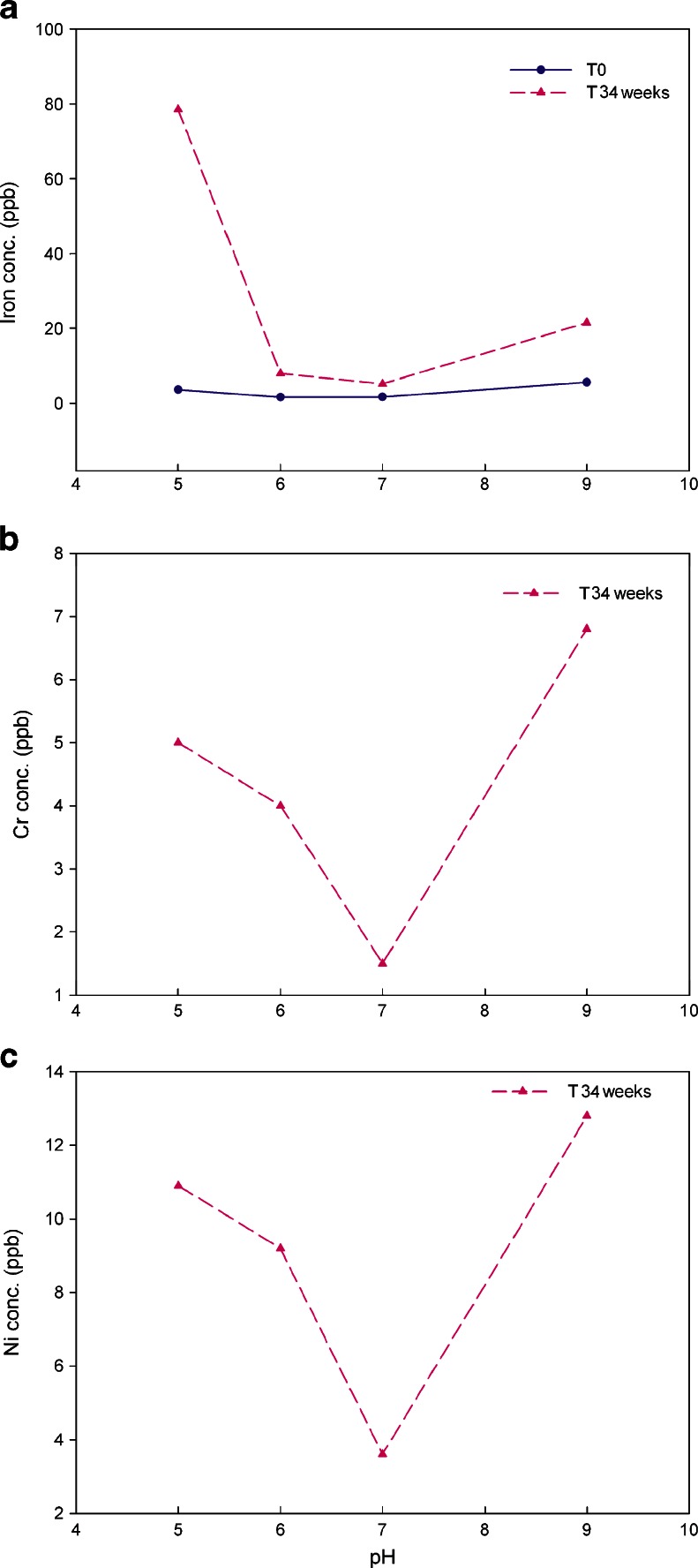
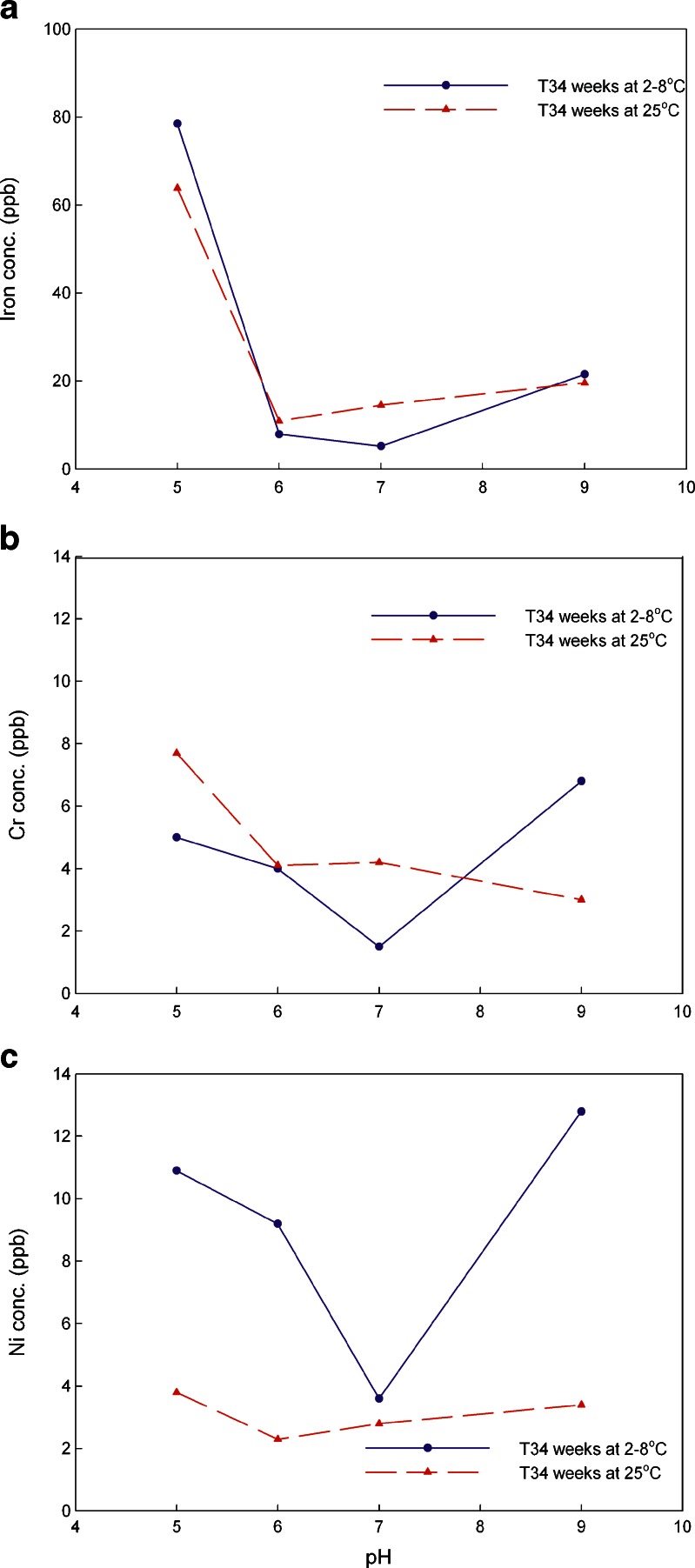
In this study, the typical pH values of 5.0, 6.0, 7.0, and 9.0 were chosen as testing conditions to evaluate the impact of pH on metal leachates. The storage temperatures were 2–8°C and 25°C at 60%RH. The amounts of iron, chromium, and nickel leaching out from the stainless steel coupons at 25°C are shown in Fig. 6 while data for 2–8°C are presented in Fig. 7. A comparison of the amounts of iron, chromium, and nickel leaching out from stainless steel at storage temperatures of 2–8°C vs. 25°C for 34 weeks is shown in Fig. 8.
Fig. 6.
a–c Iron, chromium and nickel leachates average concentration in 20 mM histidine–acetate buffer solution at pH 5.0, 6.0, 7.0, and 9.0 stored at 25°C over 34 weeks (triplicate measurement with RSD of 0.5%)
Fig. 7.
a–c Iron, chromium, and nickel leachates average concentration in 20 mM histidine–acetate buffer solution at pH 5.0, 6.0, 7.0, and 9.0 stored at 2–8°C over 34 weeks (triplicate measurement with RSD of 0.5%)
Fig. 8.
a–c Comparisons of the average concentrations of metal leachates of iron, chromium and nickel in 20 mM histidine buffer at pH 5.0, 6.0, 7.0, and 9.0 when stored at 2–8° vs. 25°C over 34 weeks time points (triplicate measurement with RSD of 0.5%)
DISCUSSION
Effect of Buffers on Metal Leachates
Chromium was quantitatively determined only at 25°C at 60%RH storage over 34 weeks. Histidine–HCl, histidine–acetate, and phosphate behaved similarly (about 3.5 ppb Cr), and better than succinate buffer (4.6 ppb Cr). The highest levels of leachables are observed for citrate buffer (7.1 ppb Cr). The leached amount of Cr did not exhibit direct relationship with its corresponding salt solubility in the buffers. Cr(III) phosphate salt is slightly soluble in water and Cr salt of histidine, succinate, and citrate are water soluble. Similarly, the leached amount of Nickel ion did not exhibit dependence on its salt solubility. Ni(II) phosphate salt is insoluble in water with solubility product constant of 4.74 × 10−32 at 25°C while its salt of histidine, citrate, and succinate are water soluble (28). Nickel levels were below the limit of quantitation for all tested conditions. Among three monitored metal ions, iron was the most abundant even though the highest concentration observed is less than 40 ppb over 34 weeks. Therefore, in this section, only the iron data were analyzed to explore each buffer behavior.
As indicated in Fig. 2, each buffer exhibited its own properties over the time course of 34 weeks studied here. Thus, temperature/time impact on iron leachates is discussed for each individual buffer first, followed by a discussion of the comparison among the five buffers.
For histidine–acetate buffer at pH 5.5 (Fig. 2a), temperature seemed to exert an effect on iron leachables. Storage temperature of −20°C exhibited the highest levels of iron leachates followed by 25°C, −40°C, and 2–8°C at time points of 12 and 22 weeks. No good explanation can be provided for this counterintuitive result. However, when the storage time increased to 34 weeks, the temperature effect diminished. Further statistical data analysis was performed by JMP to explore whether temperature or time makes a significant contribution to iron leachates. With a 95% confidence level, the probability factor of less than 0.05 indicates that the factor is significant. The calculated probability factor for time is 0.0262 while the calculated probability factor for temperature is 0.9029 which indicates that within a 95% confidence level, time is the significant factor that impacts the iron leachates.
For histidine–HCl buffer at pH 5.5 (Fig. 2b), the levels of iron leakage for the storage temperature of 25°C are significantly higher than those for lower storage temperatures of −40°C, −20°C, and 2–8°C, which was further confirmed by JMP statistical data analysis. The calculated probability factor of 0.0178 means a significant difference at the 95% confidence level. However, no significant difference in iron leachates was observed when storage temperatures range from −40°C to 2–8°C. Further data analysis by JMP indicated that, again, time plays a significant role in affecting iron leaching from the stainless steel because the calculated significant factor is 0.0909.
For 20 mM phosphate buffer at pH 5.5 (Fig. 2c), leached iron amounts at 25°C storage temperature exhibited the highest level over the tested time period, as expected, followed by the levels for a storage temperature of −40°C. The iron amount leached at the storage temperature of 2–8°C is the lowest among the tested conditions. That the iron amount detected for storage at −40°C is much higher than for storage at 2–8°C is probably due to the fact that the pH of phosphate buffer can drop significantly upon freezing (29). The significance of the temperature impact on iron leachates cannot be calculated by JMP because the iron leachates concentrations are similar for temperature of 25°C and −20°C. However, as Fig. 2c indicates, the iron leachates at 25°C or −20°C are significantly higher than those at −40°C and 2–8°C. Also, iron leachates at −40°C are three times of that at 2–8°C. The iron leachates at −40°C, 2–8°C, and 25°C did not significantly increase over the tested time course, which was confirmed by JMP data analysis. However, iron leachates at the storage temperature of −20°C increased about 2.5 times over the time period of 22 weeks. The trend is significantly different from the other three tested conditions.
For citrate buffer at pH 5.5 (Fig. 2d), the highest iron levels were seen at 25°C, followed by −20°C, 2–8°C, and −40°C. With 95% confidence level, time makes significant contribution to iron leachates observed for the four tested conditions, especially at the storage temperature of 25°C, which was suggested by JMP calculated probability factor of 0.0148.
For succinate buffer, because of the sample loss at −40°C due to vial breakage, this temperature was not evaluated. Storage temperature had a significant impact on the iron leachates: levels of iron leachates at 25°C were highest, followed by those for 2–8°C, and then −20°C (Fig. 2e). Further data analysis by JMP (probability factor of 0.0265) suggested that at the 95% confidence level, temperature is a major factor in affecting iron leaching from stainless steel. Here, within the 95% confidence level, time has no major impact on iron leachates for succinate buffer over all the tested conditions since the calculated probability factor is 0.7476.
All five buffers were tested at four different storage temperatures to explore the difference of their capacity at affecting iron leaching from stainless steel. The comparison was performed for each individual condition by calculating probability factors based on buffer species using SAS data analysis. Within the 95% confidence level, a probability factor of less than 0.05 indicates that a significant difference is observed. Over the tested time course of 34 weeks, at −40°C storage temperature, a calculated probability factor of 0.002 indicates that significant differences exists among the four buffers (no data for succinate buffer). Phosphate buffer shows the highest impact on iron leachates, followed by citrate buffer, and histidine–acetate buffer. Histidine–HCl buffer exhibited the least impact at leaching iron from stainless steel at −40°C. Similarly, significant differences with a probability factor of 0.013 was observed among five buffers at the storage temperature of 25°C. The ability to induce iron leaching from stainless steel decreases in the following order: citrate buffer > phosphate buffer, succinate buffer > histidine–HCl buffer > histidine–acetate buffer. This trend is not consistent with iron salt solubility in water at 25°C. Fe (II)/(III) phosphate salt is least soluble (Ksp for FePO4 is 1.3 × 10−22 and Fe3(PO4)2 is 10−36) while its citrate, histidine, and succinate salt are water soluble (28). However, at storage temperatures of −20°C and 2–8°C, no clear trend was observed. Thus, the effect of buffer on iron leachates is determined by multiple factors, such as buffer species, storage temperature, and contact time, etc.
Effect of Contact Surface Area on Metal Leachables
Solution fill volume per unit contact surface area between 1 and 5 mL/cm2 were investigated in this study. Figure 3 shows that, as expected, the lower the fill volume (mL) per unit stainless steel contact surface area (cm2), the higher the relative levels of metal ions leached. However, no linearity was observed at the studied relationship range between fill volume and contact surface area over the tested time period of 26 weeks. A plateau was observed when the fill volume per unit stainless steel contact surface area reached 3 mL/cm2.
Effect of Metal Chelators on Metal Leachables
The effect of the metal chelators Na2EDTA and DTPA on metal leachables was explored for the storage temperature of 25°C over 34 weeks. Both chelators at the concentrations of 0.134 and 0.268 mM showed a dramatic impact on iron leachates as shown in Fig. 4. Compared to the solutions without chelator, the concentration of leached iron from stainless steel in the buffer with Na2EDTA increased more than 20-fold over the 17 weeks, and increased more than ten fold over 34 weeks (Fig. 4a). It was reported previously that EDTA facilitated the migration of metal ions into the solution from metal contact surfaces (30). A similar trend was observed for DTPA. Compared to the solutions without any chelator, leached iron ion concentrations in the buffer containing DTPA increased more than ten fold for both the 17 and 34 weeks time period (Fig. 4b). However, increase in concentration of Na2EDTA did not have a significant impact on leached iron ion concentrations over 34 weeks unlike increased DTPA levels which exhibited a significant effect on iron leaching. Doubling the concentration of DTPA from 0.134 to 0.268 mM increased the concentration of leached iron ions by a factor of 1.7. A linear increase in leached iron ion concentration with the increased concentration of DTPA was also observed in both cases (R2 is 0.9971 when the solutions contain 0.134 mM DTPA while R2 is 0.9829 when DTPA is 0.268 mM).
The data in Fig. 5a suggest that the presence of Na2EDTA in the solutions increased chromium ions leaching from stainless steel but the concentration of the chelator did not make a difference under the tested conditions. An effect of DTPA on chromium leachates was not observed until 34 weeks (Fig. 5b). Similar to Na2EDTA, no DTPA concentration effect was observed for chromium leachates.
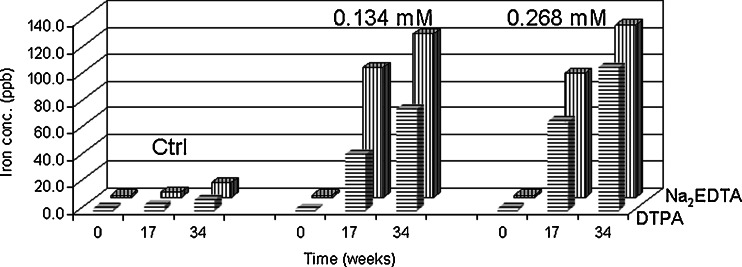
Since iron is the major metal leachate compared to chromium and nickel, it was used to explore the capacity difference affecting metal leachates between Na2EDTA and DTPA. At equal molar concentrations of Na2EDTA and DTPA over 34 weeks at 25°C, Na2EDTA facilitated iron leaching from stainless steel faster than DTPA as shown in Fig. 9. This can be of concern since it has been shown that Na2EDTA and DTPA cannot protect protein degradation if the ratio of the molar concentration of the metal chelators to iron in solution is less than 1:1 (27).
Fig. 9.
Iron leachates average concentration comparison between Na2EDTA and DTPA in 20 mM histidine-acetate buffer solutions (pH 6.0) when metal chelator concentrations at 0, 0.134, and 0.268 mM stored at 25°C over 34 weeks (triplicate measurement with RSD of 0.5%)
Effect of pH on Metal Leachables
Similar to previously discussed results, the major leached metal ions at the tested pH of 5.0, 6.0, 7.0, and 9.0 is iron, at temperatures of 2–8°C and 25°C. At 25°C, chromium and nickel could not be quantitated (levels were below the limit of quantitation) until a contact time with stainless steel of over 17 weeks. Thus, in Fig. 6b and c, only the data for 17 and 34 weeks time points were plotted. Figure 6 suggests that iron, chromium, and nickel leached most at pH 5.0 while between pH 6.0 and 9.0, no pH effect was observed. At 2–8°C, pH affected metal leaching from stainless steel differently compared to 25°C. For iron, the sequence of impact from high to low is pH 5.0 >> pH 9.0 > pH 6.0 ∼pH 7.0 (Fig. 7a). Acidic conditions of pH 5.0 produced about four fold higher iron leachate compared to pH 9.0. It has been reported that ferric/ferrous salts of sulfate, gluconate, and citrate exhibit much higher solubility in acidic conditions than neutral and basic conditions (31). At close to neutral and neutral conditions, no pH impact was observed. The effect of pH on chromium shows decreasing leakage in the following order: pH 9.0 > pH 5.0 > pH 6.0 > pH 7.0, that is, both basic and acidic conditions have impact on chromium leachables but not at neutral pH 7.0 conditions as shown in Fig. 7b. Similar to chromium, basic condition (pH 9.0) produced strongest impact on nickel leachables, followed by pH 5.0, then pH 6.0 (Fig. 7c). At neutral pH 7.0, no pH impact was observed over 34 weeks.
To further explore the temperature impact at pH 5.0–9.0, the concentrations of each leachate were compared using the 34 weeks time point as shown in Fig. 8. For iron, at the acidic pH 5.0, the lower temperature of 2–8°C storage produced a stronger impact than at 25°C while for neutral conditions of pH 7.0, the 25°C storage condition impact was stronger than 2–8°C. At pH 6.0 and 9.0, no temperature impact was observed. For chromium leachables, at acidic to neutral condition of pH 5.0–7.0, the higher temperature of 25°C exerted a stronger impact. In contrast, at basic condition of pH 9.0, the lower temperature of 2–8°C produced more leachables. For nickel leachables, lower temperatures produced much more metal leachates except at neutral condition of pH 7.0 which had no obvious impact.
CONCLUSIONS
Among the three monitored metal leachates of iron, chromium, and nickel, iron is the most abundant. However, overall metal leachates observed are at the ppb level. Multiple factors such as buffer species, the amount of testing media fill volume per unit stainless steel contact surface area, formulation excipients such as metal chelator type and/or concentration, pH, storage temperature, and contact duration with stainless steel, play an important role in affecting the concentrations of metal ions leaching from contact with stainless steel. Each studied factor exhibited its own capacity at leaching metal ions from contact with stainless steel. The commonly used buffers of histidine–acetate, histidine–HCl, phosphate, citrate, and succinate exhibited different properties at storage conditions of −40°C to 25°C with time at same concentration and pH. Furthermore, with the same buffer species and concentration, pH impacted the capacity to leach metal ions from stainless steel. Solution fill volume per unit stainless steel contact surface area above 3 mL/cm2 did not further decrease the concentration of metal iron leachates. Compared to the other factors tested in this study, commonly present metal chelators in parenteral formulations, metal chelator Na2EDTA and DTPA, produced the most significant impact on metal leachates, especially iron leachates. However, Na2EDTA and DTPA behaved differently. Na2EDTA produced a much stronger impact compared to DTPA over the tested 34 weeks at 25°C. Increase in concentration of Na2EDTA present in the formulations did not increase iron leachates. In contrast, the concentration of iron leachates increased significantly with an increase of DTPA concentration. Therefore, to minimize metal leaching from stainless steel during drug substance and/or drug product manufacturing, storage, and shipping processes, multiple factors have to be evaluated to balance the risk and benefit to achieve the desired drug product shelf life. Large-scale production tanks generally have much higher volume per unit surface areas than those tested here (of the order of 10 mL/cm2 or higher), which significantly reduces the risk if proper maintenance procedures are followed.
Acknowledgments
The 316L stainless steel coupons utilized in this study were supplied courtesy of Boehringer Ingelheim Pharma. We gratefully acknowledge Parag Kolhe (Biotherapeutic Pharmaceutical Science, Pfizer Inc.) for his help in calculating the probability factor using the JMP data analysis software and Bo Zhang (Biotherapeutic Pharmaceutical Science, Pfizer Inc.) for his help in analyzing the samples.
References
- 1.Passerini A, et al. Identifying cysteines and histidines in transition-metal-binding sites using support vector machines and neural networks. Proteins. 2006;65(2):305–316. doi: 10.1002/prot.21135. [DOI] [PubMed] [Google Scholar]
- 2.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. J Biol Chem. 2001;276(47):44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 3.Markovic I. Challenges associated with extractables and/or leachables substances in therapeutic biologic protein products. Am Pharm Rev. 2006;9(6):20–27. [Google Scholar]
- 4.Bee JS, et al. Precipitation of a monoclonal antibody by soluble tungsten. J Pharm Sci. 2009;98(9):3290–3301. doi: 10.1002/jps.21707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International conference on harmonization of technical requirements for registration of pharmaceuticals for human use (ICH). Guideline for industry: impurities in new drug substances, ICH Q3A
- 6.CDER Guidance Document: container closure systems for packaging human drugs and biologics. 1999.
- 7.EMEA, Harmonized guideline (EMEA and health Canada) on the pharmaceutical quality of inhalation and nasal products. 2005.
- 8.ICH, International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. Guideline for industry: Good manufacturing practices, ICH Q6A.
- 9.ICH, International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. Guidance for industry: impurities in new drug substances, ICH Q3B.
- 10.Arbin A, Jacobsson S, Hanninen K, Hagman A, Ostelius J. Studies on contamination of intravenous solutions from PVC-bags with dynamic headspace GC-MS and LC-diode array techniques. J Pharm Sci. 1986;28:211–218. [Google Scholar]
- 11.Goydan R, et al. High-temperature migration of antioxidants from polyolefins. Food Addit Contam. 1990;7(3):323–337. doi: 10.1080/02652039009373897. [DOI] [PubMed] [Google Scholar]
- 12.Jenke D. Extractable/leachable substances from plastic materials used as pharmaceutical product containers/devices. PDA J Pharm Sci Technol. 2002;56(6):332–371. [PubMed] [Google Scholar]
- 13.Jenke D, et al. Performance characteristics of an ion chromatographic method for the quantitation of citrate and phosphate in pharmaceutical solutions. J Chromatogr Sci. 2007;45(1):50–56. doi: 10.1093/chromsci/45.1.50. [DOI] [PubMed] [Google Scholar]
- 14.Jenke D, et al. Strategy for assessing the leachables impact of a material change made in a container/closure system. PDA J Pharm Sci Technol. 2005;59(6):360–380. [PubMed] [Google Scholar]
- 15.Jenke RD, Chess EK, Zietlow DC, Rabinow BE. Model for estimating the accumulation of solutes leaching from polymeric containers into parenteral solutions. Int J Pharm Sci. 1992;78:115–122. doi: 10.1016/0378-5173(92)90363-7. [DOI] [Google Scholar]
- 16.Jenke RD, Chess EK, Jakubowski G. Modeling of the leachables impact on the engineering of parenteral product container systems. Int J Pharm. 1994;108:1–9. doi: 10.1016/0378-5173(94)90410-3. [DOI] [Google Scholar]
- 17.Kim K, Rhee SG, Stadtman ER. Nonenzymatic cleavage of proteins by reactive oxygen species generated by dithiothreitol and iron. J Biol Chem. 1985;260(29):15394–15397. [PubMed] [Google Scholar]
- 18.Reif OW, Solkner P, Rupp J. Analysis and evaluation of filter cartridge extractables for validation in pharmaceutical downstream processing. PDA J Pharm Sci Technol. 1996;50(6):399–410. [PubMed] [Google Scholar]
- 19.Sanga SV. Review of glass types available for packaging parenteral solutions. J Parenter Drug Assoc. 1979;33(2):61–67. [PubMed] [Google Scholar]
- 20.Jenke DR, Story J, Lalani R. Extractables/leachables from plastic tubing used in product manufacturing. Int J Pharm. 2006;315(1–2):75–92. doi: 10.1016/j.ijpharm.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Nicholas K. Extractables and leachables determination: a systematic approach to select and qualify a container closure system for a pharmaceutical product. Am Pharm Rev. 2006;9(3):21–27. [Google Scholar]
- 22.Osterberg RE. Potential toxicity of extractables and leachables in drug product. Am Pharm Rev. 2005;8(2):64–67. [Google Scholar]
- 23.Taborsky CJ, Sheinin EB, Hunt DG. A critical approach to the evaluation of packaging components and the regulatory and scientific considerations in developing a testing strategy. Am Pharm Rev. 2006;9:146–150. [Google Scholar]
- 24.Allain L., Wang Q., Impact of package leachables on the stability of pharmaceutical products. Am Pharm Rev. 2007;10(4):38, 40, 42-44.
- 25.316/316L Stainless steel product data sheet. Cited 2007; available from: www.aksteel.com.
- 26.Waterman KC, et al. Stabilization of pharmaceuticals to oxidative degradation. Pharm Dev Technol. 2002;7(1):1–32. doi: 10.1081/PDT-120002237. [DOI] [PubMed] [Google Scholar]
- 27.Zhou S, Zhang B, Sturm E, Teagarden D, Schoneich C, Kolhe P, et al. Comparative evaluation of disodium EDTA adn DTPA as iron chelators to prevent metal catalyzed destabilization of a therapeutic monoclonal antibody. J Pharm Sci. 2010;99(10):4239–4250. doi: 10.1002/jps.22141. [DOI] [PubMed] [Google Scholar]
- 28.Rubinson, K.A., ed. Chemical Analysis. 1st ed. 1987, Little, Brown and Company, Boston.
- 29.Kolhe P, Amend SSE. Impact of freezing on pH of buffered solutions and consequences for monoclonal antibody aggregation. Biotech Progr. 2009 doi: 10.1002/btpr.377. [DOI] [PubMed] [Google Scholar]
- 30.Kocijan A, Milosev I, Pihlar B. The influence of complexing agent and proteins on the corrosion of stainless steels and their metal components. J Mater Sci Mater Med. 2003;14(1):69–77. doi: 10.1023/A:1021505621388. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs A, Miles PM. Role of gastric secretion in iron absorption. Gut. 1969;10(3):226–229. doi: 10.1136/gut.10.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]