Abstract
Purification of extracellular α-amylase from Bacillus subtilis KIBGE HAS was carried out by ultrafiltration, ammonium sulfate precipitation and gel filtration chromatography. The enzyme was purified to homogeneity with 96.3-fold purification with specific activity of 13011 U/mg. The molecular weight of purified α-amylase was found to be 56,000 Da by SDS-PAGE. Characteristics of extracellular α-amylase showed that the enzyme had a Km and Vmax value of 2.68 mg/ml and 1773 U/ml, respectively. The optimum activity was observed at pH 7.5 in 0.1 M phosphate buffer at 50°C. The amino acid composition of the enzyme showed that the enzyme is rich in neutral/non polar amino acids and less in acidic/polar and basic amino acids. The N-terminal protein sequence of 10 residues was found to be as Ser-Ser-Asn-Lys-Leu-Thr-Thr-Ser-Trp-Gly (S-S-N-K-L-T-T-S-W-G). Furthermore, the protein was not N-terminally blocked. The sequence of α-amylase from B. subtilis KIBGE HAS was a novel sequence and showed no homology to other reported α-amylases from Bacillus strain.
Key words: amino acids, α-amylase, Bacillus subtilis, purification, sequencing
INTRODUCTION
Alpha amylases (endo-1, 4-α-d-glucan glucanohydrolase EC 3.2.1.1) are extracellular endoenzymes that randomly cleave α-1,4 linkages between adjacent glucose units in the linear amylose chain and ultimately generate glucose, maltose, and maltotriose units. This class of industrial enzymes constitutes approximately 25% of the enzyme market. Conversion of starch into sugar syrups (glucose, maltose, maltotriose, dextrins sugar, or fructose syrups, etc.) are the major part of the starch processing industry. In addition, α-amylase have many applications in textile, paper, brewing, baking, distilling industries, preparation of digestive aids, production of cakes, and pharmaceuticals (1–3). Alpha amylases have been isolated from different sources. However, enzymes from fungal and bacterial sources have dominated applications in industrial sectors, due to advantages such as cost effectiveness, less time and space requirement and ease of process modification and optimization (4,5).
Previously, α-amylase from Bacillus subtilis KIBGE HAS been partially purified and effects of temperature, metal ions and surfactant on α-amylase activity has been investigated and enzyme showed high stability with various surfactants and detergents (6). The enzyme also showed relatively high thermostability and retained 62% of its activity when kept at 70°C for 15 min. Alpha amylase is highly stable at −18°C in 124 days of storage stability study (6).
In the present study, a novel α-amylase was purified from B. subtilis KIBGE HAS and its catalytic and molecular properties were characterized.
MATERIALS AND METHODS
Microorganism and Culture Conditions
B. subtilis KIBGE HAS been grown in liquid medium containing (g l−1): starch, 20.0; bacto-peptone, 10.0; yeast extract, 4.0; NaCl, 0.5; MgSO4·7H2O, 0.5; CaCl2·2H2O, 0.2. The pH of the medium was adjusted to 7.0 before autoclaving. The 100.0 ml of inoculum was transferred into 900.0 ml of sterile starch broth medium and incubated for 35°C for 24 h. After 24 h incubation, culture broth was centrifuged (35, 000 × g for 10 min) at 0°C to remove the cell and supernatant was stored at −20°C, which used for further studies.
Enzyme Assay and Total Protein Determination
The activity of α-amylase was assayed by incubating 0.1 ml enzyme with 1.0 ml soluble starch (1.0 w/v) prepared in 0.1 M phosphate buffer, pH 7.5. After incubation at 50°C for 5 min, the reaction was stopped and the reducing sugars released were determined by the addition of 1.0 ml of 3,5-dinitrosalicylic acid reagent with maltose as standard (7).
One unit of α-amylase activity was defined as the amount of enzyme which liberates 1 μmol of reducing sugar as maltose per min under the conditions of the assay.
Total protein was determined by the Lowry’s method with bovine serum albumin as a standard (8).
Purification of α-Amylase
Crude enzyme solution was concentrated under nitrogen stream by using ultrafiltration with 100,000 MrCO (relative molecular weight cut off) membrane and filtrate was collected and again reconcentrated with 30,000 MrCO membrane while retenate was collected for further purification. Solid ammonium sulfate was added slowly with constant stirring at 4°C to the enzyme to give a final concentration of 40% saturation and then kept for 18 h at 4°C. The precipitated proteins were separated by centrifugation at 35,000 × g for 15 min at 0°C and dissolved in minimal volume of 0.1 M phosphate buffer (pH 7.5). The enzyme solution was dialyzed against same buffer (using Molecular porous membrane tubing-Spectrum Labs.) for 18 h at 4°C.
The dialyzed α-amylase sample was then applied on to a sepharose CL 6-B column, pre-equilibrated with 0.1 M phosphate buffer (pH 7.5). The column was washed with 500 ml of equilibration buffer and the bound protein eluted with the same buffer. Fractions (2.0 ml) were collected at a flow rate of 0.5 ml/min and assayed for enzyme activity. The active fractions which showed extracellular α-amylase activity were pooled and concentrated by lypholization method and applied to high-performance liquid chromatography (HPLC) for purity analysis.
Molecular Weight Determination
The molecular weight of α-amylase was determined by comparing its electrophoretic mobility with that of standard proteins having known molecular weights. SDS-PAGE was carried out using 10% resolving gel (9).
Zymography (In Situ Electrophoresis) of α-Amylase
After electrophoresis the gel was washed twice with 0.5% (v/v) Triton X-100 for 15 min to remove SDS. After washing of SDS, the gel was incubated in 2.0% soluble starch in buffer at 50°C for 20 min. The gel was then kept in the same buffer for 10 min at 50°C and then stained with iodine solution (0.3% I2 in 3% KI) for 3 min. Enzyme activity was visible as a pale yellow band in dark colored gels.
Effect of Substrate Concentration on α-Amylase Activity
Starch concentration for optimum extracellular α-amylase activity was varied from 5.0–25.0 mg/ml in 0.1 M potassium phosphate buffer (pH 7.5) and enzyme activity was performed. Kinetic data were transformed into Lineweaver–Burk plots with graph pad prism program (version 5.0). The Km value was calculated by slope of curve.
Effect of Temperature on α-Amylase Activity
Extracellular α-amylase activity was performed at different temperatures ranging from 25°C to 80°C.
Effect of pH and Ionic Strength of Buffer on α-Amylase Activity
To determine the optimum pH, 0.05 M Glycine–HCl (3.0–4.0), acetate (4.0–5.0), succinate (6.0–6.5), potassium phosphate (6.5–8.0), Tris–HCl (7.5–9.0) and Glycine–NaOH (9.0–10.0) buffer was used. Extracellular α-amylase activity was performed at 50°C for 5 min. Ionic strength of potassium phosphate buffer (pH 7.5) was varied from 0.025–0.20 M and extracellular α-amylase activity was determined.
Amino Acid Analysis of α-Amylase
Purified α-amylase (lyophilized powder) was sent to Alta Biosciences, University of Birmingham, United Kingdom, for amino acid analysis.
N-Terminal Sequencing of α-Amylase
For N-terminal protein sequencing of purified α-amylase, the enzyme was first electrophoresed by SDS-PAGE, stained with Coomassie blue, then sample in the form of gel preparation, was sent to Alta Biosciences, University of Birmingham, United Kingdom.
Accession Numbers
The nucleotide sequence for B. subtilis KIBGE HAS is deposited at GenBank as EU819145.
RESULTS AND DISCUSSION
Selection of Strain
The Bacillus species used in this study was isolated previously from indigenous soil (6). The identification of bacterial strain was based on morphological, biochemical and 16S ribosomal ribonucleic acid (rRNA) gene sequencing. The 16S rRNA gene sequence of organism was deposited in GenBank with accession number EU819145. The general characteristics of B. subtilis KIBGE HAS have been elaborated in Table I.
Table I.
Morphological and Biochemical Characteristics of Bacillus subtilis KIBGE HAS
| Parameters | Characteristics |
|---|---|
| Morphology | Rods with rounded ends. Finely wrinkled, dull, opaque, adherent colonies on nutrient agar plate. |
| Gram’s reaction | Positive |
| Growth factors | |
| Oxygen | Strict aerobe |
| Temperature | Growth at 35°C, range, 25–45°C |
| pH | Optimum 7.0, range, 5.0–9.0 |
| Biochemical reaction | |
| Indole | Positive |
| Methyl Red | Negative |
| Vogues Prosker | Positive |
| Citrate | Utilized |
| Starch hydrolysis | Positive |
| Nitrite | Formed from nitrate |
| Fermentation reaction | |
| Glucose, fructose, galactose, lactose and sucrose | Acid |
Purification of α-Amylase
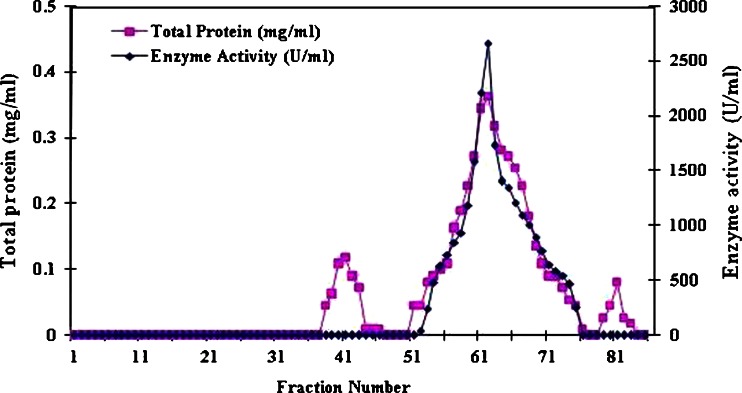
The purification scheme was summarized in Table II. Extracellular α-amylase from B. subtilis KIBGE HAS was purified by combination of ultrafiltration, ammonium sulfate precipitation and gel filtration chromatography through Sepharose CL6-B column. The active fractions from 52–75 (24 ml), which showed extracellular α-amylase activity were pooled and concentrated by using lypholization method (Fig. 1). This concentrated sample showed enzyme activity of 38385 Units with a specific activity of 13,011 U/mg (Table II).
Table II.
Steps Involved for the Purification of α-Amylase from Bacillus subtilis KIBGE HAS
| Purification steps | Enzyme activity (units) | Total protein (mg) | Specific activity (U/mg) | Fold purification |
|---|---|---|---|---|
| Crude enzyme | 684000 | 5063 | 135.0 | 1.0 |
| Ultrafiltration | 141000 | 308 | 457 | 3.4 |
| Ammonium Sulfate precipitation | 47405 | 18.3 | 2590 | 19.1 |
| Dialysis | 41080 | 14.6 | 2813 | 20.8 |
| Sepharose CL-6B | 38385 | 2.95 | 13011 | 96.3 |
Fig. 1.
Elution pattern of protein and α-amylase activity using Sepharose CL-6B column
Assessment of Homogeneity of α-Amylase by High-Performance Liquid Chromatography
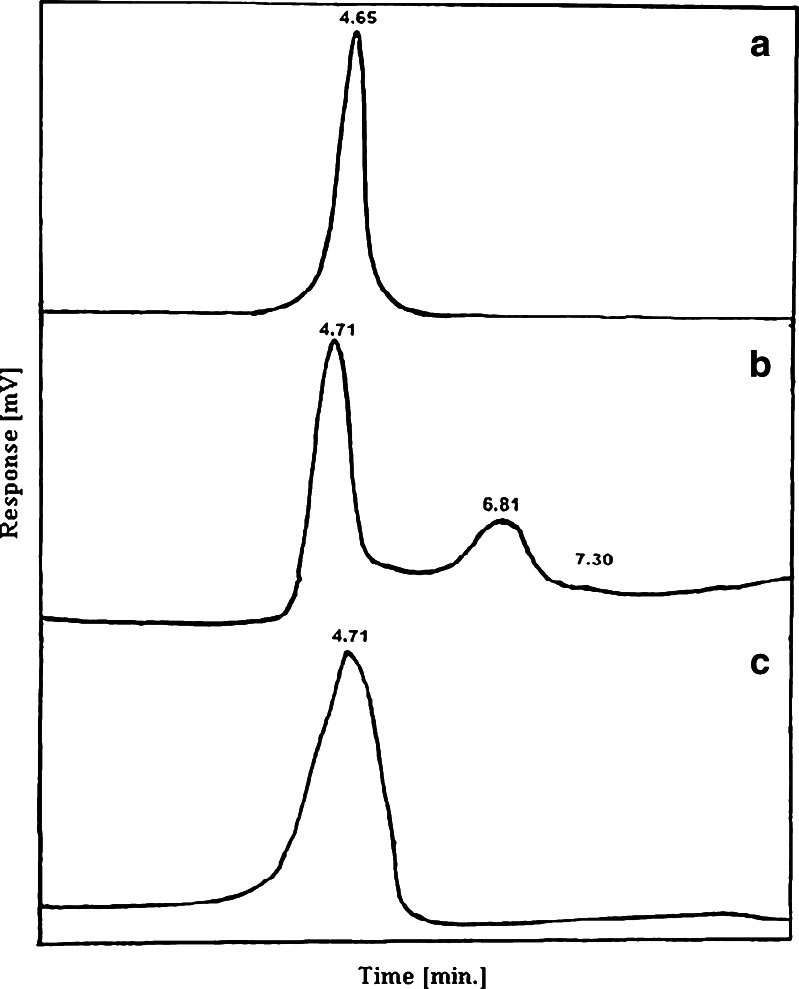
Pooled active fractions collected after fractionation through gel filtration were used for purity check analysis by HPLC. Purified α-amylase was analyzed by a C18 reversed-phase high-performance liquid chromatography column. Purified α-amylase from Sigma was used as a standard. Profile obtained from the standard shows a single peak with a retention time of 4.65 min (Fig. 2a). When partially purified α-amylase (after ammonium sulfate precipitation) was injected, the profile showed only one major peak (4.71 min) along with other minor peaks (Fig. 2b). Whereas, when purified α-amylase sample (pooled after Sepharose CL-6B fractionation) was injected, the profile showed a single peak with retention time of 4.71 min (Fig. 2c) confirming that α-amylase has been purified to homogeneity.
Fig. 2.
HPLC profile of alpha amylase from B. subtilis KIBGE HAS: a purified alpha amylase (Sigma); b partially purified alpha amylase; c purified alpha amylase after fractionation through Sepharose CL-6B
Molecular Weight Determination of α-Amylase by SDS-PAGE and In Situ Electrophoresis
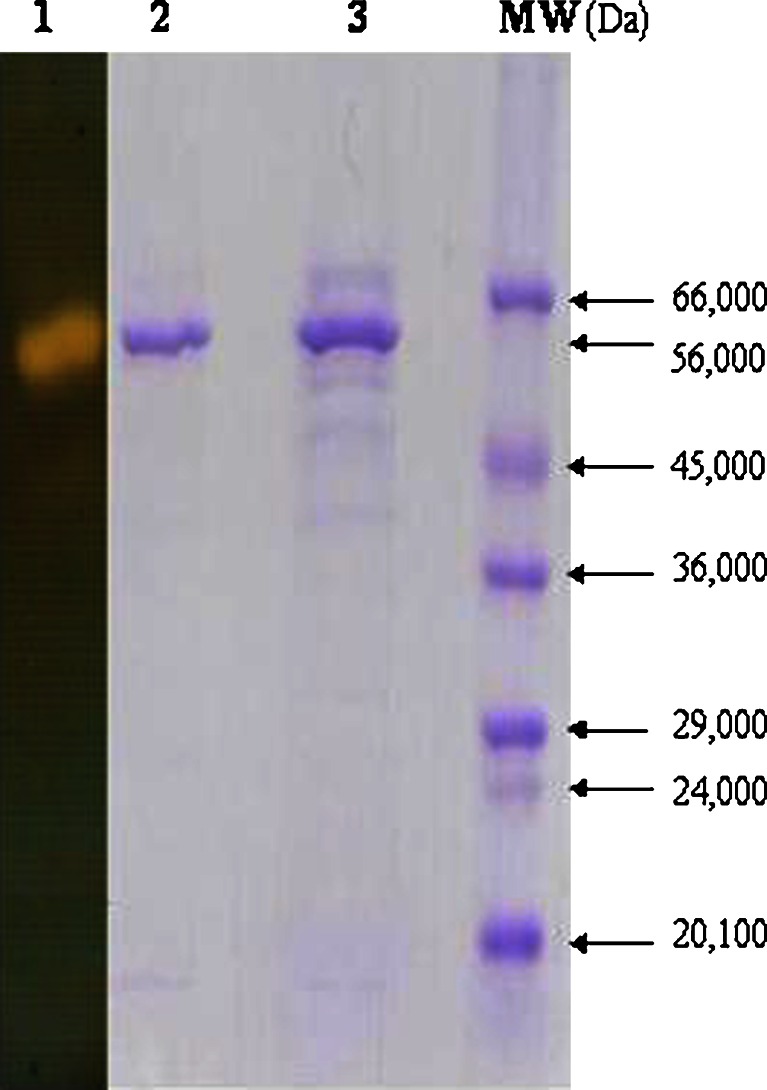
Electrophoretic analysis of extracellular α-amylase from B. subtilis KIBGE HAS was carried out after each step of purification (Fig. 3). Molecular weight was determined by comparing the relative mobility of the protein with the molecular weight markers. In situ electrophoresis was also carried out to distinguish protein bands capable of starch hydrolysis. Molecular weight of extracellular α-amylase from B. subtilis KIBGE HAS was determined as 56,000 Da. Five protein bands were detected in the partially purified sample (Fig. 3, Lane 3). After purification, SDS-PAGE profile shows a single protein band of extracellular α-amylase (Fig. 3, Lane 2) confirming that the α-amylase has been purified to homogeneity. Zymography of the purified enzyme was also performed and homogeneity of the purified enzyme was confirmed (Fig. 3, Lane 1). The molecular weight of extracellular α-amylase from B. subtilis KIBGE HAS is quite close to the molecular weight of α-amylase (55,000 Da and 57,000 Da) isolated from B. subtilis PKTH 10 and Streptomyces gulbargensis, respectively (10,11). However, it is higher than molecular weight of the same protein (42,800 and 42,000 Da) isolated from B. subtilis DM-03 and Bacillus sp. TS-23 (12,13). On the other hand, some relatively high molecular weight α-amylases have been reported from B. subtilis and B. subtilis 65 having molecular weight of 93,000 Da and 68,000 Da, respectively (14,15).
Fig. 3.
SDS-PAGE of α-amylase from B. subtilis KIBGE HAS. Lane 1 activity staining of alpha amylase; Lane 2 purified alpha amylase (Coomassie blue staining); Lane 3 partially purified alpha amylase; MW molecular weight markers
Effect of Substrate Concentration on α-Amylase Activity
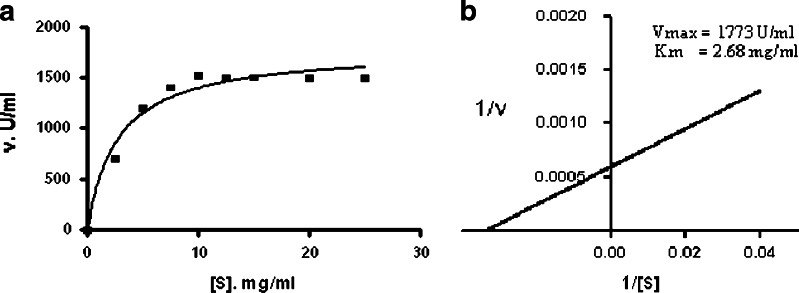
Starch was used as a substrate for α-amylase and it was observed that enzyme activity increased with increase in starch concentration and reached maximum at 10.0 mg/ml starch concentration, after that no increased in activity was observed (Fig. 4a). The Michaelis–Menten constant (Km) was determined by Lineweaver–Burk plot using starch as a substrate. The Km value of α-amylase from B. subtilis KIBGE HAS was 2.68 mg/ml (Fig. 4b), which is much lower than that of enzyme from B. stearothermophilus (16). The Km for α-amylase from different Bacillus species varies in their affinities for soluble starch as shown in Table III. The maximum reaction rate (Vmax) was calculated as 1,773 U/ml.
Fig. 4.
a Substrate saturation kinetics of α-amylase from B. subtilis KIBGE HAS b Lineweaver–Burk plot of α-amylase from B. subtilis KIBGE HAS
Table III.
Km Value of α-Amylases from Different Bacillus Species
| Bacillus species | Km (mg/ml) | Reference |
|---|---|---|
| B. subtilis KIBGE HAS | 2.6 | Current study |
| Bacillus sp. TS-23 | 2.7 | (14) |
| B. subtilis | 3.8 | (17) |
| B. subtilis KCC 103 | 2.6 | (18) |
| B. subtilis DM-03 | 1.2 | (15) |
| Bacillus sp. ANT-6 | 3.8 | (4) |
| Bacillus sp. NRRL B3881 | 1.9 | (19) |
| Bacillus sp. IMD 434 | 1.9 | (20) |
| B. stearothermophilus | 14.0 | (21) |
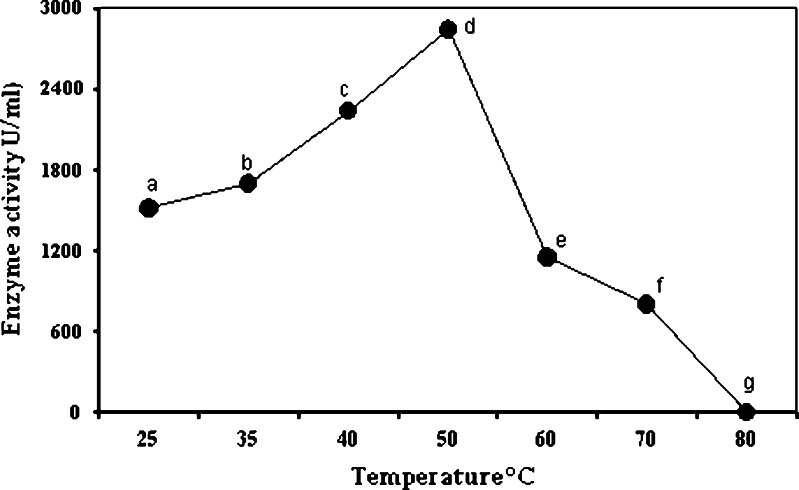
Effect of Temperature on α-Amylase Activity
To observe the effect of temperature on α-amylase, starch hydrolyzing activity was assayed at 25–80°C. The α-amylase from B. subtilis KIBGE HAS showed enzyme activity in a wide range of temperature but the optimum temperature was 50°C, with a progressive decline after that temperature and a complete inactivation at 80°C (Fig. 5).
Fig. 5.
Effect of temperature on α-amylase activity from Bacillus KIBGE HAS. Symbols (means ± S.E., n = 6) having similar letters are not significantly different from each other (Bonferroni test, P < 0.05)
It has been reported that optimal activity of α-amylase from B. subtilis and B. subtilis 65 was 50°C and 60°C, respectively (15,21). A reduction in enzyme activity (28%) was observed at 70°C, whereas at 80°C the enzyme was inactivated, it may be due to the fact that, as the temperature rises, a progressive inactivation of enzyme occurs, due to thermal inactivation of proteins in their structure, or it may also be due to incorrect conformation of protein, hydrolysis of the peptide chain, destruction of amino acid, or aggregation (17,22).
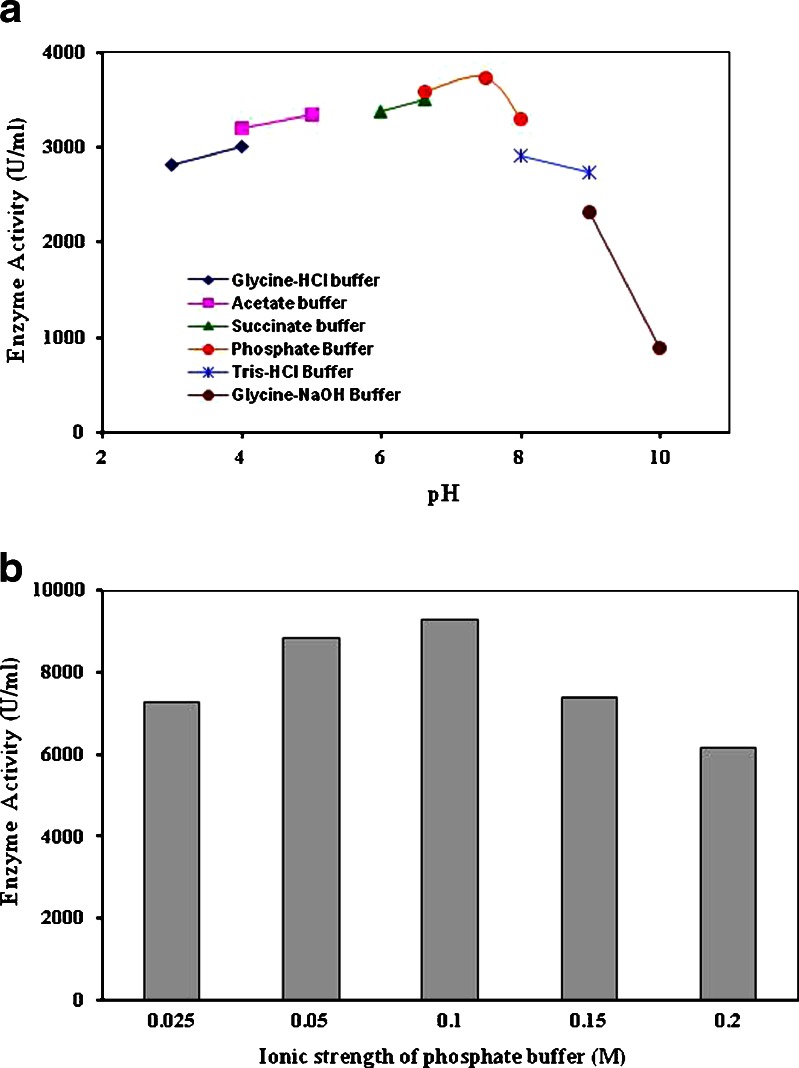
Effect of pH and Ionic Strength of Buffer on α-Amylase Activity
The effect of pH on extracellular α-amylase activity was determined at various pH ranging from pH 3.0 to pH 10.0 (Fig. 6a). The optimum pH for α-amylase activity was 7.5 (3738 U/ml). The enzyme was active at both acidic and alkaline pH, with a broad range of pH activity (pH 3–10). The enzyme retained about 73% of its original activity at pH 9.0 which suggests that enzyme is alkali tolerant; whereas amylase from Bacillus sp. TSCVKK retained only 60% activity at pH 8.5 (27). The enzyme was retained about 75% of its original activity at pH 3.0. The optima of α-amylases vary from 2–12 and amylases from most bacteria and fungi have pH optima in acidic to neutral range (5,16). Ionic strength of phosphate buffer was varied from 0.025 to 0.2 M. It was observed that the molarity of the buffer plays an important role in enzyme activity and highest α-amylase activity was determined in 0.1 M buffer (Fig. 6b).
Fig. 6.
a Effect of pH on extracellular α-amylase activity from B. subtilis KIBGE HAS b Effect of ionic strength of buffer on extracellular α-amylase activity from B. subtilis KIBGE HAS
Amino Acid Analysis of α-Amylase
The amino acid composition of purified α-amylase showed that enzyme is rich neutral/non polar amino acid and less in acidic/polar and basic amino acid (Table IV). Asparagine and glutamine were not detected in amino acid analysis, because these are completely converted to aspartic acid and glutamic acid during the acid hydrolysis. Tryptophan usually completely loss during acid hydrolysis and is not normally quantified. Amino acid analysis is expressed in nanomoles of the amino acid relative to all other residues in the protein.
Table IV.
Amino Acid Composition of The Purified α-Amylase
| Amino acids | 3 LC | 1 LC | Estimated concentration (nmol) |
|---|---|---|---|
| Aspartic acid | Asp | D | 41.1 |
| Threonine | Thr | T | 22.6 |
| Serine | Ser | S | 15.1 |
| Glutamic acid | Glu | E | 70.4 |
| Proline | Pro | P | 31.3 |
| Glycine | Gly | G | 41.2 |
| Alanine | Ala | A | 31.2 |
| Cystine | Cys | C | − |
| Valine | Val | V | 34.7 |
| Methionine | Met | M | 2.01 |
| Isoleucine | iLe | I | 23.2 |
| Leucine | Leu | L | 37.1 |
| Tyrosine | Tyr | Y | 28.6 |
| Phenylalanine | Phe | F | 15.0 |
| Histidine | His | H | 46.3 |
| Tryptophan | − | − | − |
| Lysine | Lys | K | 19.9 |
| Arginine | − | − | − |
| Totals | 460 |
N-Terminal Sequencing of α-Amylase
The N-terminal sequence of 10 residues was determined as Ser-Ser-Asn-Lys-Leu-Thr-Thr-Ser-Trp-Gly (S-S-N-K-L-T-T-S-W-G). Furthermore, the protein is not N-terminally blocked. The sequence of α- amylase from B. subtilis KIBGE HAS shows almost no homology to other reported α-amylases from Bacillus strains (Table V).
Table V.
Comparison of N-Terminal Sequences Data of α-Amylase Produced By Different Bacillus sp.
| Organism | Molecular weight (Da) | Sequence | Reference |
|---|---|---|---|
| B. subtilis HAS | 56,000 | SSNKLTTSWG | Current study |
| B. subtilis Marburg | 67,000 | LTAPSIKSGT | (23) |
| B. subtilis | 42,000 | TAPSIKSGTI | (24) |
| B. subtilis PKTH 10 | 55,000 | VNGTLMQYFE | (11) |
| B. licheniformis ERY R9 | 62,000 | ANLNGTLMEY | (25) |
| B. amyloliquifaciens N | 50,000 | VNGTLMEYFE | (26) |
It has been described earlier that there is no homology between the α-amylase from one species to others, where as the N-terminal regions of two different B. subtilis α-amylases are closely related (25). Tryptophan was detected in N-terminal amino acid sequence of α-amylase and it has been suggested to be involved in substrate binding/or catalysis for α-amylase (28).
CONCLUSIONS
Selection of microorganisms for the hyper-production of α-amylase is an important criterion in starch saccharification industries. Bacterial specifically Bacillus species are the main choice of interest over fungal species due to their several kinetic properties. Alpha amylase from B. subtilis KIBGE HAS got potential to be used in various industrial sectors where utilization of starch is concerned.
Acknowledgments
The authors would like to thank Dr. Tanzeel Haider Usmani, Director General PCSIR Laboratories Complex, Karachi for kindly providing the lab facilities.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Rao MB, Tanksale AM, Ghatge MS, Deshpande VV. Molecular and biotechnological aspects of microbial proteases. Microbiol. Mol Biol Rev. 1998;62:597–635. doi: 10.1128/mmbr.62.3.597-635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sivaramakrishnan S, Gangadharan D, Nampoothiri KM, Soccol CR, Pandey A. α-Amylases from microbial sources—an overview on recent developments. Food Technol Biotechnol. 2006;44:173–184. [Google Scholar]
- 3.Reddy NS, Nimmagada A, Sambasiva Rac RS. An over view of the microbial alpha amylase minireview. Afri J Biotechnol. 2003;2:645–648. [Google Scholar]
- 4.Burhan A, Nisa U, Gokhan C, Omer C, Ashabil A, Osman G. Enzymatic properties of novel thermostable, thermophilic, alkaline and chelator resistant amylase from alkaliphilic Bacillus sp. isolate ANT-6. Process Biochem. 2003;38:1397–1403. doi: 10.1016/S0032-9592(03)00037-2. [DOI] [Google Scholar]
- 5.Pandey A, Nigam P, Soccol CR, Soccol VT, Singh D, Mohan R. Advances in microbial amylases. Biotechnol Appl Biochem. 2000;31:135–152. doi: 10.1042/BA19990073. [DOI] [PubMed] [Google Scholar]
- 6.Bano S, Ul Qader SA, Aman A, Azhar A. Partial purification and some properties of partially purified α-amylase by Bacillus subtilis KIBGE-HAS. Ind J Biochem Biophys. 2009;46:401–404. [PubMed] [Google Scholar]
- 7.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 8.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin–phenol reagents. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 9.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 10.Takkinen K, Pettersson RF, Kalkkinen N, Palva I, Söderlund H, Kääriäinen L. Amino acid sequence of alpha-amylase from Bacillus amyloliquefaciens deduced from the nucleotide sequence of the cloned gene. J Biol Chem. 1983;258:1007–1013. [PubMed] [Google Scholar]
- 11.Syed DG, Agasar D, Pandey A. Production and partial purification of α-amylase from a novel isolate Streptomyces gulbargensis. J Ind Microbiol Biotechnol. 2009;36:189–194. doi: 10.1007/s10295-008-0484-9. [DOI] [PubMed] [Google Scholar]
- 12.Das K, Doley R, Mukherjee AK. Purification and biochemical characterization of a thermostable, alkaliphilic, extracellular α-amylase from Bacillus subtilis DM-03, a strain isolated from the traditional fermented food of India. Biotech Appl Biochem. 2004;40:291–298. doi: 10.1042/BA20040034. [DOI] [PubMed] [Google Scholar]
- 13.Lin LL, Chyau CC, Hsu WH. Production and properties of a raw-starch-degrading amylase from thermophilic and alkaliphilic Bacillus sp. TS-23. Biotechnol Appl Biochem. 1998;28:61–68. [PubMed] [Google Scholar]
- 14.Orlando AR, Ade P, Di Maggio D, Fanelli C, Vittozzi L. The purification of a novel amylase from Bacillus subtilis and its inhibition by wheat proteins. Biochem J. 1983;209:561–564. doi: 10.1042/bj2090561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashida S, Teramoto Y, Inoue T. Production and characteristics of raw potato starch digesting α-amylase from Bacillus subtilis 65. Appl Environ Microbiol. 1988;54:1516–1522. doi: 10.1128/aem.54.6.1516-1522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vihinen M, Mantsiila P. Microbial amylolytic enzymes. Crit Rev Biochem Mol Biol. 1989;24:329–418. doi: 10.3109/10409238909082556. [DOI] [PubMed] [Google Scholar]
- 17.Ciobanu A, Lascu G, Berescu V, Nicolescu L. Cooling techniques in food industry. Abacus; 1976, p. 20.
- 18.Chung H, Friedberg F. Sequence of the N-terminal half of Bacillus amyloliquefaciens alpha amylase. Biochem J. 1980;185:387–395. doi: 10.1042/bj1850387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B. Microbial α-amylases: a biotechnological perspective. Process Biochem. 2003;38:1599–1616. doi: 10.1016/S0032-9592(03)00053-0. [DOI] [Google Scholar]
- 20.Nagarajan DR, Rajagopalan G, Krishnan C. Purification and characterization of a maltooligosaccharide forming α-amylase from a new Bacillus subtilis KCC103. Appl Microbiol Biotechnol. 2006;73:591–597. doi: 10.1007/s00253-006-0513-4. [DOI] [PubMed] [Google Scholar]
- 21.Marco JL, Bataus LA, Valencia FF, Ulhoa CJ, Astolfi-Filho S, Felix CR. Purification and characterization of a truncated Bacillus subtilis α-amylase produced by Escherichia coli. Appl Microbiol Biotechnol. 1996;44:746–752. doi: 10.1007/BF00178613. [DOI] [PubMed] [Google Scholar]
- 22.Schokker EP, Van Boekel AJS. Kinetic of thermal inactivation of extracellular proteinase from Pseudomonas fluorescens 22 F influence of p H, calcium and protein. J Agric Food Chem. 1999;47:1681–1686. doi: 10.1021/jf980930q. [DOI] [PubMed] [Google Scholar]
- 23.Mäntsälä P, Zalkin H. Membrane bound and soluble extracellular α-amylase from Bacillus subtilis. J Biol Chem. 1979;254:8540–8547. [PubMed] [Google Scholar]
- 24.Hamilton LM, Kelly CT, Fogarty WM. Purification and properties of the raw starch degrading α-amylase of Bacillus sp. IMD 434. Biotechol Lett. 1999;21:111–115. doi: 10.1023/A:1005413816101. [DOI] [Google Scholar]
- 25.Kuhn H, Fietzek PP, Lampen JO. N-terminal amino acid sequence of Bacillus licheniformis α-amylases: comparison with Bacillus amyloliquifaciens and Bacillus subtilis enzymes. J Bacteriol. 1982;149:372–373. doi: 10.1128/jb.149.1.372-373.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagata Y, Suga S, Kado O, Maruo B. N-terminal amino acid sequence of α-amylase from Bacillus subtilis var. amylosacchariticus: comparison with that of “liquefying” type α-amylase. Agric Biol Chem. 1980;44:215–216. [Google Scholar]
- 27.Kiran KK, Chandra TS. Production of surfactant and detergent-stable, halophilic and alkali tolerant alpha-amylase by a moderately halophilic Bacillus sp. strain TSCVKK. Appl Microbiol Biotechnol. 2008;77:1023–1031. doi: 10.1007/s00253-007-1250-z. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura Y, Kusunoki M, Harada W, Kakudo M. Structure and possible catalytic residues of Taka-amylase A. J Biochem. 1984;95:697–702. doi: 10.1093/oxfordjournals.jbchem.a134659. [DOI] [PubMed] [Google Scholar]