Abstract
Jojoba oil-based emulgel formulations were prepared using different concentrations of various gelling agents, such as hydroxypropyl methylcellulose (HPMC) and Carbopol 934 P and combination of both. The prepared emulgels were physically evaluated for their stability after temperature cycle test, centrifugation and long-term shelf storage for 1 year at room temperature. The in vitro release at 37°C was studied to define the effect of the concentration and type of the gelling agent. A comparison between the formulated emulgels and two commercially available products, Candistan® and Canesten® creams, was carried out to judge their efficacy and stability. The prepared emulgels exhibited non-Newtonian shear thinning behavior with little or no thixotropy. Four emulgels showed excellent stability as they demonstrated consistent rheological model under different treatment conditions. The in vitro release test showed variation in the extent of percent drug released. The drug release from the commercial preparation was lower than some of the prepared emulgel formulae. One formula containing combination of the two gelling agents (HPMC and Carbopol 934 P), showed excellent stability and high extent of clotrimazole release was microbiologically evaluated against Candida albicans using cylinder and plate method. The selected formula showed superior antimycotic activity compared to the commercially available formulation. Further in vivo animal studies for the obtained stable formula is recommended.
KEY WORDS: Carbopol 934 P, clotrimazole, emulgel, emulsion gel, HPMC, microbiological evaluation, rheology, stability
INTRODUCTION
Since the mid 1980s, emulsion gels have been of growing importance in the field of pharmaceutical semisolid dosage forms. In cosmetics, such hydrophilic systems have already been known for a longer period. Their wide utilization as pharmaceutical dosage form comes from the wide utilization of emulsion systems particularly for dermatological formulae (1). Emulsion gels are gaining importance because of their many advantages. They have better application property in comparison to classical formulations such as creams and ointments. In addition, they have faster and more complete release of the drug from the vehicle to the skin and therefore, higher efficacy (c.f. creams, ointments). Furthermore, they are convenient to apply on hairy skin due to absence of greasiness and lack of residues upon application. Finally, they permit the incorporation of both aqueous and oleaginous ingredients, so poorly water-soluble drugs like antifungal agent clotrimazole (CZ) can be easily incorporated in such type of vehicles.
CZ is a widely used effective antifungal agent. It is a synthetic imidazole derivative shown to be potent and well-tolerated topical agent. It is active against dermatophytes (the causative organism of tinea infections) and yeast (Candida albicans; 2). Like other antimycotic imidazole, CZ interferes in the lipid synthesis of fungi and thus causes an alteration of the permeability of the cell walls, especially when high doses are applied causing leakage of intracellular phosphorus compounds, sodium and potassium leading to inhibition of macromolecular protein synthesis by fungi (3). The formulation of antifungal drugs in emulgel dosage could have beneficial effects on the mycological cure rate and treatment safety. Bonifaz and Saul (4) compared the efficacy and safety of topical 1% terbinafine emulsion gel versus 2% ketoconazole cream in tinea cruris and tinea corporis. The study revealed that the rates of mycological cure were 94% for terbinafine emulsion gels and 69% for ketoconazole cream. They concluded that terbinafine 1% emulsion gel is significantly more effective than ketoconazole 2% cream as regards clinical and mycological cure and treatment safety.
Jojoba oil, a liquid unsaturated wax composed of esters of long carbon chain fatty acids (C20 to C22) and long carbon chains unsaturated alcohols (C20, C22), has been used as the oily phase for our study. Dermatological research suggests that jojoba oil may help to reduce inflammation commonly associated with fungal infections (5). Habashi et al. (6) demonstrated the effectiveness of jojoba liquid wax in combating inflammation in several experimental animal models.
The aim of this work is to formulate the antifungal drug CZ into stable emulgels with good physical, rheological and release properties. These emulgels will be subjected to several evaluation tests to assess their physical characters, release properties and stability under several conditions. The prepared emulgels will be compared to two commercial preparations available in the Egyptian market Candistan® (C1) and Canesten® (C2). Microbiological evaluation of the formulations with best stability and in vitro CZ release will be carried out.
MATERIAL AND METHODS
Clotrimazole (CZ) was kindly provided by the Arab Drug Company (Cairo, Egypt). Jojoba oil purchased from Egyptian Natural Oil Company (Cairo, Egypt). Brij 35, span 60 and cellulose membrane (Mwt cutoff 12,000–14,000) were supplied from Sigma Chemical Company (USA). Propylene glycol, dimethyl formamide (DMF; analytical grade) and hydrochloric acid (HCl) were purchased from El Nasr Pharmaceutical Chemicals (Cairo, Egypt). Triethanolamine (TEA; pharmaceutical grade) is supplied from Morgan Chemicals IND. CO. (Cairo, Egypt). Hydroxypropyl methylcellulose (HPMC, Methocel E4M, and Mwt 86,000, 4,000 centipoise (cp)) is kindly gifted by Memphis Company for Pharmaceutical and Chemical Industries (Cairo, Egypt). Carbopol 934 P is kindly provided by Chemical Industries Development Company (Giza, Egypt). Candistan® B.N 160236 (C1) is purchased from Arab Drug Company (Cairo, Egypt). Canesten® B.N 2346109 (C2) is supplied from Alexandria Pharmaceutical Company (Alexandria, Egypt).
Preparation of Emulgels and Liquid Emulsion
The assigned codes and detailed compositions of the prepared formulae are given in Table I. For all preparations, the specified amount of span 60 and CZ were dissolved in the oily phase (jojoba oil) with the aid of magnetic stirrer (Thermolyne Corporation, USA) at 75 ± 0.5°C, the solution was allowed to cool, then the calculated amount of Carbopol 934 P (formulae containing Carbopol either alone or in combination) was dispersed in the formed oily solution (phase A). The aqueous phase was prepared to contain the specified amount of Brij 35 and propylene glycol (phase B). Phase A was then slowly added to phase B and emulsified using the over head mixer (Hiedolph, Germany) for 10 min at 1,400 rpm, then the prepared emulsion was introduced into the homogenizer (Erweka, type AR 401, Germany) for 5 min at 10,000 rpm. The system was then gellified by adding triethanolamine (formulae containing Carbopol either alone or in combination) and/or dispersing hydroxypropyl methylcellulose (formulae containing HPMC either alone or in combination) using the over head mixer at 200 rpm for 45 min. The final pH of preparations containing Carbopol 934 P was adjusted to pH 5.5–6.5 using TEA (7); the bases were left over night for equilibration at 20°C. Liquid emulsion formulation was prepared in similar way but without adding the gelling agents.
Table I.
Composition and Codes of Jojoba Oil Emulgel Bases
| Components | Formula’s code | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Percentage (w/w) of the components | ||||||||||
| EJa | J1 | J2 | J3 | J4 | J5 | J6 | J7 | J8 | J9 | |
| Jojoba oil | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Span 60 | 1.08 | 1.08 | 1.08 | 1.08 | 1.08 | 1.08 | 1.08 | 1.08 | 1.08 | 1.08 |
| Brij 35 | 1.92 | 1.92 | 1.92 | 1.92 | 1.92 | 1.92 | 1.92 | 1.92 | 1.92 | 1.92 |
| Triethanolamine (TEA) | – | – | – | – | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. | Q.S. |
| Hydroxypropyl methylcellulose (HPMC) | – | 1 | 1.5 | 2 | – | – | – | 0.5 | 0.75 | 1 |
| Carbopol 934 P | – | – | – | – | 0.2 | 0.3 | 0.4 | 0.1 | 0.15 | 0.2 |
| Propylene glycol | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Distilled water | 61 | 60 | 59.5 | 59 | 60.8 | 60.7 | 60.6 | 60.4 | 60.1 | 59.8 |
| CZ | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
QS quantity sufficient to adjust the pH to 5.5–6.5
aLiquid emulsion (no gelling agent)
Physical Properties Assessment
The prepared emulgels and the commercial preparations were examined for their visual as well as rheological properties.
Visual Inspection
The prepared formulae were examined for their physical characteristics, namely: color, homogeneity, and phase separation.
Rheological Properties
The rheological properties of emulgel samples were determined using cone and plate Brookfield viscometer (Brookfield, Model programmable DV2, USA). About 0.5 g of the formula to be tested was applied to the plate and left for equilibrium, measurements were made at 20°C and at shear rates ranging from 0.4 to 400 s−1 corresponding to 0.2 to 200 rpm with 10 s between each two successive speeds and then in a descending order. The hysteresis loop between the upward and downward curve was studied. The flow index was determined by linear regression of the logarithmic form of Eq. 1 (8,9).
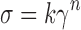 |
1 |
Where σ is the shear stress, γ is the shear rate, k is the consistency index, and n is the flow index.
n = 1 when the flow is Newtonian, if n > 1 or n < 1 indicates shear thickening or shear thinning respectively. Also the apparent viscosity at 20 s−1 was determined from the rheograms.
Stability Assessment
The prepared emulgels and the commercial preparations were subjected to temperature cycle test, centrifugation and storage for 1 year at room temperature for stability assessment.
Temperature Cycle Test
The emulgel samples were subjected to two temperature cycles for a period of 48 h, each cycle is 24 h, starting at −4°C (8 h) and 40°C (16 h) .
Centrifugation
The emulgel samples were subjected to centrifugation at 300 rpm twice, each for 15 min (10).
Long-Term Stability Assessment
The prepared formulae were shelf stored for 1 year. Visual inspection and rheological assessment previously described were conducted on the samples after exposure to the three different treatments namely; temperature cycle test, centrifugation and shelf storage. The flow behavior of the different emulgel bases was studied according to the following different mathematical models that describe the viscoplastic fluids (11–13):
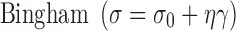 |
2 |
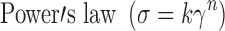 |
3 |
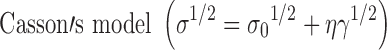 |
4 |
where σ = shearing stress, σ0 = yield value, η = viscosity, γ = shearing rate, k = consistency index, n = flow index. Curve fitting was carried out using using graphpad prism for Windows, Version 5.0 (Graphpad Software Inc).
In Vitro Drug Release Study
The release of CZ from different emulgel and liquid emulsion formulations as well as the commercially available preparation was carried out. This study was performed using the modified USP dissolution apparatus (Pharma test, type PTW 2, Germany). Samples, each of 2 g of the preparation, were spread on a cellophane membrane (Mwt cutoff 12,000–14,000) previously soaked for overnight in the receptor medium. The loaded membrane was firmly stretched over the edge of a glass cup of 2.59 cm diameter. The cup was then immersed in the dissolution vessel which contained 100 ml of the release medium (25% v/v DMF in 0.02 N HCl) previously warmed and maintained at 37 ± 0.5°C. Agitation was affected by paddle at 50 rpm and aliquots each of 5 ml were withdrawn from the release medium at different time intervals. Withdrawn samples were replaced by equal volumes of fresh release medium. The samples were assayed spectrophotometrically at 264.5 nm and the concentration of the drug was determined from the previously constructed calibration curve. Experiments were carried out in triplicates, the results were averaged and blank experiments were carried out using plain bases.
In Vitro Antimycotic Activity of Selected CZ Preparations
The microbiological evaluation of selected CZ preparation (formula that showed good stability and release) and the commercial preparation Canesten® cream was carried out according to Ibrahim et al. (14). Briefly, a single well-isolated colony of C. albicans ATCC No 60193, which is grown and maintained on sabouraud dextrose agar slants at 4°C and subcultured monthly, was picked and inoculated into a tube containing 10 mL of sterile sabouraud dextrose broth (sterilized by autoclaving at 121°C for 15 min). The broth was incubated at 35°C for 24 h. After incubation, the resulting growth was centrifuged, washed with phosphate buffer saline (PBS) and re-suspended in fresh PBS to turbidity equivalent to 0.5 McFarland. An inoculum of 1.5 mL of the above suspension was transferred to sterile petri dish (20 cm in diameter), then 30 mL of molten sabouraud dextrose agar (sterilized by autoclaving at 121°C for 15 min) were added to the inoculum and mixed well and left to solidify.
Stainless steel cylinders (11 mm internal diameter) were sterilized using hot air oven at 180°C for 1 h. Each cylinder was aseptically filled with accurately weighed 250 mg of the selected tested formulae, and put on the surface of inoculated sabouraud dextrose agar plates. The plate was incubated aerobically at 37°C for 24 h. After incubation, the inhibition zone diameter was measured using a ruler. The extent of release (zone of inhibition) was measured by taking the mean of three readings. The test was applied also for non-medicated bases.
Statistical Analysis
Statistical significance of difference was tested either using Student's t test or one-way ANOVA test (Sigma plot for Windows, version 11.0, Systat Software Inc.). The level of significance was set at α = 0.05.
RESULTS AND DISCUSSION
Preparation of Emulgels
CZ was successfully incorporated into emulgel bases containing various types and concentrations of gelling agents. HPMC and/or Carbopol 934 P were selected as gelling agents. These gelling agents increased the viscosity of emulgel by hampering the flow of the continuous phase and the particles of the internal phase within it (15). TEA was used to neutralize the formulae containing Carbopol to the pH range 5.5–6.5, as it causes the polymer chains to uncoil and forms the gel structure. This pH range was a compromise between the pH of the skin (pH 5) as reported by Langer and Wise (16), and that for maximum stability of Carbopol in water (pH 6–8) according to Lucero et al. (17).
Propylene glycol is added to the formulae as humectant, to increase the spreadability, in addition, it increases the aesthetic benefits in terms of skin feel of the product (18).
Physical Properties
Visual Inspection
Investigation of the prepared emulgel formulae indicated that they are yellowish white, smooth, homogenous of semisolid consistency without sticky skin feel. Formulae containing HPMC as gelling agent were somewhat tacky but this tackiness is reduced by combining HPMC with Carbopol 934 P. The tackiness of HPMC was reported in many literatures (19,20). It could be explained by the interaction between the polymer chains. Addition of propylene glycol or Carbopol 934 P may reduce this tackiness due to their ability to interact with HPMC chains, reducing the extent of hydrogen bonding between the polymer chains themselves and hence tackiness (19). No phase separation was reported for all the prepared formulae.
Rheological Properties
In recent years, many gelled water-soluble bases have been formulated to optimize topical drug delivery without an exhaustive rheological study (21). Nevertheless, knowledge of rheological and mechanical properties has an outstanding importance, and may lead to possible use of the resulting rheological parameters to optimize topical drug delivery from dermatological formulations.
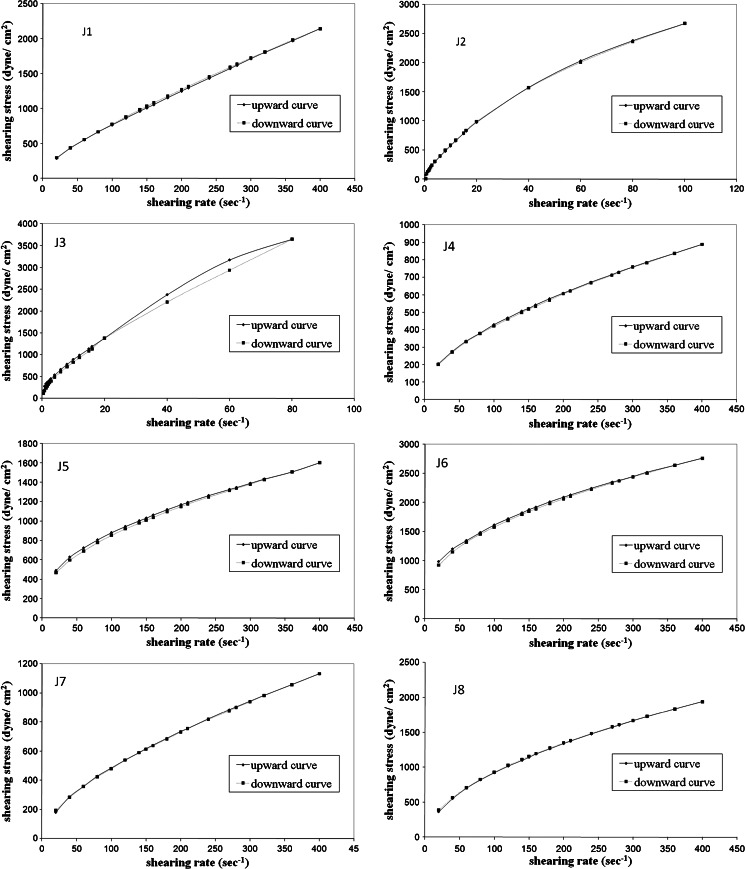
All prepared fresh samples of CZ emulgels revealed the non-Newtonian shear thinning (pseudo)plastic flow behavior described in basic rheological literature (22) with little or no thixotropy (Fig. 1). In this flow behavior, the molecules at rest entangled together with the association of immobilized solvent. Under the influence of shear, the molecules tend to become disentangled and align themselves in the direction of flow thus offering less resistance to flow and this together with the release of the entrapped inner phase accounts for the lower viscosity (23). Shear thinning behavior is a desirable property of topical semisolid preparation since it should thin during application and thicken otherwise (24).
Fig. 1.
Rheograms of different emulgels and commercial preparations
Flow patterns of all samples were nearly similar in fresh state but they differ to some extent in the consistency of the preparation and the degree of pseudoplasticity as evidenced by the values of the flow indices (n) as shown in Table II. Based on the values of n, calculated through Eq. 1 and were all <1, it could be confirmed that all the studied systems exhibited shear thinning behavior. The flow index values were ranging from 0.6795 (lowest shear thinning behavior) to 0.3522 (highest shear thinning behavior), the highest value was assigned for formula J1 (jojoba oil emulgel containing 1% HPMC) and the lowest value was assigned to the formula J6 (emulgel containing 0.4% Carbopol 934 P). The flow indices for commercial preparations C1 and C2 were 0.307 and 0.431, respectively, indicating their shear thinning behavior.
Table II.
Flow Index and Viscosity of Different CZ Preparations
| Formula’s code | Flow index (n) | Viscosity (cp)a |
|---|---|---|
| J1 | 0.6795 | 1,435 |
| J2 | 0.6657 | 4,876 |
| J3 | 0.5639 | 6,901 |
| J4 | 0.4978 | 1,101 |
| J5 | 0.3946 | 2,457 |
| J6 | 0.3522 | 6,016 |
| J7 | 0.6039 | 904.4 |
| J8 | 0.5526 | 1,848 |
| J9 | 0.4405 | 5,544 |
| C1 | 0.3067 | 6,134 |
| C2 | 0.4314 | 7,667 |
aViscosity measured at 20 s−1 at 20°C
The flow index of most formulations was dependent on the polymer concentration. By increasing the HPMC concentration from 1% (formula J1) to 2% (formula J3) the flow index decreased from 0.680 to 0.564. Similarly, increasing the concentration of a combination of HPMC and Carbopol 934 P (in formulae J7 to J9), decreased the flow index from 0.604 to 0.441. Moreover, by increasing the Carbopol 934 P concentration from 0.2% w/w (formula J4) to 0.4% w/w (formula J6) the flow index decreased from 0.498 to 0.352. Fresno et al. (25) demonstrated that there is an exponential decrease in the flow index value by increasing the polymer concentration to a constant minimum value and explained the presence of this constant minimum value by the formation of full structured three-dimensional polymer lattice due to increased polymer concentration.
It is obvious that formulae containing HPMC alone as gelling agent has a flow index range of 0.5639–0.6795. On the other hand, Formulae containing Carbopol only as gelling agent have a lower flow index range of (0.3522–0.4978). However, using a combination of HPMC and Carbopol as a gelling agent gave emulgel formulae with intermediate flow index range of (0.4405–0.6039). Emulgels containing HPMC only as the gelling agent demonstrated lower pseudoplasticity values (high mean flow index 0.6364 than those containing other gelling agents). Carbopol alone as gelling agent produced an emulgel of higher pseudoplasticity than other gelling agents as evidenced by its relatively lower mean flow index values 0.4149.
The apparent viscosity values at 20 s−1 and 20°C for different emulgel bases ranged from 904.4 to 6,901 cp with the highest value for formula J3 (emulgel with 2% HMPC) and the lowest value for formula J7 (emulgel with combination of 0.1% Carbopol and 0.5% HPMC). The type and concentration of gelling agent affected the apparent viscosity of the formulation. This variation in viscosity may be attributed to variation in shape and dimensions of crystallites of the solid fraction and their ordering in the three-dimensional structures within the resulting network where the liquid phase is held by adsorption capillarity and molecular interaction mechanisms (26,27).
The flow of commercial preparations showed thixotropic pseudoplastic pattern. The apparent viscosities of the commercial preparations C1 and C2 were also measured, and found to be 6,134 and 7,667, respectively.
Stability Assessment
The effect of accelerated stability testings (temperature cycle test and centrifugation) and long-term storage at shelf (1 year) on each of color change, phase separation and rheological properties of different formulae was assessed. No color change or signs of instability like phase separation or drop in consistency were observed.
Different mathematical models were tested by analyzing the ascending curves of the rheograms of the fresh and treated samples. The Bingham, Power’s law, and Casson mathematical models describing viscoplastic fluids (8,13,28) were selected and the best fitting model is chosen depending on the regression coefficient value (r2). Formulae that showed consistent model after different treatments were suspected to be stable from rheological point of view, in other words no structure changes have occurred.
Formulae J4, J7, J8, J9 and the commercial preparation C2 showed consistent model when fresh and after different treatments. Power’s law was the best fitting model for most of the selected formulae (r2 > 0.9531). The best-fit parameter values obtained from Power’s law, i.e., k (consistency index) and n (flow index) were determined and compared to the same values obtained with freshly prepared formulae.
The consistency index (k) is related to the gel’s real viscosity in the total absence of any shearing, consequently it provides valuable information since it is perfectly correlated with the consumer’s visual perception of the product.
It is obvious from Tables III and IV that all stable formulae, when fresh, showed a variation in consistency index (k) ranging from 30.0 to 300.2, at the same time the flow index (n) was ranging from 0.440 to 0.603.
Table III.
Consistency Index (k) (pa.sn) for the Selected CZ Formulae After Different Treatments
| Formula’s code | Consistency index (k) of the following emulgel samples | |||
|---|---|---|---|---|
| Fresh | Centrifugation | Temperature cycle | One year shelf storage | |
| J4 | 43.9 | 27.0 | 36.1 | 7.7 |
| J7 | 30.0 | 43.9 | 22.7 | 13.2 |
| J8 | 71.4 | 73.5 | 69.1 | 33.7 |
| J9 | 300.2 | 335.9 | 283.6 | 281.5 |
| C2 | 358.3 | 362.5 | 295.6 | 344.3 |
Table IV.
Flow Index (n) for Selected CZ Formulae Under Different Treatments
| Formula’s code | Flow index (n) of the following emulgel samples | |||
|---|---|---|---|---|
| Fresh | Centrifugation | Temperature cycle | One year shelf storage | |
| J4 | 0.497 | 0.543 | 0.514 | 0.638 |
| J7 | 0.603 | 0.587 | 0.647 | 0.678 |
| J8 | 0.552 | 0.571 | 0.578 | 0.681 |
| J9 | 0.440 | 0.444 | 0.452 | 0.489 |
| C2 | 0.431 | 0.400 | 0.465 | 0.407 |
It is clear that formulae with the least changes in both flow index and consistency index were those containing a combination of two gelling agents (Carbopol 934 P and HPMC). This could be explained on the basis of a synergetic rheological response commonly encountered upon combination of two gelling agents. Similar results were obtained by Abdel-Bary et al. (18) who found that chloramphenicol gel formulation containing mixture of Carbopol 940 and hydroxyethyl cellulose as gelling agent was more physically stable than those containing either carboxymethyl cellulose sodium or Carbopol 940 alone. Also, Walkenstrom and Hermansson (29) demonstrated that combination of gelatin and whey protein yields gels less sensitive to variations in shear conditions in comparison to pure gels.
At the same time, Table III showed that the studied formulations exhibited a decrease in consistency index and increase in the flow index, i.e., decrease in consistency and pseudoplasticity upon storage for 1 year, which may be explained by the coalescence of the inner oil phase droplet (30). Formula J9 showed the least change in both parameters.
In Vitro Drug Release Study
The primary objective of a topical formulation for the treatment of cutaneous disease is that the drug reaches the target site at the required concentration and achieves its therapeutic action. Thus, the clinical efficacy of the formulation depends on the ability of the vehicle to release the drug which must then penetrate the stratum corneum. Therefore, this efficiency could be somehow evaluated by measuring the in vitro release of CZ from different emulgel bases. The effect of gelling agent concentration on the extent of drug release was also studied.
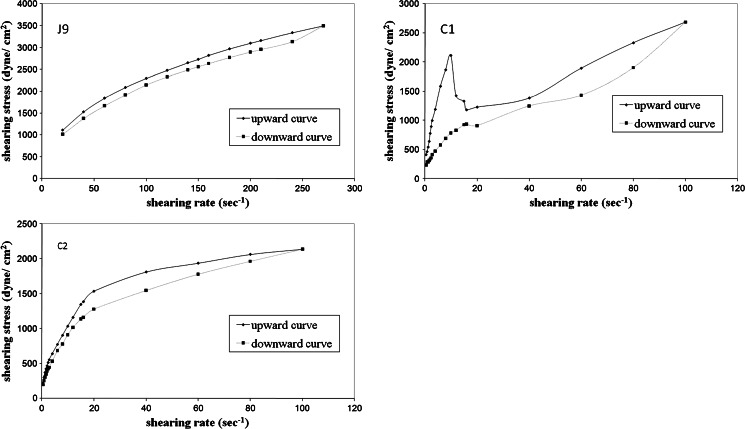
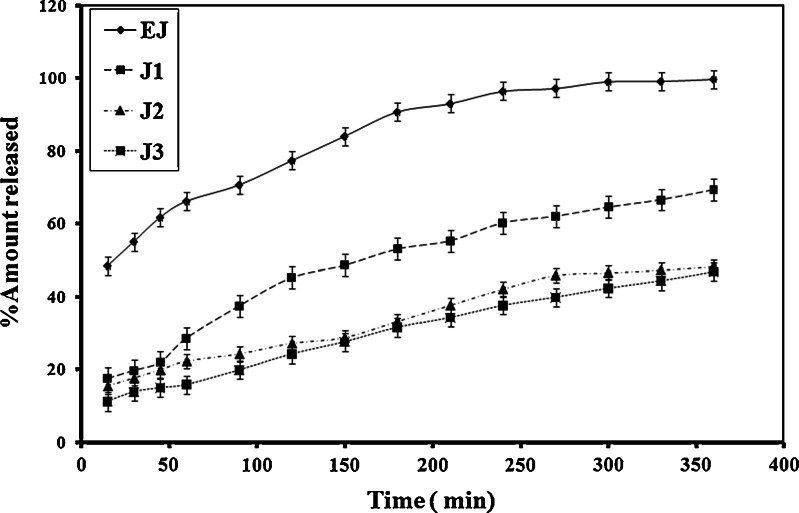
Figures 2, 3 showed that CZ released from emulgel dosage forms slowly compared to liquid emulsion formulations. The extent of CZ release was obviously affected by the gelling agent concentration. The decreased extent of CZ release at higher amounts of the gelling agent is attributed to decreased drug diffusion due to increased polymer viscosity. Mura et al. (31) showed that the viscosity increase has a negative effect on the drug diffusion rate of clonazepam from different hydrophilic ointment bases. The viscosity of the matrix may play an important role in controlling the release of the drug in the receptor compartment when the drug diffusion through the matrix is the rate limiting step (32,33). For a drug molecule to be released from a vehicle across a membrane, mass transport in a formulation occurs either by diffusion of the molecules or by diffusion and convection of the oil droplets. The apparent viscosity or macroviscosity of the formulation influences the diffusion of the droplets. The viscosity of the formulation is increased at higher concentrations of the gelling agent resulting in a decrease in molecular and droplet diffusion. Consequently, the length of diffusion pathway is increased and rate of release is decreased (34). Davis and Khanderia (35) indicated that thermodynamics and viscosity have a dominant effect on the release of active ingredient from the vehicle.
Fig. 2.
Release profiles of CZ from different emulgel bases containing HPMC
Fig. 3.
Release profiles of CZ from different emulgel bases containing Carbopol 934 P
It is noteworthy to say that although Carbopol emulgels exhibited higher viscosity than those prepared with HPMC, they showed higher drug release. This may be ascribed to the acidity of the dissolution medium (25% DMF in 0.02 N HCl) which leads to loss of ionization of Carbopol 934 P polymer chains and hence disappearance of the three-dimensional network structure necessary for the structure build up, whereas HPMC does not have any ionizable groups that could be affected by the low pH of the dissolution medium.
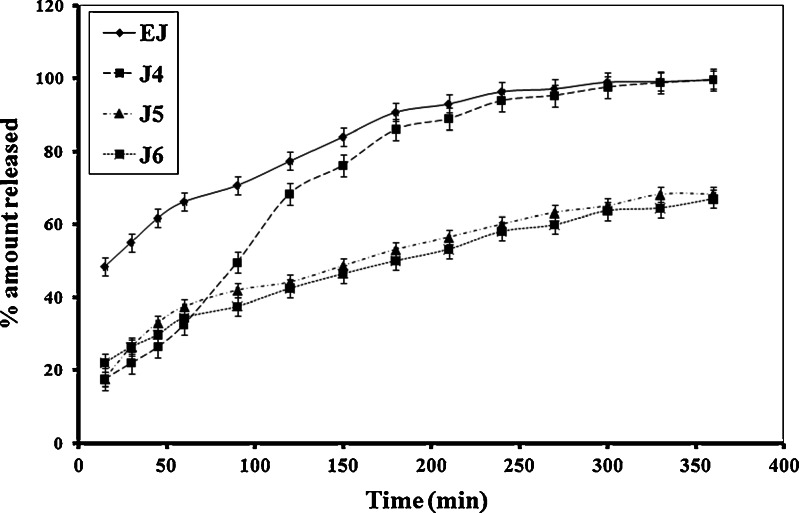
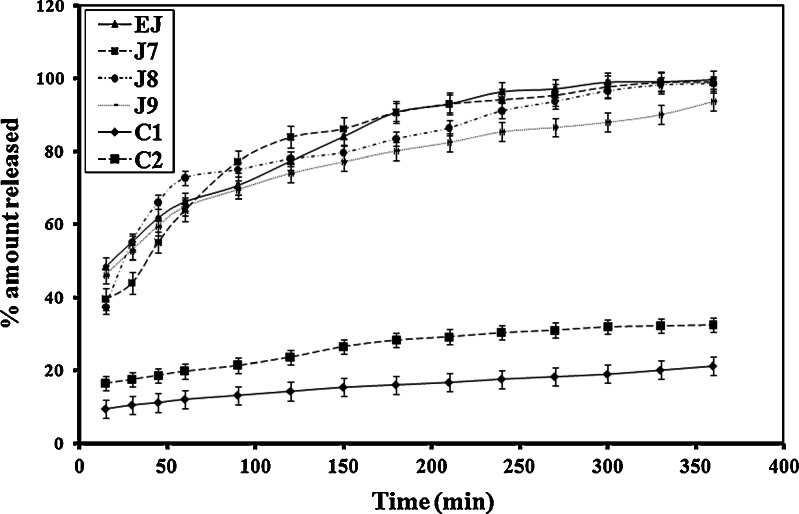
Figure 4 shows that formulations containing combination of both gelling agents released CZ as fast as the emulsion formulation.
Fig. 4.
Release profiles of CZ from different commercial preparations and emulgel bases containing HPMC and Carbopol 934 P
The release data and profiles of CZ from commercial preparations are illustrated in Fig. 4. Canesten® cream released higher amount of CZ compared to Candistan® cream. Surprisingly, the release from these creams was lower than from emulgels.
From all of the previous studies, formula J9 showed both stable rheological properties and high extent of drug release, so it has been chosen for further microbiological evaluation.
In Vitro Antimycotic Activity of Selected CZ Preparations
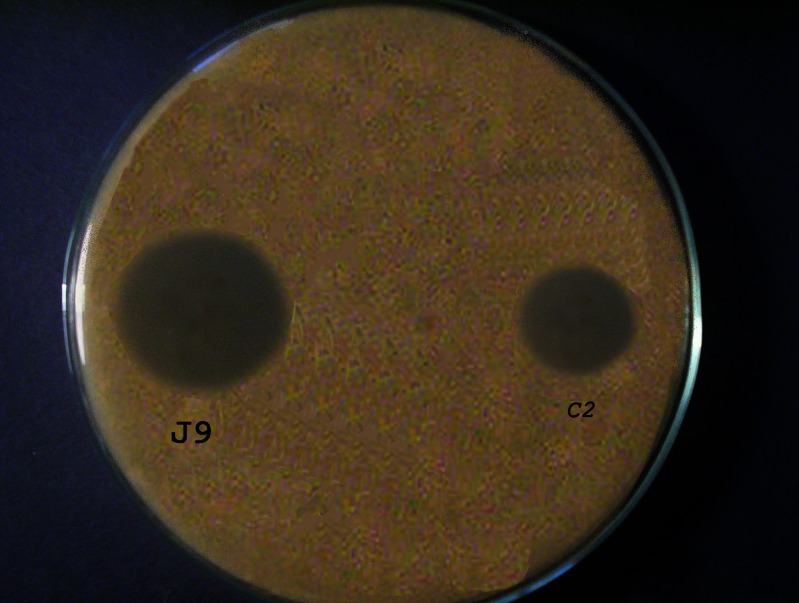
The effect of base composition on antimycotic activity of CZ against C. albicans using agar diffusion method was investigated. Plain unmedicated bases did not show any antimycotic activity (no zone of inhibition) against C. albicans. Formula J9 showed a mean zone of inhibition (3.50 ± 0.1 cm) which was significantly greater than the mean zone of inhibition exhibited by the commercial preparation Canesten® cream (2.34 ± 0.1 cm; P < 0.05; Fig. 5). This may be explained by the higher release of CZ from J9 formulation as well as the greater solubility of CZ in the emulgel (possibly due to the high ester content of jojoba oil) in comparison to the commercial preparation and hence greater partitioning of CZ at the boundary between the diffusion medium and the preparation.
Fig. 5.
In vitro antimycotic activity of 1% CZ in different formulations using cylinder plate method against C. albicans
CONCLUSIONS
Several CZ emulgels containing HPMC and/or Carbopol 934 P were prepared. They showed non-Newtonian shear thinning behavior with little or no thixotropy, and variable viscosity dependent on both the concentration and type of gelling agent. Stability testing under several conditions (centrifugation, temperature cycle test or storage for 1 year) showed that formulation containing low level of Carbopol or combination of two gelling agents have better stability compared to other formulations.
Analysis of the in vitro CZ release data showed that an inverse correlation between the concentration of gelling agent and the extent of drug released. The commercial preparations showed lower extent of drug release than some of the prepared formulae.
The formula that showed both stable rheological properties and high extent of drug release is J9, its antimicrobial activity against C. albicans has been evaluated using the cylinder and plate method and compared to the commercial formulation Canesten® cream. J9 exhibited superior antifungal activity in comparison to Canesten®. Further in vivo animal studies as well as in vitro permeation and skin retention studies using hairless rat skin to quantify the amount of CZ permeated through the skin, to calculate the local accumulation efficiency of the obtained promising stable formulation are recommended.
ACKNOWLEDGMENTS
The authors thank the Arab Drug Company, Memphis Company and Chemical Industries Development Company for their generous gifts of clotrimazole, HPMC, and Carbopol 934 P, respectively. The effort of Dr. Mohamed Hafiz and Dr. Mohamed Mabrouk for the microbiological study is greatly appreciated.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- 1.Marquardt D, Sucker H. Oil in water emulsion gels: determination and mathematical treatment of flow properties. Eur J Pharm Biopharm. 1998;46:115–24. doi: 10.1016/S0939-6411(97)00167-7. [DOI] [PubMed] [Google Scholar]
- 2.Martindale. The Extra Pharmacopiea. 29 ed. London: Pharmaceutical; 1989
- 3.Tettenborn D. Acute toxicity and local tolerance of clotrimazole. Drugs Made Ger. 1972;15(9):94–9. [Google Scholar]
- 4.Bonifaz A, Saul A. Comparative study between terbinafine 1% emulsion-gel versus ketoconazole 2% cream in tinea cruris and tinea corporis. Eur J Dermatol. 2000;10(2):107–9. [PubMed] [Google Scholar]
- 5.Borlaug N, Baldwin AR, Estefan R, Harris M, Plucknett DL. Jojoba: new crop for arid lands. New raw material for industry. Washington DC: National Academy Press; 1985. [Google Scholar]
- 6.Habashy R, Abdel-Naim A, Khalifa A, Al-Azizi M. Anti-inflammatory effects of jojoba liquid wax in experimental models. Pharmacol Res. 2005;51(2):95–105. doi: 10.1016/j.phrs.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Mura P, Faucci MT, Corti P, Bramanti G. Formulation studies on topical preparations of clonazepam. STP Pharm Sci. 1997;7(3):229–34. [Google Scholar]
- 8.Jimenez Soriano MM, Fresno Contreras MJ, Selles Flores E. Development of a cream from a self emulsifying base and moisturizing actives. IL Farmaco. 2001;56:513–22. doi: 10.1016/S0014-827X(01)01087-4. [DOI] [PubMed] [Google Scholar]
- 9.Bourret E, Ratsimbazafy V, Maury L, Brossard C. Rheological behavior of saturated polyglycolysed glycerides. J Pharm Pharmacol. 1994;46:538–41. doi: 10.1111/j.2042-7158.1994.tb03852.x. [DOI] [PubMed] [Google Scholar]
- 10.Simovic S, Milic-ASkrabic J, Vuleta G, Stupar M. Physicochemical properties of emulsion gels with different concentrations of polymeric emulsifier pemulen TR1-NF. Pharmazie. 1998;53(4):276–7. [Google Scholar]
- 11.Marquardt D, Pedrussio P, Herzog B, Sucker H. Determination of (pseudo)plastic flow properties of pharmaceutical semisolids using rheological AUC parameters. Drug Dev Tech. 1997;2(2):123–33. doi: 10.3109/10837459709022617. [DOI] [PubMed] [Google Scholar]
- 12.Krajisink D, Milic J. Polymer stabilized emulsion systems: structure characteristics and physical stability evaluation. Drug Dev Ind Pharm. 2003;29:701–11. doi: 10.1081/DDC-120021319. [DOI] [PubMed] [Google Scholar]
- 13.Grigelmo-Miguel N, Martin-Belloso O. Influence of fruit dietary fiber addition on physical and sensorial properties of strawberry jams. J Food Eng. 1999;41:13–21. doi: 10.1016/S0260-8774(99)00067-9. [DOI] [Google Scholar]
- 14.Ibrahim SA, Hafez E, El-shenawy SM, El-Gibally IS, Mohamed EA. Formulation and evaluation of clotrimazole ointment. Bull Pharm Sci Assi Univ. 1991;14(1&2):13–22. [Google Scholar]
- 15.Harry RG, Harry RG, Rieger MM. Harry’s cosmeticology. New York: Chemical Publishing; 2000. [Google Scholar]
- 16.Langer RS, Wise DS. Medical applications of controlled release. FL: CRC; 1984. [Google Scholar]
- 17.Lucero MJ, Vigo J, Leon MJ. Study of shear and compression deformations on hydrophilic gels of tretinoin. Int J Pharm. 1994;106(5):125–33. doi: 10.1016/0378-5173(94)90310-7. [DOI] [Google Scholar]
- 18.Abdel-Bary A, Shalaby S, Abdel-Aal S. Formulation and stability of chloramphenicol gel and emulgel. Bull Fac Pharm Cairo Univ. 2001;39(3):89–99. [Google Scholar]
- 19.Chan LW, Wong TW, Chua PC, York P, Heng PW. Anti-tack action of polyvinylpyrrolidone on hydroxypropyl methylcellulose solution. Chem Pharm Bull. 2003;51(2):107–12. doi: 10.1248/cpb.51.107. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs B, Merenyi G. Evaluation of tack behavior of coating solutions. Drug Dev Ind Pharm. 1990;16(15):2320–3. doi: 10.3109/03639049009043801. [DOI] [Google Scholar]
- 21.Rossi S, Ferrari F, Bonferoni MC, Caramella C. Rheological and mechanical properties of pharmaceutical gels. Part II: medicated systems: relevance to hydration properties and drug release. Boll Chim Farm. 2001;140:337–44. [PubMed] [Google Scholar]
- 22.Briceno MI. Rheology of suspensions and emulsions. In: Nelloud F, Marti-Mestres N, editors. Pharmaceutical emulsions and suspensions. New York: Marcel Dekker; 2000. pp. 577–607. [Google Scholar]
- 23.Perrot EL. Pharmaceutical technology, fundamental pharmaceutics. Minneapolis: Burgress; 1971. [Google Scholar]
- 24.Pena LE, Lee BI, Sternes JF. Structural rheology of model ointment. Pharm Res. 1994;11:875–81. doi: 10.1023/A:1018990010686. [DOI] [PubMed] [Google Scholar]
- 25.Fresno MJC, Ramirez AD, Jimenez MM. Systematic study of the flow behavior and mechanical properties of carbopol Ultrez 10 hydroalcoholic gels. Eur J Pharm Biopharm. 2002;54:329–35. doi: 10.1016/S0939-6411(02)00080-2. [DOI] [PubMed] [Google Scholar]
- 26.Huttenrauch R, Fricke S. Molecular galenics. 7. Mechanical activation of salves, an indirect proof of their quaternary structure. Pharmazie. 1976;31(6):408–9. [PubMed] [Google Scholar]
- 27.Huttenrauch R, Fricke S, Baumann V. Properties and dynamics of structure in shear crystallized ointments. Pharmazie. 1982;37(1):25–8. [PubMed] [Google Scholar]
- 28.Dickinson E, Rodford SJ, Golding M. Stability and rheology of emulsions containing sodium caseinate: combined effects of ionic calcium and non ionic surfactant. Food Hydrocoll. 2003;17:211–20. doi: 10.1016/S0268-005X(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 29.Walkenstrom P, Hermansson AM. Effect of shear on pure and mixed gels of gelatin and particulate whey protein. Food Hydrocoll. 1998;12:77–87. doi: 10.1016/S0268-005X(98)00048-4. [DOI] [Google Scholar]
- 30.Jiao J, Burgess DJ. Rheology and stability of w/o/w multiple emulsions containing span 83 and Tween 80. AAPS Pharm Sci Tech. 2003;5(1) [DOI] [PMC free article] [PubMed]
- 31.Mura P, Nassini C, Proietti D, Manderioli A. Influence of vehicle composition variation on the in vitro and ex vivo clonazepam diffusion from hydrophilic ointment bases. Pharm Acta Helv. 1996;71:147–54. doi: 10.1016/0031-6865(96)00005-2. [DOI] [PubMed] [Google Scholar]
- 32.Arellano A, Santoyo S, Martin C, Ygartua P. Influence of propylene glycol and isopropyl myristate on the in vitro percutaneous penetration of diclofenac sodium from carbopol gels. Eur J Pharm Sci. 1999;7:129–35. doi: 10.1016/S0928-0987(98)00010-4. [DOI] [PubMed] [Google Scholar]
- 33.Davis SS. Symposium on topical administration of drugs. 1989.
- 34.Petit JM, Roux B, Zhu XX. A new physical model for the diffusion of solvents and solutes probes in polymer solutions. Macromolecules. 1996;29:6031–6. doi: 10.1021/ma951159c. [DOI] [Google Scholar]
- 35.Davis SS, Khanderia MS. Viscoelastic properties of pharmaceutical semisolids: characterization of the plastibase for bioavailability studies. J Pharm Pharmacol. 1972;24:176–7. [PubMed] [Google Scholar]