Abstract
Müllerian Inhibiting Substance (MIS) expression is inversely proportional to the serum concentration of testosterone in males after birth and in vitro studies have shown that MIS can lower testosterone production by Leydig cells. Also, mice overexpressing MIS exhibited Leydig cell hypoplasia and lower levels of serum testosterone, but it is not clear whether this is a result of MIS affecting the development of Leydig cells or their capacity to produce testosterone. To examine the hypothesis that MIS treatment will result in decreased testosterone production by mature Leydig cells in vivo, we treated luteinizing hormone (LH)-stimulated adult male rats and mice with MIS and demonstrated that it can lead to a several-fold reduction in testosterone in serum and in testicular extracts. There was also a slight decrease in 17-OH-progesterone compared to the more significant decrease in testosterone, suggesting that MIS might be regulating the lyase activity of cytochrome P450c17 hydroxylase/lyase (Cyp17), but not its hydroxylase activity. Northern analysis showed that, in both MIS-treated rats and mice, the mRNA for Cyp17, which catalyzes the committed step in androgen synthesis, was down-regulated. In rats, the mRNA for cytochrome P450 side-chain cleavage (P450scc) was also down-regulated by MIS. This was not observed in mice, indicating that there might be species-specific regulation by MIS of the enzymes involved in the testosterone biosynthetic pathway. Our results show that MIS can be used in vivo to lower testosterone production by mature rodent Leydig cells and suggest that MIS-mediated down-regulation of the expression of Cyp17, and perhaps P450scc, contributes to that effect.
In mammals, expression of the testis determining factor, Sry, commits the bipotential gonad to the differentiation of the testes that produce two hormones required for normal male phenotypic development, Müllerian Inhibiting Substance (MIS) and testosterone. MIS (also known as anti-Müllerian Hormone) is a 140-kDa glycoprotein hormone member of the transforming growth factor-β (TGFβ) superfamily of growth and differentiation proteins (1). The fetal Sertoli cells express MIS, which causes regression of the Müllerian duct, the precursor of female internal reproductive tract structures. Concurrently, testosterone, produced by the fetal Leydig cells, induces the differentiation of the Wolffian duct into the vas deferens, epididymides, and seminal vesicles. In the absence of these hormones, as is the case with females, the Wolffian duct degenerates and the Müllerian duct differentiates into the uterus, Fallopian tubes, and upper vagina (2–4).
MIS expression remains long after the Müllerian duct has regressed in males and persists at very high levels until puberty. After puberty, the expression of MIS is greatly reduced in males to a level more like that of females, which express low levels of MIS at puberty (5–8). The physiological roles of MIS after Müllerian duct regression are currently being investigated and, based on experiments with mouse transgenics and knockouts, indicate that MIS is important for maintaining gonadal competence. Male mice overexpressing MIS have lowered levels of testosterone and Leydig cell hypoplasia and are undervirilized (9, 10), whereas conversely, mice with null mutations in either MIS or the MIS type II receptor (MISRII) have Leydig cell hyperplasia and other gonadal abnormalities (11, 12).
After birth, there is a reciprocal relationship between the serum concentration of MIS (5–8) and serum testosterone in males (4). As the concentration of MIS begins to decline as puberty is approached, there is a concomitant increase in serum testosterone. A likely cause for the low testosterone during the neonatal period is the paucity of remaining fetal Leydig cells after birth when this population of cells appears to die off. Prepubertally, another population of Leydig cells differentiates from mesenchymal precursors to progenitor Leydig cells, then to immature Leydig cells, and finally to adult Leydig cells at puberty. These mature Leydig cells are the source of adult testosterone (13).
Leydig cells are regulated by feedback control of the hypothalamic–pituitary–testis axis and produce testosterone in response to luteinizing hormone (LH) (14). LH, secreted from the pituitary gland, binds to its high-affinity G protein-coupled seven-transmembrane receptor on Leydig cells, activating adenylyl cyclase, which leads to the enhanced formation of cAMP and subsequent activation of protein kinase A. Phosphorylated steroid acute regulatory protein (StAR) transports cholesterol to the inner mitochondrial membrane where it is converted to pregnenolone by the activity of the cytochrome P450 side-chain cleavage enzyme (P450scc). In rodents, 3β hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase (3βHSD) converts pregnenolone to progesterone, which is then a substrate for the cytochrome P450c17 hydroxylase/lyase (Cyp17), the committed step in sex steroid synthesis.
In vitro experiments demonstrated that MIS can inhibit the production of testosterone by Leydig cells (15, 16) and that it does so, at least in part, by regulating the expression of Cyp17 (16). Our goal was to extend those in vitro studies and the results indicate that MIS delivered in vivo could lower testosterone production by Leydig cells.
Materials and Methods
Chemicals and Reagents.
Human chorionic gonadotropin (hCG) was provided by A. F. Parlow at the National Institute of Diabetes and Digestive and Kidney Diseases National Hormone and Peptide Program Harbor at UCLA Medical Center, Torrance, CA. Ketamine/xylazine were from Abbott. Radionucleotides were purchased from New England Nuclear. All other chemicals were obtained from Sigma or Fisher Scientific, unless otherwise noted. The rat P450scc cDNA was provided by JoAnne S. Richards, Baylor College of Medicine, Houston. Cyp17 and 3βHSD cDNAs were provided by Anita H. Payne, Stanford University, Palo Alto, CA. Recombinant human MIS was prepared from Chinese hamster ovary cells stably transfected with a linear construct of the human MIS gene (17). Active, secreted protein in the growth medium was then passed over an immunoaffinity column prepared with the monoclonal MIS antibody, 6E11, conjugated to Affi-Gel-10 (8). Bound MIS was then eluted off the column and protein concentrations were determined by Bradford assays (18). The bioactivity of the MIS was then verified by using an established organ culture assay, which grades the regression of the 14.5-day gestation rat urogenital ridge (19).
Animals.
All experimental protocols involving animals were reviewed and approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee. Additionally, all experiments conformed with procedures described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Rats.
For collection of blood and testicular tissue, adult male 150–175-g Sprague–Dawley rats were obtained from Charles River Breeding Laboratories. The concentrations of LH used were found to maintain high levels of testosterone up to 24 h, reproducibly (20). Rats were given single i.p. injection of LH (100 units; controls) to maximally stimulate testosterone production, or were treated with a single injection of LH and MIS (100 units LH/1 mg MIS) to monitor the effects of MIS on intratesticular and serum steroid levels. Blood was collected from the tail vein and testes were harvested for RNA preparation and for histological examination. Testicular fragments were fixed in Bouins' solution and embedded in paraffin before cutting and stained with hematoxylin/eosin.
Mice.
Male 25–30-g (6–7 weeks old) CD1 mice from Charles River Breeding Laboratories were used. Control animals were injected with 10–25 units of hCG; whereas MIS-treated animals received simultaneous injection of hCG (10–25 units) and MIS (100 μg).
Following experimental treatment, the cohorts of rats or mice were anesthetized with ketamine/xylazine 100/10 mg/kg body weight at different time-points (0.5, 1, 2, 4, 8, and 24 h). Approximately 1 ml of blood was collected by needle puncture into the heart after opening the thoracic cavity, immediately placed on ice (15 min), centrifuged (1,500 × g for 20 min at 4°C), and the serum separated and analyzed by RIA. Testes were harvested for RNA for use in northern blots and for extracts for use in the steroid RIAs. Total RNA was extracted by using the guanidine thiocyanate-cesium chloride method (21). All RNA was extracted with successive rounds of phenol, chloroform, and ether, then ethanol precipitated and quantitated by absorbance at A260. Testicular extracts were prepared by Dounce homogenization in 1 ml of 25 mM Tris, pH 7.4, with a protease inhibitor cocktail (Roche Molecular Biochemicals).
Northern Blot Analyses.
Ten micrograms of each RNA sample were denatured with dimethyl sulfoxide and glyoxal at 65°C, separated in a 1.5% agarose gel, blotted overnight onto nylon membranes, and UV-cross-linked. Blots were prehybridized with 100 μg/ml sonicated salmon sperm DNA in 50% formamide hybridization solution and hybridized overnight at 65°C with 2 × 106 cpm/ml probe with riboprobes Cyp17, P450scc, MISRII (22), and LH receptor (LHR). The murine LHR riboprobe was cloned by reverse transcription (RT)–PCR from MA-10 cells (23) with MuLHR1 5′GCACCCATCTCATTCTTTGCC3′ and MuLHR2 5′CCACCCTTTGAAGCAGGTTTC3′ primers. The random primed 3βHSD probe was used at 42°C. Blots were washed at 65°C with 0.1 × SSC (1 × SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7)/0.1% SDS and exposed to radiographic film with intensifying screens at −70°C. Blots were reprobed with a human β-actin riboprobe (24) at 65°C and washed at 55°C with 1 × SSC/0.1% SDS.
MIS ELISA and RIAs.
Following experimental treatment, mice or rat testicular tissue and serum were obtained and MIS was measured as described (8). In addition, serum and testicular extracts were assayed by RIA for total accumulated progesterone, 17-OH-progesterone, and testosterone in the Reproductive and Endocrinology Sciences RIA Laboratory at the Massachusetts General Hospital.
Data Analysis.
All experiments were repeated at least three times. An autoradiogram is presented where appropriate for qualitative analysis. Signal intensities of Northern blots were quantitated by using a Molecular Dynamics PhosphorImager. RIAs of testosterone, 17-OH-progesterone, progesterone, and MIS immunoassays (both serum and intratesticular hormone assessments) represent the mean ± SEM of combined data from replicate experiments. Statistical differences between mean values were analyzed by one-way analysis of variance followed by the student's t test. Significance was assigned at P < 0.05.
Results and Discussion
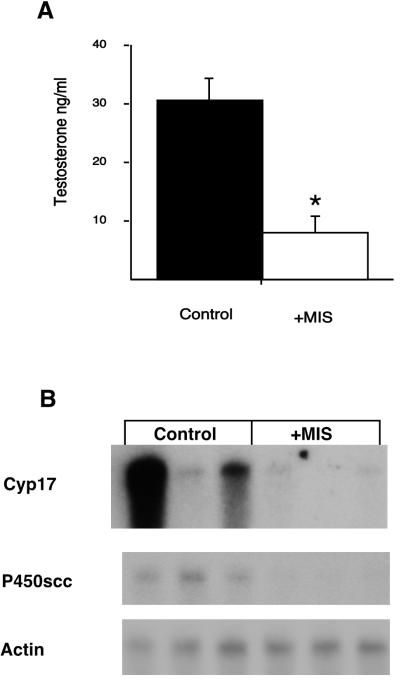
To determine whether Leydig cells could respond to MIS administration in vivo, we first studied adult male rats, which were each injected i.p. with either LH or LH and MIS as described in Materials and Methods. Both control and experimental animals were treated with LH so that a down-regulation by MIS could be observed from a maximally stimulated state and to overcome the wide variations in steroid production by Leydig cells among the animals. After 24 h, peripheral blood and testes were collected for testosterone measurement and RNA isolation, respectively. Fig. 1A shows that MIS treatment reduced LH-stimulated serum testosterone levels 3-fold. Northern analyses of testicular RNA, to evaluate the steady-state levels of the mRNAs for some of the enzymes in the testosterone biosynthetic pathway, are shown in Fig. 1B. Expression of mRNA for P450scc, the enzyme that catalyzes the conversion of the 27-carbon cholesterol molecule to 21-carbon pregnenolone in the inner mitochondria membrane, was dramatically reduced and nearly undetectable in all three animals injected with MIS. Cyp17 catalyzes androgen synthesis via its dual activities, hydroxylation of progesterone at the 17 position and conversion of the 21-carbon 17-OH-progesterone to the 19-carbon androstenedione, the immediate precursor of testosterone. Expression of the mRNA for Cyp17 was also greatly reduced in the animals injected with MIS; although, in one of the controls, its expression was unstimulated by LH, which can occur if the i.p. injection enters the bowel. The Northern blots were also probed for actin to ensure equal loading of lanes. In addition, when hematoxylin/eosin-stained histologic sections of the control and MIS-treated rat testes were compared no gross morphological differences between the two was observed (data not shown).
Figure 1.
Regulation of testosterone production by MIS in rats. (A) Rats were treated in triplicate with LH (control, black bar) or with LH and MIS (+MIS, white bar). Peripheral blood was collected and assayed for total testosterone by RIA and the results shown are plotted as mean values in ng/ml. Error bars represent the SEM. Star indicates statistical significance, P < 0.05. (B) The testes from the same animals shown in A were collected for RNA extraction. Northern analyses were done with individual samples and probed for the indicated mRNA. Actin was used to control for sample loading.
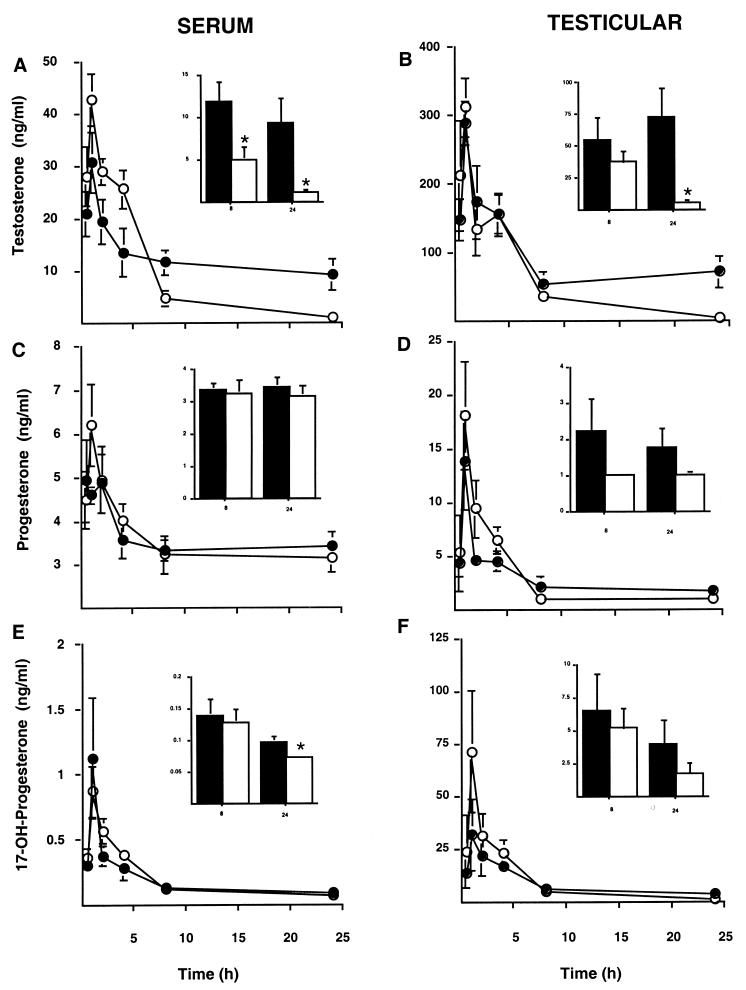
To analyze for statistical significance while still using the same concentration of MIS per animal in a given experiment, we continued our investigation with mice (1 mg/250 g rat vs. 100 μg/25 g mouse), which allowed us to collect testes and blood from nine animals at each of six time points. Fig. 2 depicts the changes observed in adult male mice injected i.p. with either hCG or hCG and MIS. At the indicated time points (0.5, 1, 2, 4, 8, and 24 h) peripheral blood (Fig. 2 A, C, and E) and testes (Fig. 2 B, D, and F) were collected and total testosterone (Fig. 2 A and B), progesterone (Fig. 2 C and D), and 17-OH-progesterone (Fig. 2 E and F) were measured. The 8 and 24 h time points for each measurement are also shown as Inset bar graphs with different y axes to highlight the effect of MIS, if any, at those time points. Although it appears that MIS elevates testosterone at the earlier time points, the differences were not statistically significant. However, the differences in serum and testicular testosterone were significant at both 8 and 24 h after MIS treatment (Fig. 2 A and B). Testosterone levels in MIS-treated mice were decreased at 8 and 24 h by 2-fold and 9-fold, respectively. Progesterone was unchanged, suggesting that any regulation by MIS would be after the StAR, P450scc, and 3βHSD steps. There was a slight, but statistically significant decrease in serum 17-OH-progesterone after 24 h treatment with MIS (Fig. 2E); however, the effect of MIS might be obscured here because all of the values for the MIS-treated animals but one were below the limit of the assay (0.07 ng/ml), whereas all of the control animals were well above the limit. It was important to assess both serum and testicular steroids to rule out the possibility that the results observed with serum, as reflective of testicular samples, were not due to MIS affecting steroid binding proteins.
Figure 2.
Serum sex steroid concentration in mice at different times following MIS treatment. Blood and testes were collected from cohorts of mice at 0.5, 1, 2, 4, 8, and 24 h following injection with either hCG (black circles and bars) or hCG and MIS (white circles and bars). The serum (A, C, and E, n = 9) and testicular extracts (B, n = 6, and D and F, n = 3) were analyzed for total testosterone (A and B), progesterone (C and D), and 17-OH-progesterone (E and F) by RIA. The means from each time point are plotted and the error bars represent the SEM. Inset bar graphs shown in each diagram are the 8- and 24-h time points replotted at a different scale to demonstrate any differences at the lower concentrations. Stars indicate statistically significant differences by the unpaired student's t test, P < 0.05.
In the testis, conversion of 17-hydroxylated steroids to sex steroids appears to be unregulated, whereas the lyase activity of Cyp17 seems to be an independently controlled event (25). The discrepancy between the observed testosterone and 17-OH-progesterone suggests that MIS might be affecting the phosphorylation state of Cyp17, which when underphosphorylated has little or no lyase activity (26). Because the lyase activity of Cyp17 in mice is greater than the hydroxylase activity (27), regulation by MIS at this step would be advantageous. The molecular mechanism that might be involved in regulation of lyase activity by MIS are currently being investigated; initial results indicate that protein kinase A is probably not involved (A.M.T., P.K.D., and J.T., unpublished observation).
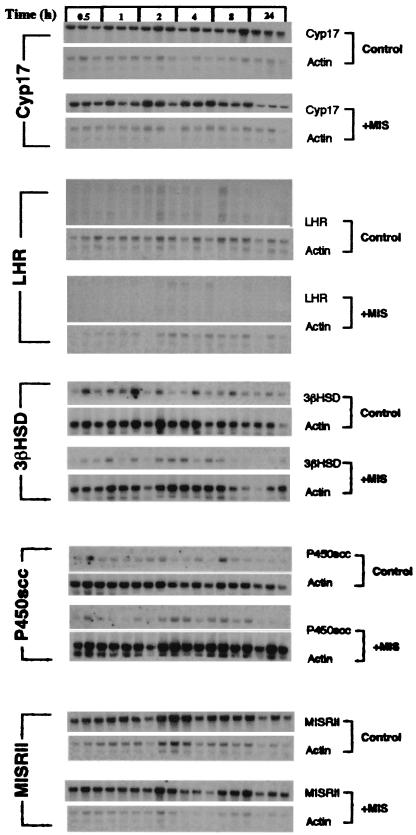
To determine whether MIS regulates the steady state levels of the mRNAs (Fig. 3) for enzymes involved in the testosterone biosynthetic pathway, groups of three RNAs for each time point in both control and MIS-treated mice were hybridized with the indicated probes. At the top of the figure are the blots hybridized with a Cyp17 probe showing that by 24 h the mRNA in MIS-treated animals was diminished 2-fold as determined by PhosphorImager analysis. This is much less than the 10-fold reduction observed when MIS was added to Leydig cells in vitro (16). In vivo, the feedback control exerted by testosterone on the hypothalamus and pituitary probably confounds the observed MIS-mediated reduction in Cyp17 mRNA, because lowered testosterone results in LH-activation of P450scc in Leydig cells to produce more testosterone (14). LHR expression also appears to be lower in the MIS-treated mice, but when the blots were reprobed with actin to control for loading and analyzed with a PhosphorImager, the lower signal detected in the MIS-treated animals also applied to actin, indicating a blotting artifact. When the blots probed with 3βHSD, P450scc, and MISRII were normalized for loading by probing with actin, MIS treatment did not significantly alter the expression of these mRNAs.
Figure 3.
Northern analysis of Leydig cell mRNAs involved in steroidogenesis. Three randomly chosen mice from the same pool of animals presented in Fig. 2 were used to obtain testicular RNA at the indicated time points. These RNAs were analyzed by separate northern blots with probes for Cyp17, LHR, 3βHSD, P450scc, and the MISRII. Blots with RNA from hCG-treated animals are shown on top of each set and labeled control and those from hCG- and MIS-treated animals are shown on the bottom of each set and labeled +MIS. To control for equal gel loading, all blots were subsequently reprobed for actin, which is shown below each respective probe.
It should be noted that, as was observed with rat and mouse Leydig cells in vitro (16), P450scc mRNA expression is regulated by MIS in rats (Fig. 1B) but is not regulated in mice (Fig. 3), indicating that the regulatory mechanisms used by MIS in the gonad might be species-specific. Additionally, although the lyase activity in mice appears to be greater than the hydroxylase activity (27), the opposite may be true in rats (28). Another example of MIS affecting mouse and rat physiology differently was the observation that MIS could inhibit oocyte meiosis in rats but not in mice (29–31). In mice, a low molecular weight cAMP-dependent protein found in follicular fluid, oocyte maturation inhibitor, accomplishes this function (29).
The dose of MIS administered was designed to produce peak serum levels in the 5–6 μg/ml range based on the dose–response of a highly specific MIS bioassay (19) in which complete regression of Müllerian ducts in fetal rat urogenital ridges is accomplished by 4–5 μg/ml purified human MIS. We measured the serum concentration of the human MIS in the injected animals, n = 3 at each time point (Table 1). Within 0.5 h the i.p. injected MIS is already detected in serum and reaches its highest level within 1–2 h. By 24 h, the MIS concentration in serum has dramatically decreased, suggesting that, because the differences observed in testosterone were after 8 h of MIS treatment, long term exposure to MIS is required. Because the ELISA we employ only detects human MIS, these results can be used to address the variability of MIS uptake after injection and, by analogy, hCG. The ranges in the results indicate that there are wide disparities between the injected amount of MIS and that observed in serum because the administration is i.p. and not i.v.; despite which there is still a statistically significant effect on testosterone. The concentrations of MIS were consistently very low 48 h after injection and not detectable after 72 h. These results indicates that immunoaffinity-purified MIS has a half-life of approximately 7.5 h after i.p. injection and is cleared from serum within 3 days.
Table 1.
Serum MIS concentrations
| Time, h | Mean ± SEM, μg/ml | Range, μg/ml |
|---|---|---|
| 0.5 | 3.2 ± 1.5 | 0.5–15 |
| 1 | 8.5 ± 4.3 | 0.5–38 |
| 2 | 6.9 ± 3.5 | 0.4–28 |
| 4 | 6.1 ± 2.6 | 0.5–21 |
| 8 | 4.9 ± 1.8 | 0.3–15 |
| 24 | 0.8 ± 0.2 | 0.1–1.8 |
| 48 | 0.06 ± 0.007 | 0.03–0.07 |
| 72 | Not detected | Not detected |
A single injection of MIS substantially lowers serum testosterone levels in vivo, and does so after the LHR, StAR, P450scc, and 3βHSD steps in the testosterone biosynthetic pathway in adult animals. These findings may have application in clinical situations where lowering serum testosterone specifically is beneficial (e.g., benign prostatic hypertrophy, prostate cancer, and perhaps even polycystic ovarian disease). Elevated testosterone states caused by constitutively activated LHR or its G protein partner, as occurs in McCune–Albright syndrome, might also be moderated by MIS.
Acknowledgments
We thank Drs. Patricia L. Morris and Alan L. Schneyer for critically reviewing the manuscript; Drs. A.F. Parlow and JoAnne S. Richards for valuable reagents; and Anita H. Payne for both helpful discussion and valuable reagents. We are grateful for the technical assistance of Nima Pahlavan, Sheila Mallette, and Paula McDonough. This work was supported by grants from the National Institute of Child Health and Human Development (U54HD28138 to P.K.D. and J.T.) and the National Cancer Institute (R01CA17393 to P.K.D. and D.T.M. and R29CA79459 to J.T.).
Abbreviations
- MIS
Müllerian Inhibiting Substance
- MISRII
MIS type II receptor
- Cyp17
cytochrome P450c17 hydroxylase/lyase
- P450scc
cytochrome P450 side-chain cleavage
- 3βHSD
3β hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase
- LH
luteinizing hormone
- hCG
human chorionic gonadotropin
- LHR
LH receptor
References
- 1.Massague J. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 2.Josso N, Cate R L, Picard J Y, Vigier B, di Clemente N, Wilson C, Imbeaud S, Pepinsky R B, Guerrier D, Boussin L, et al. Recent Prog Horm Res. 1993;48:1–59. doi: 10.1016/b978-0-12-571148-7.50005-1. [DOI] [PubMed] [Google Scholar]
- 3.Teixeira J, Donahoe P. J Androl. 1996;17:336–341. [PubMed] [Google Scholar]
- 4.Griffin J F, Wilson J D. In: Williams Textbook of Endocrinology. Wilson J D, Foster D W, Kronenberg H M, Larson P R, editors. Philadelphia: Saunders; 1998. pp. 819–875. [Google Scholar]
- 5.Josso N, Lamarre I, Picard J Y, Berta P, Davies N, Morichon N, Peschanski M, Jeny R. Early Hum Dev. 1993;33:91–99. doi: 10.1016/0378-3782(93)90204-8. [DOI] [PubMed] [Google Scholar]
- 6.Rey R, Lordereau-Richard I, Carel J C, Barbet P, Cate R L, Roger M, Chaussain J L, Josso N. J Clin Endocrinol Metab. 1993;77:1220–1226. doi: 10.1210/jcem.77.5.8077315. [DOI] [PubMed] [Google Scholar]
- 7.Lee M M, Donahoe P K, Hasegawa H, Silverman B, Crist G B, Best S, Hasegawa Y, Noto R A, Schoenfeld D, MacLaughlin D T. J Clin Endocrinol Metab. 1996;81:571–576. doi: 10.1210/jcem.81.2.8636269. [DOI] [PubMed] [Google Scholar]
- 8.Hudson P L, Dougas I, Donahoe P K, Cate R L, Epstein J, Pepinsky R B, MacLaughlin D T. J Clin Endocrinol Metab. 1990;70:16–22. doi: 10.1210/jcem-70-1-16. [DOI] [PubMed] [Google Scholar]
- 9.Behringer R R, Cate R L, Froelick G J, Palmiter R D, Brinster R L. Nature (London) 1990;345:167–170. doi: 10.1038/345167a0. [DOI] [PubMed] [Google Scholar]
- 10.Lyet L, Louis F, Forest M G, Josso N, Behringer R R, Vigier B. Biol Reprod. 1995;52:444–454. doi: 10.1095/biolreprod52.2.444. [DOI] [PubMed] [Google Scholar]
- 11.Behringer R R, Finegold M J, Cate R L. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 12.Mishina Y, Rey R, Finegold M J, Matzuk M M, Josso N, Cate R L, Behringer R R. Genes Dev. 1996;10:2577–2587. doi: 10.1101/gad.10.20.2577. [DOI] [PubMed] [Google Scholar]
- 13.Benton L, Shan L X, Hardy M P. J Steroid Biochem Mol Biol. 1995;53:61–68. doi: 10.1016/0960-0760(95)00022-r. [DOI] [PubMed] [Google Scholar]
- 14.Payne A H, Youngblood G L. Biol Reprod. 1995;52:217–225. doi: 10.1095/biolreprod52.2.217. [DOI] [PubMed] [Google Scholar]
- 15.Rouiller-Fabre V, Carmona S, Merhi R A, Cate R, Habert R, Vigier B. Endocrinology. 1998;139:1213–1220. doi: 10.1210/endo.139.3.5785. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira J M, Fynn-Thompson E, Payne A T, Donahoe P K. Endocrinology. 1999;140:4732–4738. doi: 10.1210/endo.140.10.7075. [DOI] [PubMed] [Google Scholar]
- 17.Cate R L, Mattaliano R J, Hession C, Tizard R, Farber N M, Cheung A, Ninfa E G, Frey A Z, Gash D J, Chow E P, et al. Cell. 1986;45:685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- 18.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Donahoe P K, Ito Y, Hendren W H. J Surg Res. 1977;23:141–148. doi: 10.1016/0022-4804(77)90202-5. [DOI] [PubMed] [Google Scholar]
- 20.Hakola K, Pierroz D D, Aebi A, Vuagnat B A, Aubert M L, Huhtaniemi I. Biol Reprod. 1998;59:338–343. doi: 10.1095/biolreprod59.2.338. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 22.Teixeira J, He W W, Shah P C, Morikawa N, Lee M M, Catlin E A, Hudson P L, Wing J, Maclaughlin D T, Donahoe P K. Endocrinology. 1996;137:160–165. doi: 10.1210/endo.137.1.8536608. [DOI] [PubMed] [Google Scholar]
- 23.Ascoli M. Endocrinology. 1981;108:88–95. doi: 10.1210/endo-108-1-88. [DOI] [PubMed] [Google Scholar]
- 24.Ma P T, Gil G, Sudhof T C, Bilheimer D W, Goldstein J L, Brown M S. Proc Natl Acad Sci USA. 1986;83:8370–8374. doi: 10.1073/pnas.83.21.8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller W L, Auchus R J, Geller D H. Steroids. 1997;62:133–142. doi: 10.1016/s0039-128x(96)00172-9. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L H, Rodriguez H, Ohno S, Miller W L. Proc Natl Acad Sci USA. 1995;92:10619–10623. doi: 10.1073/pnas.92.23.10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rani C S, Payne A H. Endocrinology. 1986;118:1222–1228. doi: 10.1210/endo-118-3-1222. [DOI] [PubMed] [Google Scholar]
- 28.O'Shaughnessy P J, Payne A H. J Biol Chem. 1982;257:11503–11509. [PubMed] [Google Scholar]
- 29.Takahashi M, Koide S S, Donahoe P K. Mol Cell Endocrinol. 1986;47:225–234. doi: 10.1016/0303-7207(86)90116-4. [DOI] [PubMed] [Google Scholar]
- 30.Ueno S, Manganaro T F, Donahoe P K. Endocrinology. 1988;123:1652–1659. doi: 10.1210/endo-123-3-1652. [DOI] [PubMed] [Google Scholar]
- 31.Tsafriri A, Picard J Y, Josso N. Biol Reprod. 1988;38:481–485. doi: 10.1095/biolreprod38.2.481. [DOI] [PubMed] [Google Scholar]