Sir: Cervical cancer is the second most common malignancy in women worldwide. In developing countries, most cases are still diagnosed with locally advanced disease, so the great challenge in the management of advanced cervical cancer remains the control and prevention of distant metastasis. A prominent feature of clinically advanced cervical cancers is hypoxia1,2 which is, therefore, a therapeutic and prognostic factor associated with radio-resistance and worsened clinical outcome.3–5 A central coordinator of the hypoxic cellular response is hypoxia-inducible factor-1α (HIF-1α), a master transcription factor regulating the expression of numerous downstream targets.6,7 HIF-1α binding to hypoxia response elements at enhancers of target genes increases the expression of molecules which regulate angiogenesis, glucose transport, glycolysis, tissue invasion/metastasis and cell proliferation.6 Recently, we reported the identification of human growth and transformation-dependent protein (HGTD-P) as a new HIF-1α-responsive protein.8 Although the majority of genes downstream of HIF-1α participate in survival signalling pathways against hypoxic insult, increasing oxygen delivery or reducing metabolic demands for adaptation to hypoxic conditions,9–11 little is known about the role of HGTD-P during carcinogenesis.
We investigated the expression of HGTD-P in human tissue samples of uterine cervical cancer (UCC; n = 85) and normal cervical tissue (n = 20) using immunohistochemistry with rabbit anti-HGTD-P antibody.12 As shown in Figure 1A, HGTD-P was stained strongly in the cytoplasm of the tumour cells, but no staining was observed in the nucleus or cytoplasmic membrane, whereas normal cervical mucosa showed no staining at all (Figure 1C). Most UCC tissues (70.6%) were positive for HGTD-P. Next, we aimed to determine the timing of aberrant HGTD-P expression during multistep cervical carcinogenesis and performed immunohistochemistry in cervical precancerous lesions, including cervical intraepithelial neoplasia (CIN)1 (n = 12), CIN2 (n = 7) and CIN3 (n = 13). As a result, fewer than half the precancerous lesions were stained moderately with HGTD-P, which was confined to dysplastic cells (Figure 1E–H). In fact, 33.3% of CIN1, 28.6% of CIN2 and 46.2% of CIN3 expressed cytoplasmic HGTD-P, whereas 70.6% of UCC was positive for HGTD-P (Table 1). The cases in which UCC tissue samples included normal cervical mucosa and precancerous lesions on a slide demonstrated diverse patterns of HGTD-P expression that were dependent upon the type of cervical lesion (Figure 1D). For example, HGTD-P was stained strongly in UCC and moderately in CIN3, whereas normal mucosa showed no staining. These results suggest that HGTD-P expression is a common and cancer-specific event in the uterine cervix, occurring at an early stage of cervical carcinogenesis.
Figure 1.
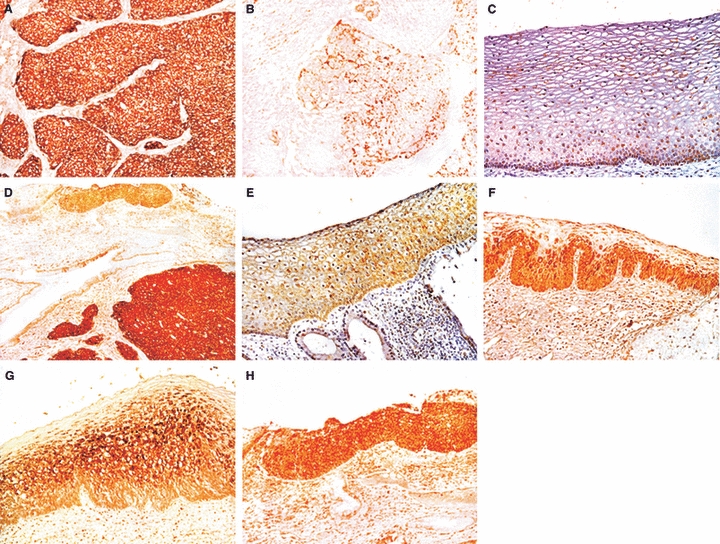
Immunohistochemical detection of human growth and transformation-dependent protein (HGTD-P) in cervical cancer (A, B), normal mucosa (C) and precancerous lesions (E–H). Representative samples of cervical cancer in which HGTD-P shows diffuse and strong staining in the cytoplasm of tumour cells (A), and no staining in tumour cells (B). C, Normal exocervical mucosa did not express HGTD-P. Among precancerous lesions including koilocytosis (E), cervical intraepithelial neoplasia (CIN)1 (F), CIN2 (G), CIN3 (H), HGTD-P expression was confined to dysplastic cells of the individual lesions. A representative case in which various cervical lesions are included within a microscopic field shows strong positive staining in cancer (c), less strong positive in CIN3 (ci) and no staining in normal cervical samples (n).
Table 1.
Comparison of human growth and transformation-dependent protein (HGTD-P) expression in cervical cancer and precancerous lesions
| HGTD-P expression | CIN1 | CIN2 | CIN3 | Cervical cancer | Total |
|---|---|---|---|---|---|
| Negative | 8 (66.7) | 5 (71.4) | 7 (53.8) | 25 (29.4) | 45 |
| Positive | 4 (33.3) | 2 (28.6) | 6 (46.2) | 60 (70.6) | 72 |
| Total | 12 | 7 | 13 | 85 | 117 |
CIN, cervical intraepithelial neoplasia.
P = 0.009, analysed by Pearson's chi-square test.
To determine the association between HGTD-P and HIF-1α in human clinical samples, we performed immunohistochemistry for HIF-1α in UCC tissues (n = 15) and compared the result of HIF-1α staining to that of HGTD-P expression. As a result, HIF-1α overexpression was detected in 60% of UCC tissues; 77.8% of HIF-1α-overexpressed tumours also expressed HGTD-P. The correlation of expression between these two proteins was statistically significant (Table 2; P = 0.04). Representative cases of UCC with positive correlation between the two proteins are shown in Figure 2. This finding suggests that HGTD-P participates in a molecular mechanism of hypoxia-regulated tumour development in association with HIF-1α, and furthermore is of great value for future investigation regarding its role in cervical carcinogenesis.
Table 2.
Comparison of human growth and transformation-dependent protein (HGTD-P) expression with hypoxia-inducible factor-1α (HIF-1α) expression in uterine cervical cancer
| HIF-1α expression (%) | |||
|---|---|---|---|
| HGTD-P expression | Negative | Positive | Total |
| Negative | 5 (83.3) | 2 (22.2) | 7 |
| Positive | 1 (16.7) | 7 (77.8) | 8 |
P = 0.042, analysed by Fisher's exact test.
Figure 2.
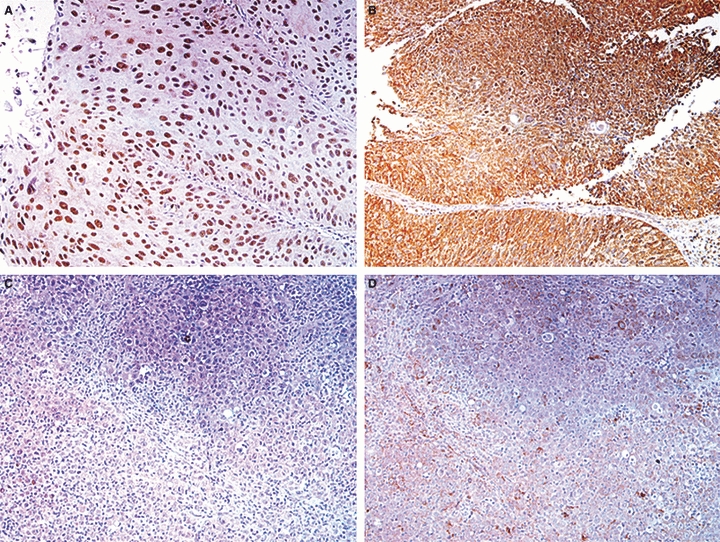
Immunohistochemistry of hypoxia-inducible factor-1α (HIF-1α) (A, C) and human growth and transformation-dependent protein (HGTD-P) (B, D) in identical tumour samples. A, B, Tumour cells strongly expressed both HIF-1 and HGTD-P. C, D, The tumour expressed neither HIF-1α nor HGTD-P.
Next, we determined any clinicopathological significance of the aberrant expression of HGTD-P in UCC and compared HGTD-P-positive tumours to -negative tumours in relation to clinicopathological parameters. HGTD-P-positive-tumours tend to be associated with advanced International Federation of Gynecology and Obstetrics (FIGO) stage, parametrial invasion and tumour recurrence, but this association did not reach statistical significance (Table 3). The overall survival rate was poorer in patients with HGTD-P expression than in those without HGTD-P expression (Figure 3). However, this difference did not reach statistical significance (P = 0.272).
Table 3.
Correlation of human growth and transformation-dependent protein (HGTD-P) expression in uterine cervical cancer with clinicopathological factors
| HGTD-P expression | ||||
|---|---|---|---|---|
| Factors | No. | −(n = 25) | +(n = 60) | P-value* |
| Age (years) | ||||
| ≤45 | 35 | 11 | 24 | 0.733 |
| >45 | 50 | 14 | 36 | |
| Histological type | ||||
| Squamous cell carcinoma | 63 | 16 | 47 | 0.355 |
| Adenocarcinoma | 15 | 7 | 8 | |
| Others | 7 | 2 | 5 | |
| FIGO stage | ||||
| IA1-IB1 | 61 | 21 | 40 | 0.122 |
| IB2-IIIB | 24 | 4 | 20 | |
| LN metastasis | ||||
| No | 71 | 20 | 51 | 0.760 |
| Yes | 12 | 4 | 8 | |
| Parametrial invasion | ||||
| No | 24 | 24 | 47 | 0.056 |
| Yes | 1 | 1 | 13 | |
| Death | ||||
| No | 75 | |||
| Yes | 10 | |||
FIGO, International Federation of Gynecology and Obstetrics; LN, lymph node.
Fisher's exact test.
Figure 3.
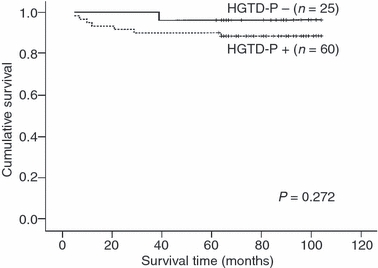
Overall survival curves in patients with uterine cervical cancer depending upon human growth and transformation-dependent protein (HGTD-P) expression. The patients with HGTD-P-expressing tumours tend to have poor overall survival rate, compared to those with tumours that do not express HGTD-P.
In summary, we have shown that HGTD-P expression is a frequent event in UCC and is associated significantly with HIF-1α expression in human cervical cancer samples. During multistep cervical carcinogenesis, the aberrant expression of HGTD-P protein occurs in CIN1 and might accumulate in the process of tumorigenesis. Moreover, HGTD-P expression is correlated with parametrial invasion. These facts suggest that HGTD-P plays an important role in cervical carcinogenesis. Moreover, HGTD-P can be considered a valuable tumour marker of cervical neoplasia. However, the molecular mechanism of HGTD-P involvement in tumour development and progression in UCC remains to be elucidated.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (grant no. R13-2002-020-02002-0).
References
- 1.Hockel M, Schlenger K, Aral B, et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 2.Dehdashti F, Grigsby PW, Mintun MA, et al. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM; relationship to therapeutic response – a preliminary report. Int. J. Radiat. Oncol. Biol. Phys. 2003;55:1233–1238. doi: 10.1016/s0360-3016(02)04477-2. [DOI] [PubMed] [Google Scholar]
- 3.Graeber TG, Osmanian C, Jacks T, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumors. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 4.Vaupel P, Kelleher DK, Hockel M. Oxygen status of malignant tumors; pathogenesis of hypoxia and significance for tumor therapy. Semin. Oncol. 2001;28:29–35. doi: 10.1016/s0093-7754(01)90210-6. [DOI] [PubMed] [Google Scholar]
- 5.Chi JH, Knudson MM, Vassar MJ, et al. Prehospital hypoxia affects outcome in patients with traumatic brain injury: a prospective multicenter study. Trauma. 2006;61:1134–1141. doi: 10.1097/01.ta.0000196644.64653.d8. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 7.Erler JT, Bennewith KL, Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 8.Lee MJ, Kim JY, Suk KH, Park JH. Identification of the hypoxia-inducible factor 1 α-responsive HGTD-P gene as a mediator in the mitochondrial apoptotic pathway. Mol. Cell. Biol. 2004;24:3918–3927. doi: 10.1128/MCB.24.9.3918-3927.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsythe JA, Jiang BH, lyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goda N, Ryan HE, Khadivi B, et al. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol. Cell. Biol. 2003;23:359–369. doi: 10.1128/MCB.23.1.359-369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 12.Cho YE, Kim JY, Kim YW, Park JH, Lee S. Expression and prognostic significance of human growth and transformation-dependent protein in gastric carcinoma and gastric adenoma. Hum. Pathol. 2009;40:975–981. doi: 10.1016/j.humpath.2008.12.007. [DOI] [PubMed] [Google Scholar]


