SUMMARY
Objective
To assess the presence, location, type and size of denuded areas of subchondral bone (dAB) in the femorotibial joint, measured quantitatively with 3 T MRI, in a large subset of OAI participants.
Methods
One knee of 633 subjects (250 men, 383 women, aged 61.7 ± 9.6 y) were studied, spanning all radiographic osteoarthritis (OA) stages. dABs were determined quantitatively using segmentations of coronal FLASHwe images, representing areas where the subchondral bone was not covered by cartilage. Post hoc visual examination of segmented images determined whether dABs represented full thickness cartilage loss or internal osteophyte.
Results
7% Of the knees were Kellgren & Lawrence (KL) grade 0, 6% grade 1, 41% grade 2, 41% grade 3, and 5% grade 4. 39% Of the participants (48% of the men and 33% of the women) displayed dABs; 61% of the dABs represented internal osteophytes. 1/47 Participants with KL grade 0 displayed ‘any’ dAB whereas 29/32 of the KL grade 4 knees were affected. Even as early as KL grade 1, 29% of the participants showed dABs. There were significant relationships of dAB with increasing KL grades (P < 0.001) and with ipsicompartimental JSN (P ≤ 0.001). Internal osteophytes were more frequent laterally (mainly posterior tibia and internal femur) whereas full thickness cartilage loss was more frequent medially (mainly external tibia and femur).
Conclusions
dABs occur already at earliest stages of radiographic OA (KL grades 1 and 2) and become more common (and larger) with increasing disease severity. Almost all KL grade 4 knees exhibited dABs, with cartilage loss being more frequent than internal osteophytes.
Keywords: Subchondral bone, Denuded, Magnetic resonance imaging, Radiography, Cartilage
Introduction
Magnetic resonance imaging (MRI) is increasingly used for obtaining quantitative endpoints of cartilage morphology in osteoarthritis (OA)1-3. Amongst these quantitative endpoints, three measures [total area of subchondral bone (tAB), cartilage thickness (ThC), denuded areas of the subchondral bone (dAB)] were shown to provide independent information4 and may therefore be used as preferred parameters, comprehensively describing the cross-sectional and longitudinal variations in osteoarthritic cartilage4.
dABs represent areas of bone not covered by cartilage and there are two potential phenotypes of dABs as measured quantitatively with MRI: full thickness cartilage loss, or intrachondral osteophytes protruding to the joint surface (Fig. 1). It is plausible that, with a calcified joint surface (dAB), the lack of adequate load distribution (hydrostatic pressurization5,6) and increased friction during joint movement5 may cause symptoms7 and interfere with normal joint function. This may be particularly true when dABs occur in regions predominantly involved in weight-bearing during common daily activities (i.e., walking). The presence and size of dABs within the knee has been shown to correlate with the presence of pain, also after adjusting for the presence of bone marrow lesions8, and the % dAB (relative to the tAB) was found to be a significant predictor of incident pain over a 2 year period in OA participants without pain at baseline8. Moreover, the presence of dAB was significantly associated with the rate of progression of cartilage loss in OA9.
Fig. 1.
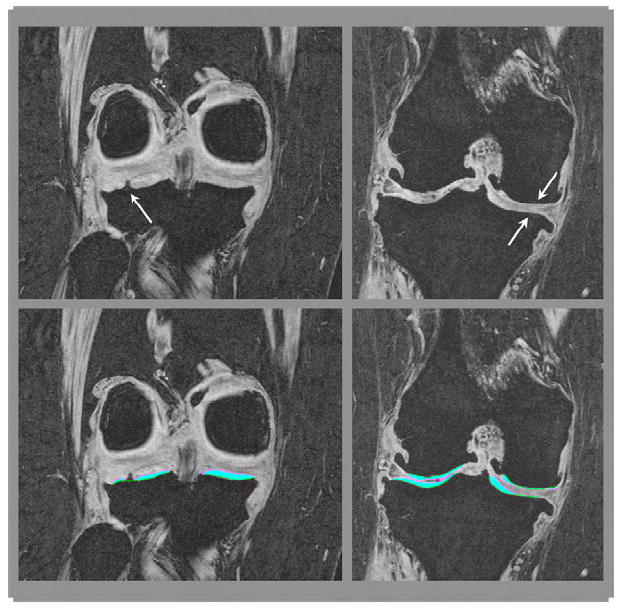
Different phenotypes of denuded areas of subchondral bone (dAB): internal osteophyte (left) and full thickness cartilage loss (right). The top row shows the un-segmented MR image, the bottom row the segmented MR image.
Little, however, is known about the prevalence, location, type and size of dABs in the femorotibial joint of OA subjects, and their relation with the radiographic stage of OA. Quantitative cartilage morphometry1-3 can be used to determine the presence and to measure the actual size of dABs. In addition, it is possible to objectively determine the location of dABs, using the computerized anatomical coordinate systems from subregional analysis of cartilage morphometry10-12.
The objective of this cross-sectional study was to determine the presence, location, type and size of dABs within the femorotibial joint, as measured quantitatively with 3 Tesla (T) MRI, in a large subset of participants of the OA Initiative13,14 (www.oai.ucsf.edu). Healthy volunteers and subjects with all radiographic stages of OA were included in order to study the relationship between dABs and radiographic knee OA status.
Methods
The Osteoarthritis Initiative (OAI) is a large ongoing cohort study, targeted at characterizing risk factors associated with the onset and progression of symptomatic knee OA, and at identifying sensitive biomarkers of the disease. Fixed flexion radiographs15,16 and 3 T MRIs14,15 are acquired at baseline and at one through 4-year follow ups and are available for public use. Patients were recruited at four clinical sites: the University of Maryland School of Medicine (Baltimore), the Ohio State University (Columbus), the University of Pittsburgh, and the Memorial Hospital of Rhode Island (Pawtucket). The participants were between 45 and 79 years old at the time of recruitment and include a diversity of ethnic minorities. They either exhibit both frequent knee symptoms (pain, aching or stiffness on most days of a month in past year) and radiographic OA (definite osteophytes in the posteroanterior fixed flexion radiographs) at baseline in at least one of their knees based upon initial radiograph reading at the clinical sites (progression subcohort), or they have an elevated risk of developing symptomatic knee OA in at least one knee during the course of the study (incidence cohort). General exclusion criteria for participation in the OAI were rheumatoid or inflammatory arthritis, bilateral, end stage knee OA, inability to walk without aids, and 3 T MRI contraindications.
The present analysis was based on a convenience sample of OAI participants from a consortium initiative of pharmaceutical industry partners (Pfizer Inc., Eli Lilly & Co, Merck Serono SA, Glaxo Smith Kline, Wyeth Research, Centocor, and Novartis Pharma AG), the OAI coordinating center at the University of California San Francisco (UCSF), and an image analysis company (Chondrometrics GmbH) with an overall aim of analyzing images from a large data set of OAI participants, to study various research questions. The subcohort studied here included baseline knee MRIs from four subsets of participants selected for different analysis projects:
158 Right knees from an age and gender stratified subsample from the progression subcohort. This was the first sample made public by the OAI (public-use data sets 0.1.1, 0.B.1)9,17, including participants with symptomatic and radiographic knee OA in at least one knee. Because the coronal fast low angle shot (FLASH) water excitation (we) acquisitions of these participants were studied17, in order to allow for a better comparison with previously published literature, and because the coronal FLASHwe was only acquired in the right knees of the OAI participants, the knees studied included both symptomatic and asymptomatic knees and spanning all ROA grades (cKLG 0–4, central adjudicated readings described below).
418 right knees from participants with a calculated Kellgren & Lawrence (cKL) grade (described below) 2 or 3, selected consecutively from a table of ascending OAI release identification numbers (IDs) from the first half of the OAI cohort, without any other inclusion or exclusion criteria (public data releases 0.1.1 and 0.C.1).
13 Right knees from participants with cKL grade 4 selected from the first half of the cohort (public data releases 0.1.1 and 0.C.1)
44 Right knees selected from the healthy reference cohort, i.e., subjects without knee pain, without radiographic signs of OA (cKL grade 0) and without risk factors of OA (public data releases 0.2.1 and 0.F.1)
Thus, a total of 633 subjects were studied, including participants from the healthy reference subcohort, from the progression subcohort, or from the incidence subchort. Information on demographic and radiographic data was obtained from the 0.2.1 release (www.oai.ucsf.edu).
Radiographic grading
The radiographic readings used in this study relied on the cKL grades, aggregated from the clinical site readings for the purpose of recruitment, since central adjudicated readings was only provided for the image data set B17,18 (160 participants), but not for the entire cohort studied here. At the clinical sites, the osteoarthritis research society international (OARSI) atlas19,20 was used to determine osteophytes and joint space narrowing (JSN) and cKL grades were assigned according to the following algorithm (www.oai.ucsf.edu):
-
➢
cKL grade 0 = Normal (no osteophytes, no JSN)
-
➢
cKL grade 1 = Doubtful (possible or minute osteophyte of doubtful significance, no JSN)
-
➢
cKL grade 2 = Minimal (OARSI grades 1–3 osteophyte [definite], no JSN)
-
➢
cKL grade 3 = Moderate (definite osteophyte, OARSI grade 1 or 2 medial or lateral JSN)
-
➢
cKL grade 4 = Severe (definite osteophyte, OARSI grade 3 medial or lateral JSN [severe or bone to bone contact]).
We also used JSN grades in this study since cKL grades are not specific to a given (medial or lateral) femorotibial compartment. The OARSI JSN grades were assigned at the clinical recruiting sites. Data available in the OAI database, however, was collapsed in the following way (www.oai.ucsf.edu):
-
➢
OAI JSN grade 0 = normal joint space width [no JSN];
-
➢
OAI JSN grade 1 = OARSI JSN grades 1 or 2;
-
➢
OAI JSN grade 2 = OARSI JSN grade 3.
Because few subjects displayed OAI JSN grade 2, we further collapsed the grades to: presence of JSN (OARSI grades 1–3) or absence of JSN (OARSI grade 0) for the statistical analysis.
MRI analysis
MR images were acquired using four different 3 T scanners (Siemens Magnetom Trio, Erlangen, Germany) and quadrature transmit-receive knee coils (USA Instruments, Aurora, OH) at the four sites of the OAI13,14,17,18. The analysis was based on the double oblique coronal 3D FLASH MR images with water excitation (we) acquired in all right knees (Fig. 1), rather than on the sagittal dual echo steady state (DESS) with water excitation (we) sequence obtained in both knees21 as the FLASHwe sequence was recently validated2,22,23. After quality control at the image analysis center (Chondrometrics GmbH, Ainring, Germany), segmentation of the femorotibial cartilages was performed by seven expert readers12,17,21 with at least 3 years of experience in cartilage segmentation from MR images. The tAB (according to the proposed nomenclature1) and the area of the cartilage surface (AC) were traced manually in each section for the medial (MT) and lateral tibia (LT) as well as the weight-bearing portion of the medial (cMF) and lateral femoral condyle (cLF)12,17,21 (Figs. 1 and 2). The region of interest on the femoral condyles was defined by a 60% distance between the intercondylar notch and the posterior end of the femoral condyles, as seen in the coronal images21. All segmentations were quality controlled by a single expert (Dr Susanne Maschek, Chondrometrics GmbH, Ainring, Germany). The readers and the QC reader were blinded to the aim of this study and to all other (clinical or radiographic) data. The percentage of subchondral bone covered with cartilage (% cAB) and the percentage of subchondral bone denuded (% dAB) were computed using in house software12,17. To determine the location of dAB, we applied a recently developed algorithm for subregional femorotibial cartilage analysis11,12, which computed the % dAB in five tibial (central, external, internal, anterior, and posterior), and three femoral (central, external, internal) subregions, both in the medial and lateral compartment (16 subregions in total; Fig. 2)11,12. The subregions were automatically defined based on the tAB of each cartilage plate where the central tibial subregions occupy 20% and the central femoral subregions 33% of the tAB11.
Fig. 2.
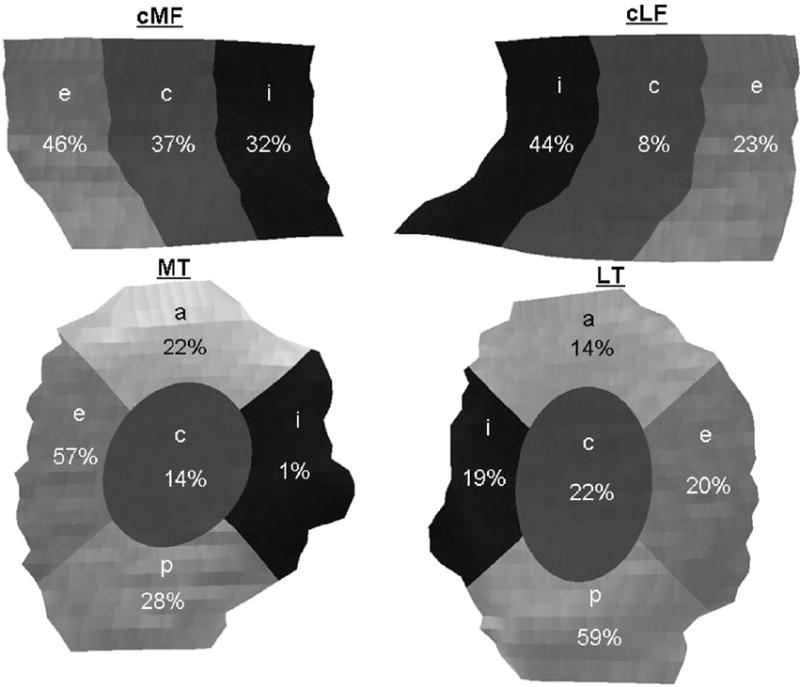
Frequency distribution of the main location of discontinuous denuded areas of subchondral bone (dAB) in the femorotibial subregions. The values give the percentage of cases in which a given subregion displayed dAB >10% of the subregion size in the 245 cases affected by “any” subregion. The total number of plates affected by dABs was 94 (cMF), 89 (cLF), 90 (MT) and 116 (LT). Note that any one knee could have dAB affecting >10% in more than one subregion (or no subregion affected by dAB > 10%) in each cartilage plate. cMF, weight-bearing medial femoral condyle; cLF, weight-bearing lateral femoral condyle; MT; LT; e, external, c, central; i, internal; a, anterior; p, posterior.
Analysis strategy
Knees with a % dAB > 0 in any of the four femorotibial cartilage plates were considered as knees with “any dAB”.
To determine the (average) size of dAB in each cartilage plate, the median size was determined across all plates with % dAB > 0. The computation algorithm did not differentiate between one large or several small discontinuous dABs per plate, and thus the output was % dAB relative to tAB of each plate.
To determine the main location of dABs, we assessed the number (and location) of subregions affected by dAB. Since the methodology did not differentiate between one and several dABs in each plate but did present the size of dAB within each subregion, a 10% threshold was chosen to determine the main location of dAB within each cartilage plate (i.e., a subregion was only considered being affected by dAB if more than 10% of the tAB of this subregion was denuded). Several subregions in a given cartilage plate may thus be affected by one or several dABs.
To determine the type and number of dABs per cartilage plate, the segmentations of all cartilage plates affected by dAB were reviewed by two readers (SC, RF). In each cartilage plate with “any dAB”, the number of discontinuous dABs was noted and classified as either full thickness cartilage loss or an internal osteophyte (allowing for combinations of both within one plate). Any discrepancies in classifying dABs were resolved in consensus between the two readers.
Statistical analyses
All statistical analyses were performed using SPSS (version 15.0). Differences in gender between subjects with “any dAB” or without dAB were analyzed using a Chi2 (χ2) test and differences in age and body mass index (BMI) were analyzed using the t test. The relationship between the presence of dAB and cKL grades were performed using the Mann Whitney U test and the relationship between the presence of medial or lateral JSN and “any dAB” with a χ2 test. Correlations of the size of dAB with age and BMI were computed using the Pearson correlation coeffcient in each plate affected by dAB; correlations with sex and cKL grades using Spearman’s Rho, and the relationship with medial and lateral JSN using the Mann Whitney U test. Comparisons between dAB size and type were made using the Kruskal Wallis (three types) test and the Mann Whitney U test (two types).
Results
Demographics and radiographic status
One knee from each of 250 men and 383 women were included in the study, their demographics being given in Table I. Of the 633 participants, 47 (7%) were cKL grade 0, 34 (6%) were cKL grade 1, 259 (41%) were cKL grade 2, 261 (41%) were cKL grade 3, and 32 (5%) were cKL grade 4. 232 (37%) displayed medial JSN and 126 (20%) lateral JSN (Table I).
Table I.
Demographics and radiographic characterization of the total sample (N = 633)
| Men (n = 250) | Women (n = 383) | Difference between men and women; P-value | |
|---|---|---|---|
| Age, years, mean (SD) | 61.9 (9.9) | 61.6 (9.4) | 0.716 |
| BMI, kg/m2, mean (SD) | 29.3 (3.9) | 29.4 (5.2) | 0.743 |
| cKL grade, n (%) | 0.715 | ||
| 0 | 21 (8) | 26 (7) | |
| 1 | 22 (9) | 13 (3) | |
| 2 | 91 (36) | 168 (44) | |
| 3 | 97 (39) | 164 (43) | |
| 4 | 19 (8) | 13 (3) | |
| JSN (OARSI grades 1–3) | |||
| Medial | 103 (41) | 129 (34) | 0.052 |
| Lateral | 45 (18) | 81(21) | 0.340 |
SD, standard deviation; calculated, composed from osteophyte and JSN readings; n, number of observations.
Presence of dABs and their relationship with age, sex, BMI and radiographic knee status
There were 245 knees (39% of the total sample) with “any” dAB, 33% of the women and 48% of the men being affected. 72 Knees (29%) had dABs exclusively localized in the medial compartment, 102 (42%) exclusively in the lateral compartment, and 72 (29%) in both compartments. Of those with dABs exclusively in the medial compartment, 31% had dABs in both plates, 38% exclusively in MT, and 31% exclusively in cMF. Of those with dABs exclusively in the lateral compartment, 17% had dABs in both plates, 43% in LT, 40% in cLF. The MT, cMF, and cLF showed similar frequency of dABs, but LT was more frequently affected (Table II). In terms of subregional main location of dABs, the external subregion was most frequently affected in MT and cMF, the internal subregion in cLF, and the posterior subregion in LT (Fig. 2).
Table II.
Number of plates affected by denuded areas of bone, median size of the dAB (expressed as percent of tAB), 25th and 75th percentile of the size, and P-values for differences in total size between types of dAB (cartilage, internal osteophyte, or a combination of both*)
| n | Median size % of tAB | 25th, 75th Percentiles | P-value 3 groups | P-value 2 groups | |
|---|---|---|---|---|---|
| MT (n = 90) | <0.001 | ||||
| Cartilage loss | 43 | 6.8 | 4.1, 11.1 | <0.001 | |
| Internal osteophytes | 30 | 1.9 | 1.4, 3.5 | ||
| Combination* | 17 | 16.2 | 10.4, 31.0 | ||
| cMF (n = 94) | <0.001 | ||||
| Cartilage loss | 42 | 19.1 | 8.9, 44.6 | <0.001 | |
| Internal osteophytes | 50 | 5.3 | 3.3, 9.2 | ||
| Combination* | 2 | 11.1 | 8.9, 13.4 | ||
| LT (n = 116) | <0.001 | ||||
| Cartilage loss | 32 | 7.3 | 4.4, 13.9 | 0.001 | |
| Internal osteophytes | 67 | 4.0 | 2.5, 6.4 | ||
| Combination* | 17 | 11.0 | 7.7, 24.2 | ||
| cLF (n = 89) | 0.001 | ||||
| Cartilage loss | 17 | 7.9 | 6.0, 24.2 | 0.001 | |
| Internal osteophytes | 67 | 4.3 | 2.8, 7.6 | ||
| Combination* | 5 | 10.2 | 6.8, 23.7 |
cMF, weight-bearing medial femoral condyle; cLF, weight-bearing lateral femoral condyle.
A combination of both internal osteophyte and cartilage loss within the same cartilage plate.
There were 483 discontinuous dABs in a total of 389 cartilage plates. Up to four discontinuous dABs were observed in MT, the breakdown for all plates being shown in Fig. 3.
Fig. 3.
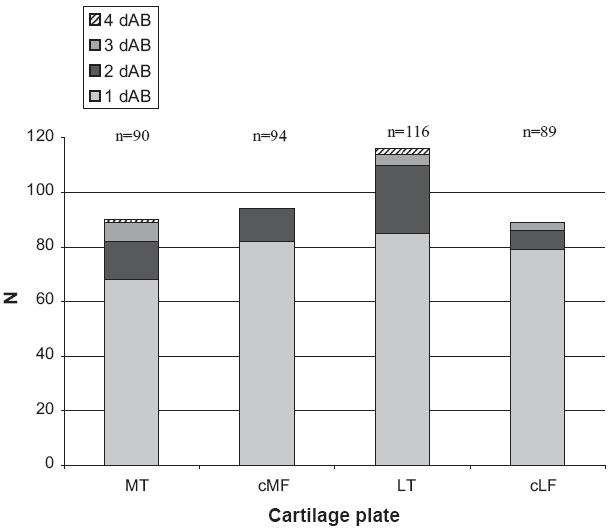
Frequency of the number of discontinuous areas (1–4) of denuded subchondral bone (dAB) observed in each cartilage plate (N = 245); MT; cMF, weight-bearing medial femoral condyle; LT; cLF, weight-bearing lateral femoral condyle.
61% Of the 483 dABs were classified as internal osteophytes (Fig. 4) and only 39% as full thickness cartilage loss, with the numbers per cartilage plate being shown in Fig. 5. Internal osteophytes had a higher relative frequency in the lateral than in the medial compartment. When only considering full thickness cartilage loss, this was more frequent in MT than LT, and more frequent in cMF than cLF (Fig. 5). Combinations of full thickness cartilage loss and internal osteophytes (within the same plate) occurred more often in MT and LT (17%) than in cLF (5%) and cMF (2%, Table II).
Fig. 4.
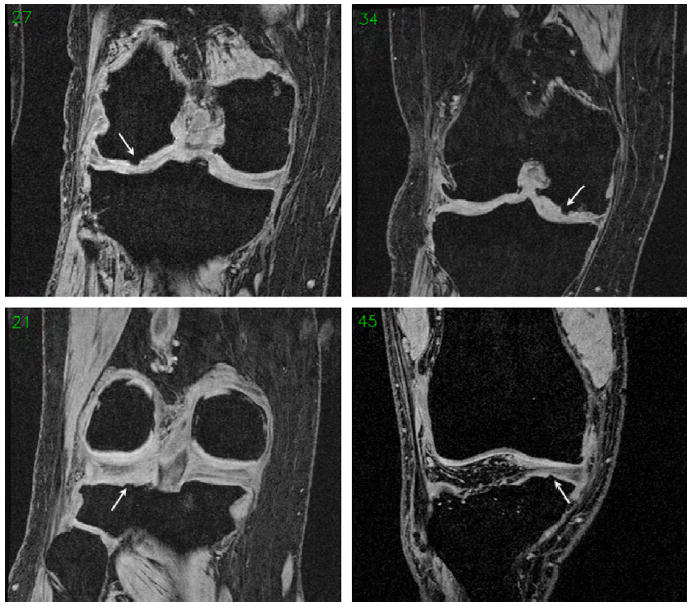
Example images showing internal osteophytes without segmentation (arrows) in central lateral femur (cLF, top left), LT (bottom left), central medial femur (cMF, top right) and MT (bottom right).
Fig. 5.
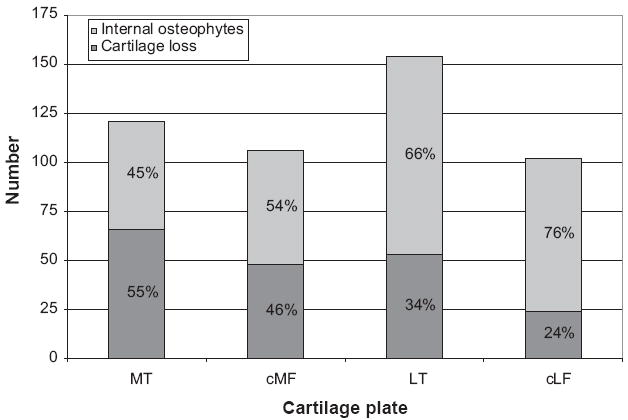
Relation between frequency (number, n) and of type of denuded areas of subchondral bone (dAB) within each cartilage plate.
Subjects with “any dAB” were significantly older (P = 0.001) and had a higher BMI (P = 0.011) than those not having dAB, although these differences were not clinically relevant (64 vs 61 years; 29 vs 30 kg/m2). Male knees were more likely to have dAB than female knees (P < 0.001). In those with “any dAB”, there was no relation between the specific type of dAB and BMI or sex for any cartilage plate.
Presence of “any dAB” was significantly related to greater cKL grades (P < 0.001 for both men and women, Fig. 6) as well as with the presence of medial (P = 0.001) and lateral (P = 0.001) JSN. Whereas only 1/47 participants with cKL grade 0 displayed any dAB, almost all with cKL grade 4 (29/32) were affected. Even as early as cKL grade 1, 29% of the participants showed dABs (Figs. 6 and 7). In MT, cMF, and cLF of cKL grade 4 knees, dabs represented full thickness cartilage loss substantially more often than internal osteophytes, but this was not the case for LT (Fig. 7).
Fig. 6.
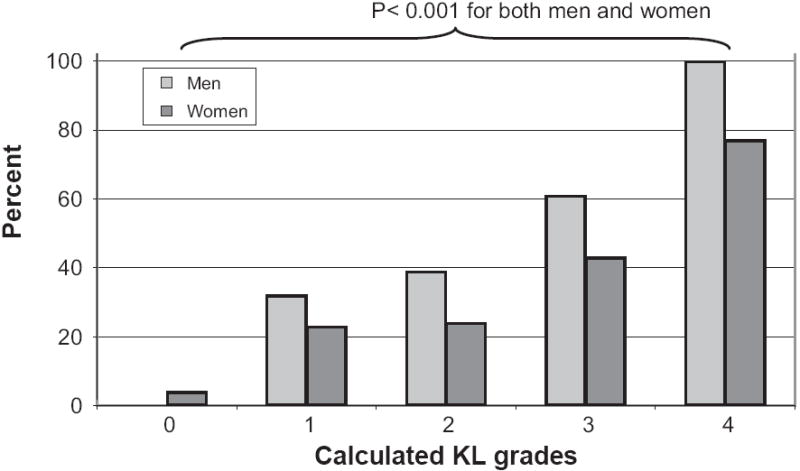
Relation between the relative frequency (%) of knees affected by any denuded area of subchondral bone (dAB) and cKL grades for men and women, respectively. The frequency differed significantly across cKL grades.
Fig. 7.
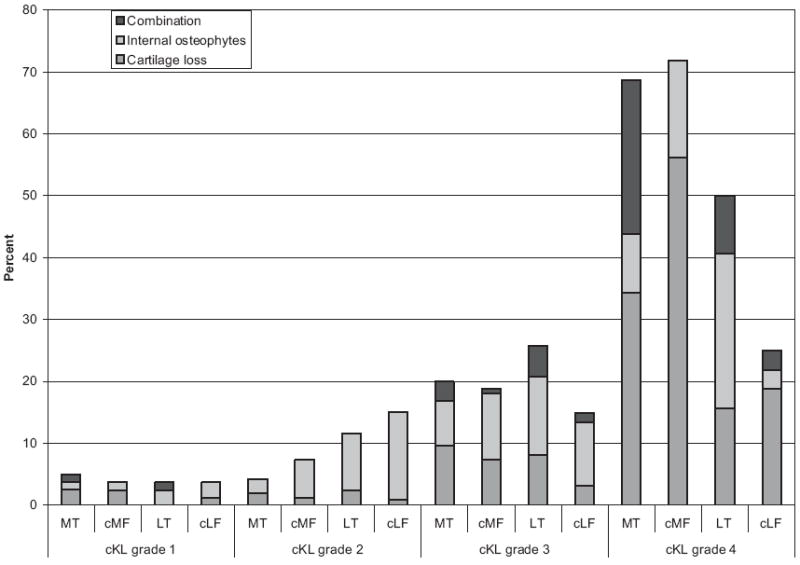
Relation between the relative frequency (%) of knees affected by denuded area of subchondral bone (dAB) exclusively representing internal osteophytes, or full thickness cartilage loss, or a combination of both type with cKL grades 1–4; cMF, weight-bearing medial femoral condyle; cLF, weight-bearing lateral femoral condyle.
The presence of exclusive full thickness cartilage loss and internal osteophytes (i.e., the combination was not analyzed) showed a significant independent relationship with increasing cKL grades in MT, LT and cMF (P < 0.001), whereas in cLF increasing cKL grade was related only to full thickness cartilage loss (P < 0.001) but not to internal osteophytes (Fig. 7). With regard to cartilage plates, the presence of dAB in MT, LT or cMF was significantly associated with greater cKL grades (P < 0.001), while no respective relation was found for cLF. The presence of dAB in MT or cMF was associated with increased medial JSN (P < 0.001), but not with increased lateral JSN, and presence of dAB in LT or cLF was associated with increased lateral JSN (P ≤ 0.001), but not with increased medial JSN.
Size of dABs and their relationship with age, sex, BMI and radiographic knee status
The maximal size of dABs observed (expressed as the percent of the tAB in each plate covered by dAB) was 54% in MT, 65%, in cMF, 71% in LT and 39% in cLF, with the highest median for dABs being observed in the cMF (Table II). There was no significant relationship between the size of dAB (in knees with “any dAB”) with age, gender or BMI. In all cartilage plates, dABs representing full thickness cartilage loss (or from a combination of cartilage loss and internal osteophytes) were larger than those originating from internal osteophytes alone (P ≤ 0.001, Table II). In all cartilage plates but cLF (P = 0.110, r = 0.17), a significant correlation was found between the size of all dABs combined and cKL grades (P ≤ 0.001). The correlation coefficients for MT (Spearman’s Rho, r = 0.50) and cMF (r = 0.55) were higher than for LT (r = 0.39). Further, the size of dABs in MT (P = 0.001) and cMF (P = 0.003), but not that in LT (P = 0.570) and cLF (P = 0.181) was significantly and positively associated with medial JSN. The size of dAB in cLF (P = 0.045) and LT (P < 0.001), but not that in MT (P = 0.846) and cMF (P = 0.126) was significantly associated with lateral JSN.
Discussion
In this cross-sectional study of 633 subjects from the OAI we have assessed the presence, location, type and size of dABs within the femorotibial joint. 39% Of the participants were found to display dABs, the majority of those being internal osteophytes. The tibiae were more frequently affected by dAB than the weight-bearing portion of the femoral condyles, and the lateral femorotibial compartment more often than the medial. However, when only considering full thickness cartilage loss, the medial compartment had a more frequent involvement than the lateral one, and cMF had the largest relative area of cartilage loss. To our surprise, the central subregions were not the main location of dABs in any of the cartilage plates as determined by the 10% threshold used in this study. A potential explanation is that because 60% of all dABs were internal osteophytes, the central locations may be less involved due to continuous mechanical loading of these areas. dABs were more frequent in men, and there was a statistically significant, albeit not clinically relevant, relationship with increasing age and BMI. As expected, there was a strong relationship between the presence of dAB and advanced radiographic disease stages, i.e., cKL grade and JSN in the same compartment. In all plates but cLF, there was also a strong correlation between the size of dAB and increasing cKL grades.
Denuded area of subchondral bone (dAB) is a term derived from morphometry where measurements of cartilage are quantified from MR images, dAB representing an area where the subchondral bone is not covered by cartilage1. Thus, dAB represents an area where the ThC is 0 mm and that could hence represent either full thickness cartilage loss or internal osteophytes. To our knowledge there are no comprehensive reports on the numbers of internal osteophytes in OA subjects by MRI or arthroscopy, and comparative studies between MRI and arthroscopy have so far focussed on focal cartilage lesions/defect24,25, but not on dABs in general. Further, none of the semi-quantitative methods currently in use to assess knee OA on MR images assesses internal osteophytes26,27. Therefore, dABs remain an MRI finding and need to be further assessed in the context of their clinical relevance.
Recent studies have shown a significant relationship between dABs and the presence and incidence of pain8 as well as between dABs and the rate of progression of cartilage loss9. A recent paper in a small cohort of 61 participants with medial femorotibial OA, where one knee per subject was studied, showed that about a third of the participants displayed a dAB in both the medial and the lateral femorotibial compartment, and that dABs were more frequent amongst KL grade 3 than two participants28. However, little is known about the location, type and size of dABs in the femorotibial joint of participants with medial or lateral disease, and their relation with the entire spectrum of radiographic stages of OA. We therefore performed a comprehensive and descriptive study on regional distribution patterns of dABs in a large sample from the OAI and investigated their relationship with radiographic OA stages.
A limitation of the current study is that only the weight-bearing part of the femoral condyles was assessed in a region of interest covering 60% of the distance between the intercondylar notch and the posterior end of the femoral condyles21, but did not take into account the patella, femoral trochlea or posterior parts of the femoral condyles. However, these other regions cannot be reliably measured on coronal views. We have given preference to the selection of the coronal FLASHwe in this study, as it has been specifically validated not only for measuring cartilage volume and thickness, but also for the purpose of measuring cAB and dAB23. Moreover, recent MRI-based studies have shown that the weight-bearing regions of the femoral condyles are more frequently affected by cartilage loss than the posterior regions10,29. Another limitation was that the cohort was not specifically selected for the purpose of the study question. However, we obtained a sample spanning from healthy knees to severe radiographic OA stages although few participants were cKL grades 0, 1 and 4. Nevertheless, it was interesting to note that despite the uncertainty in assigning a KL 0 grade (no osteophyte) or a KL 1 grade (possible or minute osteophyte) to a knee, those with cKL grade 1 had substantially more dABs than those with cKL grade 0.
In all cartilage plates, internal osteophytes were more commonly seen than actual cartilage loss in cKL grade 2 or 3 knees, whereas cartilage loss was more commonly seen (than internal osteophytes) in cKL grade 4 knees of all plates but LT. This supports the assumption that osteophytes precede cartilage loss, but the natural history of both cartilage loss and osteophytes (including internal osteophytes) needs to be confirmed in longitudinal trials. The external subregion was the most commonly affected subregion on the medial side, where a majority of the dABs represented full thickness cartilage loss (data not shown). On the lateral side, however, the internal- (cLF) and posterior subregion (LT) was most commonly affected by dAB, and in these two subregions dABs most frequently represented internal osteophytes (data not shown).
Since dABs represent a calcified surface (either from internal osteophyte or from missing cartilage), it is plausible to assume that they interfere with load transmission under both static (lack of hydrostatic pressurization) and dynamic conditions (i.e., increased friction), especially when located in the weight-bearing area (i.e., central subregion). Unlike the articular cartilage, the subchondral bone is highly innervated by pain receptors, and focal exposure of subchondral bone is thus likely to produce pain30. In support, a recent publication showed a significant relationship between the presence of dABs in OA subjects and the presence and incidence of pain8. In the current study we show that only a minority of the dABs affected central subregions of the femorotibial cartilage plates. Future studies may therefore investigate whether the relationship between dABs and pain becomes stronger when only considering those cases with centrally located dABs, where the highest pressure can be assumed to occur during normal daily activities. Further, only 40% of all dABs represented actual exposure of subchondral bone (i.e., full thickness cartilage loss). We are not aware of studies investigating whether internal osteophytes are or are not innervated with pain receptors or whether they form prior to or after some kind of cartilage lesion. Therefore, it would also be of interest to further investigate relationship of full thickness cartilage loss dABs and internal osteophyte dABs with pain, respectively.
In conclusion, this paper shows that dABs occur already at the earliest stages of radiographic OA and that they become more common with increasing disease severity. The lateral femorotibial compartment was more often affected by “any dAB” than the medial one, this because of the high frequency of internal osteophytes, particularly located in the internal part of the lateral femoral condyle and in the posterior part of the LT. When considering full thickness cartilage loss only, the medial compartment, however, had a more frequent involvement than the lateral one, the external tibia and femur being more frequently affected than other medial subregions. Presence and size of dABs was related to radiographic disease stages, i.e., increasing cKL grade and JSN were in the same compartment as the dAB. Almost all KL grade 4 knees exhibited dABs, with cartilage loss being more frequent than internal osteophytes.
Acknowledgments
We would like to thank the following readers: Gudrun Gold-mann, Linda Jakobi, Manuela Kunz, Dr Susanne Maschek, Jana Matthes, Sabine Mühlsimer, Annette Thebis, and Dr Barbara Wehr for dedicated data segmentation. The image analysis of this study was funded by an industry consortium consisting of Pfizer Inc., Eli Lilly & Co, Merck Serono SA, Glaxo Smith Kline, Wyeth Research, Centocor, and Novartis Pharma AG. The OAI is a public–private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Footnotes
Conflict of interest RBF has no competing interest. WW has a part time employment with Chondrometrics GmbH. MN has no competing interest. BTW is a full time employee of Pfizer. OB is a full time employee of Eli Lilly. DD is a full time employee of Merck Serono. RYD is a full time employee of GlaxoSmithKline. JHL is a full time employee of Wyeth. FB is a full time employee of Centocor. AG is a full time employee of Novartis. MH has a part time employment with Chondrometrics GmbH. SC has a part time employment with Chondrometrics GmbH. FE is CEO of Chondrometrics GmbH, a company providing MR image analysis services. He provides consulting services to Pfizer, Merck Serono, Novo Nordisk, Wyeth, and Novartis.
References
- 1.Eckstein F, Ateshian G, Burgkart R, Burstein D, Cicuttini F, Dardzinski B, et al. Proposal for a nomenclature for magnetic resonance imaging based measures of articular cartilage in osteoarthritis. Osteoarthritis Cartilage. 2006;14(10):974–83. doi: 10.1016/j.joca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14(Suppl A):A46–75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Guermazi A, Burstein D, Conaghan P, Eckstein F, Hellio Le Graverand-Gastineau MP, Keen H, et al. Imaging in osteoarthritis. Rheum Dis Clin North Am. 2008;34(3):645–87. doi: 10.1016/j.rdc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Buck R, Wyman B, Hellio Le Graverand-Gastineau MP, Wirth W, Eckstein F. An efficient subset of morphological measures for articular cartilage in the healthy and diseased human knee. Magn Reson Med. doi: 10.1002/mrm.22207. in press. [DOI] [PubMed] [Google Scholar]
- 5.Ateshian GA, Lai WM, Zhu WB, Mow VC. An asymptotic solution for the contact of two biphasic cartilage layers. J Biomech. 1994;27(11):1347–60. doi: 10.1016/0021-9290(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 6.Soltz MA, Basalo IM, Ateshian GA. Hydrostatic pressurization and depletion of trapped lubricant pool during creep contact of a rippled indenter against a biphasic articular cartilage layer. J Biomech Eng. 2003;125(5):585–93. doi: 10.1115/1.1610020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niv D, Gofeld M, Devor M. Causes of pain in degenerative bone and joint disease: a lesson from vertebroplasty. Pain. 2003;105(3):387–92. doi: 10.1016/S0304-3959(03)00277-X. [DOI] [PubMed] [Google Scholar]
- 8.Moisio KC, Eckstein F, Song J, Cahue S, Marshall M, Dunlop D, et al. The relationship of denuded subchondral bone area to knee pain severity and incident frequent knee pain. Osteoarhritis Cartilage. 2008;16(Suppl 4):S31. Abstract. [Google Scholar]
- 9.Eckstein F, Wirth W, Hudelmaier MI, Maschek S, Hitzl W, Wyman BT, et al. Relationship of compartment-specific structural knee status at baseline with change in cartilage morphology: a prospective observational study using data from the osteoarthritis initiative. Arthritis Res Ther. 2009;11(3):R90. doi: 10.1186/ar2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. doi: 10.1186/ar2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27(6):737–44. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 12.Wirth W, Hellio Le Graverand MP, Wyman BT, Maschek S, Hudelmaier M, Hitzl W, et al. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage. 2009;17(3):291–7. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–41. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider E, NessAiver M, White D, Purdy D, Martin L, Fanella L, et al. The osteoarthritis initiative (OAI) magnetic resonance imaging quality assurance methods and results. Osteoarthritis Cartilage. 2008;16(9):994–1004. doi: 10.1016/j.joca.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nevitt MC, Peterfy C, Guermazi A, Felson DT, Duryea J, Woodworth T, et al. Longitudinal performance evaluation and validation of fixed-flexion radiography of the knee for detection of joint space loss. Arthritis Rheum. 2007;56(5):1512–20. doi: 10.1002/art.22557. [DOI] [PubMed] [Google Scholar]
- 16.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test–retest reproducibility. Skeletal Radiol. 2003;32(3):128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 17.Eckstein F, Maschek S, Wirth W, Hudelmaier M, Hitzl W, Wyman B, et al. One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative progression subcohort: association with sex, body mass index, symptoms and radiographic osteoarthritis status. Ann Rheum Dis. 2009;68(5):674–9. doi: 10.1136/ard.2008.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter DJ, Niu J, Zhang Y, Totterman S, Tamez J, Dabrowski C, et al. Change in cartilage morphometry: a sample of the progression cohort of the Osteoarthritis Initiative. Ann Rheum Dis. 2009;68(3):349–56. doi: 10.1136/ard.2007.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):A1–A56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Altman RD, Hochberg M, Murphy WA, Jr, Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3(Suppl A):3–70. [PubMed] [Google Scholar]
- 21.Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65(4):433–41. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgkart R, Glaser C, Hyhlik-Durr A, Englmeier KH, Reiser M, Eckstein F. Magnetic resonance imaging-based assessment of cartilage loss in severe osteoarthritis: accuracy, precision, and diagnostic value. Arthritis Rheum. 2001;44(9):2072–7. doi: 10.1002/1529-0131(200109)44:9<2072::AID-ART357>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Graichen H, von Eisenhart-Rothe R, Vogl T, Englmeier KH, Eckstein F. Quantitative assessment of cartilage status in osteoarthritis by quantitative magnetic resonance imaging: technical validation for use in analysis of cartilage volume and further morphologic parameters. Arthritis Rheum. 2004;50(3):811–6. doi: 10.1002/art.20191. [DOI] [PubMed] [Google Scholar]
- 24.Bredella MA, Tirman PF, Peterfy CG, Zarlingo M, Feller JF, Bost FW, et al. Accuracy of T2-weighted fast spin-echo MR imaging with fat saturation in detecting cartilage defects in the knee: comparison with arthroscopy in 130 patients. AJR Am J Roentgenol. 1999;172(4):1073–80. doi: 10.2214/ajr.172.4.10587150. [DOI] [PubMed] [Google Scholar]
- 25.Disler DG, McCauley TR, Kelman CG, Fuchs MD, Ratner LM, Wirth CR, et al. Fat-suppressed three-dimensional spoiled gradient-echo MR imaging of hyaline cartilage defects in the knee: comparison with standard MR imaging and arthroscopy. AJR Am J Roentgenol. 1996;167(1):127–32. doi: 10.2214/ajr.167.1.8659356. [DOI] [PubMed] [Google Scholar]
- 26.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67(2):206–11. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 27.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Hellio Le Graverand MP, Buck RJ, Wyman BT, Vignon E, Mazzuca SA, Brandt KD, et al. Subregional femorotibial cartilage morphology in women – comparison between healthy controls and participants with different grades of radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:1177–85. doi: 10.1016/j.joca.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Eckstein F, Benichou O, Wirth W, Nelson DR, Maschek S, Hudelmaier M, et al. Magnetic resonance imaging-based cartilage loss in painful contralateral knees with and without radiographic joint space narrowing: Data from the Osteoarthritis Initiative. Arthritis Rheum. 2009;61:1218–25. doi: 10.1002/art.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ateshian G, Mow VC. Friction, Lubrication, and Wear of Articular Cartilage and Diathrodial Joints. (3) 2005:447–94. [Google Scholar]


