Abstract
Colon cancer is a common epithelial malignancies worldwide. Epidemiologic evidence has shown that nutrition and dietary components are important environmental factors involved in the development of this disease. We investigated the biological activity of 6,7,4′-trihydroxyisoflavone (6,7,4′-THIF, a metabolite of daidzein) in in vitro and in vivo models of human colon cancer. 6,7,4′-THIF suppressed anchorage-dependent and -independent growth of HCT-116 and DLD1 human colon cancer cells more effectively than daidzein. In addition, 6,7,4′-THIF induced cell cycle arrest at the S and G2/M phases in HCT-116 human colon cancer cells. Western blot analysis revealed that 6,7,4′-THIF effectively suppressed the expression of cyclin-dependent kinase (CDK) 2, but had no effect on other S- or G2/M-phase regulatory proteins such as cyclin A, cyclin B1 or CDK1. Daidzein did not affect the expression of any of these proteins. In kinase and pull-down assays, 6,7,4′-THIF, but not daidzein, inhibited CDK1 and CDK2 activities in HCT-116 cells by directly interacting with CDK1 and CDK2. In a xenograft mouse model, 6,7,4′-THIF significantly decreased tumor growth, volume and weight of HCT-116 xenografts. 6,7,4′-THIF bound directly to CDK1 and CDK2 in vivo, resulting in the suppression of CDK1 and CDK2 activity in tumors corresponding with our in vitro results. Collectively, these results suggest that CDK1 and CDK2 are potential molecular targets of 6,7,4′-THIF to suppress HCT-116 cell proliferation in vitro and in vivo. These findings provide insight into the biological actions of 6,7,4′-THIF and might establish a molecular basis for the development of new cancer therapeutic agents.
Introduction
Colon cancer is one of the world’s most common epithelial malignancies (1), and epidemiologic evidence has demonstrated that nutrition and dietary components are key environmental factors involved in the development of this disease (2,3). Although gradual progress has been made in the treatment of colon cancer, it still remains one of the most common epithelial malignancies in both men and women and is essentially incurable when it reaches the most advanced stages (4). Thus, novel preventive treatment approaches are needed to reduce its incidence.
Epidemiologic data have indicated that the incidence of colon cancer is relatively low in Asian populations, perhaps because soy-based foods are a principal component of Asian diets (5). Isoflavones, including daidzein and genistein, are major bioactive molecules present in soybeans and soy-based foods, and although daidzein is regarded as a less active component than genistein, several studies have indicated that metabolites of daidzein exert a chemopreventive effect (6,7). For example, equol is one of the most biologically active metabolites of daidzein (7). We previously reported that equol, but not daidzein, exerts an antitumor effect due to the inhibition of cell transformation mainly by targeting the mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1 signaling pathway (8). Recently, several studies have shown that isoflavones are subject to oxidative biotransformation in the rat liver (9) and in humans (10) through hepatic metabolism. One of the major products of the hepatic metabolism of daidzein is 6,7,4′-trihydroxyisoflavone (6,7,4′-THIF) (11). However, the anticancer effects and molecular mechanism of this hepatic metabolite in colon cancer have not been reported.
Deregulated growth is a unique characteristic of cancer cells and a primary requirement for processes in cancer progression, including angiogenesis and metastasis (12). Numerous reports suggest that the existence of a strong link between cell cycle deregulation and carcinogenesis (13). Proliferation is caused by deregulation of cyclin-dependent kinases (CDKs), which are serine/threonine kinases activated by formation of complexes with proteins known as cyclins. The levels of CDKs remain constant during the cell cycle, whereas the levels of cyclins fluctuate, and the phases of the cell cycle are controlled by the activation of different CDK/cyclin complexes (14), the importance of which in cell proliferation is supported by the finding that deregulation of CDK activity is observed in a variety of human tumors. The CDK4/cyclin D, CDK6/cyclin D and CDK2/cyclin E complexes regulate progression from G1 to S phase of the cell cycle. CDK2/cyclin A and CDK1/cyclin A act specifically during S phase and control progression through G2. The CDK1/cyclin B complex is a critical initiator of mitosis (15). Therefore, regulating the activities of CDKs poses a promising strategy for cancer prevention and therapy.
Here, we investigated the biological activity of 6,7,4′-THIF, a metabolite of daidzein (Figure 1A), in in vitro and in vivo models of human colon cancer. 6,7,4′-THIF exerted antiproliferative effects stronger than those of daidzein in HCT-116 human colon cancer cells. We also report that 6,7,4′-THIF, but not daidzein, was a potent inhibitor of CDK1 and CDK2, and subsequently induced cell cycle arrest at the S and G2/M phases in HCT-116 cells. In a xenograft model, 6,7,4′-THIF greatly reduced the volume and weight of tumors in nude mice. Consistent with the in vitro data, 6,7,4′-THIF bound directly to CDK1 and CDK2 in vivo, resulting in the suppression of CDK1 and CDK2 activity in these tumors. The results of this investigation suggest a molecular mechanism that underlies the anticancer activity of 6,7,4′-THIF and might partially account for the chemopreventive effects of soy-based foods against cancer.
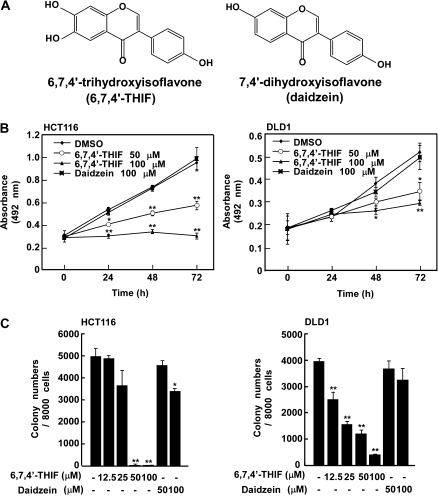
Fig. 1.
6,7,4′-THIF, but not 7,4′-dihydroxyisoflavone (daidzein), inhibits anchorage-dependent and -independent growth of HCT-116 and DLD1 human colon cancer cells. (A) Chemical structures of 6,7,4′-THIF and daidzein. (B) HCT-116 and DLD1 cells were treated with 6,7,4′-THIF or daidzein (0, 50 or 100 μM) for the indicated times. Proliferation was determined by MTT assay. (C) HCT-116 and DLD1 cells were subjected to a soft agar assay as described in Materials and Methods. Cell colonies were counted 10 days later under a microscope with the aid of the Image-Pro Plus software program (v4). Data are presented as means ± standard deviations of the number of colonies determined from three independent experiments. The asterisks, (*) and (**), indicate a significant decrease, (P < 0.05) and (P < 0.01), respectively, in the number of colonies after treatment with 6,7,4′-THIF compared with the untreated control group.
Materials and methods
Chemicals
RPMI 1640 medium, Basal Medium Eagle (BME), gentamicin and L-glutamine were purchased from Invitrogen (Carlsbad, CA). 6,7,4′-THIF was from Indofine Chemical Company (Hillsborough, NJ). Daidzein and anti-β-actin were purchased from Sigma–Aldrich (St Louis, MO) and the antibodies against cyclin B1, CDK1, cyclin A and CDK2 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Histone H1 was obtained from Millipore (Billerica, MA), CNBr–Sepharose 4B, Glutathione–Sepharose 4B, [γ-32P] adenosine triphosphate (ATP) and the chemiluminescence detection kit were purchased from Amersham Pharmacia Biotech (Piscataway, NJ), and the protein assay kit was from Bio-Rad Laboratories (Hercules, CA).
Cell culture
HCT-116 and DLD1 human colon cancer cell lines were cultured in monolayers at 37°C in a 5% CO2 incubator in RPMI 1640 containing 10% fetal bovine serum, 2 mM L-glutamine and 25 μg/ml gentamicin.
MTT assay
To estimate cell proliferation, HCT-116 or DLD1 cells were seeded (8 × 103 cells per well) in 96-well plates. After culturing for various times, 10 μl of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] solution (final concentration, 1 mg/ml) were added to each well, and the cells were incubated for 1 h. The dark blue formazan crystals that formed in intact cells were dissolved in dimethyl sulfoxide and the absorbance at 570 nm was measured with a microplate reader. The results are expressed as the percentage of MTT reduction relative to the absorbance of control cells.
Anchorage-independent growth assay
The effects of daidzein or 6,7,4′-THIF on colony formation were investigated in HCT-116 or DLD1 cells. Cells (8 × 103/ml) were exposed to daidzein (0–100 μM) or 6,7,4′-THIF (0–100 μM) in 1 ml of 0.33% Basal Medium Eagle agar containing 10% fetal bovine serum or in 3.5 ml of 0.5% Basal Medium Eagle agar containing 10% fetal bovine serum. The cultures were maintained at 37°C in a 5% CO2 incubator for 10 days, and cell colonies were counted under a microscope with the aid of the Image-Pro Plus software (v.4) program (Media Cybernetics, Silver Spring, MD) as described by Colburn et al. (16).
Cell cycle analysis
Cell cycle analysis was conducted using a published method (17) with slight modifications. Cells (7 × 104) were seeded in a 60 mm dish and cultured for 24 h and then treated with various concentrations of 6,7,4′-THIF for 48 h. The cells were harvested with trypsin, fixed with ethanol, stained with propidium iodide and then analyzed for cell cycle phase by flow cytometry.
Western blot analysis
Cells (2 × 105) were cultured in a 10 cm dish for 24 h and then treated with 6,7,4′-THIF for 48 h. Cell lysates were harvested and sodium dodecyl sulfate–polyacrylamide gel electrophoresis and western blotting were performed as described previously (8).
Immunoprecipitation and kinase assays
CDK1 and CDK2 immunoprecipitation and kinase assays were performed in accordance with the instructions provided by Millipore. Briefly, 500 μg of cell lysate or 700 μg of mouse tumor lysate were mixed with protein-A/G beads (20 μl) for 1 h at 4°C and a CDK1 or CDK2 antibody (20 μl) was added to the supernatant fraction, which was gently rocked overnight at 4°C. After washing, immunoprecipitated CDK1 or CDK2 was incubated with 2.5 μl of 1 mg/ml histone H1 (Millipore), 7.5 μl of H2O, 5 μl of ×5 reaction buffer and 10 μl of diluted [γ-32P] ATP solution at 30°C for 30 min and then 15 μl aliquots were transferred onto p81 paper and washed (8). The incorporation of radioactivity was determined using a scintillation counter. Each experiment was performed three times.
Pull-down assays
The supernatant fractions of HCT-116 cell lysates (500 μg) or mouse tumor lysates (700 μg) were incubated with 6,7,4′-THIF–Sepharose 4B or daidzein–Sepharose 4B (or Sepharose 4B as a negative control) beads (100 μl, 50% slurry) in reaction buffer (8). Beads were incubated overnight at 4°C and washed five times with buffer (8). Proteins bound to the beads were analyzed by western blotting as described above.
HCT-116 cell tumor xenografts in mice
Female athymic nude mice (5 weeks old; mean body weight, 20 g) were purchased from Orient (Seoul, Republic of Korea). Animals were acclimated for 1 week before the study and had free access to food and water. Mice were housed in climate-controlled quarters (24°C at 50% humidity) with a 12 h light/12 h dark cycle. Each animal was subcutaneously injected with HCT-116 cells (2 × 106 cells/200 μl) in the left flank and cells allowed to form tumors. When tumors reached a size of 100 mm3, the mice were randomly assigned to groups (six mice/group) and treated with 6,7,4′-THIF (5 or 25 mg/kg body wt) in 50% dimethyl sulfoxide/phosphate-buffered saline buffer, given intraperitoneally every other day for 20 days. Tumor size was measured every other day using calipers and tumor volume was calculated according to a standard formula: width × length × height/2. Mice were killed after 20 days of treatment when control tumors reached ∼1600 mm3. All procedures were conducted in accordance with the accepted guidelines for the use and care of laboratory animals. The tumors were collected, photographed and weighed. Some tumor tissues were harvested and used for kinase and pull-down assays. To obtain lysates from mouse tumors, the tumors of each mouse were excised and blended on ice with a homogenizer (IKA T10 basic; IKA Laboratory Equipment, Staufen, Germany) and the homogenate was centrifuged at 12 000 r.p.m. for 20 min. The protein content was determined using the Bio-Rad protein assay kit.
Statistical analysis
When applicable, data are expressed as means ± standard deviations. The Student’s t-test was used for single statistical comparisons. A P value of <0.05 was considered statistically significant.
Results
6,7,4′-THIF inhibits anchorage-dependent and -independent growth of HCT-116 cells
Uncontrolled proliferation is a characteristic of cancer cells that accelerates steps in progression such as angiogenesis and metastasis. We evaluated the effect of 6,7,4′-THIF on anchorage-dependent and -independent growth of HCT-116 and DLD1 human colon cancer cells. After 24, 48 or 72 h of 6,7,4′-THIF treatment, anchorage-dependent growth of HCT-116 and DLD1 cells was clearly suppressed in a dose- and time-dependent manner (Figure 1B) without cytotoxicity (data not shown). In contrast, daidzein had only a slight inhibitory effect on anchorage-dependent growth (Figure 1B). Because anchorage-independent growth is a specific feature of cancer cells compared with normal cells and strongly corresponds with tumorigenesis in vivo, we determined the effect of daidzein and its metabolite, 6,7,4′-THIF, on anchorage-independent growth of HCT-116 and DLD1 cells using a soft agar assay. HCT-116 and DLD1 cells formed colonies on agar after 10 days of incubation and treatment with 6,7,4′-THIF showed a much stronger inhibitory effect on colony formation of HCT-116 and DLD1 cells compared with daidzein (Figure 1C).
6,7,4′-THIF induces cell cycle arrest of HCT-116 cells at S and G2/M phases
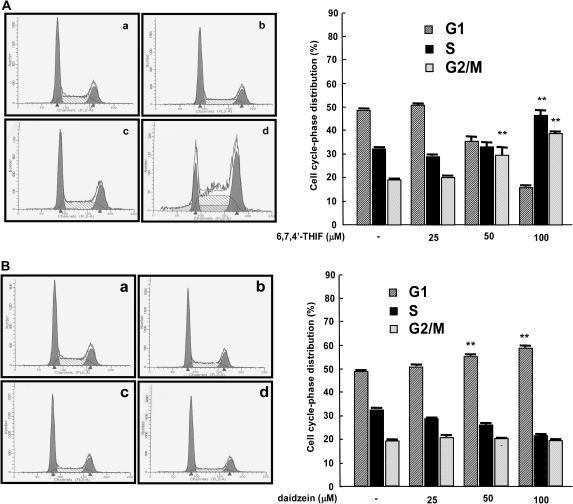
To determine whether the inhibition of growth is associated with cell cycle arrest, HCT-116 cells were treated for 48 h with 6,7,4′-THIF (0, 25, 50 or 100 μM) and analyzed by flow cytometry. The results of cell cycle analysis revealed that 6,7,4′-THIF significantly induced cell cycle arrest at the S and G2/M phases. The percentage of cells in S phase was clearly higher in the 100 μM 6,7,4′-THIF-treated group compared with the control group (46.4 versus 32.1%, respectively), and the same pattern was observed in G2/M phase (29.5% in the 100 μM 6,7,4′-THIF-treated group versus 19.1% in the control group; Figure 2A). Daidzein showed a slight inhibitory effect on cell cycle progression at G1 phase (Figure 2B). Furthermore, 6,7,4′-THIF treatment had no effect on the percentage of cells in sub-G1 phase, suggesting that the antiproliferative effect of 6,7,4′-THIF results from growth inhibition without cell death (data not shown).
Fig. 2.
Comparison of the effects of 6,7,4′-THIF and daidzein on cell cycle distribution. (A) 6,7,4′-THIF causes arrest of HCT-116 cells at S and G2/M phases. (B) daidzein causes a slight arrest of HCT-116 cells at G1 phase. Cells were treated with 6,7,4′-THIF or daidzein at the indicated concentrations for 48 h. Cell cycle analysis was performed as described in Materials and Methods: a, untreated control; b, 25 μM 6,7,4′-THIF or daidzein; c, 50 μM 6,7,4′-THIF or daidzein; d, 100 μM 6,7,4′-THIF or daidzein. The effects of 6,7,4′-THIF or daidzein on cell cycle distribution are presented as the percent of 6,7,4′-THIF or daidzein-treated cells in G1, S or G2/M phases of cell cycle compared with untreated control cells. Data are shown as means ± standard deviations of three samples from three independent experiments. The asterisks (**) indicate a significant difference (P < 0.01) in the number of cells after treatment with 6,7,4′-THIF (or daidzein) compared with untreated control group as determined by Student’s t-test.
Comparison of the effects of 6,7,4′-THIF and daidzein on the expression and activity of S- and G2/M-phase regulatory proteins
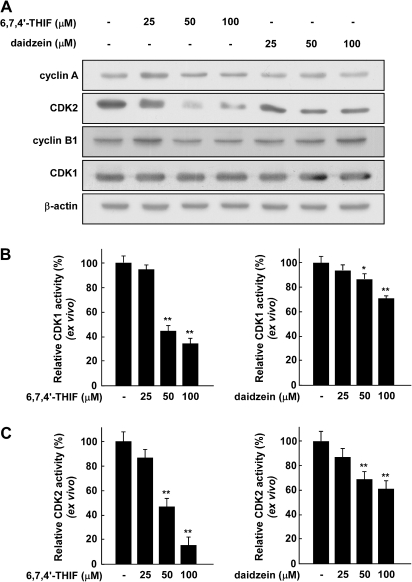
To determine the mechanism responsible for cell cycle arrest at the S and G2/M phases, we examined the expression of cyclin B1, CDK1, cyclin A and CDK2, which are involved in the progression from S phase to G2/M phase. The treatment of HCT-116 cells for 48 h with 6,7,4′-THIF (0, 25, 50 or 100 μM) clearly suppressed expression of CDK2 in a dose-dependent manner but did not affect the expression of cyclin B1, CDK1 or cyclin A (Figure 3A). Daidzein had no effect on the expression of cyclin B1, CDK1, cyclin A or CDK2 (Figure 3A). Because the activity of CDKs is also very important for regulating the cell cycle, we next evaluated the effect of daidzein and 6,7,4′-THIF on the kinase activity of CDK1 and CDK2 in S and G2/M phases in HCT-116 cells. The results of the kinase assay revealed that 6,7,4′-THIF suppressed CDK1 activity in a dose-dependent manner (Figure 3B, left panel), whereas daidzein showed a smaller inhibitory effect (Figure 3B, right panel). Moreover, 6,7,4′-THIF inhibited CDK2 activity much more strongly in HCT-116 cells compared with daidzein (Figure 3C). These results indicate that the induction of cell cycle arrest by 6,7,4′-THIF involves its suppression of CDK2 expression and both CDK1 and CDK2 activity leading to cell cycle arrest at the S and G2/M phases.
Fig. 3.
Effect of 6,7,4′-THIF or daidzein on the expression and activity of S- and G2/M-phase regulatory proteins in HCT-116 cells. (A) Cells were treated with 6,7,4′-THIF or daidzein at the indicated concentrations (0, 25, 50 or 100 μM) for 48 h. Protein abundance was determined by western blot analysis as described in Materials and Methods using specific antibodies against cyclin B1, cyclin A, CDK1 and CDK2. Data are representative of three independent experiments. (B and C) 6,7,4′-THIF inhibits cyclin B1/CDK1 (B) or cyclin A/CDK2 (C) complex activity more effectively than daidzein in HCT-116 cells. For the ex vivo CDK1 or CDK2 assay, cells were treated with 6,7,4′-THIF or daidzein at the indicated concentrations for 48 h. Kinase activity is expressed as percent inhibition relative to untreated control cells. The average 32P count was determined from three independent experiments, and data are presented as means ± standard deviations. The asterisk(s) indicates a significant decrease in kinase activity between groups treated with 6,7,4′-THIF or daidzein and the untreated control group (*P < 0.05; **P < 0.01).
6,7,4′-THIF interacts directly with CDK1 or CDK2 but does not compete with ATP for binding with CDK1 or CDK2
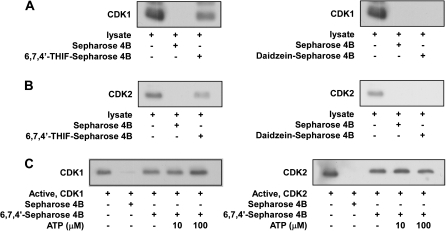
The results above indicated that the induction of cell cycle arrest by 6,7,4′-THIF involves the suppression of CDK1 or CDK2 activity. To determine whether 6,7,4′-THIF exerts its effects by interacting directly with CDK1 or CDK2, we performed an ex vivo pull-down assay using 6,7,4′-THIF–Sepharose 4B beads and HCT-116 cell lysates. The precipitated cell lysates were collected and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis. CDK1 (Figure 4A, left panel, third lane) or CDK2 (Figure 4B, left panel, third lane) was detected in association with 6,7,4′-THIF–Sepharose 4B beads, but not with Sepharose 4B-only beads. The results indicate that 6,7,4′-THIF binds directly to CDK1 or CDK2, whereas daidzein (Figure 4A and B, right panel, third lane) does not interact with either CDK1 or CDK2 in HCT-116 cells. The first lane in Figure 4A and B shows the results for unprecipitated cell lysates, indicating that the bands observed represent specific detection of the CDK1 or CDK2 protein. We also confirmed that 6,7,4′-THIF, but not daidzein, binds directly to a recombinant CDK1 or CDK2 protein, and results in suppression of CDK1 and CDK2 activity in vitro (data not shown). Moreover, ATP does not appear to compete with 6,7,4′-THIF for binding with CDK1 (Figure 4C, left panel) or CDK2 (Figure 4C, right panel) because the binding of 6,7,4′-THIF to CDK1 or CDK2 did not decrease with increasing amounts of ATP. Therefore, these results suggest that 6,7,4′-THIF inhibits CDK1 and CDK2 activity through direct binding that does not involve competition with ATP.
Fig. 4.
Direct binding of 6,7,4′-THIF or daidzein to CDK1 and CDK2. (A) 6,7,4′-THIF, but not daidzein, specifically binds to the cyclin B1/CDK1 complex ex vivo. CDK1–6,7,4′-THIF (left panel) and CDK1–daidzein (right panel) binding ex vivo was assessed by immunoblotting using a specific antibody against CDK1: lane 1 (input control), whole cell lysate from HCT-116 cells; lane 2 (control), whole cell lysate from HCT-116 cells precipitated with Sepharose 4B; lane 3, whole cell lysate from HCT-116 cells precipitated with 6,7,4′-THIF–Sepharose 4B (left panel) and daidzein–Sepharose 4B (right panel). (B) 6,7,4′-THIF, but not daidzein, specifically binds to the cyclin A/CDK2 complex ex vivo. CDK2–6,7,4′-THIF (left panel) and CDK2–daidzein (right panel) binding ex vivo was assessed by immunoblotting using a specific antibody against CDK2. First lane (input control), whole cell lysate from HCT-116 cells; second lane (control), whole cell lysate from HCT-116 cells precipitated with Sepharose 4B; third lane, whole cell lysate from HCT-116 cells precipitated by 6,7,4′-THIF–Sepharose 4B (left panel) and daidzein–Sepharose 4B (right panel). Data are representative of three independent experiments. (C) 6,7,4′-THIF does not compete with ATP for binding with cyclin B/CDK1 or cyclin A/CDK2. The active cyclin B1/CDK1 complex (2 μg) (left panel) and cyclin A/CDK2 complex (2 μg) (right panel) were incubated with different concentrations of ATP (0, 10 or 100 μM) and 100 μl of 6,7,4′-THIF–Sepharose 4B or 100 μl of Sepharose 4B (as a negative control) in reaction buffer to a final volume of 500 μl. The mixtures were incubated at 4°C overnight with shaking. After washing, the pulled down proteins were detected by western blotting. Lane 2 (negative control): cyclin B1/CDK1 or cyclin A/CDK2 complex does not bind with Sepharose 4B alone; lane 3: positive control; lanes 4 and 5: increasing the amount of ATP has no effect on 6,7,4′-THIF binding to the cyclin B1/CDK1 complex or the cyclin A/CDK2 complex.
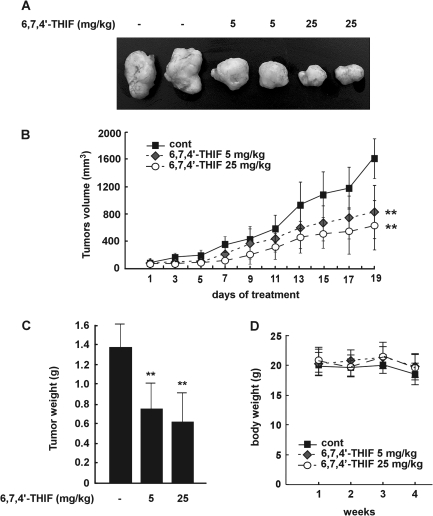
6,7,4′-THIF inhibits tumorigenicity of HCT-116 cells in a xenograft mouse model
The xenograft mouse model is very useful for the study of human tumor biology and anticancer agents (18). Because 6,7,4′-THIF was observed to be effective in suppressing the proliferation of HCT-116 cells, we studied the effects of 6,7,4′-THIF in an in vivo xenograft mouse model. Photographic data show that 6,7,4′-THIF treatment-suppressed tumor development in mice (Figure 5A). The average volume of tumors in non-6,7,4′-THIF-treated mice increased as a function of time and reached a volume of 1689 mm3 at 4 weeks postinoculation. However, at this time, in mice treated with 5 or 25 mg/kg 6,7,4′-THIF, the average tumor volume was only 750 or 522 mm3, respectively (Figure 5B). At the end of the study, we removed the tumors and measured their weight for each group. Treatment with 6,7,4′-THIF (5 or 25 mg/kg) clearly reduced tumor weight compared with the control group (Figure 5C). 6,7,4′-THIF treatment did not cause any loss in body weight, indicating that the dosages used were not toxic to the animals (Figure 5D). Collectively, these results suggest that 6,7,4′-THIF might serve as an effective anticancer treatment with the potential to inhibit or delay the tumorigenicity of HCT-116 cells in an in vivo system.
Fig. 5.
6,7,4′-THIF inhibits the growth of colon cancer tumor xenografts in vivo. (A) photographs of excised tumors from each group. (B) the average volume of tumors from control and 6,7,4′-THIF-treated mice was plotted >20 days after tumor cell inoculation. Points, mean of six tumors; bars, standard deviation; P values indicate statistical significance of the inhibition of tumor growth by 6,7,4′-THIF, **P < 0.01. (C) at the end of the study, the excised tumors from each group were weighed (n = 6). The asterisks indicate a significant decrease in tumor weight between the control group and the group treated with 6,7,4′-THIF (**P < 0.01). (D) The weight of nude mice from each group did not change significantly during the experiment.
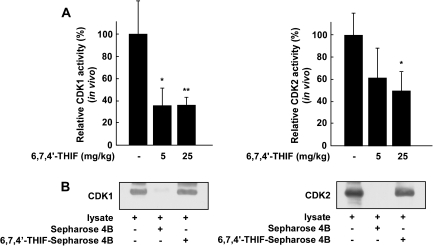
6,7,4′-THIF inhibits CDK1 and CDK2 activity by binding directly to CDK1 and CDK2 in HCT-116 cell-derived tumors in athymic nude mice
To further confirm the anticancer effect of 6,7,4′-THIF in an in vivo xenograft model, we examined CDK1 and CDK2 activity in xenograft tumors. Consistent with our results with the HCT-116 cell line, the results of the in vivo kinase assay showed that CDK1 or CDK2 activity in the 6,7,4′-THIF-treated tumor groups was significantly inhibited compared with CDK1 (Figure 6A, left panel) or CDK2 (Figure 6A, right panel) activity in the untreated group. Moreover, the in vivo pull-down assay results showed that 6,7,4′-THIF bound directly to CDK1 (Figure 6B, left panel, third lane) and CDK2 (Figure 6B, right panel, third lane). Overall, these results further support the hypothesis that the inhibition of CDK1 or CDK2 activity by 6,7,4′-THIF is critically involved in the suppression of tumor growth in an in vivo model.
Fig. 6.
Effect of 6,7,4′-THIF on CDK1 or CDK2 activity in vivo. (A) 6,7,4′-THIF inhibits CDK1 and CDK2 activity in tumor extracts. Data are shown as means ± standard deviations of the 32P count from three mice in each group from three separate experiments. The asterisk (*) indicates a significant difference (P < 0.05) between groups treated with 6,7,4′-THIF and untreated control groups. (B) 6,7,4′-THIF directly interacts with CDK1 and CDK2 in tumors. CDK1– and CDK2–6,7,4′-THIF binding was confirmed by immunoblotting using an antibody against CDK1 (left panel) or CDK2 (right panel): lane 1 (input control), whole tumor lysates from nude mice; lane 2 (control), whole tumor lysates from nude mice precipitated with Sepharose 4B beads and lane 3, whole tumor lysates from nude mice precipitated by 6,7,4′-THIF–Sepharose 4B beads.
Discussion
Soybean and its processed products are major food sources that provide a high concentration of isoflavones (19), which are a class of bioactive phytochemicals that have been widely reported to have a potential role in the prevention of various chronic diseases, such as cancer, cardiovascular disease, obesity and osteoporosis (20–22). Daidzein is one of the major isolavone in soybeans along with genistein. It is metabolized by intestinal microflora (23) or hepatic enzymes (9,10) and its metabolites exert biological activity. Indeed, <10% of daidzein remains unchanged in human urine after oral ingestion of isoflavones (24). Therefore, the effect of daidzein metabolites might contribute to the anticarcinogenic effects associated with soybean intake. We thus evaluated the antitumor activity of the isoflavone 6,7,4′-THIF, a metabolite of daidzein, and found that 6,7,4′-THIF clearly suppressed the proliferation of HCT-116 colon cancer cells much more effectively than daidzein and acts through cell cycle arrest at S the and G2/M phases.
The continuous activation of CDKs in cancer has stimulated the development of small-molecule inhibitors of CDKs for the treatment of cancer, with several targeted CDK inhibitors entering clinical trials. Results of a previous study results showed that genistein, 4′,5,7-trihydroxyisoflavone, induced G2 arrest by inhibiting CDK2 activity in the human choroidal melanoma cell line OCM-1 (25). Recently, however, several studies employing mutation or knockout of single CDKs in mice have demonstrated that CDKs are dispensable for cell proliferation due to their high level of functional redundancy and other compensatory mechanisms (26,27). Therefore, several CDK inhibitors in preclinical development are designed to suppress more than a single CDK. In contrast to the results for CDK4 or CDK2 single mutants, however, the CDK1 knockout mouse is not viable, and CDK1 is required for the interaction of other CDKs with their complementary cyclin partners (28). Further data revealed that depletion of CDK1 or CDK2 alone by small RNA interference in human cancer cells was insufficient to completely suppress cell cycle progression. On the other hand, combined depletion of the CDKs effectively induces cell cycle arrest at the G2/M phase (29).
In this study, we found that 6,7,4′-THIF potently suppressed the kinase activities of two CDKs, CDK1 and CDK2, at concentrations that corresponded to the inhibitory effects of 6,7,4′-THIF on the proliferation of HCT-116 cells. Moreover, an ex vivo pull-down assay and kinase assay showed that 6,7,4′-THIF inhibited CDK1 and CDK2 activity through specific binding of CDK1 and CDK2 proteins. Also, 6,7,4′-THIF inhibited expression of CDK2 but not other S- and G2/M-phase regulatory proteins such as cyclin A, cyclin B1 or CDK1. This inhibition might have caused cell cycle arrest at the S and G2/M phases in HCT-116 cells. In contrast, daidzein produced only slight inhibition of growth and only a small effect on HCT-116 cell cycle arrest. Moreover, daidzein did not inhibit the expression level of S- and G2/M-phase regulatory proteins. Although daidzein resulted in slight inhibition of CDK1 and CDK2 activity in HCT-116 cells, it did not bind to either CDK1 or CDK2. These results imply that the slight inhibitory effect of daidzein on CDK1 and CDK2 activity does not result from its specific binding to these proteins because daidzein does not interact directly with CDK1 or CDK2. A possible explanation is that daidzein might upregulate the expression of an endogenous CDK inhibitor such as p21 or p27. Indeed, we observed that daidzein slightly induced the expression of p21 in HCT-116 cells (data not shown) without affecting the expression of other proteins. In contrast, the present study showed that 6,7,4′-THIF modulated the expression and kinase activity of several cell cycle regulatory proteins. Therefore, we concluded that by targeting multiple proteins, 6,7,4′-THIF exerts an anticancer effect that is stronger than daidzein.
In summary, 6,7,4′-THIF prevented the anchorage-dependent and -independent growth of HCT-116 cells much more effectively than daidzein by inducing cell cycle arrest at S and G2/M phases. 6,7,4′-THIF effectively suppressed the expression of CDK2 without affecting the expression of other S- and G2/M-phase regulatory proteins such as cyclin A, cyclin B1 or CDK1, whereas daidzein had no effect. 6,7,4′-THIF was effective at inhibiting CDK1 and CDK2 activity in HCT-116 cells through its direct interaction with CDK1 and CDK2. In contrast, daidzein did not bind to either CDK1 or CDK2. The in vivo data showed that 6,7,4′-THIF significantly decreased tumor growth, volume and weight of HCT-116 xenografts. 6,7,4′-THIF bound directly to CDK1 and CDK2 in vivo, resulting in the suppression of CDK1 and CDK2 activity in 6,7,4′-THIF-treated tumors corresponding with our in vitro results. Collectively, these results suggest that CDK1 and CDK2 are potential molecular targets of 6,7,4′-THIF in the suppression of HCT-116 colon cancer cell proliferation. These results provide insight into the biological actions of 6,7,4′-THIF and might establish a molecular basis for the development of new cancer therapeutic agents.
Funding
This work was supported in part by The Hormel Foundation; National Institutes of Health grants CA027502, CA120388, CA111536, CA077646 and CA081064; and by grants from World Class University Program (R31-2008-00-10056-0), World Class Institute Program (2009-0093824), Leap Research Program (2010-0029233) and Basic Science Research Program (2009-0090797) through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology, Republic of Korea.
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ATP
adenosine triphosphate
- CDK
cyclin-dependent kinase
- 6,7,4′-THIF
6,7,4′-trihydroxyisoflavone
References
- 1.Fahy B, et al. Epidemiology and molecular genetics of colorectal cancer. Surg. Oncol. 1998;7:115–123. doi: 10.1016/s0960-7404(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 2.Forte A, et al. Dietary chemoprevention of colorectal cancer. Ann. Ital. Chir. 2008;79:261–267. [PubMed] [Google Scholar]
- 3.Garay CA, et al. Chemoprevention of colorectal cancer: dietary and pharmacologic approaches. Oncology (Williston Park) 1999;13:89–97. discussion 97–100, 105. [PubMed] [Google Scholar]
- 4.Greenlee RT, et al. Cancer statistics, 2000. CA Cancer J. Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 5.Toyomura K, et al. Soybeans, soy foods, isoflavones and risk of colorectal cancer: a review of experimental and epidemiological data. Asian Pac. J. Cancer Prev. 2002;3:125–132. [PubMed] [Google Scholar]
- 6.Choi EJ, et al. Equol induces apoptosis through cytochrome c-mediated caspases cascade in human breast cancer MDA-MB-453 cells. Chem. Biol. Interact. 2009;177:7–11. doi: 10.1016/j.cbi.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Lampe JW. Is equol the key to the efficacy of soy foods? Am. J. Clin. Nutr. 2009;89:1664S–1667S. doi: 10.3945/ajcn.2009.26736T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang NJ, et al. Equol, a metabolite of the soybean isoflavone daidzein, inhibits neoplastic cell transformation by targeting the MEK/ERK/p90RSK/activator protein-1 pathway. J. Biol. Chem. 2007;282:32856–32866. doi: 10.1074/jbc.M701459200. [DOI] [PubMed] [Google Scholar]
- 9.Kulling SE, et al. Oxidative in vitro metabolism of the soy phytoestrogens daidzein and genistein. J. Agric. Food Chem. 2000;48:4963–4972. doi: 10.1021/jf000524i. [DOI] [PubMed] [Google Scholar]
- 10.Kulling SE, et al. Oxidative metabolism of the soy isoflavones daidzein and genistein in humans in vitro and in vivo. J. Agric. Food Chem. 2001;49:3024–3033. doi: 10.1021/jf0012695. [DOI] [PubMed] [Google Scholar]
- 11.Kulling SE, et al. Oxidative metabolism and genotoxic potential of major isoflavone phytoestrogens. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;777:211–218. doi: 10.1016/s1570-0232(02)00215-5. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen-Ba G, et al. Epigenetic events during the process of cell transformation induced by carcinogens (review) Oncol Rep. 1999;6:925–932. doi: 10.3892/or.6.4.925. [DOI] [PubMed] [Google Scholar]
- 13.Katrien Vermeulen DRVB, et al. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malumbres M, et al. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro GI. Cyclin-dependent kinase pathways targets for cancer treatment. J. Clin. Oncol. 2006;24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 16.Colburn NH, et al. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc. Natl Acad. Sci. USA. 1981;78:6912–6916. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad N, et al. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Natl Cancer Inst. 1997;89:1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 18.Drees M, et al. Flavopiridol (L86-8275): selective antitumor activity in vitro and activity in vivo for prostate carcinoma cells. Clin. Cancer Res. 1997;3:273–279. [PubMed] [Google Scholar]
- 19.Mateos-Aparicio I, et al. Soybean, a promising health source. Nutr. Hosp. 2008;23:305–312. [PubMed] [Google Scholar]
- 20.Messina MJ, et al. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr. Cancer. 1994;21:113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 21.Montenegro MF, et al. Isoflavone genistein inhibits the angiotensin-converting enzyme and alters the vascular responses to angiotensin I and bradykinin. Eur. J. Pharmacol. 2009;607:173–177. doi: 10.1016/j.ejphar.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Orgaard A, et al. The effects of soy isoflavones on obesity. Exp. Biol. Med. 2008;233:1066–1080. doi: 10.3181/0712-MR-347. [DOI] [PubMed] [Google Scholar]
- 23.Atkinson C, et al. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp. Biol. Med. 2005;230:155–170. doi: 10.1177/153537020523000302. [DOI] [PubMed] [Google Scholar]
- 24.Lu L, et al. Sex and long-term soy diets affect the metabolism and excretion of soy isoflavones in humans. Am. J. Clin. Nutr. 1998;68:1500S–1504S. doi: 10.1093/ajcn/68.6.1500S. [DOI] [PubMed] [Google Scholar]
- 25.Casagrande F, et al. p21CIP1 is dispensable for the G2 arrest caused by genistein in human melanoma cells. Exp. Cell Res. 2000;258:101–108. doi: 10.1006/excr.2000.4914. [DOI] [PubMed] [Google Scholar]
- 26.Carlson BA, et al. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 1996;56:2973–2978. [PubMed] [Google Scholar]
- 27.De Azevedo WF, Jr, et al. Structural basis for specificity and potency of a flavonoid inhibitor of human CDK2, a cell cycle kinase. Proc. Natl Acad. Sci. USA. 1996;93:2735–2740. doi: 10.1073/pnas.93.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DePinto W, et al. In vitro and in vivo activity of R547: a potent and selective cyclin-dependent kinase inhibitor currently in phase I clinical trials. Mol. Cancer Ther. 2006;5:2644–2658. doi: 10.1158/1535-7163.MCT-06-0355. [DOI] [PubMed] [Google Scholar]
- 29.Squires MS, et al. Biological characterization of AT7519, a small-molecule inhibitor of cyclin-dependent kinases, in human tumor cell lines. Mol. Cancer Ther. 2009;8:324–332. doi: 10.1158/1535-7163.MCT-08-0890. [DOI] [PubMed] [Google Scholar]