We are writing in response to the letter by Sloan et al.1 regarding our review of ‘Kangaroo mother care’.2 We are happy to see the ongoing interest in this publication and thank these colleagues for their letter. We fully agree that meta-analysis is frequently used inappropriately, especially given the ease of use of Revman® (Review Manager (RevMan) [Computer program]. Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008). The published paper was not such a ‘quick and easy’ meta-analysis, but developed through an extensive process for over 18 months with data kindly provided by investigators, review at meetings of the Child Health Epidemiology Reference Group (CHERG) and by United Nations colleagues, as well as by external reviewers for the journal, including members of the Cochrane Collaboration.
We would again like to make it clear that this review is not intended to be a Cochrane review. As stated in the paper and in the introductory papers to the supplement, all the Lives Saved Tool (LiST) reviews follow a standard methodology developed by CHERG and WHO using GRADE criteria.3 The reviews are designed to inform inputs to LiST, which is a software tool designed to encourage evidence-based programme investment for mortality effect in a given country. The goal of this exercise is not to duplicate the work of the Cochrane collaboration, but to provide transparent estimates of the cause-specific mortality effects of priority interventions that are being scaled up in low- and middle-income countries. That said, it should be noted that the LiST review process identified limitations in previous Cochrane reviews. For example, the Cochrane review meta-analysis for Kangaroo mother care (KMC) combined mortality outcomes at 12 months, 6 months and pre-discharge.4 Any review is likely to have limitations and the goal for us all is to minimize these.
Intervention definition for RCT mortality meta-analysis
In the paper, we clearly defined, a priori, that for mortality outcomes we were examining the intervention of KMC commencing in the first week of life. Sloan et al. suggest that studies were excluded based on results. As stated in the paper, mortality studies were included or excluded based on explicit criteria—recruitment of babies with birth weight ≤2000 g and on their median day of initiating KMC (Figure 1 and Table 1 in the original article2). We did not use terms mentioned in her letter such as ‘early KMC’ and ‘traditional KMC’, as these may mean different things to different expert groups—we preferred a specific, reproducible measure regarding median day of initiation of KMC. We included studies in which the median day of KMC initiation was ≤7 (Charpak,5 4 days; Suman,6 3.7 days; Worku,7 <1 day) and we excluded those studies in which the median day of KMC initiation was >7 days (Sloan et al.,8 12.4 days; Cattaneo et al.,9 10 days). We also excluded the more recent Sloan study from Bangladesh10 since birth weight was not measured for most neonates in the study, and there were other limitations in implementing this trial in a challenging setting in rural Bangladesh as discussed in the paper and as noted in Sloan et al.’s communication now. In the paper, we reported a sensitivity analysis including the two late initiation studies in the meta-analysis (Sloan et al.8 and Cattaneo et al.9). The mortality result remained significant {relative risk (RR) 0.64 [95% confidence interval (CI) 0.42–0.96]}.2
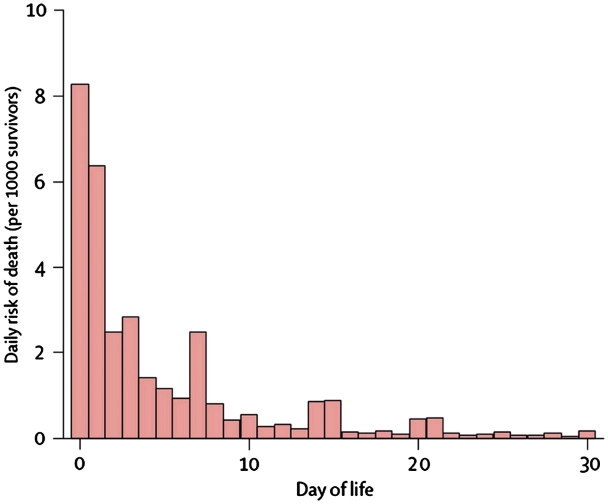
Figure 1.
Daily risk of death during first month of life based on analysis of 47 DHS data sets (1995–2003) with 10 048 neonatal deaths. Deaths in first 24 h recorded as occurring on Day 0, or possibly Day 1, depending on interpretation of question and coding of response. Preference for reporting certain days (7, 14, 21 and 30) is apparent. Between 73 and 76% of neonatal deaths occur in the first 7 days after birth. Source: The Lancet.12
The reason for excluding studies of KMC starting after the first week of life is that the mortality effect of any intervention starting after the first week could not be compared with those starting in the first week since 75% of neonatal deaths occur in the first week. Sloan suggests that the distribution of neonatal mortality by day is ‘extremely variable’. However, the proportion of neonatal deaths that occur in the first week is actually remarkably stable in both Vital Registration11 and Household Survey data12 (Figure 1). Even with data limitations such as age heaping on Day 7 and misclassification of Day 0, there is a predictable distribution.13 We even considered a cut-off before the first week of life because ∼50% of deaths occur in the first 48 h, and >75% occur in the first week. However, we applied a conservative and clear cut-off for median commencement of KMC by day 7.
We do not accept that studies were selected based on their results. They were selected on explicit, preset criteria, which in this case related to the usual PICO format of Patient, Intervention Comparison Outcome.
Outcome definition for RCT mortality meta-analysis
Our review focused on ‘neonatal’ mortality, which differs from the earlier Cochrane review (Conde Aguedelo 2003)4 in which the mortality meta-analysis combined infant mortality,5 6-month mortality8 and pre-discharge mortality.9 Both Charpak and Suman kindly provided us with their unpublished neonatal mortality data. The Worku trial7 was based on pre-discharge neonatal mortality and we recognized this as a limitation and discussed this in the text and the detailed GRADE webtable marks down the quality based on this and other limitations. Although some post-discharge neonatal deaths may have been missed, given the steep survival curve, such deaths are likely to have been a small proportion and given the expectation of fewer deaths in the KMC group this loss to follow-up bias will be expected to weaken the effect size and is conservative. In the Suman trial,6 where cases were followed after discharge, no further neonatal deaths were identified post-discharge in control or intervention arms.
We do not agree with Sloan et al.’s point that it is incorrect to undertake meta-analysis of trials for an outcome (in this instance neonatal mortality) that those trials were not individually powered to examine. Indeed, we would argue that one of the most important uses of meta-analysis is precisely for such situations.
Neither do we accept their argument that these studies are too heterogeneous to be combined. We followed standard meta-analysis rules to examine for heterogeneity using the I2 statistic as laid out in our methods section under the subtitle ‘Analyses, and summary measures’. For these three RCTs, the I2 = 0.0%, P = 0.539 and so a fixed effects meta-analysis was appropriate. Mantel–Haenszel weights were used to combine the studies. The weight given to Worku simply reflects lower standard error in their estimates, as shown by the narrower confidence interval. This is the standard approach to weighting. No special weighting was applied.
Mortality outcome observational study meta-analysis
Sloan et al. state that a meta-analysis of observation studies ‘is almost a contradiction in terms’ and should not be done. Once again we emphasize that this review is not a Cochrane review—our purpose is to examine the data to inform implementation at scale. As stated in the paper, the purpose was to examine whether the effect estimate obtained from small, RCTs measuring efficacy would be consistent with the effect estimate obtained from larger, observational studies with weaker designs. The paper explicitly allocates these observational studies a low level of evidence. This meta-analysis is of relevance to programmatic planners since the results suggest that wide-scale, routine implementation of KMC is still associated with considerable mortality reduction of around 32% reduction in deaths for babies under 2000 gms.
Morbidity outcome RCT meta-analysis
We agree that our paper should have had more detailed discussion of the inputs for the morbidity meta-analysis compared with the mortality analysis. The reported morbidity outcomes in most of these trials are beyond the neonatal period and morbidity, notably infections in pre-term infants tends to peak later than mortality. The paper presented a meta-analysis with studies of both early and late initiation of KMC [RR 0.34 (95% CI 0.17–0.65)].2 We should also have reported a meta-analysis excluding the KMC studies with later initiation as per the criteria set out for the mortality RCT meta-analysis. The results of this analysis are not very different in terms of the point estimate [RR 0.25 (95% CI 0.06–1.07)].
Weight cut-off of <2000 g
We state in the paper which of the studies included had a weight cut-off of ≤2000 g. A lower cut-off will dilute the effect in the morbidity meta-analysis (Udani,14 1800 g) and the observational study meta-analysis (Kambarini,15 1600 g and Lincetto,16 1800 g) as smaller babies have a higher risk, and hence this bias is conservative. The exclusion of babies <1000 g in Pattinson et al. may increase the effect in the observational meta-analysis in the observational study meta-analysis. However, these biases are discussed in the paper and none of these effect sizes is being used in LiST—the analysis for morbidity and observational trials was to examine consistency of effect between these studies and the RCT meta-analysis.
Misinterpretation of study statistics
Finally we were surprised to read the section entitled ‘Misinterpretation of study statistics’, which refers to an e-mail notice regarding the paper circulated by others.1 This e-mail suggested that KMC could halve all neonatal deaths, instead of halving of neonatal deaths in stable neonates <2000 g as we clearly stated in the paper. Such e-mails are, unfortunately, beyond our control, and do not seem appropriate material for a journal letter.
Conclusions
Sloan et al. agree with our statement that there is insufficient evidence to recommend community initiation of KMC.1 Although Sloan et al. state that KMC, especially started early ‘ … has potential for averting some neonatal mortality associated with prematurity … ’, their conclusion is that there is ‘no single adequately designed and implemented trial to demonstrate the effect of early KMC on newborn or infant mortality’, even for facility-based KMC. We all agree more trials are needed, especially for community initiation, and we all agree future trials should learn from the limitations of the ones included and excluded here. We also all agree that there is as yet no one ‘perfect’ trial even for facility KMC, and as stated in our paper, all of the three RCTs in our meta-analysis have limitations. However, we do not agree with Sloan et al. that policy and programme investment in facility KMC should wait for a ‘perfect’ trial. We, and the many scientists who reviewed this paper, believe that this review is transparent in providing a mortality effect size of KMC and in discussing the potential biases in both directions. Indeed, many of the biases are likely to result in under-estimation of effect for the real question for public health relevance—namely, how much better is KMC than no care at all.
Funding
This work was supported in part by a grant to the US Fund for UNICEF from the Bill & Melinda Gates Foundation (grant 43386) to ‘Promote evidence-based decision making in designing maternal, neonatal and child health interventions in low- and middle-income countries’, and by a grant to Save The Children USA from the Bill & Melinda Gates Foundation (Grant 50124) for ‘Saving Newborn Lives’. We also acknowledge the Global Alliance for Prevention of Prematurity and Stillbirths. www.gappsseattle.org.
References
- 1.Sloan N, Anderson G, Moore E. Letter to the Editor re ‘Kangaroo mother care' to prevent neonatal deaths due to preterm birth complications’. Int J Epidemiol. 2011;40:521–25. doi: 10.1093/ije/dyq174. [DOI] [PubMed] [Google Scholar]
- 2.Lawn JE, Mwansa-Kambafwile J, Horta BL, Barros FC, Cousens S. 'Kangaroo mother care' to prevent neonatal deaths due to preterm birth complications. Int J Epidemiol. 2010;39(Suppl. 1):i144–54. doi: 10.1093/ije/dyq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker N, Fischer-Walker C, Bryce J, Bahl R, Cousens S. Standards for CHERG reviews of intervention effects on child survival. Int J Epidemiol. 2010;39(Suppl. 1):i21–31. doi: 10.1093/ije/dyq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conde-Agudelo A, az-Rossello JL, Belizan JM. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2003;(2) doi: 10.1002/14651858.CD002771. [DOI] [PubMed] [Google Scholar]
- 5.Charpak N, Ruiz-Pelaez JG, Figueroa de CZ, Charpak Y. Kangaroo mother versus traditional care for newborn infants ≤2000 grams: a randomized, controlled trial. Pediatrics. 1997;100:682–88. doi: 10.1542/peds.100.4.682. [DOI] [PubMed] [Google Scholar]
- 6.Suman RP, Udani R, Nanavati R. Kangaroo mother care for low birth weight infants: a randomized controlled trial. Indian Pediatr. 2008;45:17–23. [PubMed] [Google Scholar]
- 7.Worku B, Kassie A. Kangaroo mother care: a randomized controlled trial on effectiveness of early kangaroo mother care for the low birthweight infants in Addis Ababa, Ethiopia. J Trop Pediatr. 2005;51:93–7. doi: 10.1093/tropej/fmh085. [DOI] [PubMed] [Google Scholar]
- 8.Sloan NL, Camacho LW, Rojas EP, Stern C. Kangaroo mother method: randomised controlled trial of an alternative method of care for stabilised low-birthweight infants. Maternidad Isidro Ayora Study Team. Lancet. 1994;344:782–85. doi: 10.1016/s0140-6736(94)92341-8. [DOI] [PubMed] [Google Scholar]
- 9.Cattaneo A, Davanzo R, Worku B, Surjono A, Echeverria M, Bedri A, et al. Kangaroo mother care for low birthweight infants: a randomized controlled trial in different settings. Acta Paediatr. 1998;87:976–85. doi: 10.1080/080352598750031653. [DOI] [PubMed] [Google Scholar]
- 10.Sloan NL, Ahmed S, Mitra SN, Choudhury N, Chowdhury M, Rob U, et al. Community-based kangaroo mother care to prevent neonatal and infant mortality: a randomized, controlled cluster trial. Paediatr. 2008;121 doi: 10.1542/peds.2007-0076. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Neonatal and Perinatal Mortality: Country, Regional and Global Estimates. Geneva, Switzerland: WHO; 2006. [Google Scholar]
- 12.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 13.Hill K, Choi Y. Neonatal mortality in the developing world. Demographic Res. 2006;14:429–52. [Google Scholar]
- 14.Udani. Innovation: KEM Kangaroo Bag – For Kangaroo Mother Care. http://kangaroo.javeriana.edu.co/encuentros/7encuentro/posters/Rekha%20Udani%204.pdf.
- 15.Kambarami RA, Chidede O, Kowo DT. Kangaroo care versus incubator care in the management of well preterm infants. A pilot study. Ann Trop Paediatr. 1998;18:81–6. doi: 10.1080/02724936.1998.11747932. [DOI] [PubMed] [Google Scholar]
- 16.Lincetto O, Nazir AI, Cattaneo A. Kangaroo mother care with limited resources. J Trop Pediatr. 2000;46:293–5. doi: 10.1093/tropej/46.5.293. [DOI] [PubMed] [Google Scholar]