Abstract
Background Despite the perceived importance of early life nutrition for mental development, few studies have related gestational undernutrition to later-life cognitive functioning. We investigated the consequences of gestational exposure to the Dutch famine of 1944–45 for cognitive functioning at the age of 59 years.
Methods We recruited men and women who were (i) born in birth clinics in Amsterdam, Rotterdam and Leiden, between January 1945 and March 1946, whose mothers experienced famine during or immediately preceding pregnancy (n = 354); (ii) born in the same three institutions during 1943 and 1947, whose mothers did not experience famine during this pregnancy (n = 292); or (iii) same-sex siblings of those in the first two categories (n = 311). We assessed cognitive performance at the age of 59 years by means of a comprehensive test battery.
Results All cognitive functioning test scores were within normal ranges for this age group. There were no differences in cognitive performance at the age of 59 years between individuals exposed to gestational undernutrition and those without this exposure. For the general cognitive index, a summary measure across six functional domains (mean 100, standard deviation (SD) 15 points), famine exposure was associated with a decrease of 0.57 points [95% confidence interval (95% CI) −2.41 to 1.28] points. Individuals exposed to famine in gestational weeks 1–10 had a cognitive functioning index 4.36 (95% CI 8.04–0.67) points lower than those without this exposure. Within-sibling-pair analyses gave consistent results.
Conclusion We found no overall association between maternal exposure to acute famine in pregnancy and cognitive performance of the offspring at the age of 59 years, but cannot rule out an association specific to early pregnancy exposure.
Keywords: Prenatal exposures, nutrition, mental development, developmental origins, Dutch famine
Introduction
During the first half of pregnancy, when neurogenesis (6–18 weeks of gestation) and cell migration (until 26 weeks of gestation) take place,1 the brain is particularly sensitive to ionizing irradiation,2 alcohol use,3 smoking,4 drug abuse,5 maternal influenza6 and stress.7 In addition, nutrient availability may affect the timing or quality of neural development and/or alter neuronal membrane function and, thus, potentially affect brain functioning and cognitive development.8 Well-controlled studies have shown that periconceptional folic acid supplementation can prevent neural tube defects,9 that the risk of schizophrenia is elevated among individuals whose mothers were exposed to severe famine in both The Netherlands10 and China,11 and that post-natal nutritional intervention among chronically undernourished populations improves reading performance comparable with an additional year of schooling.12
To date, the influence of overall maternal undernutrition during pregnancy on cognitive functioning of the offspring has not been well examined. Cognitive functioning is the net result of complex interactions among genetic, biological and environmental exposures experienced over the life course.13,14 Studies that relate pre-natal and early post-natal undernutrition to delayed cognitive and psychosocial development15,16 are difficult to interpret, as the observed associations may be confounded by social, economic and family factors, including post-natal compensation.17
The Dutch famine in World War II (the ‘Hunger Winter’ of 1944–45) provides a unique opportunity to investigate the long-term consequences of undernutrition at defined stages of gestation. In earlier studies, there was no detectable adverse effect of gestational undernutrition on IQ, assessed by Raven Progressive Matrices scores among 19-year-old men,18 and a change in one out of four selected measures of cognition in 59-year-old men and women.19 We investigate in the current study whether severe undernutrition of the mother immediately preceding or during pregnancy affects cognitive function of their offspring in late middle age, including a wide array of functional assessments.
We hypothesized that undernutrition during sensitive periods of pregnancy, specifically exposure to famine at Weeks 1–10 and Weeks 11–20 of gestation, would be associated with lower cognitive performance compared with controls. This hypothesis is based upon the knowledge that the neurogenesis of cerebral cortical neurons and migration is largely completed in the first half of pregnancy.1 Conversely, lack of an association of gestational undernutrition with cognitive functioning at the age of 59 years would suggest that adaptive mechanisms in pregnancy can protect the fetus from maternal food shortage.20
Materials and Methods
Setting
During the Dutch Hunger Winter of 1944–45, the per capita food availability was reduced dramatically as a result of a German embargo on rail transport and a relatively extreme winter, which led to frozen canals.21 The availability of food before, during and after the famine has been reported widely elsewhere.22–24 Prior to this period, the Dutch population had access to adequate food supplies. By November 26, 1944, official rations in the western Netherlands had fallen below 900 kcal/day. They were as low as 500 kcal/day by April 1945. The famine ended immediately after the liberation on May 5, 1945.
Selection of participants
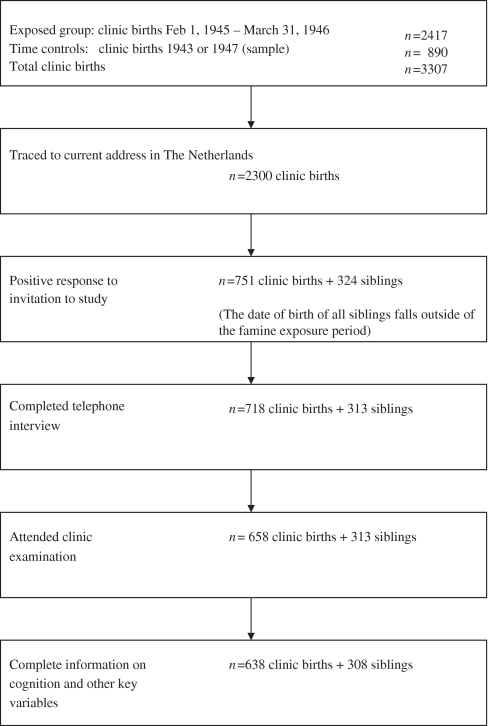
We identified 3307 live-born singleton births at three institutions in famine-exposed cities: the midwifery training schools in Amsterdam and Rotterdam and the university hospital in Leiden. We selected (i) all 2417 births between February 1, 1945 and March 31, 1946 (infants whose mothers were already ∼2 months pregnant when the famine started, or who conceived during the famine or in the month following its end) and hence were exposed to a ration of <1000 kcal/day for at least 3 months of pregnancy and (ii) a sample of 890 births from 1943 and 1947 (infants whose mothers did not experience famine during this pregnancy) as time controls (Figure 1). The sample of controls included an equal number of births for each month, allocated across the three institutions according to their size. At that time, the large majority of deliveries (≥70%) were scheduled to occur at home. The client mix at the two midwifery schools consisted of low-risk pregnancies to women of lower socio-economic status whose home environment was unsuitable for delivery. The client mix in Leiden also included higher-risk pregnancies identified during pre-natal care and emergency admissions following complications of labour or delivery.
Figure 1.
Flowchart of participant selection, recruitment, and examination
Recruitment and examination
We provided the names and addresses at birth of all 3307 persons to the population register in the municipality of birth. According to the registry records, 308 (9%) had already died in The Netherlands and 275 (8%) had emigrated. The population registry in Rotterdam refused to trace 130 (4%) persons born out of wedlock and for 294 subjects (9%) a current address could not be located. Final address information was obtained for 2300 offspring (68% of the exposed individuals and 73% of the time controls). All 2300 traced individuals were sent a letter of invitation signed by the current director of the institution in which they were born, a brochure describing the study and a response card. We mailed one reminder letter to non-responders. Initially, our study design called for the recruitment of same-sex sibling pairs; hence the lack of an available sibling was a reason for ineligibility. We received a reply from 1767 people, 347 of whom expressed willingness to participate in a telephone interview and clinical examination together with a same-sex sibling. In the end, 324 of the siblings agreed to participate. Among the 1420 people who declined, 951 reported not having a same-sex sibling available for study. To increase the overall number of participants, we then attempted to enrol these 951 individuals and 381 agreed to participate in full and 23 by responding to a mailed questionnaire. This results in 1075 positive responses: 751 from the 2300 traced individuals and 324 same-sex siblings. We confirmed that the date of birth of all siblings fell outside the famine exposure period, for their use as unexposed controls.21
We conducted a telephone interview and followed this with an examination at the Leiden University Medical Center in 2003–05. We scheduled each member of a sibling pair independently; they were permitted to attend together. Most of the examinations were conducted within 6 weeks of the telephone interview. The study protocol was approved by the medical ethics committees of the participating institutions. Participants provided verbal consent at the start of the telephone interview and written informed consent at the start of the clinical examination.
Cognitive test battery
We administered a well-established battery of tests of cognitive functioning.25–27
Visual verbal word learning task
This test assesses learning capacity as well as recall and retrieval from long-term memory.28 A set of 15 words was presented in a fixed order at 2-s intervals in each of three trials. The words were all monosyllabic meaningful Dutch words that occur frequently, are acquired early in life and easily evoke a mental image. At the end of each trial, respondents were asked to recall as many words as possible, regardless of order. The total recall [word learning task (WLT) (WLTtot)] over the three trials was used as a measure of encoding. Twenty minutes after the last trial (during which time other tasks were performed), the subjects were asked to reproduce the set of words [delayed recall (WLTdr)] to determine memory retrieval. We used two parallel test versions of the WLT in order to prevent exchange between siblings and controlled for WLT test version in the analyses.
The Stroop colour–word interference test
This measures selective attention.29,30 The test consists of three subtests, involving three cards displaying 40 stimuli each: colour names printed in black (subtask I), coloured patches (subtask II) and colour names printed in one of the other colours (subtask III). For subtask I, subjects had to read aloud the printed items, for subtask II they had to name the colour of the patches and for subtask III they had to name the ink colour of the printed words. The outcome of this test was the time (in seconds) needed to complete each subtest. The mean speed for subtasks I and II was used as a measure of general information processing speed (Str12). The speed for subtask III was used as a measure of colour word interference susceptibility (Str3).
The letter-digit-substitution-test
This is related to the Symbol-Digit Modalities Test developed by Smith,31 which originates from the Digit Symbol Test developed by Wechsler.32 The letter-digit-substitution-test (LDST) measures the efficiency of operations in working memory.33 At the top of the test sheet, a box was presented in which nine numbers were linked with nine letters in a random order. Subjects were asked to copy as many corresponding numbers as possible to boxes on the rest of the page which contained only the letters. The total number of correctly copied items in 1 min was used.
Verbal fluency
The participants completed a verbal fluency task34 as a measure of retrieval from long-term semantic memory. Subjects had to name as many animals as possible within 1 min. Verbal fluency is considered a measure of the adequate, strategy-driven retrieval of information from semantic memory. The number of animals named was used.
General cognitive index
We constructed a single compound measure based on the WLTtot, WLTdr, Str12, Str3, LDST and verbal fluency (FLU). The WLTtot, WLTdr LDST and FLU were direct-coded, with higher scores denoting higher cognitive functioning, whereas the Str12 and Str3 are measures of time and hence a higher score reflects poorer functioning. The signs of the scores for the Str12 and Str3 were reversed before computing the general cognitive index (GCI). The GCI was calculated as the sum of the standardized test scores and was then itself standardized to a mean of 100 and a standard deviation (SD) of 15.35
Protocol adherence was scored on a six-point scale, varying from ‘complete and reliable’ to ‘test not administered’. The tests were administered according to procedures developed for the Maastricht Aging Study.36,37
Other measures
Schooling attainment was coded according to an eight-level classification:38 (i) elementary, (ii) lower vocational, (iii) intermediate secondary, (iv) intermediate vocational, (v) higher secondary, (vi) higher vocational, (vii) university and (viii) scientific (PhD level). For the present study, we categorized schooling as low (levels 1–4) or high (levels 5–8).
Smoking status and alcohol consumption were ascertained by questionnaire. We coded smoking as current vs other. We categorized alcohol consumption as 21 or more drinks per week vs fewer than 21 drinks per week.
We used the Mini-Mental State Examination (MMSE)39 to screen for an increased risk for dementia and excluded the seven participants with an MMSE score lower than 24.
Exposure to famine
As previously described,21,40 we defined the start of each gestation by the date of the mother’s last menstrual period (LMP) as listed on the birth record, unless this information was missing or implausible (12%). In these cases, we approximated the date of LMP from the date of birth and estimates of gestational age recorded on the birth record or from a gestational age estimate based on sex- and parity-specific birth weights of singleton live births at the Amsterdam midwives school and the University of Amsterdam Department of Obstetrics between 1931 and 1965 at each gestation between 24 and 46 weeks.41
We characterized exposure to famine during gestation by determining the gestational ages (in weeks after the LMP) during which the mother was exposed to an official ration of <900 kcal/day between November 26, 1944 and May 12, 1945. We considered the mother exposed in gestational weeks 1–10, 11–20, 21–30 or 31 weeks until delivery if these gestational time windows were entirely included in this period. Thus, pregnancies with LMP between November 26, 1944 and March 4, 1945 were exposed in Weeks 1–10: between 19 September 19, 1944 and December 24, 1944, in Weeks 11–20; between July 10, 1944 and October 15, 1944 in weeks 21–30; and between May 2, 1944 and August 24, 1944, in weeks 31 through to delivery.
Because these time windows overlap, the participants can be considered exposed during none, one or (at most) two 10-week periods; those exposed in at least one 10-week period were considered to have some exposure to famine. In addition, we defined a period of exposure immediately prior to conception as exposure to a ration of <900 kcal/day for at least 10 weeks prior to the date of LMP. Conceptions between February 3, 1945 and May 11, 1945 met this definition.
Statistics
All data were entered in the database twice and inconsistencies were checked against the original coding sheets. No data transformations were considered necessary as the unstandardized residuals were normally distributed. Statistical analyses were performed using Stata (StataCorp, College Station, TX, USA) using a significance level of P < 0.05.
We used analysis of variance (ANOVA) and Chi-squared tests to evaluate the differences in continuous and categorical distributions, respectively, among the three groups (exposed, time controls, sibling controls). We used linear regression analyses to estimate the association between famine exposure and cognitive performance at the age of 59 years. We used the combined population of time and sibling controls as the reference category and controlled for family clustering using the cluster () option. We controlled for age (centered), age2 (centered; to account for non-linear age effects), hospital of birth of the hospital-born sibling, sex, alcohol, smoking and test version (for WLTtot and WLTdr). We did not control for attained schooling as this may be intermediate in any pathway.
We used linear regression with dummy variables for the four different exposure groups (exposed in Weeks 1–10, 11–20, 21–30 or 31 weeks until delivery) to investigate whether specific vulnerable or critical periods could be identified. We tested for overall significance of the periods of gestational exposure using a 4-degree of freedom (df) Wald test.
We replicated the above analyses using a within-sibling-pair analysis. We computed the within-pair difference in each measure of cognitive functioning and used this as the dependent variable in regression models together with the hospital of birth of the hospital-born sibling and the within-pair differences of the above covariates.
Results
Birth characteristics by follow-up status
Cumulative mortality was highest among probands born in 1943 (10.4%) and lowest among probands born in 1947 (6.0%). Emigrant status or other reasons why a current address was not found did not differ by year of birth or period of exposure to famine. There were no clinically significant differences in mean birth weight, crown-to-heel length, placental weight, maternal age at delivery or birth order between the birth records of participants traced to a current address and those who had either died, emigrated or had not been located. Similarly, there were no meaningful differences between traced and untraced study subjects or between interviewed and non-interviewed subjects among those who were traced.21
Final sample for analysis
Nine hundred and seventy-one participants attended the clinic for examination. We excluded 25 participants (MMSE <24 as an indication for dementia (2 exposed, 3 time controls, 2 siblings), not motivated (1 exposed, 2 time controls), not having Dutch as native language (1 time control), having physical limitations (1 exposed, 1 time control) or incomplete data on one or more cognitive measures (5 exposed, 3 time controls, 3 siblings). The final sample consisted of 946 individuals (Figure 1).
There were differences across the three groups in age (Table 1). As reported previously, famine-exposed participants were heavier than both the time and the sibling control groups.40,42 Other characteristics were similar across the three groups and were within the ranges observed in other Dutch populations.28,30,33
Table 1.
Selected characteristics of 946 persons who completed cognitive testing in 2002–04, by exposure to the Dutch famine of 1944–45
| Exposed during gestation (n = 349) | Time controls (n = 289) | Sibling controls (n = 308) | P-valuea | |
|---|---|---|---|---|
| Age (years), mean ± SD | 58.9 ± 0.5 | 58.8 ± 1.6 | 57.3 ± 6.3 | <0.001 |
| Male gender (%) | 44.7 | 46.4 | 41.9 | 0.57 |
| Education ≥ category 5b (%) | 26.4 | 31.5 | 30.2 | 0.41 |
| Weight, mean ± SD (kg) | 83.0 ± 15.1 | 80.5 ± 15.3 | 79.7 ± 14.2 | 0.01 |
| Height, mean ± SD (cm) | 170.6 ± 8.9 | 171.5 ± 9.0 | 171.7 ± 8.9 | 0.37 |
| BMI, mean ± SD (kg/m2) | 28.5 ± 5.0 | 27.3 ± 4.3 | 27.0 ± 4.2 | <0.001 |
| Current smoker (%) | 25.5 | 23.2 | 22.4 | 0.71 |
| Alcohol consumption ≥21 drinks/week (%) | 9.5 | 7.3 | 9.7 | 0.43 |
aANOVA (2 df) was used to evaluate the differences in age, weight, length and BMI. Chi-squared tests (2 df) were used for binary variables.
bSchooling attainment was coded according to an eight-level classification and recoded into low (levels 1–4) and high (levels 5–8).38
Exposure to famine and cognitive functioning
Table 2 shows the cognitive test results for each of the three exposure groups. There were no substantive differences among the groups on any test and the GCI for all three groups was within 0.8 points. Table 3 presents the results for the four gestational windows. For each test and for the GCI, individuals exposed in Weeks 1–10 had somewhat poorer scores than did those with exposure in other periods and participants exposed in later gestation had scores somewhat better than those with exposure in other periods, but these differences did not reach statistical significance (Wald score for each test P > 0.05). The difference in GCI between individuals exposed in early vs late gestation was 5.33 points (∼1/3 SD).
Table 2.
Performance on tests of cognitive functioning among 946 Dutch individuals who completed testing in 2002–04, by study status
| Domain | Test | Exposed during gestation (n = 349) | Time controls (n = 289) | Sibling controls (n = 308) |
|---|---|---|---|---|
| mean ± SD | mean ± SD | Mean ± SD | ||
| Memory | ||||
| Encoding | WLTtot (number of words recalled) | 23.9 ± 5.8 | 24.0 ± 5.7 | 24.3 ± 6.4 |
| Retrieval | WLTdr (number of words recalled after delay) | 8.0 ± 2.9 | 7.9 ± 2.7 | 8.3 ± 3.0 |
| Semantic memory/fluency | FLU (number of words in 1 min) | 24.8 ± 5.8 | 25.1 ± 5.8 | 25.0 ± 5.5 |
| Speed | ||||
| General information processing speed | Str12 (s) | 19.0 ± 3.0 | 18.8 ± 3.0 | 19.2 ± 3.1 |
| Colour word interference susceptibility | Str3 (s) | 44.5 ± 11.7 | 43.1 ± 11.1 | 44.0 ± 12.1 |
| Coding | LDST (number filled in 1 min) | 33.1 ± 6.4 | 33.9 ± 6.1 | 33.6 ± 7.0 |
| Overall | ||||
| General cognitive index | 99.3 ± 14.7 | 100.6 ± 14.1 | 100.2 ± 16.1 |
WLTtot, 15-words Learning Task total recall; WLTdr, 15-Words Learning Task delayed recall; Str, Stroop test; FLU, fluency task; LDST, Letter Digit Substitution Test.
For WLTtot, WLTdr, FLU, LDST and general cognitive index, higher scores denote higher cognitive functioning; for Str12 and Str3, higher scores denote poorer cognitive functioning.
Table 3.
Performance on tests of cognitive functioning among 946 Dutch individuals who completed testing in 2002–04, by exposure period
| Domain | Measurea | Week 1–10 (n = 74) | Week 11–20 (n = 122) | Week 21–30 (n = 139) | Week 31 to delivery (n = 129) |
|---|---|---|---|---|---|
| mean ± SD | mean ± SD | mean ± SD | mean ± SD | ||
| Memory | |||||
| Encoding | WLTtot (number of words recalled) | 23.1 ± 6.5 | 23.9 ± 6.1 | 23.9 ± 5.7 | 24.7 ± 5.4 |
| Retrieval | WLTdr (number of words recalled after delay) | 7.5 ± 2.7 | 7.9 ± 2.9 | 8.1 ± 2.9 | 8.4 ± 2.9 |
| Semantic memory | FLU (number of words in 1 min) | 23.9 ± 6.7 | 24.7 ± 6.2 | 24.8 ± 5.4 | 25.3 ± 5.2 |
| Speed | |||||
| General information processing speed | Str12 (s) | 19.6 ± 3.5 | 18.9 ± 2.9 | 19.1 ± 2.9 | 18.7 ± 2.8 |
| Colour word interference susceptibility | Str3 (s) | 44.9 ± 10.5 | 44.7 ± 11.3 | 45.0 ± 11.2 | 44.1 ± 13.3 |
| Coding | LDST (number filled in 1 min) | 32.1 ± 6.4 | 33.2 ± 6.7 | 33.4 ± 6.5 | 33.8 ± 6.5 |
| Overall | |||||
| General cognitive index | 96.2 ± 15.4 | 99.2 ± 15.2 | 99.3 ± 13.7 | 101.5 ± 14.4 |
WLTtot, 15-Words Learning Task total recall; WLTdr, 15-Words Learning Task delayed recall; Str, Stroop test; FLU, fluency task; LDST, Letter Digit Substitution Test.
For WLTtot, WLTdr, FLU, LDST and general cognitive index, higher scores denote higher cognitive functioning; for Str12 and Str3, higher scores denote poorer cognitive functioning.
Exposure to famine at any point during gestation was associated with a 0.57 (95% CI −2.41 to 1.28) point decrease in the GCI (Table 4). In the sibling-pair analysis, any exposure to famine was associated with a 1.03 (95% CI −4.97 to 2.91) point decrease in the CGI.
Table 4.
Association of maternal exposure to the Dutch famine during gestation with performance on measures of cognitive functioning at mean age 59 years among 946 persons examined between 2003 and 2005
| Domain | Measure | Whole sample (n = 946) | Within-sibling pairs (n = 300 pairs) |
|---|---|---|---|
| Estimatea (95% CI) | Estimateb (95% CI) | ||
| Memory | |||
| Encoding | WLTtot (number of words recalled) | 0.44 (−0.34 to 1.22) | −0.22 (−2.02 to 1.57) |
| Retrieval | WLTdr (number of words recalled after delay) | 0.07 (−0.31 to 0.46) | −0.02 (−0.86 to 0.82) |
| Semantic memory | FLU (number of words in 1 min) | −0.13 (−0.90 to 0.64) | −0.28 (−1.84 to 1.29) |
| Speed | |||
| General information processing speed | Str12 (s) | −0.03 (−0.41 to 0.36) | −0.06 (−0.96 to 0.85) |
| Colour word interference susceptibility | Str3 (s) | 0.82 (−0.71 to 2.35) | 1.49 (−1.59 to 4.56) |
| Coding | LDST (number filled in 1 min) | −0.42 (−1.25 to 0.41) | −0.45 (−2.21 to 1.32) |
| Overall | |||
| General cognitive index | −0.57 (−2.41 to 1.28) | −1.03 (−4.97 to 2.91) |
WLTtot, 15-Words Learning Task total recall; WLTdr, 15-Words Learning Task delayed recall; Str, Stroop test; FLU, fluency task; LDST, Letter Digit Substitution Test.
For WLTtot, WLTdr, FLU, LDST and general cognitive index, higher scores denote higher cognitive functioning; for Str12 and Str3, higher scores denote poorer cognitive functioning.
aValues represent differences from unexposed. Estimates were obtained by linear regression and were adjusted for age, age2, educational level, sex, alcohol intake, smoking, hospital of birth (Amsterdam, Leiden, Rotterdam, sibling) and where necessary test version and clustering of siblings.
bValues represent within-pair difference (exposed–unexposed) in respective measure. Estimates were obtained by linear regression and were adjusted for hospital of birth of the hospital-born sibling and within-pair differences in the above covariates.
When the whole sample was considered in a single model, maternal exposure in early gestation appeared to be associated with somewhat poorer cognitive functioning across all measures, although only some estimates reached statistical significance (Table 5). For the CGI, the estimate was a reduction of 4.37 (95% CI 0.67–8.04) points. We observed increases in measures of cognitive functioning with exposure in later gestation, which was for WLTtot statistically significant [an increase of 1.44 (95% CI 0.37–2.51) points]. The within-sibling-pair analysis was consistent with these results (Table 6).
Table 5.
Association of exposure to the Dutch famine (overall or in specific periods of gestation) with performance on measures of cognitive functioning among 946 persons examined between 2003 and 2005
| Domain | Measure | Period of gestational exposure |
|||||
|---|---|---|---|---|---|---|---|
| Weeks 1–10 (n = 74) | Weeks 11–20 (n = 122) | Weeks 21–30 (n = 139) | Week 31 to delivery (n = 129) | ||||
| Estimatea (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | P-valueb | |||
| Memory | |||||||
| Encoding | WLTtot (number of words recalled) | −0.48 (−2.02 to 1.06) | 0.42 (−0.76 to 1.60) | −0.02 (−1.12 to 1.07) | 1.44 (0.37 to 2.51) | 0.09 | |
| Retrieval | WLTdr (number of words recalled after delay) | −0.29 (−0.97 to 0.39) | −0.00 (−0.59 to 0.58) | −0.01 (−0.57 to 0.56) | 0.55 (−0.02 to 1.11) | 0.26 | |
| Semantic memory | FLU (number of words in 1 min) | −1.04 (−2.62 to 0.54) | 0.18 (−1.11 to 1.46) | −0.44 (−1.54 to 0.66) | 0.42 (−0.66 to 1.50) | 0.62 | |
| Speed | |||||||
| General information processing speed | Str12 (s) | 0.95 (−0.11 to 1.79) | −0.56 (−1.16 to 0.04) | 0.37 (−0.19 to 0.94) | −0.52 (−1.06 to 0.03) | 0.04 | |
| Colour word interference susceptibility | Str3 (s) | 1.73 (−0.83 to 4.29) | −0.15 (−2.39 to 2.08) | 1.36 (−0.80 to 3.53) | −0.16 (−2.74 to 2.42) | 0.55 | |
| Coding | LDST (number filled in 1 min) | −1.64 (−3.24 to −0.05) | 0.50 (−0.81 to 1.80) | −0.37 (−1.56 to 0.82) | 0.59 (−0.65 to 1.83) | 0.29 | |
| Overall | |||||||
| General cognitive index | −4.37* (−9.04 to −0.67) | 1.32 (−1.70 to 4.34) | −1.62 (−4.31 to 1.08) | 2.39 (−0.41 to 5.19) | 0.08 | ||
WLTtot, 15-Words Learning Task total recall; WLTdr, 15-Words Learning Task delayed recall; Str, Stroop test.
For WLTtot, WLTdr, FLU, LDST and general cognitive index, higher scores denote higher cognitive functioning; for Str12 and Str3, higher scores denote poorer cognitive functioning.
aValues represent differences from unexposed (n = 603). Estimates were obtained by linear regression and were adjusted for age, age2, educational level, sex, alcohol intake, smoking, hospital of birth (Amsterdam, Leiden, Rotterdam, sibling) and where necessary test version and clustering of siblings.
bTest of association of all four periods of exposure considered as a group (Wald test, 4 df).
*P < 0.05.
Table 6.
Association of exposure to the Dutch famine (overall or in specific periods of gestation) with performance on tests of cognitive functioning within 300 sibling pairs examined between 2003 and 2005
| Domain | Measurea | Period of gestational exposure of hospital-born sibling |
|||||
|---|---|---|---|---|---|---|---|
| Weeks 1–10 (n = 74) | Weeks 11–20 (n = 122) | Weeks 21–30 (n = 139) | Week 31 to delivery (n = 129) | ||||
| Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | P-valueb | |||
| Memory | |||||||
| Encoding | WLTtot (number of words recalled) | −0.87 (−4.02 to 2.28) | −0.13 (−2.44 to 2.20) | −0.19 (−2.37 to 1.99) | 1.36 (−1.10 to 3.81) | 0.76 | |
| Retrieval | WLTdr (number of words recalled after delay) | 0.57 (−0.91 to 2.04) | −0.46 (−1.54 to 0.63) | 0.27 (−0.75 to 1.29) | 0.12 (−1.02 to 1.27) | 0.84 | |
| Semantic memory | FLU (number of words in 1 min) | 0.32 (−2.41 to 3.07) | 0.13 (−1.89 to 2.15) | −1.41 (−3.31 to 0.49) | 0.83 (−1.31 to 2.97) | 0.61 | |
| Speed | |||||||
| General information processing speed | Str12 (s) | 0.37 (−1.19 to 1.93) | −0.97 (−2.13 to 0.19) | 0.92 (−0.17 to 2.01) | −0.70 (−1.92 to 0.53) | 0.32 | |
| Colour word interference susceptibility | Str3 (s) | 0.18 (−5.17 to 5.53) | −0.72 (−4.69 to 3.25) | 2.91 (−0.82 to 6.64) | 0.46 (−3.74 to 4.66) | 0.62 | |
| Coding | LDST (number filled in 1 min) | 0.11 (−2.96 to 3.18) | 0.41 (−1.87 to 2.69) | −1.25 (−3.39 to 0.89) | 1.18 (−1.23 to 3.59) | 0.74 | |
| Overall | |||||||
| General cognitive index | −1.05 (−7.91 to 5.81) | 1.41 (−3.67 to 6.50) | −3.49 (−8.28 to 1.29) | 2.82 (−2.56 to 8.21) | 0.60 | ||
FLU, fluency task; LDST, Letter Digit Substitution Test.
aValues represent within-pair difference in respective measure. Estimates were obtained by linear regression and were adjusted for the sex of the sibling pair, within-pair difference in age and alcohol intake, exposure to famine of the hospital-born sibling in any other period of gestation and hospital of birth of the hospital-born sibling.
bTest of association of all four periods of exposure considered as a group (Wald test, 4 df).
All the previous results were consistent with simpler models that controlled only for age and sex. Finally, there was no evidence of a statistical interaction between sex and maternal exposure to famine on cognitive performance of the offspring (P for heterogeneity all > 0.2; data not shown).
Discussion
We investigated whether severe maternal undernutrition during pregnancy would predict cognitive function of their offspring in late middle age. We observed no association with exposure in pregnancy in general, but cannot exclude that exposure to famine early in gestation is associated with a small change in cognitive performance at the age of 59 years.
Our study may be best understood within the framework of the ‘cognitive reserve’ hypothesis, which states that the cognitive performance of people with reduced reserve may be more vulnerable to age-related changes of the brain as compared with people with normal reserve. Both biological and psychological factors are important for development of greater cognitive reserve.43 For optimal development, the brain requires an adequate nutritional status in order to provide suitable circumstances for dendritic outgrowth, the formation of synaptic spines and development of more efficient neuronal networks. A brain that has developed optimally is better suited to help the organism to adapt to a changing environment, to process information as efficiently as possible and also to learn from experience. We hypothesized that gestational undernutrition in crucial phases of fetal development would have a major impact on the development of essential structural elements of the brain and thus constrain the biological boundaries within which the brain develops. The resulting lower capacity would lead to a lower brain reserve capacity and hence to lower cognitive performance especially in conditions in which the physiological processing of the brain is compromised such as at older ages. Our population was tested at average age of 59 years, an age at which cognitive variability between individuals has become substantial.44,45
The current study was well controlled and involved a large sample (n = 946), providing adequate power to observe moderate effect sizes. The quasi-experimental exposure is a particular strength, as is the use of sibling controls, who share childhood home and other environments that influence cognitive development. For within-pair analyses, the available sample is more limited.
Some potential threats to validity must nevertheless be considered. Persons who were already suffering from more cognitive dysfunction might have been less willing to participate in the current study compared with persons who were not. This potential bias is unlikely, however, as our data show no difference in important baseline characteristics comparing traced and untraced persons or interviewed and non-interviewed persons and do not show any difference in participation rates by time-period of pre-natal exposure. Still we cannot exclude a possible underreporting effect of this factor. In the current study, we focus on maternal famine exposure during pregnancy as the primary risk factor for offspring cognitive performance in late adulthood. Famine exposure is not just a period of undernutrition and relevant exposures might also include mental stress and exposure to cold. Study participants will have been exposed, furthermore, to additional risks and protective factors during life. In the current study, these aspects were controlled by including both time controls and sibling controls, achieving considerable control for genetic as well as social and economic factors. Finally, the pregnant women in our study were exposed to an acute famine of limited duration brought about by conditions of war.
Although we have no direct birth or childhood measures of brain functioning, our results seem to reaffirm the understanding that the developing brain is somewhat protected against maternal nutritional deprivation in later gestation, provided that the psychomotor stimulation of the baby is adequate.46 Our results speak only to famine exposure during gestation and hence should not be generalized to the effects of chronic undernutrition as occurs in developing countries, nor with chronic malnutrition in Western populations in special categories such as in anorexia patients.
Our results extend the findings of Stein and colleagues who investigated 125 000 male recruits at age 19 years18 and did not find an effect of exposure to the Dutch famine on adult mental performance. The absence of significant associations in the study of Stein could have been due to the age of the participants as, at the age 19 years, cognitive development is still a dynamic ongoing process, as brain development is now known to proceed until the third decade of life;47 or it could have been due to the limited sensitivity of the assessment tool, the Raven Progressive Matrices test, to assess executive functioning. The rationale behind the present study was that a subtle effect of severe maternal undernutrition on brain development during pregnancy might eventually become evident in a phase where age-related deterioration in the physiological functioning of many organs is occurring.
In a recent report of individuals born during the Dutch famine, 737 men and women from Amsterdam were examined at the age of ∼59 years on four measures of cognition.19 No association was seen with a general intelligence test, a memory task or a perceptual motor learning task. A Stroop-like selective attention task was associated with pre-natal famine exposure. This was interpreted as an early manifestation of accelerated cognitive aging after pre-natal famine. This test was differently operationalized in comparison with the original Stroop test as used in our study which might explain the inconsistent results.
It remains uncertain whether these results would differ if the study were to be repeated at a later age, when individual differences are likely to be larger. Therefore, there are good reasons to repeat this study again in the future and to then also evaluate other, more sensitive, indicators of limited brain reserve capacity such as neuroenergetic problems, depressed mood states or mild cognitive impairments and complaints.
In conclusion, we found no overall association between maternal exposure to acute famine in pregnancy and cognitive performance of the offspring at the age of 59 years, but cannot rule out an association specific to early pregnancy exposure.
Funding
National Institutes of Health (grant HL067914 to L.H.L.), USA.
Acknowledgements
The authors thank the midwifery schools of Amsterdam and Rotterdam and the Obstetrics Department of the Leiden University Medical Center in Leiden for their help in accessing the archived delivery and birth records, the population registers for assistance in tracing and all study participants.
Conflict of interest: None declared.
KEY MESSAGES.
Individuals exposed to famine during gestation do not differ in cognitive functioning at mean age of 58 years compared with individuals born before the famine or conceived after it, or compared with unexposed same-sex siblings.
This analysis confirms earlier observations of no changes in measures of intelligence among military recruits examined at the age of 18 years, extending the period of observation through middle age.
We can not rule out that exposure to famine very early in gestation may be associated with a small deficit in cognitive functioning.
References
- 1.Uylings HBM. Development of the human cortex and the concept of “critical” or “sensitive” periods. Human Devel Lang Learning. 2006;56:59–90. [Google Scholar]
- 2.Shigematsu I, Ito C, Kamada N, Akiyama M, Sasaki H. Effects of A-Bomb Radiation on the Human Body. Langhorne, PA: Harwood Academic; 1995. [Google Scholar]
- 3.Malanga CJ, Kosofsky BE. Effect of drugs of abuse on brain development. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 2004. pp. 720–39. [Google Scholar]
- 4.Winzer-Serhan UH. Long-term consequences of maternal smoking and developmental chronic nicotine exposure. Front Biosci. 2008;13:636–49. doi: 10.2741/2708. [DOI] [PubMed] [Google Scholar]
- 5.Williams JH, Ross L. Consequences of prenatal toxin exposure for mental health in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry. 2007;16:243–53. doi: 10.1007/s00787-006-0596-6. [DOI] [PubMed] [Google Scholar]
- 6.Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13:241–56. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- 7.Laplante DP, Barr RG, Brunet A, et al. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr Res. 2004;56:400–10. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- 8.Reisbick S. Neural development. In: Carlson SE, Neuringer M, editors. Assessment of Infant Visual and Cognitive Function in relation to Long Chain Polyunsaturated Fatty Acids. Basel, Switserland: F. Hoffmann-La Roche AG; 1996. [Google Scholar]
- 9.Berry RJ, Li Z, Erickson JD, Li S, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341:1485–90. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- 10.Susser E, Neugebauer R, Hoek HW, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 11.St Clair D, Xu M, Wang P, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA. 2005;294:557–62. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- 12.Stein AD, Wang M, DiGirolamo A, et al. Nutritional supplementation in early childhood, schooling, and intellectual functioning in adulthood: a prospective study in Guatemala. Arch Pediatr Adolesc Med. 2008;162:612–18. doi: 10.1001/archpedi.162.7.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–53. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 14.Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. J Alzheimers Dis. 2007;12:11–22. doi: 10.3233/jad-2007-12103. [DOI] [PubMed] [Google Scholar]
- 15.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–71. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- 16.Stoch MB, Smythe PM, Moodie AD, Bradshaw D. Psychosocial outcome and CT findings after gross undernourishment during infancy: a 20-year developmental study. Dev Med Child Neurol. 1982;24:419–36. doi: 10.1111/j.1469-8749.1982.tb13647.x. [DOI] [PubMed] [Google Scholar]
- 17.Lumey LH, Susser ES. Long-term effects of prenatal and early postnatal nutrition on adult psychosocial outcomes. In: Tremblay RE, Barr RG, Peters RDV, editors. Encyclopedia on Early Childhood Development [online] Montreal, Quebec: Centre of Excellence for Early Childhood Development; 2003. pp. 1–7. [Google Scholar]
- 18.Stein Z, Susser M, Saenger G, Marolla F. Nutrition and mental performance. Science. 1972;178:708–13. doi: 10.1126/science.178.4062.708. [DOI] [PubMed] [Google Scholar]
- 19.de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ. Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci USA. 2010;107:16881–86. doi: 10.1073/pnas.1009459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobbing J. Maternal nutrition in pregnancy and later achievement of offspring: a personal interpretation. Early Hum Dev. 1985;12:1–8. doi: 10.1016/0378-3782(85)90130-6. [DOI] [PubMed] [Google Scholar]
- 21.Lumey LH, Stein AD, Kahn HS, et al. Cohort profile: the Dutch Hunger Winter families study. Int J Epidemiol. 2007;36:1196–204. doi: 10.1093/ije/dym126. [DOI] [PubMed] [Google Scholar]
- 22.Burger GCE, Drummond JC, Sandstead HR. Malnutrition and Starvation in Western Netherlands, September 1944 to July 1945. Pt 1 & 2. 's-Gravenhage: Staatsuitgeverij; 1948. [Google Scholar]
- 23.Stein ZA, Susser M, Saenger G, Marolla F. Famine and Human Development: The Dutch Hunger Winter of 1944-1945. New York: Oxford University Press; 1975. [Google Scholar]
- 24.Trienekens G. The food supply in the Netherlands during the Second World War. In: Smith DF, Phillips J, editors. Food, Science, Policy and Regulation in the Twentieth Century. International and Comparative Perspectives. Routledge: London; 2000. [Google Scholar]
- 25.Bohnen N, Twijnstra A, Jolles J. Performance in the Stroop color word test in relationship to the persistence of symptoms following mild head injury. Acta Neurol Scand. 1992;85:116–21. doi: 10.1111/j.1600-0404.1992.tb04009.x. [DOI] [PubMed] [Google Scholar]
- 26.de Groot RHM, Vuurman EF, Hornstra G, Jolles J. Differences in cognitive performance during pregnancy and early motherhood. Psychol Med. 2006;36:1023–32. doi: 10.1017/S0033291706007380. [DOI] [PubMed] [Google Scholar]
- 27.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 28.van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J. Rey's verbal learning test: normative data for 1855 healthy participants aged 24-81 years and the influence of age, sex, education, and mode of presentation. J Int Neuropsychol Soc. 2005;11:290–302. doi: 10.1017/S1355617705050344. [DOI] [PubMed] [Google Scholar]
- 29.Stroop J. Studies of interference in serial verbal reaction. J Exp Psychol. 1935;18:643–62. [Google Scholar]
- 30.van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J. The Stroop color-word test: influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment. 2006;13:62–79. doi: 10.1177/1073191105283427. [DOI] [PubMed] [Google Scholar]
- 31.Smith A. The symbol Digit Modalities Test: A neuropsychologic test for economic screening of learning and other cerebral disorders. Learning Disorders. 1968;3:83–91. [Google Scholar]
- 32.Wechsler D. The Measurement and Appraisal of Adult Intelligence. Baltimore: Williams & Wilkins; 1958. [Google Scholar]
- 33.van der Elst W, van Boxtel MP, van Breukelen GJ, Jolles J. The Letter Digit Substitution Test: normative data for 1,858 healthy participants aged 24-81 from the Maastricht Aging Study (MAAS): influence of age, education, and sex. J Clin Exp Neuropsychol. 2006;28:998–1009. doi: 10.1080/13803390591004428. [DOI] [PubMed] [Google Scholar]
- 34.Lezak MD. Neuropsychological Assessment. 3rd. New York: Oxford University Press; 1995. [Google Scholar]
- 35.Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J. A large-scale cross-sectional and longitudinal study into the ecological validity of neuropsychological test measures in neurologically intact people. Arch Clin Neuropsychol. 2008;23:787–800. doi: 10.1016/j.acn.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Jolles J, Houx P, van Boxtel MP, Ponds R. The Maastricht Aging Study; Determinants of Cognitive Aging. Maastricht: Neuropsych Publishers; 1995. [Google Scholar]
- 37.Adam JJ, Paas FG, Teeken JC, et al. Effects of age on performance in a finger-precuing task. J Exp Psychol Hum Percept Perform. 1998;24:870–83. doi: 10.1037//0096-1523.24.3.870. [DOI] [PubMed] [Google Scholar]
- 38.de Bie SE. Standaardvragen 1987: Voorstellen Voor Uniformering Van Vraagstellingen Naar Achtergrondkenmerken En Interviews [Standard Questions 1987: Proposal for Uniformisation of Questions Regarding Background Variables and Interviews] 2nd. Leiden, The Netherlands: Leiden University Press; 1987. [Google Scholar]
- 39.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 40.Stein AD, Kahn HS, Rundle A, Zybert PA, van der Pal-de Bruin K, Lumey LH. Anthropometric measures in middle age after exposure to famine during gestation: evidence from the Dutch famine. Am J Clin Nutr. 2007;85:869–76. doi: 10.1093/ajcn/85.3.869. [DOI] [PubMed] [Google Scholar]
- 41.Kloosterman GJ. On intrauterine growth. Int J Gynaecol Obstet. 1970;8:895–912. [Google Scholar]
- 42.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–16. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 43.Stern Y. Cognitive Reserve: Theory and Application. New York & London: Taylor & Francis; 2007. [Google Scholar]
- 44.Salthouse TA. What and when of cognitve aging. Current Direction Psychol Sci. 2004;13:140–44. [Google Scholar]
- 45.Schaie KW. The course of adult intellectual development. Am Psychol. 1994;49:304–13. doi: 10.1037//0003-066x.49.4.304. [DOI] [PubMed] [Google Scholar]
- 46.Guesry P. The role of nutrition in brain development. Prev Med. 1998;27:189–94. doi: 10.1006/pmed.1998.0292. [DOI] [PubMed] [Google Scholar]
- 47.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–79. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]